Abstract
To evaluate postextubation swallowing dysfunction (PSD) 21 days after endotracheal extubation and to examine whether PSD is time-limited and whether age matters.
For this prospective cohort study, we evaluated 151 adult critical care patients (≥20 years) who were intubated for at least 48 hours and had no pre-existing neuromuscular disease or swallowing dysfunction. Participants were assessed for time (days) to pass bedside swallow evaluations (swallow 50 mL of water without difficulty) and to resume total oral intake. Outcomes were compared between younger (20–64 years) and older participants (≥65 years).
PSD, defined as inability to swallow 50 mL of water within 48 hours after extubation, affected 92 participants (61.7% of our sample). At 21 days postextubation, 17 participants (15.5%) still failed to resume total oral intake and were feeding-tube dependent. We found that older participants had higher PSD rates at 7, 14, and 21 days postextubation, and took significantly longer to pass the bedside swallow evaluations (5.0 vs 3.0 days; P = 0.006) and to resume total oral intake (5.0 vs 3.0 days; P = 0.003) than their younger counterparts. Older participants also had significantly higher rates of subsequent feeding-tube dependence than younger patients (24.1 vs 5.8%; P = 0.008).
Excluding patients with pre-existing neuromuscular dysfunction, PSD is common and prolonged. Age matters in the time needed to recover. Swallowing and oral intake should be monitored and interventions made, if needed, in the first 7 to 14 days postextubation, particularly for older patients.
Keywords: aging, dysphagia, intensive care unit, oral intake level, oral intubation, swallowing functions
1. Introduction
Endotracheal intubation is life-sustaining, but it may contribute to postextubation swallowing dysfunction (PSD), delaying oral intake.[1,2] In particular, patients with prolonged intubation, often defined as ≥48 hours of intubation,[3] were at greater risk of developing PSD.[4] Moreover, as acute and intensive care have advanced, critically ill patients are increasingly intubated, regardless of their age.[1] It is reasonable to assume that aging-related changes, coupled with multiple comorbidities, are likely to change the incidence of PSD, its recovery trajectories, and subsequent oral intake status between older and younger patients.
Age, however, has been both supported and refuted as a potential risk factor for incidence and severity of PSD.[3,5] Briefly, older age has been associated with a higher incidence of PSD in several studies,[1,5–7] but other work has shown age to be unrelated.[2,8–15] In a retrospective study, the odds of 70 critically ill patients having severe PSD increased by 7.5% for each additional year of age,[15] but age was unrelated in another study.[10] Comorbidity data are sparse for extubated patients. Congestive heart failure (CHF), diabetes mellitus (DM), and chronic kidney disease (CKD) were examined in few studies.[4,12,13] Higher rates of CHF were found in 2 retrospective studies involving cardiac surgery patients with PSD,[4,12] but another retrospective study on patients in intensive care units (ICUs) found that CHF was unrelated to PSD.[13] Furthermore, DM did not discriminate ICU and cardiac surgery patients with and without PSD in 2 retrospective studies,[4,13] but CKD was higher among patients with PSD who survived cardiac surgery.[4] For the recovery trajectory, fewer studies followed up these postextubation patients. Older patients in 1 study were less likely to recover and 14% had persistent PSD at 2 weeks postextubation,[11] whereas 40% of patients in another study had persistent PSD by hospital discharge, but age did not matter.[14]
Thus, little is known about whether PSD is prolonged, when recovery can be expected, and whether age matters in terms of incidence rates and the time needed to recover. Furthermore, many studies did not exclude patients with pre-existing neuromuscular pathologies (i.e., stroke, head and neck structural deformity, or swallowing dysfunction before intubation) that compromise swallowing functions. As such, the incidence of PSD may be inflated. In addition, PSD that is independent of pre-existing neuromuscular pathologies is more likely to be modifiable with tailored swallowing and intake therapeutics. Thus, the objectives of this study were to evaluate PSD (defined as inability to swallow 50 mL of water within 48 hours after extubation) and to follow PSD and oral intake levels for 21 days after endotracheal extubation in critical care patients with prolonged intubation, but without pre-existing neuromuscular disease or swallowing dysfunction. We also compared younger (20–64 years) and older (≥65 years) participants’ time (days) to pass the bedside swallow evaluation (BSE; swallow 50 mL of water without difficulty) and to resume total oral intake.
2. Methods
2.1. Participants
Upon approval from the Research Ethics Committee of National Taiwan University Hospital, a prospective cohort study was conducted at a 2000-bed, tertiary medical center in Taipei, Taiwan. Study participants were recruited from consecutive adult patients (aged 20 years and older) who had been admitted to the medical center's 6 ICUs from October 2012 to December 2014 and received emergency oral endotracheal intubation for at least 48 hours. Patients were excluded if they met the following criteria: (1) had pre-existing neuromuscular diseases (e.g., stroke, parkinsonism, or head and neck deformities; (2) had pre-existing swallowing dysfunction; (3) were delirious or unable to respond to questions; (4) received a tracheostomy,; or (5) were isolated for infectious disease. All participants and/or their family members signed a written informed consent to participate in the study.
2.2. Data collection
Data on PSD and oral intake 21 days after extubation were collected from participants in face-to-face assessments by 2 trained research nurses using validated instruments, as described below. Participants’ demographics (age, sex, education [years], marital status, tobacco use) and medical characteristics (admission diagnosis, comorbidities, disease severity, admission body mass index [BMI], endotracheal tube size, and lengths of intubation, ICU stay, and hospital stay) were collected from the medical record. Comorbidities were based on the Charlson comorbidity index, in which weighted comorbidities are summed to obtain a score, with higher scores indicating higher comorbid burden.[16] Disease severity was measured by the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, with higher scores corresponding to more severe disease and a higher risk of mortality.[17]
2.3. PSD and oral intake measures
Postextubation swallowing dysfunction was assessed daily by an established 3-step BSE protocol[18,19] and documented as time (days) to pass the BSE. Participants were first assessed for prior history of dysphagia/feeding-tube dependence and signs of consciousness change, poor oxygen saturation (i.e., SaO2<90%; oxygen-mask dependence; reintubation), obvious drooling, or frequent choking on saliva. If negative, participants were then asked to swallow 3 mL of water. If laryngeal elevation was identified with no signs of choking or wet voice, participants were asked to swallow 50 mL of water; those without any signs of choking, wet voice, or slow swallowing were considered to pass the BSE. The sensitivity, specificity, positive predictive value, and negative predictive values of this BSE protocol were 70%, 88%, 83%, and 77%, respectively, for predicting aspiration.[19]
Participants’ oral intake levels were recorded daily using an ordinal scale of the Functional Oral Intake Scale (FOIS).[20] The FOIS is a validated tool with established validity (81%–98%) and interexaminer reliabilities of 0.86 to 0.91.[20] Scores range from 1 to 7, with lower scores indicating greater intake limitation. Specifically, FOIS scores 1 through 3 indicate varying degrees of nonoral feeding; FOIS scores 4 through 7 indicate varying degrees of oral feeding related to patient compensations and dietary modifications. In this study, an FOIS score of 4 (total oral intake of a single consistency) was considered as the patient's readiness to “resume total oral intake.” Nonetheless, the time (days) taken to reach each FOIS level (from 1 to 7) was assessed and recorded daily by trained nurses.
2.4. Statistical analysis
Statistical tests were carried out using SPSS software (SPSS Statistics 20.0; SPSS Inc., Chicago, IL). Statistical significance was set at P < 0.05. Data are described as percentages, median, or mean ± interquartile range (IQR) or standard deviation (SD). Demographic and medical characteristics, and also swallowing dysfunction of older and younger participants were compared and tabulated. The Student t test or Mann–Whitney statistical test was used to compare continuous variables, and the chi-square test or Fisher exact test was used for categorical variables. Correlation matrices were also produced to examine correlations between variables. Between-group differences in postextubation oral intake levels (1–7) were compared using a repeated-measures method of the generalized estimating equations (GEE). Using a public-access Optitxs program, an a priori power analysis indicated that a sample size of at least 116 participants was necessary to have 80% power for detecting a small-sized effect when alpha was set at 0.05. Considering a 25% of attrition rate for critical care patients, at least 145 participants were needed to ensure the power of the study.[21]
3. Results
Of the 151 participants enrolled in this study, 110 completed 21-day follow-ups (72.8%). The remaining 41 participants did not complete all follow-ups because of death (n = 10), reintubation (n = 15), change in consciousness (n = 2), hospital transfer (n = 2), refusal to continue (n = 1), or discharge and no longer followed at the study hospital (n = 11). These 41 participants did not differ significantly from those who completed the study (n = 110) in comorbidities (Charlson index; P = 0.14), disease severity (APACHE II; P = 0.11), length of intubation (P = 0.20), BMI (P = 0.40), length of hospital stay (P = 0.89), or length of ICU stay (P = 0.48). Details of participant flow are presented in Fig. 1.
Figure 1.
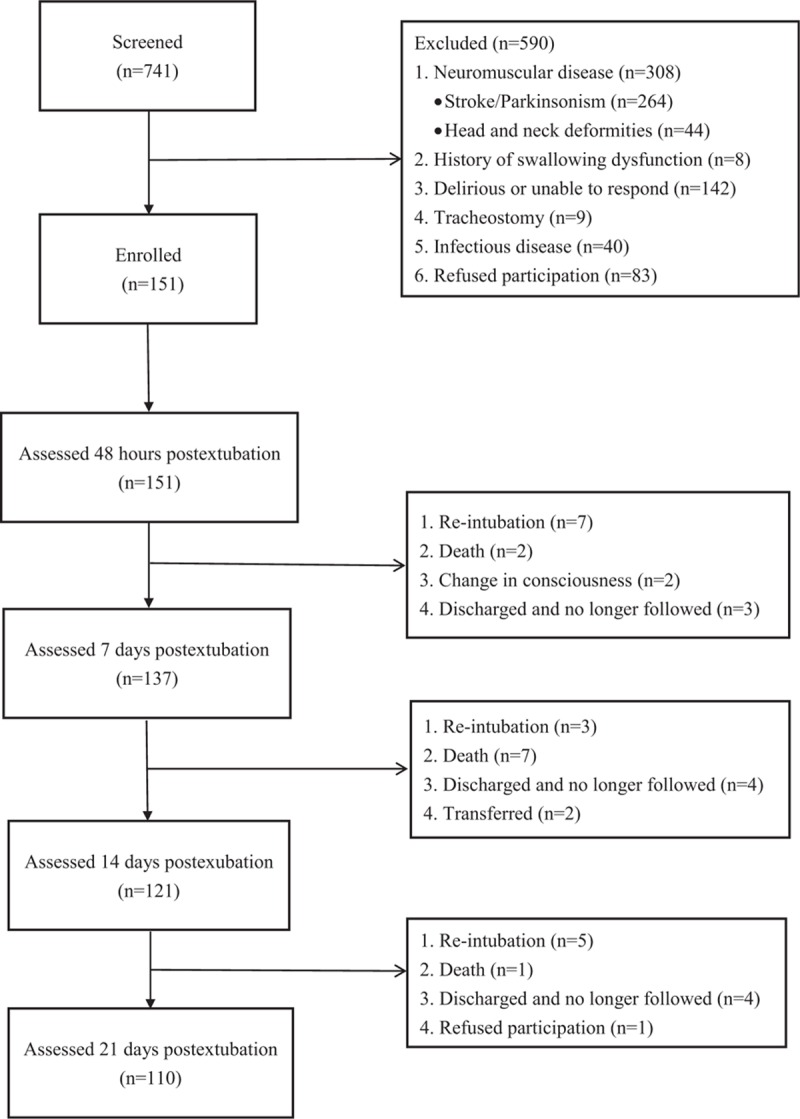
Participants’ flowchart.
3.1. Baseline characteristics
Participants’ demographic and clinical characteristics are shown in Table 1. Baseline characteristics are presented for all participants (N = 151) and for the subsets of participants who were older (n = 79; mean age = 75.0 ± 6.5 years) and younger (n = 72; 50.5 ± 9.9 years old).
Table 1.
Participants’ demographic and clinical characteristics.

Overall, the majority of participants was male and had been intubated for 8.4 ± 6.8 days with a 7.5 Fr. endotracheal tube. Except for age and years of education, older and younger participants did not differ significantly in sex, marital status, tobacco use, admission diagnosis, Charlson index, APACHE II score, admission BMI, endotracheal tube size, and length of intubation, ICU, and hospital stays. Notably, this critically ill sample presented with high comorbidities; 12.6% had CHF, 42.4% had DM, 17.9% had CKD, and 17.9% had solid tumors. Specifically, the younger and older groups had comparable prevalence rates for most comorbidities, except for peripheral vascular disease (38.9% vs 68.4%, respectively), chronic obstructive pulmonary disease (2.8% vs 22.8%, respectively), and liver disease (19.4% vs 7.6%, respectively).
3.2. PSD and oral intake levels
Postextubation swallowing dysfunction affected 61.7% of our sample. Despite recovery being noted in the first week after extubation, 30.4%, 12.0%, and 10.9% of participants still failed to pass the BSE at 7, 14, and 21 days postextubation, respectively (Table 2). Longitudinally, participants took a median of 4.0 days (IQR 7.5) to pass the BSE. For oral intake levels, participants took a median of 2.0 days (IQR 3.0) to try water. Total oral intake (of a single consistency) was resumed a median of 4.0 days (IQR 7.0) after extubation. Notably, time to total oral intake was highly correlated with time to pass the BSE (Pearson r = 0.89, P < 0.001). Furthermore, specific comorbid conditions were not associated with higher rates of PSD at 2, 7, 14, and 21 days postextubation, except for participants with solid tumors (results not shown). Compared with overall PSD incidences, consistently higher rates of PSD over time were experienced only by participants with solid tumors. However, our results cannot be compared with those of other studies because the association of comorbidities with pathology is understudied.
Table 2.
Swallowing dysfunction and resuming total oral intake postextubation.

For the age effect, 68.4% of older participants (n = 54) versus 54.3% of younger participants (n = 38) failed to drink 50 mL water without choking, wet voice, or slow in swallow (Table 2). The difference, at 2 days postextubation, was not significant (P = 0.11). The gap, however, became greater as time passed. BSE failure rates were significantly higher for older participants at subsequent time points (40.0% vs 20.0%; 18.0% vs 5.4%; and 17.2% vs 3.9% by 7, 14, and 21 days postextubation, respectively). Longitudinally, the median time to pass the BSE was significantly longer for older participants than for their younger counterparts (5.0 vs 3.0 days; P = 0.006).
For oral intake, older participants took a median of 5.0 days (IQR 9.0) to resume total oral intake, whereas the younger group took a median of 3.0 days (IQR 5.0; P = 0.003). At 21 days after extubation, 24.1% of older participants (n = 14) had prolonged PSD, committing them to feeding-tube dependence, whereas only 5.8% of younger participants (n = 3) did so.
Moreover, the Charlson index and length of intubation were significantly correlated with time to resume total oral intake (Pearson r = 0.31 and 0.25, respectively), suggesting the need to adjust for these 2 confounding factors. Indeed, with adjustment for participants’ Charlson index and length of intubation, older participants’ trajectory for recovery of oral intake levels was significantly longer (GEE, P < 0.001) than that for younger participants (Fig. 2).
Figure 2.

Trajectories of oral intake level per postextubation day between age groups. FOIS level 1: nothing by mouth; FOIS level 4: total oral diet of a single consistency; FOIS level 7: multiple food consistencies without specific food limitations. Curves differ significantly by generalized estimating equation analysis, after adjusted for Charlson index and length of intubation (P < 0.001). FOIS = Functional Oral Intake Scale.
4. Discussion
Postextubation swallowing dysfunction was prevalent in our participants, affecting 61.7% within 48 hours postextubation. Our finding of 61.7% for PSD incidence with prolonged intubation is at the higher end of previous studies, which ranged from 10% to 67.5%, hovering around 50%, despite different timing and methods (assessed instrumentally or by the BSE) of evaluation.[1,3,4,8–12,22,23] This difference might be due to our sample being older (mean age = 63.4 years with 52.3% being ≥65 years). We also found that such swallowing dysfunctions were prolonged and that age was associated with higher rates of PSD at 7, 14, and 21 days after extubation, longer times to resume total oral intake, and subsequent tube-feeding dependence. Thus, PSD recovery is not spontaneous, particularly for patients aged 65 years and older. Our results raise 2 points of interest.
First, PSD is prolonged even at 21 days after extubation. At the end of follow-ups, 15.5% of our sample had prolonged PSD and were still unable to resume total oral intake, committing them to feeding-tube dependence. Our findings are similar to those of 3 studies.[12,13,24] For example, 33.9% of 254 cardiac surgery patients had a quick recovery, but 8.3% with an average age of 64.5 years and intubated for 4.8 days required >10 days to resume total oral intake after extubation.[12] Similarly, 29% of 446 ICU patients (some were intubated <48 hours) had prolonged PSD at hospital discharge.[13] Recently, 21.6% of 37 ICU survivors were reported to still be unable to resume total oral intake 4 months after ICU admission.[24] Since recovery is not spontaneous, swallowing and intake therapeutics are clinical priorities. Future studies are warranted to test whether oral motor exercise, postural changes, and dietary texture modification can reduce time needed to recover from PSD.
Second, PSD incidence within 48 hours of extubation did not significantly differ between older and younger participants (68.4% vs 54.3%; P = 0.11). Rather, age mattered in PSD recovery and its rates at 7, 14, and 21 days postextubation. The difference in PSD rates between older and younger participants became even greater as time passed. Our finding might partially explain why previous cross-sectional studies had inconsistent findings on age as a risk factor. Age might not matter for PSD incidence right after extubation, but it matters significantly as time passes. Indeed, older participants took significantly more time than younger participants to pass the BSE (5.0 vs 3.0 days) and to resume total oral diet with a single consistency (5.0 vs 3.0 days).
Why age matters in the time needed to recover is not fully understood. It may be due simply to less capacity for compensation or reduced cognitive reserves with older age. Specifically, neurogenic and myogenic factors might contribute to a prolonged recovery of PSD in older patients. For example, delayed swallow response in older patients is related to neural alteration that prolongs responses to pharyngeal reconfiguration and increases oropharyngeal residue.[25] Weak muscular tongue strength caused by sarcopenia (age-related muscle wasting) may impair bolus propulsion, thus reducing swallowing efficacy.[25]
Nevertheless, recovery (passing the BSE and resuming total oral intake) reached a plateau by 7 days after extubation, especially for younger participants. Even for older participants, little improvement was noted 14 days after extubation. We therefore would recommend that 7 to 14 days postextubation, if not earlier, patients who cannot pass the BSE or resume total oral intake should be referred for possible swallowing or intake therapeutics. Furthermore, whether this PSD is due to oral intubation or disease severity and whether recovery was complicated by sarcopenia[26,27] should be further investigated. More study is also suggested to determine whether interventions can reduce rates of PSD and time needed to resume total oral intake postextubation.[28–30]
4.1. Study limitations
This study's strengths are its prospective design, and also its evaluation of PSD and oral intake levels 21 days after extubation in 151 participants who were orally intubated ≥48 hours. Nevertheless, the study had important limitations. First, our identification of PSD was based on the 50-mL water BSE and not on a diagnostic gold standard such as videofluoroscopy of swallowing (VFS). This limitation is minimized by the widespread use of the BSE to reliably identify swallowing dysfunction in lieu of VFS-based clinical diagnosis.[8,9,11] In addition, given the exploratory nature of this study, we did not use fiber endoscopy to identify postextubation-related vocal palsy and test the pharyngeal reflex, all of which might have helped to explain the PSD etiology and study findings. Second, attrition by 21 days postextubation was 27% (n = 41), including 10 patients who died, 15 who were reintubated, and 13 who were discharged or transferred. Since data were not imputed, inferences were conditional on patient survival. Third, the sample was too small for more advanced subgroup analyses and was limited by recruiting patients from 1 medical center with exclusion criteria and by male predominance (64.2% males). These factors might limit the generalizability of our findings. Studies are warranted to identify opportunities for better prevention and management of PSD.
5. Conclusions
Postextubation swallowing dysfunction was common and prolonged even for patients without prior swallowing difficulties or known pathologies such as stroke or neuromuscular deficits. Age matters in the time needed to recover. Nearly 1 in 4 older participants (24.1%) undergoing oral endotracheal intubation for 48 hours and longer had prolonged PSD, committing them to feeding-tube dependence. In fact, PSD has been strongly linked with poor patient outcomes, including pneumonia,[31] reintubation, institutionalization, and death.[13,32,33] As a first step, swallowing and oral intake should be monitored in the first 7 to 14 days postextubation to identify opportunities to manage PSD, particularly for patients aged 65 years and older.
Footnotes
Abbreviations: APACHE II = acute physiology and chronic health evaluation II, BMI = body mass index, BSE = bedside swallow evaluation, FEES = fiber optic endoscopic evaluation of swallowing, FOIS = Functional Oral Intake Scale, GEE = generalized estimating equations, ICU = intensive care unit, PSD = postextubation swallowing dysfunction, VFS = videofluoroscopy of swallowing.
Authorship: M-HT participated in the design, collected data, analyzed data, and drafted the manuscript. S-CK participated in the design, assisted with study enrollment and data collection, and revised the manuscript. T-GW participated in the design and helped to revise the manuscript. T-YH participated in the design and helped to revise the manuscript. J-JL participated in the design and assisted with study enrollment and data collection. D-CC participated in the design and assisted with study enrollment. G-HH assisted with statistical analysis. CC-HC designed the study, assisted with study enrollment and data collection, assisted with statistical analysis, and helped to revise the manuscript. All authors read and approved the final manuscript.
Funding: The study was funded by the Taiwan Ministry of Science and Technology (Grant no. NSC-101–2314-B-002–131-MY3) in Taiwan.
The authors have no conflicts of interest to disclose.
References
- [1].Bordon A, Bokhari R, Sperry J, et al. Swallowing dysfunction after prolonged intubation: analysis of risk factors in trauma patients. Am J Surg 2011;202:679–83. [DOI] [PubMed] [Google Scholar]
- [2].de Larminat V, Montravers P, Dureuil B, et al. Alteration in swallowing reflex after extubation in intensive care unit patients. Crit Care Med 1995;23:486–90. [DOI] [PubMed] [Google Scholar]
- [3].Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation. Chest 2010;137:665–73. [DOI] [PubMed] [Google Scholar]
- [4].Skoretz S, Yau T, Ivanov J, et al. Dysphagia and associated risk factors following extubation in cardiovascular surgical patients. Dysphagia 2014;29:647–54. [DOI] [PubMed] [Google Scholar]
- [5].Macht M, Wimbish T, Bodine C, et al. ICU-acquired swallowing disorders. Crit Care Med 2013;41:2396–405. [DOI] [PubMed] [Google Scholar]
- [6].Brown CVR, Hejl K, Mandaville AD, et al. Swallowing dysfunction after mechanical ventilation in trauma patients. J Crit Care 2011;26:108.e9–e13. [DOI] [PubMed] [Google Scholar]
- [7].Kwok AM, Davis JW, Cagle KM, et al. Post-extubation dysphagia in trauma patients: it's hard to swallow. Am J Surg 2013;206:924–8. [DOI] [PubMed] [Google Scholar]
- [8].Leder SB, Cohn SM, Moller BA. Fiberoptic endoscopic documentation of the high incidence of aspiration following extubation in critically ill trauma patients. Dysphagia 1998;13:208–12. [DOI] [PubMed] [Google Scholar]
- [9].Ajemian MS, Nirmul GB, Anderson MT, et al. Routine fiberoptic endoscopic evaluation of swallowing following prolonged intubation: implications for management. Arch Surg 2001;136:434–7. [DOI] [PubMed] [Google Scholar]
- [10].Barquist E, Brown M, Cohn S, et al. Postextubation fiberoptic endoscopic evaluation of swallowing after prolonged endotracheal intubation: a randomized, prospective trial. Crit Care Med 2001;29:1710–3. [DOI] [PubMed] [Google Scholar]
- [11].El Solh A, Okada M, Bhat A, et al. Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med 2003;29:1451–5. [DOI] [PubMed] [Google Scholar]
- [12].Barker J, Martino R, Reichardt B, et al. Incidence and impact of dysphagia in patients receiving prolonged endotracheal intubation after cardiac surgery. Can J Surg 2009;52:119–24. [PMC free article] [PubMed] [Google Scholar]
- [13].Macht M, Wimbish T, Clark B, et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care 2011;15:R231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moraes DP, Sassi FC, Mangilli LD, et al. Clinical prognostic indicators of dysphagia following prolonged orotracheal intubation in ICU patients. Crit Care 2013;18:R243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nizolek KN. Risk factors for dysphagia in critically-ill patients with prolonged orotracheal intubation. Columbia University, 2014. Available at: http://academiccommons.columbia.edu/catalog/ac%3A176095 Accessed September 20, 2015. [Google Scholar]
- [16].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [17].Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- [18].Yeh SJ, Huang KY, Wang TG, et al. Dysphagia screening decreases pneumonia in acute stroke patients admitted to the stroke intensive care unit. J Neurol Sci 2011;306:38–41. [DOI] [PubMed] [Google Scholar]
- [19].Tohara H, Saitoh E, Mays KA, et al. Three tests for predicting aspiration without videofluorography. Dysphagia 2003;18:126–34. [DOI] [PubMed] [Google Scholar]
- [20].Crary MA, Mann GDC, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 2005;86:1516–20. [DOI] [PubMed] [Google Scholar]
- [21].Basagaña X, Spiegelman D. Power and sample size calculations for longitudinal studies comparing rates of change with a time-varying exposure. Stat Med 2010;29:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Padovani AR, Moraes DP, de Medeiros GC, et al. Orotracheal intubation and dysphagia: comparison of patients with and without brain damage. Einstein 2008;6:343–9. [Google Scholar]
- [23].Medeiros GC, Sassi FC, Mangilli LD, et al. Clinical dysphagia risk predictors after prolonged orotracheal intubation. Clinics 2014;69:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zielske J, Bohne S, Brunkhorst F, et al. Acute and long-term dysphagia in critically ill patients with severe sepsis: results of a prospective controlled observational study. Eur Arch Otorhinolaryngol 2014;271:3085–93. [DOI] [PubMed] [Google Scholar]
- [25].Rofes L, Arreola V, Romea M, et al. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol Motil 2010;22:851–8. [DOI] [PubMed] [Google Scholar]
- [26].Robbins JA, Gangnon RE, Theis SM, et al. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc 2005;53:1483–9. [DOI] [PubMed] [Google Scholar]
- [27].Shiozu H, Higashijima M, Koga T. Association of sarcopenia with swallowing problems, related to nutrition and activities of daily living of elderly individuals. J Phys Ther Sci 2015;27:393–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].O’Rourke F, Vickers K, Upton C, et al. Swallowing and oropharyngeal dysphagia. Clin Med 2014;14:196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rassameehiran S, Klomjit S, Mankongpaisarnrung C, et al. Postextubation dysphagia. Proc (Bayl Univ Medi Cent) 2015;28:18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Macht M, White SD, Moss M. Swallowing dysfunction after critical illness. Chest 2014;146:1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cabré M, Serra-Prat M, Force L, et al. Oropharyngeal dysphagia is a risk factor for readmission for pneumonia in the very elderly persons: observational prospective study. J Gerontol A Biol Sci Med Sci 2014;69:330–7. [DOI] [PubMed] [Google Scholar]
- [32].Reza Shariatzadeh M, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc 2006;54:296–302. [DOI] [PubMed] [Google Scholar]
- [33].Altman KW, Yu GP, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg 2010;136:784–9. [DOI] [PubMed] [Google Scholar]


