Abstract
It remains to be seen whether S-1 can be a replacement for infusional fluorouracil (5-FU) for advanced gastric cancer (AGC). The aim of this study was to compare the efficacy and safety of S-1 with 5-FU in AGC.
PubMed and Cochrane Library were searched. Randomized controlled trials and meta-analyses comparing S-1 with 5-FU for AGC were eligible. Meta-analysis was performed using RevMan 5.2.
Seven trials involving 2443 patients were included. Compared with 5-FU, S-1 showed no significant prolongation of overall survival (OS) (hazard ratio [HR] = 0.91, 95% confidence interval [CI] [0.83–1.01], P = 0.07) and progression-free survival (HR = 0.89, 95% CI [0.70–1.13], P = 0.35), but longer time to treatment failure (HR = 0.74, 95% CI [0.56–0.97], P = 0.03). The objective response rates were comparable (risk ratio [RR] = 1.36, 95% CI [0.95, 1.96], P = 0.10). Regarding treatment-related deaths and hematological toxicities, there was significant heterogeneity between Asian and non-Asian trials, and subgroup analysis was applied. In Asian patients, there was a significant increase in hematological toxicities such as leukopenia (grade 1–4: RR = 1.22, 95% CI [1.08, 1.37], P = 0.001; grade 3–4: RR = 2.21, 95% CI [1.52, 3.21], P < 0.0001), neutropenia (grade 1–4: RR = 1.29, 95% CI [1.11, 1.48], P = 0.0005; grade 3–4: RR = 1.87, 95% CI [1.11, 3.17], P = 0.02), and thrombocytopenia (grade 1–4: RR = 1.71, 95% CI [1.22, 2.41], P = 0.002) in S-1-containing regimens compared with 5-FU-containing regimens, but without significant difference in treatment-related mortality rate (risk difference [RD] = 0.00, 95% CI [−0.01, 0.01], P = 0.68). In non-Asian patients, S-1-containing regimens were, however, associated with significantly fewer treatment-related deaths (RD = −0.02, 95% CI [−0.05, −0.00], P = 0.04), as well as less all grade 1–4 and grade 3–4 hematological toxicities except anemia. There was no significant heterogeneity in nonhematologic toxicities between Asian and non-Asian trials. Lower incidence of grade 1–4 nausea, diarrhea, mucositis, grade 3–4 mucositis, increased creatinine, and decreased calculated creatinine clearance was observed in S-1-containing regimens.
S-1 could not improve OS, but increase some hematological toxicities in Asian patients. Therefore, special attention on hematological toxicities should be paid to Asian patients because S-1 is administered on an outpatient basis.
Keywords: Efficacy, fluorouracil, heterogeneity, meta-analysis, S-1, stomach neoplasms, toxicity
1. Introduction
Gastric cancer is the fifth most common malignancy and the third leading cause of cancer mortality worldwide.[1] Because an early detection strategy through population-based screening is not yet widely practiced, the majority of patients are diagnosed with advanced disease. Surgical resection is the only treatment modality that is potentially curative for patients with local or locoregional disease. However, palliative chemotherapy should be considered for patients with unresectable locally advanced, metastatic, or recurrent disease, as it prolongs overall survival (OS) and improves quality of life (QOL) compared with best supportive care alone.[2]
At present, infusional fluorouracil (5-FU) remains the mainstay for the treatment of advanced gastric cancer (AGC) due to its broad antitumor activity, as well as its synergism with other anticancer drugs.[3] Nevertheless, its efficacy is relatively low, whereas its hematological and gastrointestinal toxicities are relatively common. S-1 is a novel oral fluoropyrimidine, comprising tegafur, gimeracil, and oteracil in a molar ratio of 1:0.4:1. Tegafur is a pro-drug of 5-FU and converted into 5-FU after absorption; gimeracil prolongs the half-life of 5-FU by decreasing its degradation in the liver; and oteracil improves gastrointestinal tolerability through decreasing 5-FU phosphorylation in the gastrointestinal tract.[4]
Data from a series of phase II studies of S-1-containing regimens showed promising efficacy and favorable toxicity profiles.[4] Based on the evidence obtained by the Japan Clinical Oncology Group trials, S-1 plus cisplatin has been recommended as the first-line regimen for AGC in Japan.[5] In addition, more randomized trials outside of Japan comparing S-1 with 5-FU in mono or combined therapies have been carried out in recent years, with not completely consistent results regarding the efficacy and safety of these 2 treatments. It remains to be seen whether S-1 can be a replacement for infusional 5-FU.
A published meta-analysis of 4 trials indicated that S-1-based therapy showed better OS and nearly equivalent objective response rate (ORR) and safety profiles, compared with 5-FU-based therapy.[6] After careful reading of the full text, we, however, found that this meta-analysis contained duplicate material, which led to unreliable results and conclusions. During our preparation of this manuscript, another meta-analysis including 6 trials reported similar results to the previous one.[7] However, a few trials were not included and some pivotal information, such as heterogeneity between Asian and non-Asian, has been neglected in these analyses. In addition, the results of some trials have been updated recently. Therefore, we considered that it was deemed important to finish this meta-analysis to clarify the issue.
2. Materials and methods
Because no individual patient data were involved in this systematic review and meta-analysis, ethical approval from an ethics committee was not required.
2.1. Search strategy
Two reviewers (X-DC and F-QH) independently searched the following online electronic databases: PubMed and Cochrane Library using the following search terms: (((((((((((gastric cancer) OR gastric carcinoma) OR gastric adenocarcinoma) OR stomach cancer) OR stomach carcinoma) OR stomach adenocarcinoma)) OR ((((((esophagogastric junction cancer) OR esophagogastric junction carcinoma) OR esophagogastric junction adenocarcinoma) OR gastroesophageal junction cancer) OR gastroesophageal junction carcinoma) OR gastroesophageal junction adenocarcinoma))) AND fluorouracil) AND ((S-1[Title/Abstract]) OR TS-1[Title/Abstract])) AND random∗. Searches were updated until December 2015. After identifying relevant citations, the abstracts of these studies were read to decide if the studies were eligible. The full text was retrieved when the information in the title and/or abstract seemed to meet the objective of this review. A manual search of reference lists of studies was conducted to identify any relevant articles not found in the computerized search.
2.2. Inclusion and exclusion criteria
Randomized controlled trials (RCTs) comparing S-1-containing chemotherapy with 5-FU-containing chemotherapy, mono or combined chemotherapy with S-1 versus 5-FU, and not confounded by additional agents or interventions (i.e., the experimental and control arms in the combined chemotherapy differed only in S-1 and 5-FU) were eligible for inclusion. Abstracts or unpublished work with sufficient information were also included. Included patients were diagnosed with histologically confirmed unresectable, recurrent, or metastatic gastric or esophagogastric junction carcinoma. Reports of survival outcomes, response rates, or toxicities were mandatory for inclusion. Studies containing duplicate material were excluded, and the studies containing the best documented data were included for analysis.
2.3. Selection, quality assessment, and data extraction
Two reviewers (X-DC and F-QH) independently read the title and abstract of each searched citation to select eligible studies for further assessment. The full text of potentially eligible citations was retrieved and evaluated for inclusion. The Jadad scale was used to assess the quality of the selected studies.[8] Two reviewers independently extracted all relevant information in a data collection form, including study characteristics (author, country, sample size, regimen details, and methodological characteristics), efficacy, and safety data.
2.4. Outcomes of interest
The primary efficacy endpoint used for this study was OS. Secondary efficacy measures were endpoints based on tumor assessments, including ORR, defined as the sum of partial and complete response rates assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST), progression-free survival (PFS), time to treatment failure (TTF), and time to progression (TTP). Safety analysis was based on adverse events (AEs), including treatment-related deaths and toxicities graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI-CTCAE).
2.5. Statistical analysis
Statistical analyses of the pooled hazard ratio (HR) for OS, PFS, and TTF, the risk ratio (RR) or risk difference (RD) for ORR and AEs were calculated using Review Manager (RevMan) Version 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). We also compared the pooled estimates of OS and ORR for subgroups stratified by region (Asia vs non-Asia) and regimen (different combined cytotoxic drugs). Statistical heterogeneity was evaluated by the χ2 test and the I2 statistic and was considered high when P < 0.1 or I2 > 50%. In meta-analysis, the fixed-effects method was used when the statistical heterogeneity was low, and the random-effects model and subgroup analysis were applied when it was high. The result stability was evaluated by performing a sensitivity analysis, in which one study was removed at a time. The presence of publication bias was evaluated by using the Begg and Egger tests in StataSE version 12.0 (Stata Corporation, College Station, TX).[9,10] An HR < 1 indicated a favorable outcome of S-1-containing regimens for OS, PFS, and TTF. A RR > 1 or RD > 0 indicated a favorable ORR or more AEs in S-1-containing regimens. A 2-tailed P < 0.05 was considered statistically significant.
3. Results
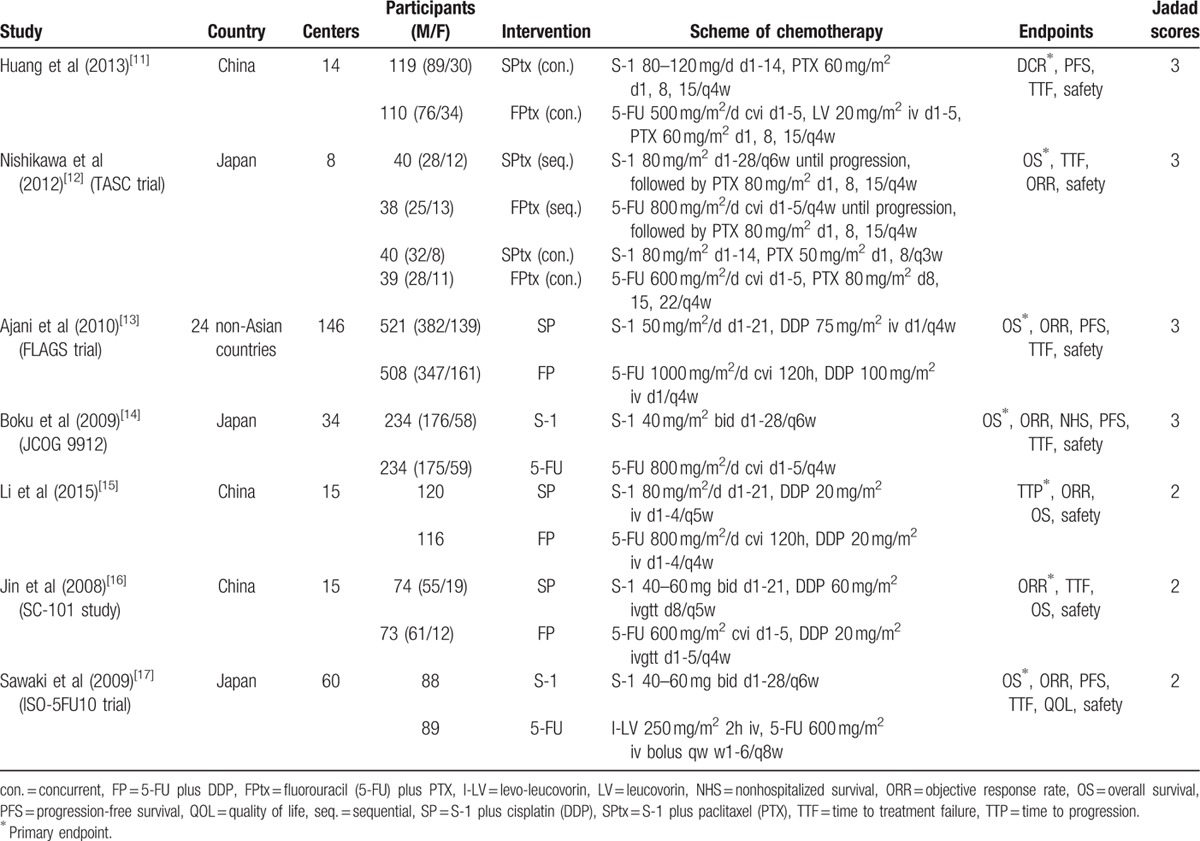
The literature search and selection procedure are shown in Figure 1. A total of 7 RCTs were eligible for analysis, including 5 full texts,[11–15] 1 abstract from ASCO Annual Meeting,[16] and 1 abstract from the ECCO/ESMO Congress.[17] The main characteristics of the included trials were shown in Table 1. Six studies were performed in East Asia (3 in Japan[12,14,17] and 3 in China[11,15,16]), and the remaining study was a non-Asian global phase III trial performed in 24 countries.[13] All studies were multicenter in nature, involving 8 to 146 centers. Of the 2443 patients in those studies, 1236 patients received S-1-containing chemotherapy and 1207 patients received 5-FU-containing chemotherapy. The sample size of individual trials ranged from 147 to 1029 patients. Two studies compared S-1 alone with 5-FU alone (S-1 vs 5-FU),[14,17] 3 compared S-1 plus cisplatin with 5-FU plus cisplatin (SP vs FP),[13,15,16] and 2 compared S-1 plus paclitaxel with 5-FU plus paclitaxel (SPtx vs FPtx).[11,12] Regimens were similar with respect to doses and schedules in every trial. There were no significant differences in the baselines between S-1-containing and 5-FU-containing groups in these studies as reported.
Figure 1.
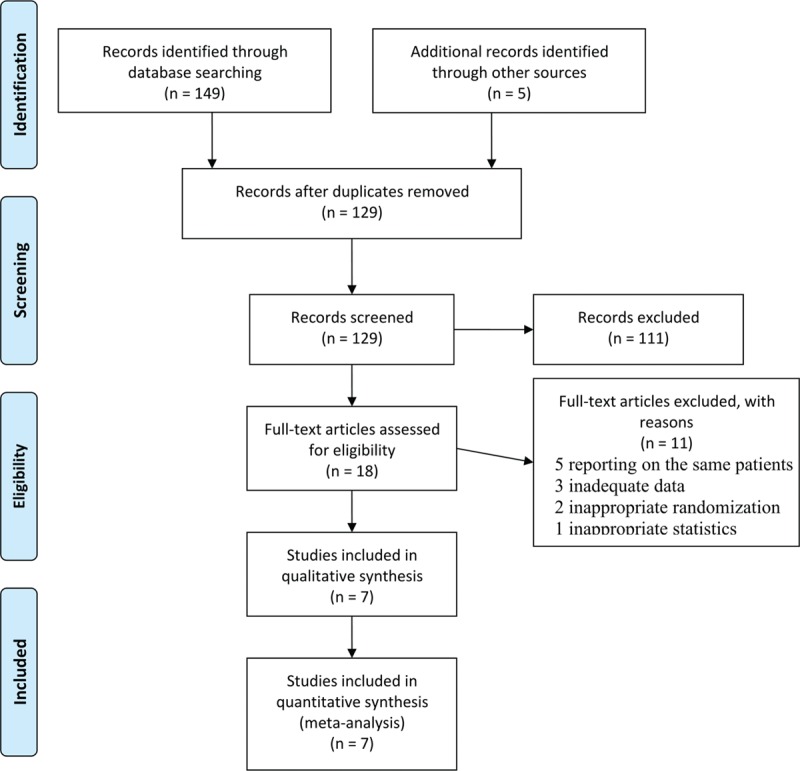
Flow diagram of literature search and selection procedure.
Table 1.
Characteristics of included trials.
3.1. Efficacy
3.1.1. OS
Six studies reported OS data. Median OS ranged from 8.3 to 15.2 months in the S-1-containing groups and 7.9 to 14.2 months in the 5-FU-containing groups (Table 2).[12–17] Two studies reported that S-1-containing regimens were superior to 5-FU-containing regimens,[14,16] whereas the others reported that S-1-containing regimens were comparable or noninferior to 5-FU-containing regimens. The meta-analysis showed no statistically significant improvement of OS with S-1-containing regimens versus 5-FU-containing regimens (HR = 0.91, 95% confidence interval [CI] [0.83–1.01], P = 0.07; P of heterogeneity = 0.18, I2 = 34%; Fig. 2). The subgroup analyses of OS by region (Asia and non-Asia) and regimen (S-1 vs 5-FU, SP vs FP, and SPtx vs FPtx) found no differences, and tests for subgroup differences showed no significant difference in heterogeneity among subgroups (Figs. 3 and 4, respectively).
Table 2.
Detailed information of OS, PFS, TTF, and TTP reported by some included trials.
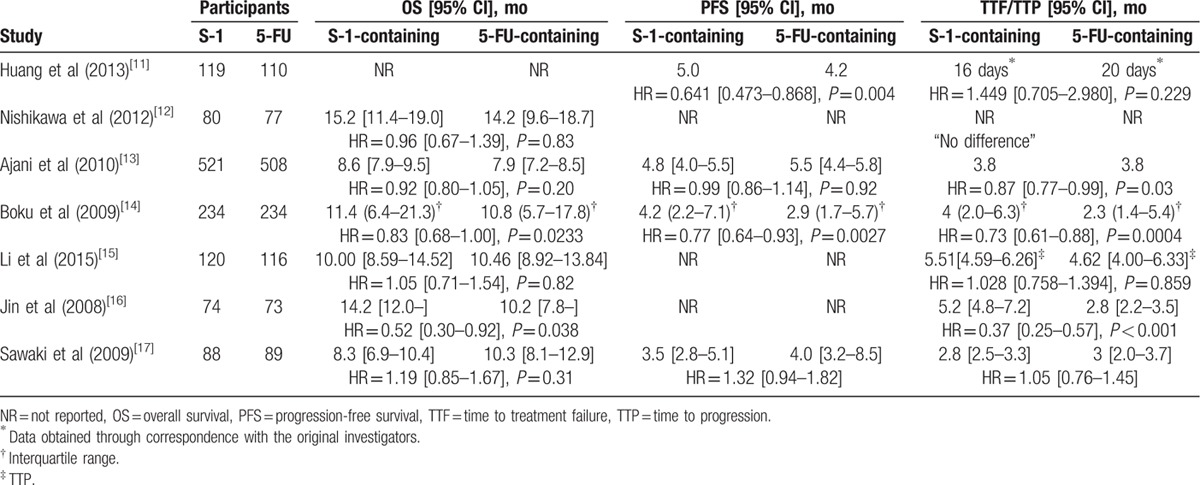
Figure 2.
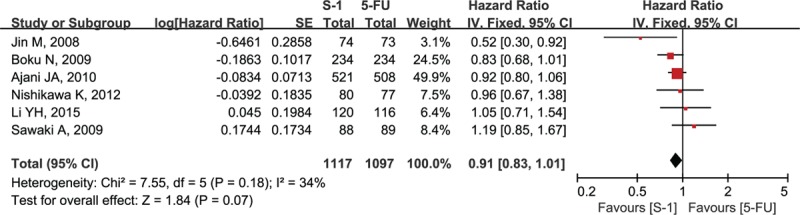
Forest plot of overall survival associated with the S-1-containing chemotherapy compared with the 5-FU-containing chemotherapy.
Figure 3.
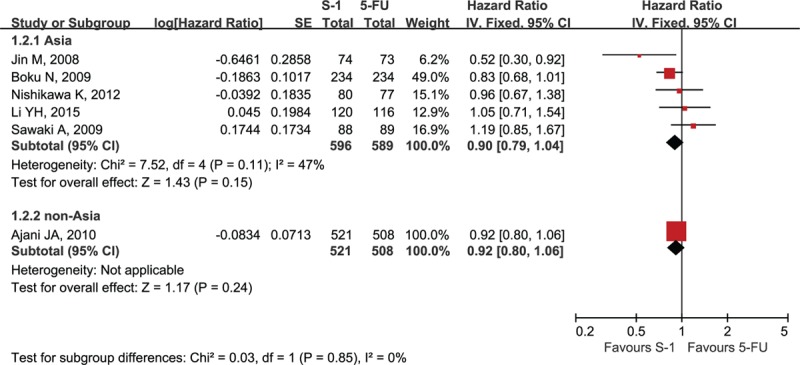
Forest plot of subgroup analysis of overall survival by region.
Figure 4.
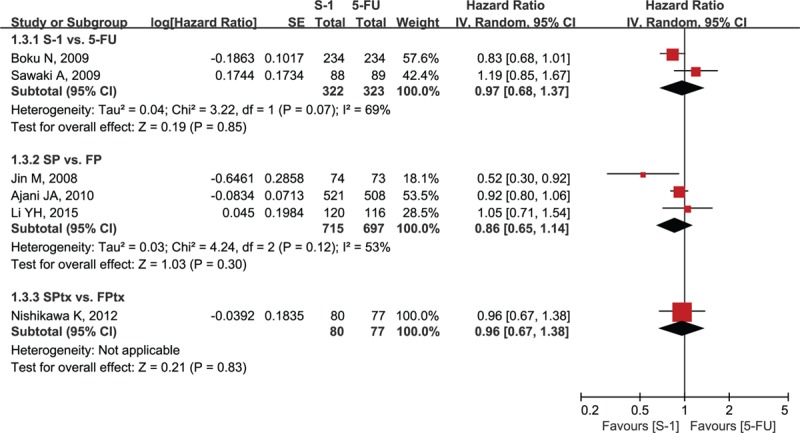
Forest plot of subgroup analysis of overall survival by regimen. SP = S-1 plus cisplatin, FP = 5-FU plus cisplatin, SPtx = S-1 plus paclitaxel, FPtx = 5-FU plus paclitaxel.
3.1.2. PFS
Four studies reported PFS, which ranged from 3.5 to 6.0 months in the S-1-containing groups and 2.9 to 5.5 months in the 5-FU-containing groups (Table 2).[11,13,14,17] Two Asian studies reported that S-1-containing regimens significantly prolonged PFS compared with 5-FU-containing regimens,[11,14] whereas the meta-analysis showed there were no significant benefits in PFS for the S-1-containing groups (HR = 0.89, 95% CI [0.70–1.13], P = 0.35; P of heterogeneity = 0.003, I2 = 79%; Fig. 5).
Figure 5.
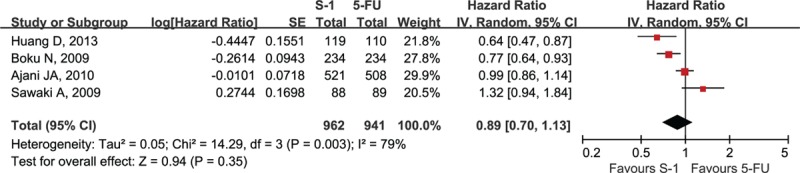
Forest plot of progression-free survival associated with the S-1-containing chemotherapy compared with the 5-FU-containing chemotherapy.
3.1.3. TTF
Six studies reported TTF, which ranged from 2.8 to 5.2 months in the S-1-containing groups and 2.8 to 3.8 months in the 5-FU-containing groups (Table 2).[11–14,16,17] One study reported that no difference in TTF was observed between S-1-containing and 5-FU-containing regimens but did not provide exact data for meta-analysis.[12] Another study reported the Kaplan-Meier curve and HR of TTF; however, the median of TTF obtained through correspondence with the original investigators was only 16 days and 20 days which were observed in 17 patients and 19 patients of the S-1-containing and 5-FU-containing groups, respectively.[11] Consequently, these 2 studies were excluded from the meta-analysis. The pooled data of the remaining 4 studies by the random-effects model were significantly in favor of the S-1-containing group (HR = 0.74, 95% CI [0.56–0.97], P = 0.03; P of heterogeneity = 0.0004, I2 = 84%; Fig. 6).
Figure 6.
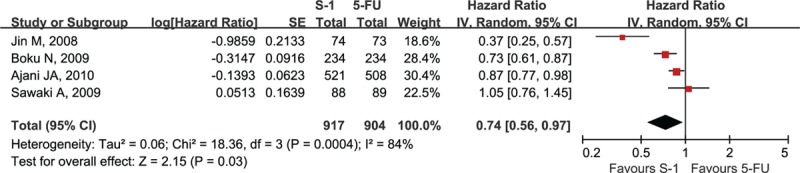
Forest plot of time to treatment failure associated with the S-1-containing chemotherapy compared with the 5-FU-containing chemotherapy.
3.1.4. TTP
Only one study demonstrated no significant difference in TTP between the 2 groups.[15]
3.1.5. ORR
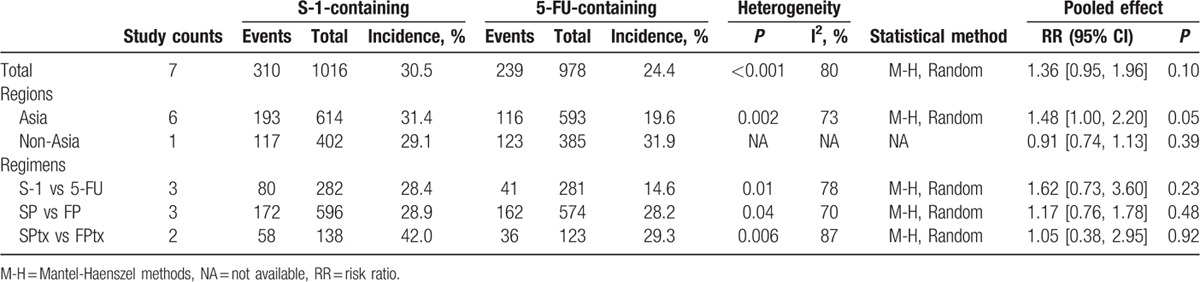
All studies provided information on response rate. ORR was 30.5% (310/1016) in the S-1-containing groups versus 24.4% (239/978) in the 5-FU-containing groups (Table 3). However, the meta-analysis showed no significant difference between the 2 groups (RR = 1.36, 95% CI [0.95, 1.96], P = 0.10; P of heterogeneity <0.001, I2 = 80%). The subgroup meta-analyses by region and regimen are shown in Table 3. The response rate in Asian patients seemed higher in S-1-containing groups than 5-FU-containing groups (31.4% vs 19.6%, RR = 1.48, 95% CI [1.00, 2.20], P = 0.05); however, no difference was observed in non-Asian patients. The comparisons between S-1 alone and 5-FU alone, SP and FP, SPtx and FPtx showed no significant differences.
Table 3.
Objective response rate by region and regimen.
3.2. Safety
We compared grade 1–4 and grade 3–4 toxicities in both groups according to reported information (Tables 4 and 5). The major toxicities of S-1-containing regimens were hematological toxicities including anemia, leukopenia, neutropenia, febrile neutropenia, thrombocytopenia, and gastrointestinal toxicities including nausea, vomiting, anorexia, diarrhea, constipation, and mucositis. Other relatively common toxicities were liver impairment (increased aminotransferases and bilirubin), renal impairment (increased creatinine and decreased calculated creatinine clearance), fatigue, infection, weight loss, and neuropathy.
Table 4.
Comparisons of treatment-related deaths and hematological toxicities between S-1-containing and 5-FU-containing regimens.
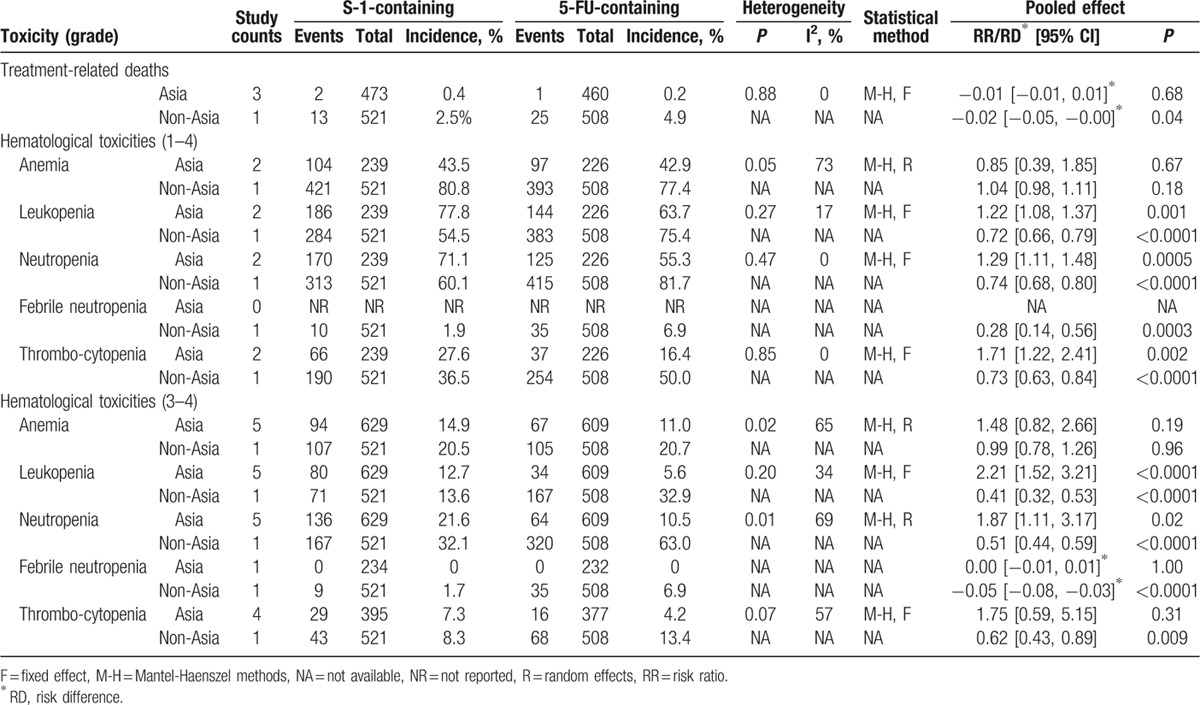
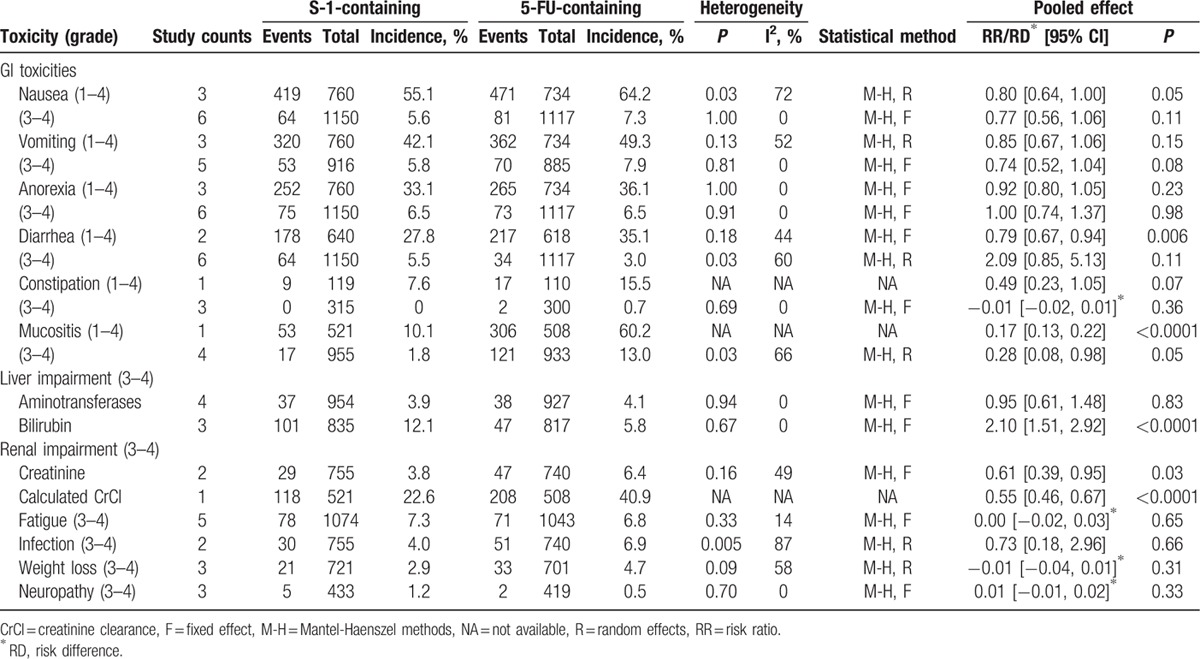
Table 5.
Comparisons of nonhematological toxicities between S-1-containing and 5-FU-containing regimens.
3.2.1. Treatment-related deaths
Treatment-related deaths were reported in 4 studies.[11,13–15] The accumulated treatment-related mortality rate in Asian patients was 0.4% (2/473) in the S-1-containing group versus 0.2% (1/460) in the 5-FU-containing group, without significant difference (RD = 0.00, 95% CI [−0.01, 0.01], P = 0.68; Table 4); however, the mortality rate in non-Asian patients was 2.5% (13/521) in the S-1-containing group versus 4.9% (25/508) in the 5-FU-containing group, which was a significant difference (RD = −0.02, 95% CI [−0.05, −0.00], P = 0.04; Table 4). Testing for subgroup differences was then performed, showing a significant difference in heterogeneity between Asian and non-Asian subgroups (P of heterogeneity = 0.04, I2 = 76.5%).
3.3. Hematological toxicities
The profile of hematological toxicities was distinct between Asian and non-Asian trials (Table 4). Testing for subgroup differences demonstrated significant difference in heterogeneity between Asian and non-Asian subgroups. In Asian patients, there was a significant increase of hematological toxicities in S-1-containing regimens such as leukopenia (grade 1–4: RR = 1.22, 95% CI [1.08, 1.37], P = 0.001; grade 3–4: RR = 2.21, 95% CI [1.52, 3.21], P < 0.0001), neutropenia (grade 1–4: RR = 1.29, 95% CI [1.11, 1.48], P = 0.0005; grade 3–4: RR = 1.87, 95% CI [1.11, 3.17], P = 0.02), and thrombocytopenia (grade 1–4: RR = 1.71, 95% CI [1.22, 2.41], P = 0.002). Conversely, in non-Asian patients, S-1-containing regimens were found to be associated with lower toxicities for leukopenia (grade 1–4: RR = 0.72, 95% CI [0.66, 0.79], P < 0.0001; grade 3–4: RR = 0.41, 95% CI [0.32, 0.53], P < 0.0001), neutropenia (grade 1–4: RR = 0.74, 95% CI [0.68, 0.80], P < 0.0001; grade 3–4: RR = 0.51, 95% CI [0.44, 0.59], P < 0.0001), febrile neutropenia (grade 1–4: RR = 0.28, 95% CI [0.14, 0.56], P = 0.0003; grade 3–4: RD = −0.05, 95% CI [−0.08, −0.03], P < 0.0001), and thrombocytopenia (grade 1–4: RR = 0.73, 95% CI [0.63, 0.84], P < 0.0001; grade 3–4: RR = 0.62, 95% CI [0.43, 0.89], P = 0.009). However, no significant difference in anemia was found in both subgroups.
3.4. Gastrointestinal toxicities
With regard to nonhematological toxicities, there was no significant heterogeneity between Asian and non-Asian subgroups. Gastrointestinal toxicities were frequent in both groups (Table 5). The incidence of nausea, vomiting, anorexia, diarrhea, and constipation demonstrated no significant differences between groups except that S-1-containing regimens were associated with a significantly decreased incidence of grade 1–4 nausea (RR = 0.80, 95% CI [0.64, 1.00], P = 0.05) and grade 1–4 diarrhea (RR = 0.79, 95% CI [0.67, 0.94], P = 0.006) (Table 4). Of note, S-1-containing regimens were associated with less frequent and less severe mucositis (stomatitis and/or mucosal inflammation), with an incidence of grade 1–4 mucositis of 10.1% versus 60.2% with 5-FU-containing regimens (RR = 0.17, 95% CI [0.13, 0.22], P < 0.001), and grade 3–4 of 1.9% versus 14.7%, respectively (RR = 0.23, 95% CI [0.06, 0.88], P = 0.03).
3.4.1. Other toxicities
Liver impairment (increased bilirubin) was significantly more frequent in S-1-containing than 5-FU-containing regimens (12.1% vs 5.8%, RR = 2.10, 95% CI [1.51, 2.92], P < 0.001). However, the incidence of renal impairment was significantly less frequent in S-1-containing regimens, with 3.8% of patients exhibiting increased creatinine versus 6.4% in 5-FU-containing regimens (RR = 0.61, 95% CI [0.39, 0.95], P = 0.03) and 22.6% of S-1 patients having decreased calculated creatinine clearance versus 40.9% in 5-FU (RR = 0.55, 95% CI [0.46, 0.67], P < 0.001). Some toxicities, including fatigue, infection, weight loss, and neuropathy, were reported infrequently and had similar incidence in both groups (Table 5).
3.5. Sensitivity analysis
Sensitivity analysis showed that the corresponding pooled HRs and ORs were not significantly altered when one study was removed at a time, suggesting stability of the results.
3.6. Publication bias
Begg funnel plot and Egger test were performed to assess the publication bias of the analyzed studies. The shapes of the Begg funnel plots did not reveal evidence of obvious asymmetry (P = 1.00 and 0.76 for OS and ORR, respectively; Fig. 7). Egger test was then used to provide statistical evidence of funnel plot symmetry. The results still suggested no evidence of publication bias (P = 0.91 and 0.20 for OS and ORR, respectively).
Figure 7.
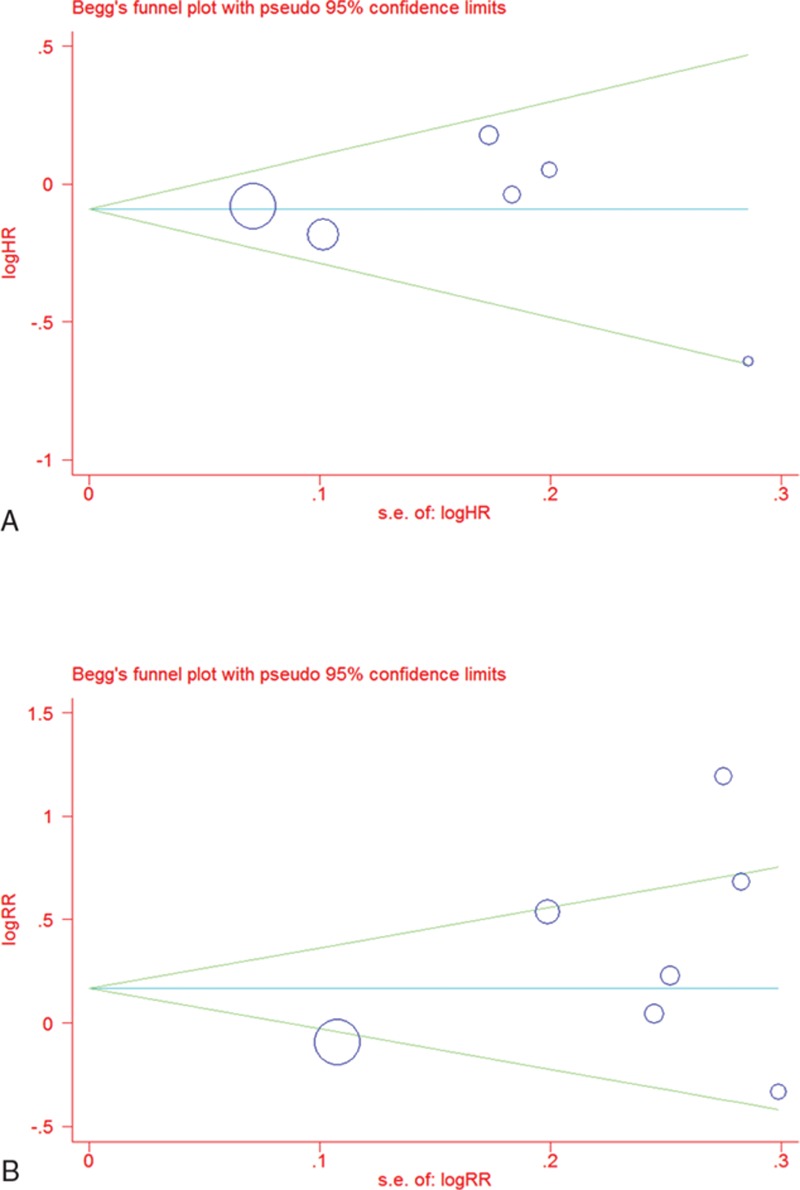
Begg funnel plot of publication bias test: (A) overall survival (OS) and (B) objective response rate (ORR). Each point represents a separate study for the indicated association; log = natural logarithm, s.e. = standard error.
4. Discussion
Five meta-analyses were identified in the literature search.[6,7,18–20] The main characteristics and results of these analyses were compared with the present study in Table 6. Although 2 published meta-analyses showed the superiority of S-1-containing regimens compared with 5-FU-containing regimens in terms of OS, one contained duplicate material[6] and the other missed a trial which was included in the present analysis.[7] In the present study, the pooled data revealed no significant improvement in OS for S-1-containing regimens (HR = 0.91, 95% CI [0.83–1.01], P = 0.07). It seems difficult to draw firm data-driven conclusion as to survival benefit of S-1. The median OS of S-1 monotherapy was approximately 11 months and the addition of other cytotoxic drugs, such as cisplatin,[16,21] oxaliplatin,[22] paclitaxel,[23] docetaxel,[24,25] or irinotecan[26,27] to S-1, even prolonged median OS beyond 12 months. Several trials comparing these doublets with S-1 monotherapy were conducted and demonstrated that the doublets resulted in a longer OS than that resulting from S-1 monotherapy.[16,21,23,25] However, there are no data indicating which doublets are superior as first-line treatments for AGC.[28–30] Therefore, we suggest that the emphasis should be on further developing S-1 with different cytotoxic drugs and/or biologic agents to anticipate the prolongation of OS in patients with AGC.
Table 6.
Comparisons of the main characteristics and results of the identified analyses with the present study.
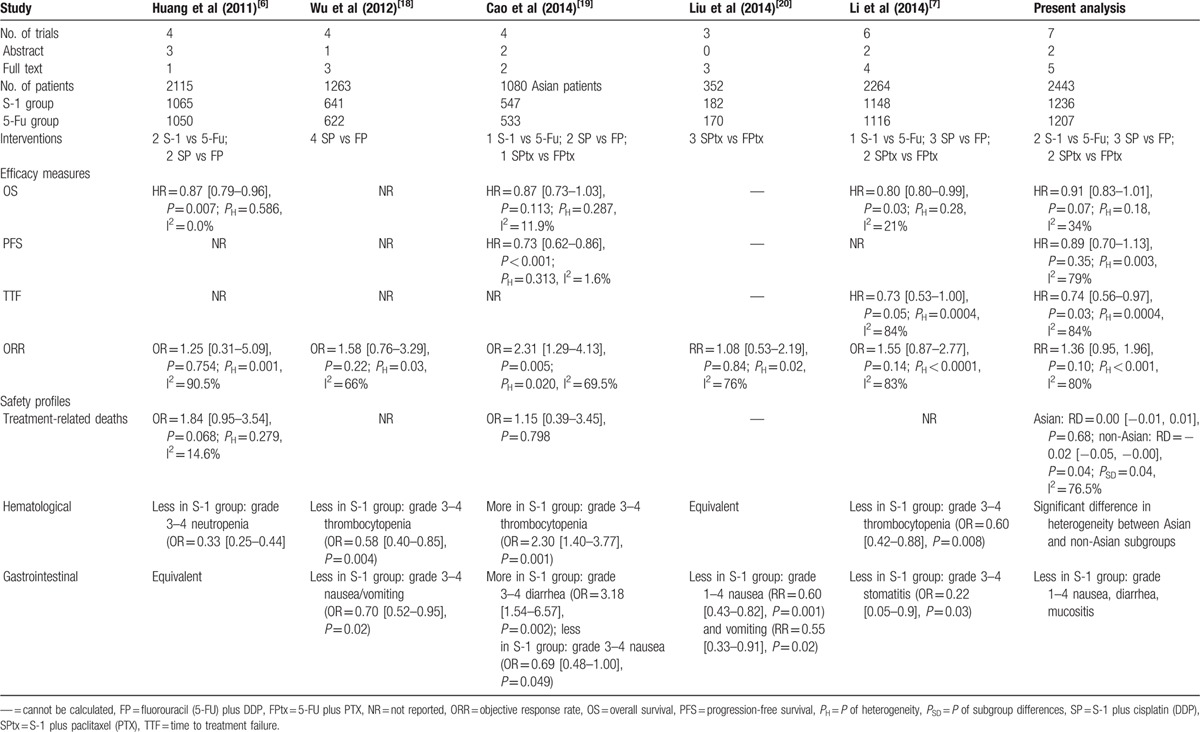
Regarding endpoints based on tumor assessments, the pooled data showed that there were no significant differences in ORR and PFS between the 2 groups, whereas S-1-containing regimens were associated with much longer TTF than 5-FU-containing regimens. However, there was significant heterogeneity among trials; therefore, these results should be interpreted with caution. The subgroup meta-analyses showed that there was higher ORR in Asian patients with S-1-containing regimens than 5-FU-containing regimens, but no such difference was found in non-Asian patients. Different stage presentation may affect the efficacy; however, there was no obvious difference in the extent of disease between Asian and non-Asian patients. The profile of stage presentation was remarkably similar in all trials, that is, the majority (above 95%) of patients had metastatic disease and over two-thirds had more than one site of metastasis. In fact, according to the analyzed data in the present study (Tables 2 and 3), the efficacy of S-1 was closely comparable between Asian and non-Asian patients. The median OS was 8.6 months in non-Asian patients versus 8.3 to 15.2 months in Asian patients, PFS 4.8 months versus 3.5 to 5.0 months, TTF 3.8 months versus 2.8 to 5.2 months, ORR 29.1% versus 31.4%; nevertheless, the ORR of 5-FU-containing regimens was evidently high in non-Asian patients (31.9% vs 19.6%), which might be responsible for the difference.
With respect to safety profiles, S-1 monotherapy had a low incidence of grade 3–4 toxicities (usually <5% for each toxicity)[31]; however, the addition of other cytotoxic drugs significantly increased the incidence of grade 3–4 toxicities, which remained manageable.[16,21,23,25,27] It is noteworthy that there was significant heterogeneity in the incidence of treatment-related deaths and hematological toxicities between Asian and non-Asian patients. The reported treatment-related mortality was <1% in Asian patients, but was >2.5% in non-Asian patients. Treatment-related deaths were mainly caused by myelosuppression and infection.[11,13] Accordingly, the incidences of grade 3–4 leukopenia, neutropenia, and febrile neutropenia were markedly higher in non-Asian patients, especially in those assigned to 5-FU-containing regimens. Actually, according to the data from Table 4, the incidences of hematological toxicities for S-1-containing regimens were comparable between Asian and non-Asian patients; by contrast, the incidences for 5-FU-containing regimens were relatively low in Asian patients whereas remarkably high in non-Asian patients. As a result, the incidences of hematological toxicities in Asian patients were significantly higher in S-1-containing regimens than those in 5-FU-containing regimens. Conversely, the rates of hematological toxicities in non-Asian patients were significantly less frequent in S-1-containing regimens than 5-FU-containing regimens.
The reason for these differences is complicated and may be related to geographic region, dose, and schedule of cytotoxic drugs and gene polymorphism. A meta-analysis indicated that the Asian trials were associated with lower incidences of grade 3–4 neutropenia and febrile neutropenia, and concluded that geographic region was an independent predictor of safety in chemotherapy for gastric cancer.[32] The dose and schedule of cytotoxic drugs varied among studies due to the individual distinctions and practice culture in different regions. In every chemotherapy cycle, the daily dose of S-1 was usually 80 mg/m2 in Asian patients versus 50 mg/m2 in non-Asian patients; the total dose of 5-FU varied widely among studies, from 2500 mg/m2 to 4000 mg/m2 in Asian patients versus 5000 mg/m2 in non-Asian patients. We observed that the non-Asian patients were administered noticeably lower single doses of S-1 but higher total doses of 5-FU. In addition, the total dose of cisplatin was lower, from 60 mg/m2 to 80 mg/m2 in S-1-containing arms versus 80 mg/m2 to 100 mg/m2 in 5-FU-containing arms; moreover, cisplatin was administered daily at a dose of 20 mg/m2/d intravenously over 4 to 5 days in Asian studies, whereas at the total dose was administered intravenously over 1 to 3 hours in non-Asian studies. However, the metabolic rate of conversion of tegafur to 5-FU differs in various ethnic populations,[33] Cytochrome P450 2A6 (CYP2A6) enzyme is now identified as the principal enzyme responsible for this conversion process.[34] The efficacy of CYP2A6 enzyme is higher in non-Asian patients than in Asian patients, which is attributed to different polymorphisms in the CYP2A6 gene.[35–37] Thus, the conversion rate of tegafur to 5-FU was faster in non-Asian patients, which would cause a lower dose of S-1 in non-Asian patients to achieve a comparable area under the curve of 5-FU with that in Asian patients and provide considerable improved safety without compromising efficacy. Taking all into consideration, the clinical heterogeneity of hematological toxicities between Asian and non-Asian patients would be mainly attributed to higher doses of 5-FU and cisplatin in 5-FU-containing regimens.
With respect to the nonhematological toxicities, S-1-containing regimens significantly reduced the frequency and severity of mucositis compared with 5-FU-containing regimens. A grade 3–4 increase in total bilirubin was the only notable nonhematological toxicity observed more frequently in S-1-containing regimens. However, there were no differences with respect to grade 3–4 elevations in aminotransferases (ALT/AST) or reports of death due to drug-related hepatic toxicity, indicating that there was no evidence of direct hepatotoxicity by S-1.[31,38] Ajani et al acknowledged that the significantly lower rate of renal function abnormalities, such as elevated serum creatinine and impairment of renal clearance in the cisplatin/S-1 arm, could be attributed to lower cisplatin dose (75 mg/m2) than in the cisplatin/infusional fluorouracil arm (100 mg/m2).[13]
Although the data on QOL and cost-effectiveness analysis were limited, S-1-containing regimens seemed to be associated with longer nonhospitalized survival, fewer hospitalizations for drug administration, and lower monetary costs. One abstracts mentioned QOL as secondary endpoints, but no detailed data were reported.[17] Because infusional chemotherapy is commonly performed with hospitalization, we would presume that the nonhospitalized survival reflects a patient's benefit from a QOL point of view.[5] Boku et al reported that S-1 was associated with longer nonhospitalized survival compared with 5-FU (9.3 [interquartile range 4.2–18.0] vs 7.2 [2.7–13.3] months, HR = 0.77, 95% CI [0.63–0.92], P = 0.0025).[14] Moreover, Ajani et al reported that the total number of hospitalizations and percentage of patients hospitalized were lower and the median number of days hospitalized was shorter in S-1-containing arm.[38] In addition to efficacy and safety concerns, expenditure on chemotherapy drugs has recently become a main concern. Unfortunately, few trials compared S-1 with 5-FU from an economic point of view. Only Boku et al offered some information in their discussion section, reporting that in Japan, the cost of S-1 was cheaper than that of 5-FU.[14]
Some limitations should be considered when interpreting the data presented here. First, 2 included studies were abstracts from international conferences with insufficient data on methodological and patient characteristics. In addition, many important estimates, such as PFS, TTF, TTP, and some AEs, were not reported in many of the studies analyzed. These omissions might potentially limit detection of difference. Furthermore, heterogeneity problems were frequently found among the included studies and subgroups, which may have influenced our results. Therefore, we chose to use the random-effects model as well as subgroup analysis to calculate the estimates and explain the causes of heterogeneity, such as differences in geographic region, dose, and schedule of cytotoxic drugs. In addition, most of the studies included were performed in Asia, with only one non-Asian study. We noticed significant differences in efficacy and safety profiles, such as median OS, treatment-related mortality, and incidence of hematological toxicities. However, future studies are needed to determine the mechanism underlying this phenomenon.
In conclusion, S-1-containing regimens could not improve survival outcomes, but increase some hematological toxicities in Asian patients, compared with 5-FU-containing regimens. Therefore, special attention on hematological toxicities should be paid to Asian patients because S-1 is administered on an outpatient basis. Moreover, whether S-1 could provide advantages in terms of QOL and monetary costs needs to be clarified by further trials.
Footnotes
Abbreviations: 5-FU = fluorouracil, AEs = adverse events, AGC = advanced gastric cancer, CI = confidence interval, HR = hazard ratio, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, QOL = quality of life, RCTs = randomized controlled trials, RD = risk difference, RR = risk ratio, TTF = time to treatment failure, TTP = time to progression.
X-DC and F-QH equally contributed to this work.
Funding: This research was funded by Health and Family Planning Commission of Sichuan Province (No. 130226) and Sichuan Provincial Science and Technology Project (No. 2014FZ0089). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; Available at: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx Accessed January 17, 2016. [Google Scholar]
- [2].Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903–9. [DOI] [PubMed] [Google Scholar]
- [3].Wöhrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol 2004;15:1585–95. [DOI] [PubMed] [Google Scholar]
- [4].Shirasaka T. Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 2009;39:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boku N. JCOG trials of systemic chemotherapy for unresectable or recurrent gastric cancer. Gastric Cancer 2009;12:43–9.19390931 [Google Scholar]
- [6].Huang J, Cao Y, Wu L, et al. S-1-based therapy versus 5-FU-based therapy in advanced gastric cancer: a meta-analysis. Med Oncol 2011;28:1004–11. [DOI] [PubMed] [Google Scholar]
- [7].Li DH, Pan ZK, Ye F, et al. S-1-based versus 5-FU-based chemotherapy as first-line treatment in advanced gastric cancer: a meta-analysis of randomized controlled trials. Tumour Biol 2014;35:8201–8. [DOI] [PubMed] [Google Scholar]
- [8].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [9].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [10].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huang D, Ba Y, Xiong J, et al. A multicentre randomised trial comparing weekly paclitaxel + S-1 with weekly paclitaxel + 5-fluorouracil for patients with advanced gastric cancer. Eur J Cancer 2013;49:2995–3002. [DOI] [PubMed] [Google Scholar]
- [12].Nishikawa K, Morita S, Matsui T, et al. A randomized phase-II trial comparing sequential and concurrent paclitaxel with oral or parenteral fluorinated pyrimidines for advanced or metastatic gastric cancer. Gastric Cancer 2012;15:363–9. [DOI] [PubMed] [Google Scholar]
- [13].Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 2010;28:1547–53. [DOI] [PubMed] [Google Scholar]
- [14].Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009;10:1063–9. [DOI] [PubMed] [Google Scholar]
- [15].Li YH, Qiu MZ, Xu JM, et al. S-1 plus cisplatin versus fluorouracil plus cisplatin in advanced gastric or gastro-esophageal junction adenocarcinoma patients: a pilot study. Oncotarget 2015;6:35107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jin M, Lu H, Li J, et al. Randomized 3-armed phase III study of S-1 monotherapy versus S-1/CDDP (SP) versus 5-FU/CDDP (FP) in patients (pts) with advanced gastric cancer (AGC): SC-101 study. J Clin Oncol 2008;26suppl 15:4533. [Google Scholar]
- [17].Sawaki A, Yamaguchi K, Nabeya Y, et al. 5-FU/l-LV (RPMI) versus S-1 as first-line therapy in patients with advanced gastric cancer: a randomized phase III non-inferiority trial. (ISO-5FU10 study group trial). Eur J Cancer Supplements 2009;7:364. [Google Scholar]
- [18].Wu HJ, Waqng JR, Xu CA. Meta-analysis of S-1 plus cisplatin chemotherapy versus 5-fluorouracil plus cisplatin chemotherapy for advanced gastric cancer [Chinese]. Chin J Cancer Prev Treat 2012;19:134–8. [Google Scholar]
- [19].Cao C, Zhang X, Kuang M, et al. Survival benefit from S-1 as compared to fluorouracil in Asian patients with advanced gastrointestinal cancer: a meta-analysis. Cancer Sci 2014;105:1008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu H, Chen X, Sun J, et al. The efficacy and toxicity of paclitaxel plus S-1 compared with paclitaxel plus 5-FU for advanced gastric cancer: a PRISMA systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2014;93:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215–21. [DOI] [PubMed] [Google Scholar]
- [22].Koizumi W, Takiuchi H, Yamada Y, et al. Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study). Ann Oncol 2010;21:1001–5. [DOI] [PubMed] [Google Scholar]
- [23].Wang X, Wang ML, Zhou LY, et al. Randomized phase II study comparing paclitaxel with S-1 vs. S-1 as first-line treatment in patients with advanced gastric cancer. Clin Transl Oncol 2013;15:836–42. [DOI] [PubMed] [Google Scholar]
- [24].Park SR, Kim HK, Kim CG, et al. Phase I/II study of S-1 combined with weekly docetaxel in patients with metastatic gastric carcinoma. Br J Cancer 2008;98:1305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol 2014;140:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Uedo N, Narahara H, Ishihara R, et al. Phase II study of a combination of irinotecan and S-1 in patients with advanced gastric cancer (OGSG0002). Oncology 2007;73:65–71. [DOI] [PubMed] [Google Scholar]
- [27].Narahara H, Iishi H, Imamura H, et al. Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002). Gastric Cancer 2011;14:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mochiki E, Ogata K, Ohno T, et al. Phase II multi-institutional prospective randomised trial comparing S-1+paclitaxel with S-1+cisplatin in patients with unresectable and/or recurrent advanced gastric cancer. Br J Cancer 2012;107:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Higuchi K, Koizumi W, Yamada Y, et al. Randomized phase III study of S-1 plus oxaliplatin versus S-1 plus cisplatin for first-line treatment of advanced gastric cancer. J Clin Oncol 2013;30suppl 34: abstr 60. [Google Scholar]
- [30].Sugimoto N, Fujitani K, Imamura H, et al. Randomized phase II trial of S-1 plus irinotecan versus S-1 plus paclitaxel as first-line treatment for advanced gastric cancer (OGSG0402). Anticancer Res 2014;34:851–7. [PubMed] [Google Scholar]
- [31].Yamanaka T, Matsumoto S, Teramukai S, et al. Safety evaluation of oral fluoropyrimidine S-1 for short- and long-term delivery in advanced gastric cancer: analysis of 3,758 patients. Cancer Chemother Pharmacol 2008;61:335–43. [DOI] [PubMed] [Google Scholar]
- [32].Hsu C, Shen YC, Cheng CC, et al. Geographic difference in safety and efficacy of systemic chemotherapy for advanced gastric or gastroesophageal carcinoma: a meta-analysis and meta-regression. Gastric Cancer 2012;15:265–80. [DOI] [PubMed] [Google Scholar]
- [33].Ajani JA, Faust J, Ikeda K, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 2005;23:6957–65. [DOI] [PubMed] [Google Scholar]
- [34].Ikeda K, Yoshisue K, Matsushima E, et al. Bioactivation of tegafur to 5-fluorouracil is catalyzed by cytochrome P-450 2A6 in human liver microsomes in vitro. Clin Cancer Res 2000;6:4409–15. [PubMed] [Google Scholar]
- [35].van der Weide J, Steijns LS. Cytochrome P450 enzyme system: genetic polymorphisms and impact on clinical pharmacology. Ann Clin Biochem 1999;36:722–9. [DOI] [PubMed] [Google Scholar]
- [36].Yoshida R, Nakajima M, Nishimura K, et al. Effects of polymorphism in promoter region of human CYP2A6 gene (CYP2A6∗9) on expression level of messenger ribonucleic acid and enzymatic activity in vivo and in vitro. Clin Pharmacol Ther 2003;74:69–76. [DOI] [PubMed] [Google Scholar]
- [37].Daigo S, Takahashi Y, Fujieda M, et al. A novel mutant of the CYP2A6 gene (CYP2A6∗11) found in a cancer patient who showed poor metabolic phenotype towards tegafur. Pharmacogenetics 2002;12:299–306. [DOI] [PubMed] [Google Scholar]
- [38].Ajani JA, Buyse M, Lichinitser M, et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer 2013;49:3616–24. [DOI] [PubMed] [Google Scholar]


