Abstract
As choosing wisely has raised the issue of whether some individuals with type 2 diabetes may be overtreated, we examined the intensity of glycemic control across health status strata defined by comorbidities or frailty.
This is a retrospective cohort study of commercially insured patients from 50 US states (Clinformatics Data Mart). We evaluated treated HbA1c levels in adults with new diabetes diagnosed between January 2004 and December 2009 who had HbA1C measured after at least 1 year of follow-up.
Of 191,590 individuals with diabetes, 78.5% were otherwise healthy, 10.6% had complex health status (3 or more chronic conditions), and 10.9% were very complex (Johns Hopkins Adjusted Clinical Groups frailty marker or end-stage chronic disease). The proportion of patients who were tightly controlled (HbA1C <7%) was similar in otherwise healthy patients (66.1%) and in complex patients (65.8%, P = 0.37), and although it was lower (60.9%, P < 0.0001) in very complex patients, the magnitude of the difference was small. A substantial proportion of complex/very complex patients were taking sulfonylurea or insulin despite being at an increased risk for adverse effects from these agents and having tightly controlled HbA1C: 40.6% had HbA1C <7% and 24% had HbA1C <6.5%. Among patients with HbA1C <7%, use of insulin or sulfonylureas was associated with an increased risk for all-cause hospitalization [aHR 1.54, 95% confidence interval (95% CI) 1.45–1.64] and for emergency room visits (aHR 1.44, 95% CI 1.35–1.53) over the subsequent median 6 months follow-up.
Diabetic control was similar regardless of comorbidity burden and frailty status. Despite being at a higher risk for adverse effects, nearly half of complex and very complex patients were still receiving insulin or sulfonylureas despite having treated HbA1C levels <7%, and these patients did exhibit higher risk of all-cause hospitalizations or emergency visits subsequently.
Keywords: diabetes mellitus, targets, treatment
1. Introduction
Although intensive glucose control [glycosylated hemoglobin (HbA1c) <7%] produces microvascular and mortality benefits in younger patients with type 1 diabetes mellitus (DM),[1,2] the benefits of intensive control in patients with type 2 DM are less certain with conflicting trial results and pooled data suggesting no impact on all-cause mortality and indeterminate results for micro or macrovascular events.[3–5] Moreover, observational studies suggest that comorbidities increase the risks and lessen the benefits of pursuing intensive glycemic control in patients with Type 2 DM, especially older patients.[6–10] Thus, current guidelines from the American Diabetes Association,[11] the American Geriatrics Society,[12] the Canadian Diabetes Association,[13] and the European Diabetes Working Group[14] recommend individualization of treatment intensity based on each patient's burden of comorbidities, frailty status, and life expectancy. However, a recent analysis of NHANES data suggested that most older individuals with diabetes may be overtreated in that nearly two-third had HbA1c <7% with no differences between healthy individuals and those with extensive comorbidities and/or foreshortened life expectancy.[15]
The guidelines recommend HbA1c goals of less than 7.0% in healthy individuals (<7.5% in healthy patients older than 65 years), less than 8.0% in those who are “complex” (defined as having 3 or more chronic conditions), and less than 8.5% for patients who are very complex (defined as having at least 1 end-stage chronic illness or impairments in 2 or more activities of daily living).[11–14] The purpose of this study is to examine blood glucose control in a large cohort of insured patients with incident diabetes and explore whether control varies by health status to confirm or refute the findings from the NHANES analysis done in less than 1300 patients.[15]
2. Methods
As previously described,[16] we conducted a population-based retrospective cohort study using a large US claims and integrated laboratory database that included employed, commercially insured patients and their dependents from all 50 States (Clinformatics Data Mart Database; OptumInsight, Eden Prairie, MN). Clinformatics Drug Mart is a research affiliate of United Healthcare, 1 of the largest health care companies in the United States, with over 13 million lives included per annum and 340,000 affiliated physicians among all 50 states. Clinformatics collects complete health service utilization data, including US medical, pharmacy, and laboratory results. All patients are in standard commercial or managed care plans. Clinformatics places a significant emphasis on the quality of the data and uses a series of internal data evaluation and reconciliation steps to ensure the completeness, validity, and consistency of the data. Indeed, Clinformatics (formerly i3) is one of the few data sources approved by the FDA's Mini-Sentinel Program to perform active surveillance of the safety of marketed medical products, including drugs and biologics because of the internal strength and representativeness of the US population. Patient-level data are collected directly from each clinical encounter (inpatient and outpatient) and the database includes de-identified longitudinal patient clinical data, all laboratory tests and results, and pharmacy claims data (de-identified prescribing physician, drug dispensed based on National Drug Codes, quantity and date dispensed, drug strength, days supply, cost of service). The database contains over 13 million annual lives and data are updated every 90 days.
All patients in our cohort were followed prospectively until death, termination of medical insurance, or December 31, 2010, providing a maximum follow-up of 6 years. All data were de-identified and accessed with protocols compliant with the Health Insurance Portability and Accountability Act. The study was approved by the ethics review board of the University of Alberta, Edmonton, Alberta, Canada, and the New England Ethics Institutional Review Board, Massachusetts.
2.1. Cohort selection
We identified all patients aged 20 years or older with at least 1 hospitalization or 2 physician claims within 2 years for ICD-9 250.x (DM) using physician claims, hospital discharge abstracts, and/or ambulatory care visits, or a first claim for an oral antihyperglycemic drug or insulin, between January 1, 2004, and December 31, 2009. These were all incident cases, as none had a history of diabetes visits or diabetes drug therapy in the previous 2 years. This definition of incident diabetes has been widely utilized and validated, and has a specificity of 92% to 97% for correctly identifying patients with diabetes.[17,18] In order to be eligible for this study, patients in the cohort had to have had at least 1 HbA1C measurement after the diagnosis of diabetes and had to have at least 1 year of coverage before the last HbA1C (to ensure that we had sufficient data to accurately classify patients’ health status at the time of the index HbA1C measurement as described below).
2.2. Definition of health status
For each patient, we calculated the Johns Hopkins Adjusted Clinical Groups (ACG) Case-Mix System score (a single comorbidity score weighted by the 32 Adjusted Diagnostic Groups that performs equally or better than the Charlson and Elixhauser comorbidity scores) using all visits up to and including the date that the HbA1C we used to define their DM control was drawn (see below).[19] In addition, the Johns Hopkins ACG System includes a frailty marker for any patients with notations of malnutrition, abnormal weight loss, difficulty walking, fecal/urinary incontinence, morbid obesity, dementia, falls, or decubitus ulcer.
To mirror the NHANES study[15] and the recommendations from the ADA and AGS,[11,12] we defined complex health status if a patient had 3 or more chronic conditions: arthritis, heart failure but without hospitalization as most responsible diagnosis in the past year, chronic obstructive pulmonary disease (COPD) without hospitalization as most responsible diagnosis in the past year, chronic kidney disease but not requiring dialysis, coronary heart disease (prior MI or angina), stroke, urinary incontinence, cancer, depression, or hypertension.
We defined very complex health status if a patient had the frailty marker derived by the Johns Hopkins ACG system (which corresponds to the variable “impairments in activities of daily living”[20] suggested by the ADA and AGS) or if they had end-stage renal disease requiring dialysis, heart failure with a most responsible heart failure hospitalization in the past year, COPD with a most responsible COPD hospitalization in the past year, metastatic cancer, or severe cognitive impairment.
Finally, mirroring a recent publication from the Veterans Health Administration,[21] we examined HbA1C control among patients being treated with sulfonylureas and/or insulin who had comorbidities, which made them high-risk for hypoglycemia (such as advanced age, chronic kidney disease, dementia, or advanced diabetes complications).
2.3. Definition of diabetic control
We used the last recorded HbA1C for each patient. Thus, health status, medications, and covariates were all drawn from the database up to, and including, the date of that HbA1C measurement.
2.4. Covariates
The specific variables included were age; sex; socioeconomic status [type of medical insurance (Health Maintenance Organization insurance agreements, preferred provider plans, exclusive provider plans, point of service plans, etc) and median household income based on Census region and according to the 2010 US census]; clinical laboratory data [HbA1c, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, estimated glomerular filtration rate (eGFR) (according to the modified diet in renal disease calculation: ≥90; 89.9–60; 59.9–30; <30), albuminuria]; and glucose-lowering medications. For all covariates, we used values closest to the index HbA1C measurement date (up to and including the index date) and for medications we used all prescriptions in the 90 days before and including the index date. To further control for comorbidities, we used the Johns Hopkins ACG System score. In addition, we included the Expanded Diagnosis Cluster for Diabetes embedded within the Johns Hopkins Adjusted Clinical Groups Case-Mix System, which includes validated algorithms for 16 diabetes complications (ranging from ketoacidosis to renal/retinal manifestations) to further control for diabetes-specific complications.
2.5. Other outcomes
In those patients with index HbA1C <7%, we examined the frequency of all-cause hospitalizations or emergency department (ED) visits and hospitalizations/ED visits for major cardiovascular events (acute coronary syndrome, stroke/transient ischemic attack, heart failure) after the index HbA1C measurement as secondary outcomes. We conducted multivariable Cox proportional hazards regression to examine which factors predicted hospitalizations or ED visits.
2.6. Statistical analysis
Patient characteristics were reported as means and standard deviations for continuous variables and proportions for categorical variables. Analysis of variance (ANOVA) and Chi-squared tests were used to compare characteristics between health status tiers. Diabetic control was determined by calculating the proportion of patients having HbA1C within specified cut-points (i.e., <7%; 7.0–7.9%, 8.0–8.9%, and >9%) and compared among the health status groups using Chi-squared tests. For the secondary outcomes (hospitalizations and ED visits), multivariable Cox proportional hazards models were used to calculate adjusted hazard ratios, including covariates for health status, age, sex, type of insurance, ADG comorbidity score, lab measurements, time between incident diagnosis of diabetes and HbA1c measurement, and diabetes medications. Only lab values contained any missing values, and in such cases, the missing indicator approach was used so that no patients were excluded from the analysis. In addition to our primary analyses involving all incident diabetes patients over age 20, we restricted our analyses to only those patients 65 years and older (at the time of HbA1c measurement) in a sensitivity analysis. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
Of 311,940 individuals with incident diabetes during the study period, 191,590 had a HbA1C drawn after diabetes diagnosis and had at least 1 year of coverage before that HbA1C being drawn and thus formed our study cohort (Fig. 1). The median time until the HbA1C measurement (used to define diabetic control) after the diagnosis of diabetes was 744 days [interquartile range (IQR) 436–1191].
Figure 1.
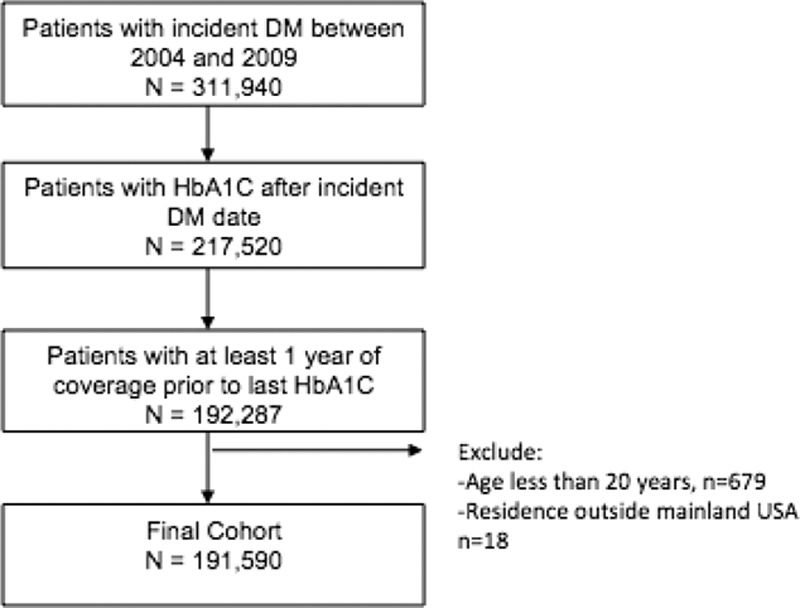
Derivation of study cohort.
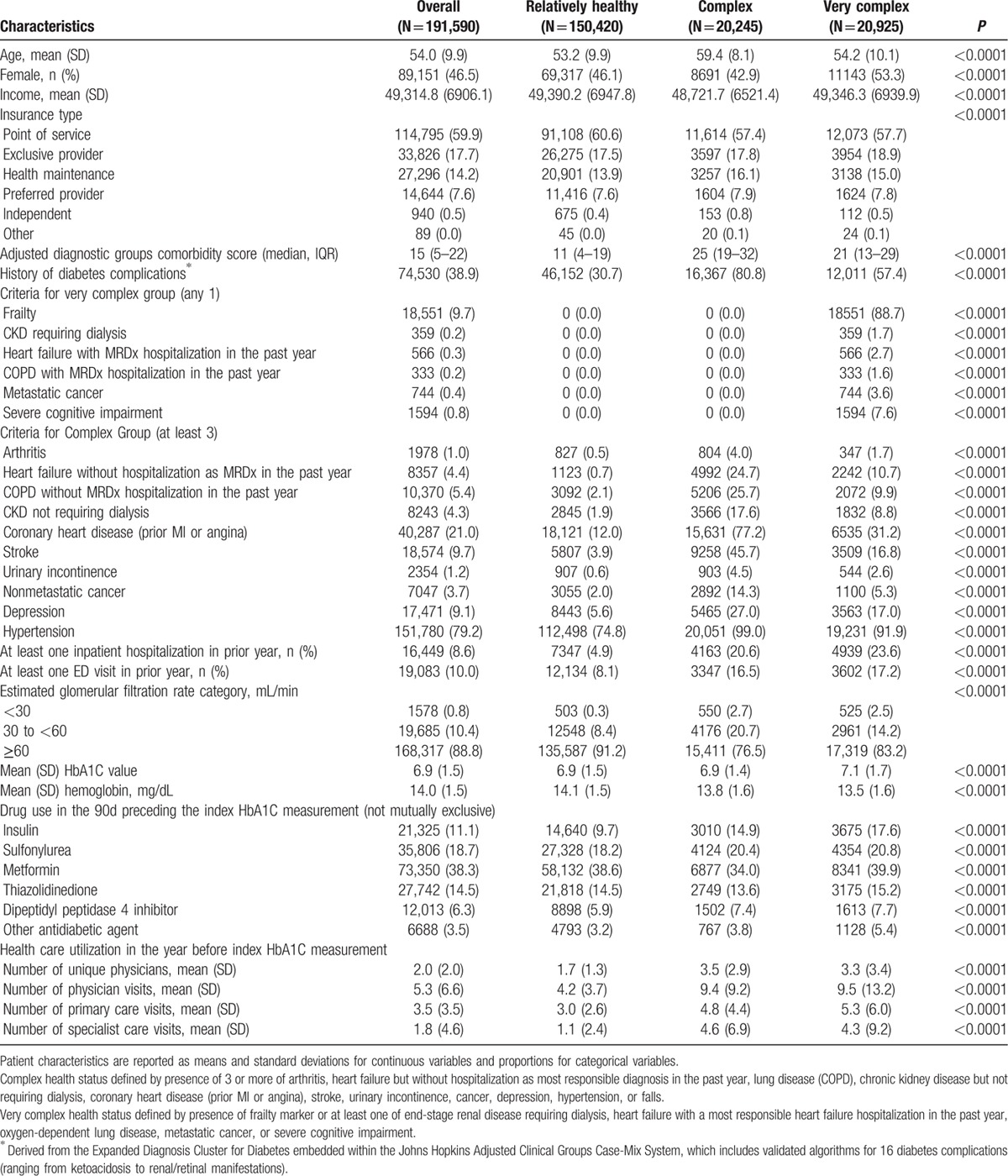
Most patients (78.5%) in the study cohort were relatively healthy, but 10.6% met the definition for complex and 10.9% for very complex health status. Not surprisingly, the frequency of comorbidities, diabetes complications, lower eGFR, prior hospitalizations or ED visits, number of outpatient visits in prior year, and number of physicians seen in prior year were all greater in the complex and very complex patients, although it is noteworthy that even in the relatively healthy subgroup, 30.7% had a diabetes complication recorded by the time the HbA1C we used to define diabetic control was measured (Table 1). Interestingly, although use of metformin and thiazolidinediones was similar across patient subgroups, insulin and sulfonylurea use was greater in complex and very complex patients (Table 1). Patterns were identical when we restricted the analysis to patients 65 years or older (data available upon request).
Table 1.
Patient socio-demographics, health care utilization, lab results, and prescription drug use up until the time of the index HbA1C measurement, stratified according to patient health status.
Diabetic control was identical in patients who were relatively healthy compared with those with complex health status (mean HbA1C 6.9% in both groups) but was marginally less intensive in patients with very complex health status (mean 7.1%, P < 0.0001). The proportion of patients who were tightly controlled (HbA1C <7%) was similar in relatively healthy patients (66.1%) and in complex patients (65.8%, P = 0.37), and although it was lower (60.9%, P < 0.0001) in very complex patients, the magnitude of the difference was small (Fig. 2). In fact, on-treatment HbA1C was <6.5% not only in 49.4% of relatively healthy patients but also in 48.8% of those meeting the definition for complex health status and in 44.8% of the very complex status patients.
Figure 2.
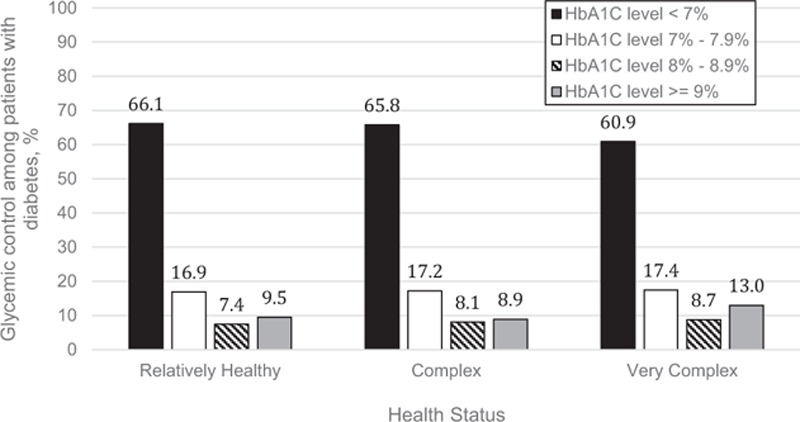
Achieved glycemic control among adults with diabetes mellitus across 3 health status categories.
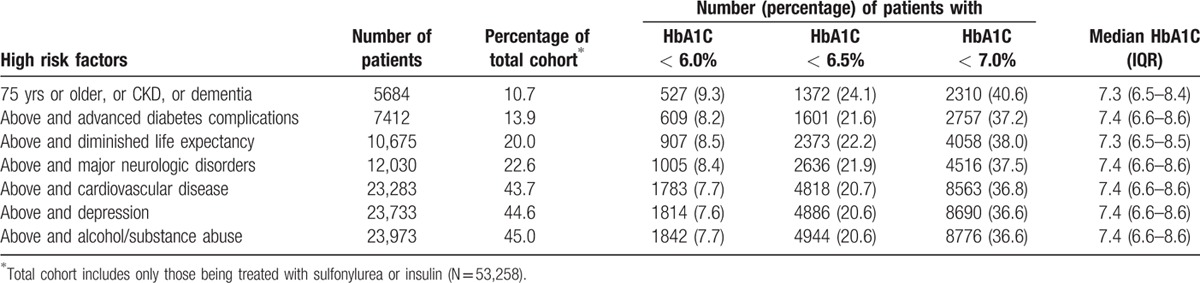
Of the complex/very complex patients who were using sulfonylurea or insulin despite being at an increased risk for adverse effects from these agents (Table 2), a substantial proportion had very tightly controlled HbA1C. For example, 40.6% of patients aged 75 years or older, or with dementia, or with chronic kidney disease were still taking sulfonylurea or insulin even though their HbA1C was <7% (and 24% were still taking those agents despite having HbA1C <6.5%).
Table 2.
Evaluation of rates of possible overtreatment in high-risk patients being treated with sulfonylurea or insulin.
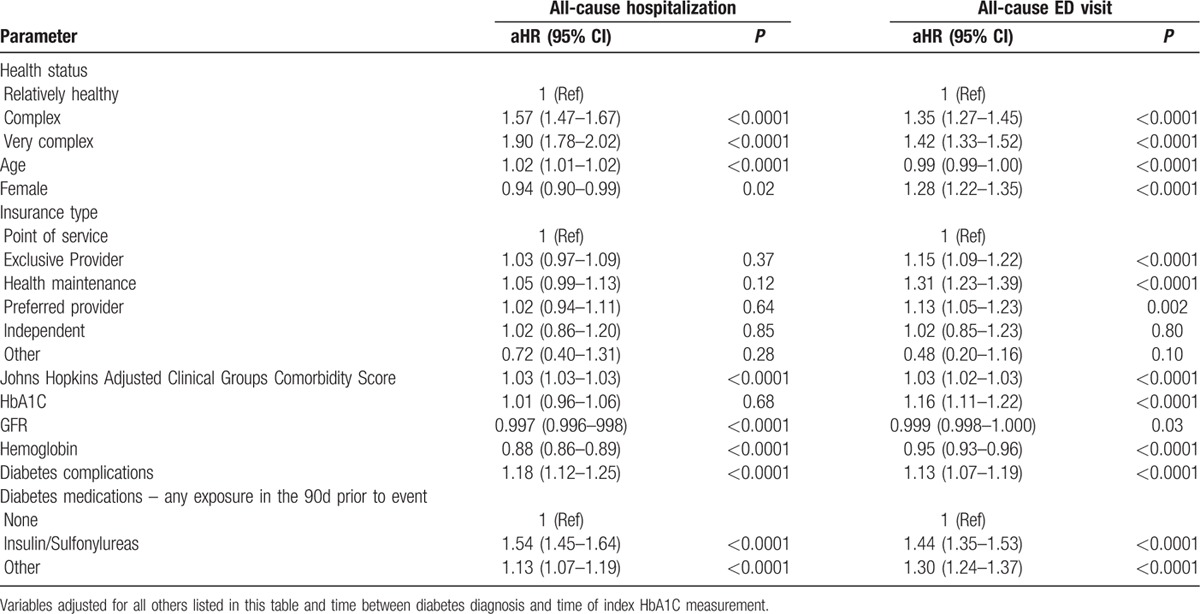
As expected, higher proportions of patients with complex or very complex health status had all-cause hospitalizations or ED visits (Table 3) over a median follow-up of 6 months after the index on-treatment HbA1C measurement. Rates were highest in patients with poor glycemic control and lowest in those with on-treatment HbA1C within guideline-defined optimal ranges (Table 3). Among those patients with on-treatment HbA1C <7%, use of insulin or sulfonylureas was associated with an increased risk for both outcomes (Table 4) even after adjusting for other prognostic factors.
Table 3.
Hospitalization and emergency department visits after index HbA1C measurement, median follow-up 6 months.
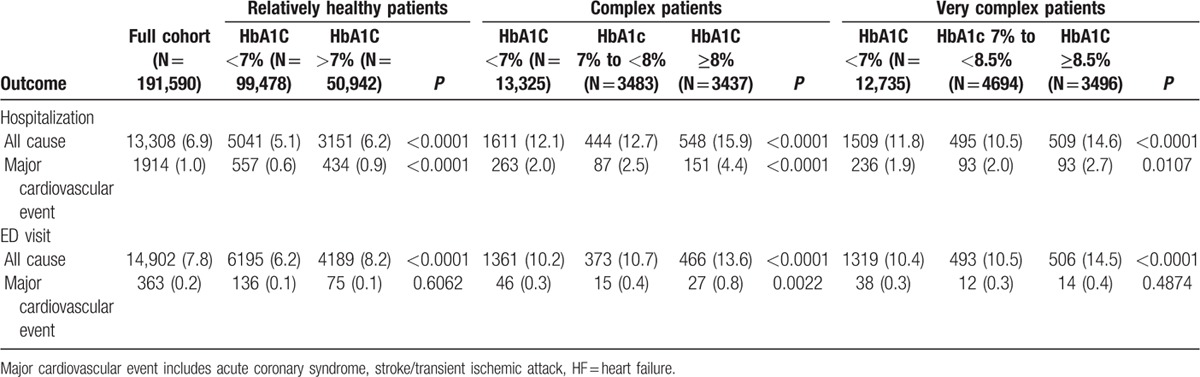
Table 4.
Results of multivariable time to event Cox models for patients with HbA1C < 7% (n = 125,538).
4. Discussion
4.1. Overall findings
Classifying individual health status on the basis of comorbidity burden and presence/absence of frailty, we found that diabetic control was similar for relatively healthy patients, complex patients, and very complex patients, with nearly two-thirds of all 3 groups having HbA1C <7% and nearly one half having HbA1C < 6.5%. Complex and very complex patients were more likely to be treated with insulin or sulfonylureas, and nearly 40% of those at a high risk for hypoglycemia from these agents were still receiving them despite the fact their HbA1C was <7% (and nearly one quarter had HbA1C <6.5%). All-cause hospitalizations or ED visits were more likely in patients with HbA1C <7% being treated with insulin/sulfonylureas even after adjusting for other covariates.
4.2. Comparison with other studies
Recent reports from an elderly subgroup of NHANES participants[15] and the Veterans Health Administration[21] (mean age 66 years, but 48% were younger than 65) have suggested substantial rates of potential overtreatment of diabetic individuals, especially those who are elderly or have multiple comorbidities. Indeed, overtreatment of diabetes in the elderly is one of the conditions highlighted by the Choosing Wisely Campaign.[22] Another recent report from 31,545 patients with well controlled type 2 diabetes not being treated with insulin in the Optum Labs Data Warehouse documented that over 60% had their HbA1C measured more frequently than recommended in guidelines and that excessive testing was associated with treatment intensification despite these patients already being well controlled—raising the spectre of overtreatment.[23] Others have pointed to the imbalance in pay-for-performance measures for diabetes, which have focused on detecting undertreatment of diabetes and have ignored the issue of potential overtreatment.[24–26] Although diabetes performance measures frequently exclude patients older than 75 years, we speculate that Continuing Medical Education events emphasizing the tight control of blood glucose in young patients with Type 1 diabetes may have encouraged a culture of intensification in the management of diabetes at the expense of the concept of individualization based on the health status of each individual patient. Moreover, pay-for-performance programs often provide monetary incentives for meeting treatment targets and we are not aware of any such programs that currently reward de-implementation of therapy or treating some patients to less stringent targets.[27]
Although intensive control in type 2 diabetes does not necessarily mean “overtreatment,” the substantial proportion of complex/very complex patients who were still utilizing sulfonylureas or insulin despite having HbA1C <7% (and even <6%) is concerning, particularly as this has been proposed as a potential marker for overtreatment in type 2 diabetes.[28] Insulin is already one of the most common causes of adverse drug reactions and ED visits.[29–31] Both insulin and sulfonylureas can also cause weight gain, which may be problematic in patients with existing comorbidities or frailty. There is also substantial debate over the cardiovascular safety of these drugs, with several studies suggesting harm compared with other glucose-lowering therapies,[32] and increasing recognition of the potential hazards from polypharmacy in patients with type 2 diabetes.[33]
4.3. Strengths and limitations
Despite several strengths of our study, including the availability of detailed clinical data and the relatively large population-based sample size of patients with incident diagnoses of diabetes, there are several potential limitations to our work. First, to the extent that the clinical records may have undercaptured comorbidities (particularly likely for conditions such as dementia or frailty), we may have underestimated the proportion of individuals with complex health status and thus the true proportion who are potentially overtreated may be even higher. Second, although we acknowledge that some comorbidities have more profound effects on life expectancy or functional status than others, we used the ADA and AGS framework to define complex/very complex health status to replicate the work done with NHANES data.[15] Third, as the ADA only advocated individualization of treatment targets in 2012, the intensity of treatment may have declined since then, although the AGS guidelines endorsed individualization of treatment targets for almost a decade earlier, including during the years we had access to data for. Fourth, this dataset only included commercially insured patients and thus the results may not be generalizable to other patient populations, but our patient characteristics are similar to those from another US national administrative claims database[23] and our findings are similar to an earlier report from the VA system.[21] Fifth, as with any study using laboratory data, HbA1C measurements demonstrate measurement variability and measurement error can be up to ±0.5%.[34] Sixth, we did not evaluate patient adherence rates and thus cannot definitively say that lower HbA1C levels in patients taking insulin or sulfonylureas were due to those agents. Seventh, the association between use of insulin and higher all-cause hospitalizations and ED visits may be due to residual bias, as other hypoglycemic medications are relatively contraindicated in patients with some comorbidities such as advanced kidney disease or heart failure. Finally, it should be acknowledged that there may have been clinical reasons why a particular patient might appropriately be treated to an intensive HbA1C goal even if they had conditions that placed them in the complex or very complex health status groups and that intensive control does not mean overtreatment in all cases.
5. Conclusion and implications
Nearly two-thirds of all diabetic individuals in this cohort of commercially insured patients had treated HbA1C <7%, and the intensity of glycemic control was similar regardless of the number and severity of comorbidities and/or markers of frailty—this raises the spectre of potential overtreatment. Although they were at a higher risk for adverse effects, complex and very complex patients were more likely to be treated with insulin or sulfonylureas, and nearly half were still receiving these agents despite having treated HbA1C levels <7% (and nearly one quarter had HbA1C <6.5%)—meeting another proposed indicator for potential overtreatment. In the era of Choosing Wisely, the issue of potential overtreatment needs to be considered in future diabetes quality improvement initiatives.
Footnotes
Abbreviations: ACG = adjusted clinical groups, ADA = American Diabetes Association, AGS = American Geriatrics Association, aHR = adjusted hazard ratio, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, ED = emergency department, EDWG = European Diabetes Working Group, eGFR = estimated Glomerular Filtration Rate, HbA1C = Glycosylated hemoglobin, HF = heart failure, ICD-9 = International Classification of Disease-9th Revision, IQR = Interquartile Range, NHANES = National Health And Nutrition Examination Survey, TIA = transient ischemic attack, VA = Veterans Affairs.
Authorship: All authors researched data. EY did statistical analysis. FM wrote manuscript. DE reviewed/edited manuscript.
Funding: This work was funded by an operating grant from the Canadian Diabetes Association (OG-2–09–2691-DE) and the Canadian Institutes of Health Research (MOP 126093). DTE receives salary support from Alberta Innovates Health Solution (AIHS) and the Canadian Institutes for Health Research (CIHR). FAM is a Senior Health Scholars with AIHS and holds the University of Alberta Chair in Cardiovascular Outcomes Research.
The study sponsors played no role in study design or conduct; collection, analysis, interpretation of data; writing of the report; or in the decision to submit the paper for publication. All authors declare that they have no financial interests that may be relevant to the submitted work. Dr. McAlister is guarantor of this work.
The authors have no conflicts of interest to disclose.
References
- [1].The Diabetes Control, Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. [DOI] [PubMed] [Google Scholar]
- [2].The Diabetes Control, Complications Trial/Epidemiology of Diabetes Interventions, Complications Study Research Group. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Montori VM, Fernández-Balsells M. Glycemic control in type 2 diabetes: time for an evidence-based about-face? Ann Intern Med 2009;150:803–8. [DOI] [PubMed] [Google Scholar]
- [4].Hemmingsen B, Lund SS, Gluud C, et al. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ 2011;343:d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rodriguez-Gutierrez R, Lipska KJ, McCoy RG. Intensive glycemic control in type 2 diabetes mellitus – a balancing act of latent benefit and avoidable harm. A teachable moment. JAMA Intern Med 2016;176:300–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang ES, Zhang Q, Gandra N, et al. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes. Ann Intern Med 2008;149:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Greenfield S, Billimek J, Pellegrini F, et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med 2009;151:854–60. [DOI] [PubMed] [Google Scholar]
- [8].Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med 2014;174:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huang ES, Laiteerapong N, Liu JY, et al. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med 2014;174:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Feil DG, Rajan M, Soroka O, et al. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc 2011;59:2263–72. [DOI] [PubMed] [Google Scholar]
- [11].American Diabetes Association. Standards of medical care in diabetes–2016. Diabetes Care 2016;39suppl 1:S1–06. [DOI] [PubMed] [Google Scholar]
- [12].Moreno G, Mangione CM, Kimbro L, et al. Guidelines abstracted from the American Geriatrics Society Guidelines for improving the care of older adults with diabetes mellitus. J Am Geriatr Soc 2013;61:2020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2013;37suppl 1:S1–212. [DOI] [PubMed] [Google Scholar]
- [14].Sinclair AJ, Paolisso G, Castro M, et al. European Diabetes Working Party for Older People. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011;37suppl 3:S27–38. [DOI] [PubMed] [Google Scholar]
- [15].Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eurich DT, Simpson S, Senthilselvan A, et al. Comparative safety and effectiveness of sitagliptin in patients with type 2 diabetes: retrospective population based cohort study. BMJ 2013;346:f2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].The Public Health Agency of Canada. Report from the National Diabetes Surveillance System: Diabetes in Canada. Ottawa, Canada: Public Health Agency of Canada; 2009. [Google Scholar]
- [18].Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–6. [DOI] [PubMed] [Google Scholar]
- [19].Austin PC, Walraven CV. The Mortality Risk Score and the ADG Score: two points-based scoring systems for the Johns Hopkins aggregated diagnosis groups to predict mortality in a general adult population cohort in Ontario, Canada. Med Care 2011;49:940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Greysen SR, Cenzer IS, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med 2015;175:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tseng CL, Soroka O, Maney M, et al. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med 2014;174:259–68. [DOI] [PubMed] [Google Scholar]
- [22].American Geriatrics Society. Five Things Physicians and Patients Should Question. Choosing Wisely: An Initiative of the American Board of Internal Medicine. Available at: http://www.choosingwisely.org/doctor-patient-lists/american-geriatrics-society/ (Accessed June 1, 2016). [Google Scholar]
- [23].McCoy RG, van Houten HK, Ross JS, et al. HbA1C overtesting and overtreatment among US adults with controlled type 2 diabetes, 2001-13: observational population based study. BMJ 2015;351:h6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pogach L, Aron D. The other side of quality improvement in diabetes for seniors: a proposal for an overtreatment glycemic measure. Arch Intern Med 2012;172:1510–2. [DOI] [PubMed] [Google Scholar]
- [25].Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ali MK, Bullard KM, Saaddine JB, et al. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–24. [DOI] [PubMed] [Google Scholar]
- [27].Houle S, McAlister FA, Jackevicius C, et al. Does performance-based remuneration for individual health care practitioners affect patient care? A systematic review. Ann Intern Med 2012;157:889–99. [DOI] [PubMed] [Google Scholar]
- [28].Pogach L, Aron D. The other side of quality improvement in diabetes for seniors: a proposal for an overtreatment glycemic measure. Arch Intern Med 2012;172:1510–2. [DOI] [PubMed] [Google Scholar]
- [29].Ben Salem C, Fathallah N, Hmouda H, et al. Drug-induced hypoglycaemia: an update. Drug Safety 2011;34:21–45. [DOI] [PubMed] [Google Scholar]
- [30].Geller AI, Shehab N, Lovegrove MC. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med 2014;174:678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011;365:2002–12. [DOI] [PubMed] [Google Scholar]
- [32].Nissen SE. Cardiovascular effects of diabetes drugs: emerging from the dark ages. Ann Intern Med 2012;157:671–2. [DOI] [PubMed] [Google Scholar]
- [33].Lipska KJ, Krumholz H, Soones T, et al. Polypharmacy in the aging patient. A review of glycemic control in older adults with type 2 diabetes. JAMA 2016;315:1034–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Little RR, Rohlfing CL, Sacks DB. National Glycohemoglobin Standardization Program (NGSP) Steering Committee. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem 2011;57:205–14. [DOI] [PubMed] [Google Scholar]


