Abstract
Actinomycosis is a rare heterogeneous anaerobic infection with misleading clinical presentations that delay diagnosis. A significant number of misdiagnosed cases have been reported in specific localizations, but studies including various forms of actinomycosis have rarely been published.
We performed a multicenter retrospective chart review of laboratory-confirmed actinomycosis cases from January 2000 until January 2014. We described clinical characteristics, diagnostic procedures, differential diagnosis, and management of actinomycosis of clinical significance.
Twenty-eight patients were included from 6 hospitals in France. Disease was diagnosed predominately in the abdomen/pelvis (n = 9), orocervicofacial (n = 5), cardiothoracic (n = 5), skeletal (n = 3), hematogenous (n = 3), soft tissue (n = 2), and intracranially (n = 1). Four patients (14%) were immunocompromised. In most cases (92 %), the diagnosis of actinomycosis was not suspected on admission, as clinical features were not specific. Diagnosis was obtained from either microbiology (50%, n = 14) or histopathology (42%, n = 12), or from both methods (7%, n = 2). Surgical biopsy was needed for definite diagnosis in 71% of cases (n = 20). Coinfection was found in 13 patients (46%), among which 3 patients were diagnosed from histologic criteria only. Two-thirds of patients were treated with amoxicillin. Median duration of antibiotics was 120 days (interquartile range 60–180), whereas the median follow-up time was 12 months (interquartile range 5.25–18). Two patients died.
This study highlights the distinct and miscellaneous patterns of actinomycosis to prompt accurate diagnosis and earlier treatments, thus improving the outcome. Surgical biopsy should be performed when possible while raising histologist's and microbiologist's awareness of possible actinomycosis to enhance the chance of diagnosis and use specific molecular methods.
Keywords: Actinomyces spp., actinomycosis, differential diagnosis, immunocompetent, internal medicine
1. Introduction
Actinomycosis is a rare, chronic granulomatous disease, caused by Gram-positive filamentous organisms, mainly anaerobic or microaerophilic bacteria from the Actinomycetaceae family (genus Actinomyces).[1]Actinomyces spp. are pigment-producing rods that form branching filaments, commensal in human open cavities, mainly oropharynx, gastrointestinal tract, and female genital tract. The most frequent pathogen species encountered is Actinomyces israelii,[1,2] but many different species have been described and are associated with pathogenic conditions specific to anatomical sites.[3]
The infection occurs as a contiguous growth through anatomic barriers when mucosal tissue is damaged, leading to the formation of abscesses and fistula. Coinfection with other organisms is common, and may contribute to infection by inhibiting host defenses, as suggested in animal studies.[4,5] The role of immunosuppression is unclear and it is important to note that most reported cases were described in immunocompetent individuals. The disease is characterized by a wide range of clinical presentations that include nonspecific symptoms; therefore, it is often misdiagnosed with other conditions such as tuberculosis, nocardiosis, or malignancies.[6] Distinct clinical features are reported according to the anatomical location, but misleading forms are frequent. Usual presentations include orocervicofacial abscesses in patients with poor oral hygiene and dentition,[2] thoracic infection after aspiration of oropharyngeal secretion or gastric content,[7] abdominal infection after surgery or gastrointestinal perforation, and pelvic infection in patient with intra uterine contraceptive devices (IUDs).[8] Actinomycosis is readily treatable and the bacteria are susceptible to most of the β-lactam agents.[9]
In the literature, most reported cases of actinomycosis are not suspected at the clinical onset of the symptoms. Failure to diagnose the disease in a timely and reliable way seems to increase mortality and unnecessary costly diagnostic investigations. Most studies present cases of a distinct clinical form, whereas broader studies including diverse and multiorgan forms of actinomycosis have sparsely been published. The aim of this study is to highlight the miscellaneous clinical forms to diagnose and treat actinomycosis earlier. In this retrospective multicenter study, we report a series of 28 patients with various clinical presentations.
2. Methods
The study involved 6 participating centers in France, mainly teaching hospitals (Aurillac, Bordeaux, Marseille, Metz, Poitiers, and La Reunion). Adult patients with actinomycosis admitted to internal medicine departments were identified retrospectively through French hospital discharge databases of participating centers from January 2000 to January 2014.
Inclusion criteria were a microbiological or histological confirmation from tissue biopsies, and pus or blood sample specimens. Microbiological confirmation was defined as the conjunction of several factors: presence of nonspore-forming Gram-positive rods on direct examination, slow growing organisms in anaerobic culture conditions (chocolate blood agar media at 35–37°C), and specific biochemical profiling with commercial kit (API A 20, bioMérieux, SA, France). Histological confirmation was defined as the presence of Gram-positive filamentous organism nonpositive on acid-fast staining concomitant with sulphur granules, which are of significant diagnostic relevance.[3]
Exclusion criteria were: age under 18, diagnosis of actinomycosis documented before admission to internal medicine ward, cases including positive microbiological identification of Actinomyces spp. from superficial site without clinical or radiological hallmarks of infection, and medical records without sufficient data. For each case, we reviewed demographic characteristics, underlying diseases, clinical and radiological manifestations, microbiological results, histological assessment, and treatment and outcome data. Two cases have already been reported in literature.[10,11]
Owing to the restricted number of individuals included in the study, the quantitative variables were described as medians and interquartile ranges. Categorical data were described as numbers and percentages. Comparisons of quantitative variables were performed with nonparametrical Mann–Whitney U test. A P value under 0.05 was considered to indicate statistical significance.
This study was conducted in compliance with good clinical practices and the Declaration of Helsinki principles. All collected data were anonymized in standardized forms. In accordance with French law, formal approval from an ethics committee, and also written informed consent, is not required for this type of study.
2.1. Definitions
We used the following definitions:
Coinfection: Concomitant presence of another microorganism identified by microbiological methods on the same sample(s) that identified Actinomyces spp.
Fever: Axillary temperature ≥38°C measured with a mercury thermometer.
Sepsis: Sepsis and septic shock were defined as proposed by American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee.[12]
3. Results
Twenty-eight patients were included in our study. The median age at presentation was 52 years (interquartile range [IQR] 45–58), and 50% were female. Patients’ characteristics are summarized in Table 1. A local risk factor was found in 20 patients (71%), mainly local tissue injury including dental procedure or poor dentition, radiotherapy, IUDs, surgery, or trauma. Common risk factors were present in 20 patients, including diabetes mellitus in 4 patients (14%), immunosuppressive agent intake in 4 patients (14%), malignancies in 6 patients (22%) previously treated by chemotherapy (3 patients) and radiotherapy (1 patient), and 1 patient with alcoholism. None of the patients were HIV-positive.
Table 1.
Summary of 28 cases of actinomycosis.
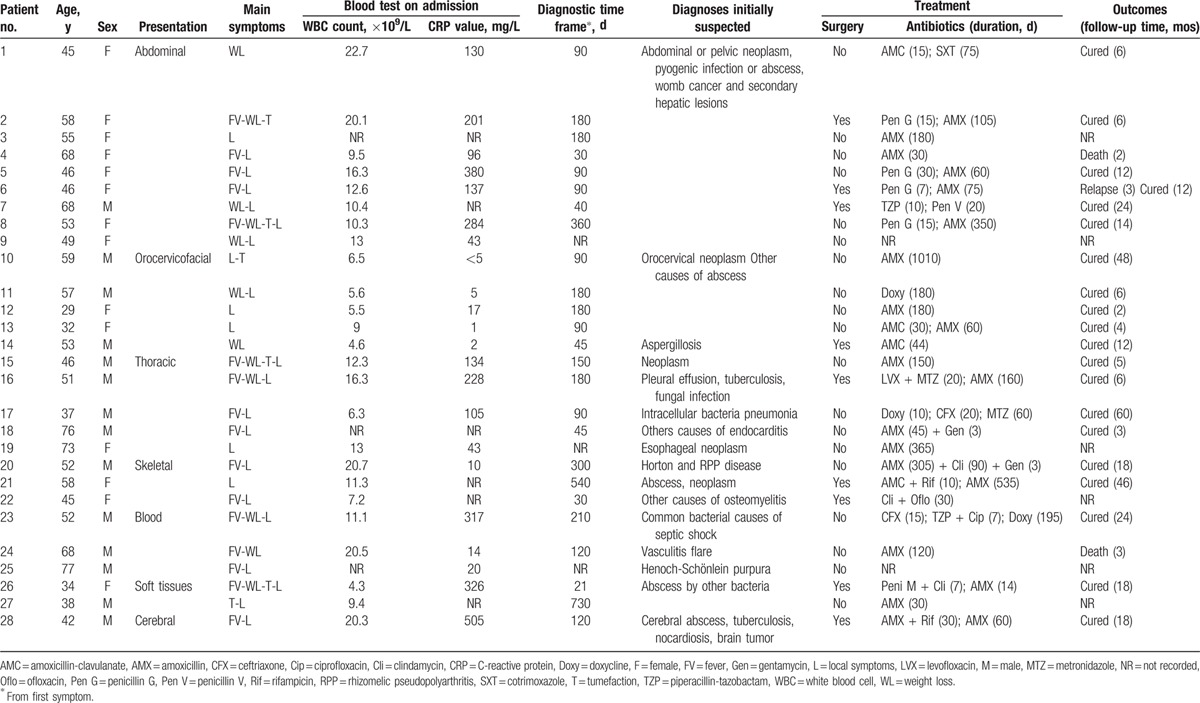
Concerning general symptoms, fever was observed in 16 patients (57%), whereas weight loss and anorexia were present in 12 (43%) and 14 (50%) patients, respectively. C-reactive protein elevation and leukocytosis were observed in 19 patients (67%) and 10 patients (30%), respectively. Median diagnosis time frame from first symptoms was 30 days (IQR 20–157).
With respect to anatomical location of infection, abdominopelvic location was the most frequent with 9 patients (32%). An IUD was present in 7 patients, among whom 3 patients had the IUD for more than 10 years. Presenting complaints were abdominal pain (90%), fever (60%), weight loss (60%), and, to a lesser extent, lower urinary tract symptoms. Seven patients suffered from abdominal abscesses (including patient 1; Fig. 1), among which 2 patients had concomitant hepatic actinomycosis (patients 2 and 8). Patient 8 presented with a rectal fistula from tubo-ovarian uterine abscess. Patient 7 presented with a pseudotumoral gastric tumor in which low-grade mucosa-associated lymphoid tissue (MALT) lymphoma was associated with numerous Actinomyces on histological examination. Hydronephrosis was present in 2 patients requiring ureteral stent placement.
Figure 1.
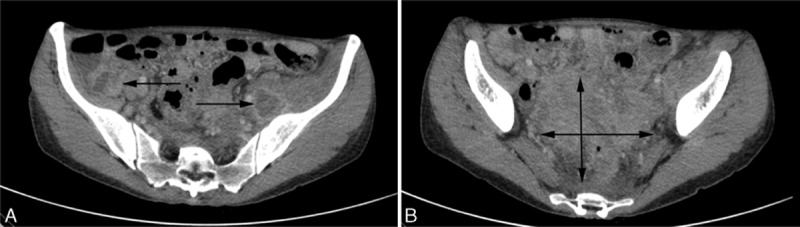
Abdominal and pelvic abscesses (black arrows) consecutive to actinomycosis in a patient with intrauterine device carried for more than 10 years (patient 1). Part A, CT-scan at pelvic level showing numerous abscesses. Part B, CT-scan at abdominal level. CT = computed tomography.
Among 5 patients with orocervicofacial localization, 3 patients (patients 11, 12, and 13) had mandibular or maxillary osteomyelitis. Mandibular osteonecrosis was seen in patient 11 who had a recent history of radiotherapy and surgery for a gingival cancer. Patients 14 and 10 had sinuses and pharyngeal abscess, respectively.
Among cardiothoracic forms, patient 15 had a voluminous chest wall tumor without rib lysis. Patient 16 had a history of tuberculosis and presented with a cavitary mass with an air-fluid level, surgically removed and complicated by postpneumectomy empyema. Patient 17 presented with a pneumonia with nonspecific interstitial infiltrates. Patient 18 presented with a mitral valve endocarditis from hematogenous spread from an infected implantable port. Patient 19 presented with esophageal actinomycosis and had a history of esophageal neoplasm with a stent.
Patient 20 was diagnosed with cervical spondylitis and spinal abscess. Patient 21 presented with paraplegia and ataxia. He had T1-T2 spondylitis with spinal abscess responsible for cord compression. Patient 22 had osteomyelitis of his foot, with history of type 2 diabetes.
Three patients were diagnosed with actinomycosis directly from blood sample microbiological identification. Patients 24 and 25 presented with sepsis on admission and both had 1 positive blood culture set indicating bacteremia. A concomitant vasculitis flare secondary to chronic myelomonocytic leukemia was present in patient 24. Patient 25 had an acute renal failure secondary to Henoch–Schönlein purpura. Patient 23 had septic shock complicated by resuscitated cardiac arrest. Actinomycosis was the only bacteria growing from his blood samples. Transesophageal echocardiography and sinus imaging were normal. Culture negativity was obtained at 1 month of treatment.
Two patients presented with isolated soft tissue infection from local trauma. Patient 26 had an inguinal abscess in the setting of hidradenitis suppurativa. Patient 27 presented with a slow-growing thigh mass after human bite when practicing rugby.
Patient 28 presented with a 3 cm temporal lobe abscess with peripheral edema and herniation signs (Fig. 2). Stereotaxic surgery was performed to assess diagnosis, and the patient was treated for a total duration of 120 days. Ventriculoperitoneal shunt was used to treat postinfection hydrocephalus.
Figure 2.
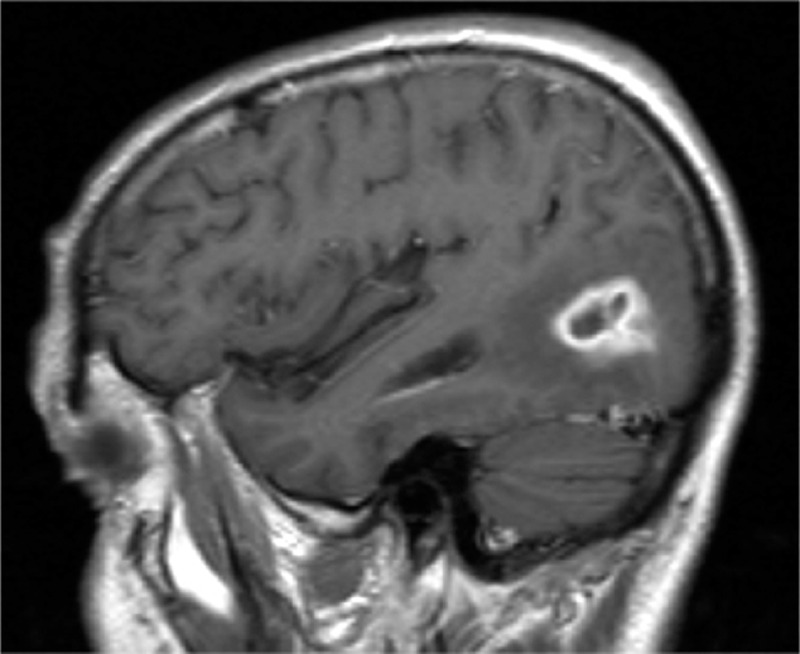
Brain abscess due to Actinomyces meyeri in patient 28. Brain MRI T1 sequence showing a 3 cm tumefaction in the right posterior temporal region, with annular homogeneous contrast enhancement and peripheral edema. MRI = magnetic resonance imaging.
In most cases (92 %), the diagnosis of actinomycosis was not suspected on admission. The most frequent initial diagnosis suspected were malignancies and widespread infections such as tuberculosis (Table 1). Imaging was performed in 82% of patients, including computed tomography (CT)-scan (19/28 [67%]), magnetic resonance imaging (MRI) (6/28 [21%]), ultrasound (14%), and positron emission tomography (PET) scan (n = 1). Findings were those of nonspecific abscesses with accurate anatomical location.
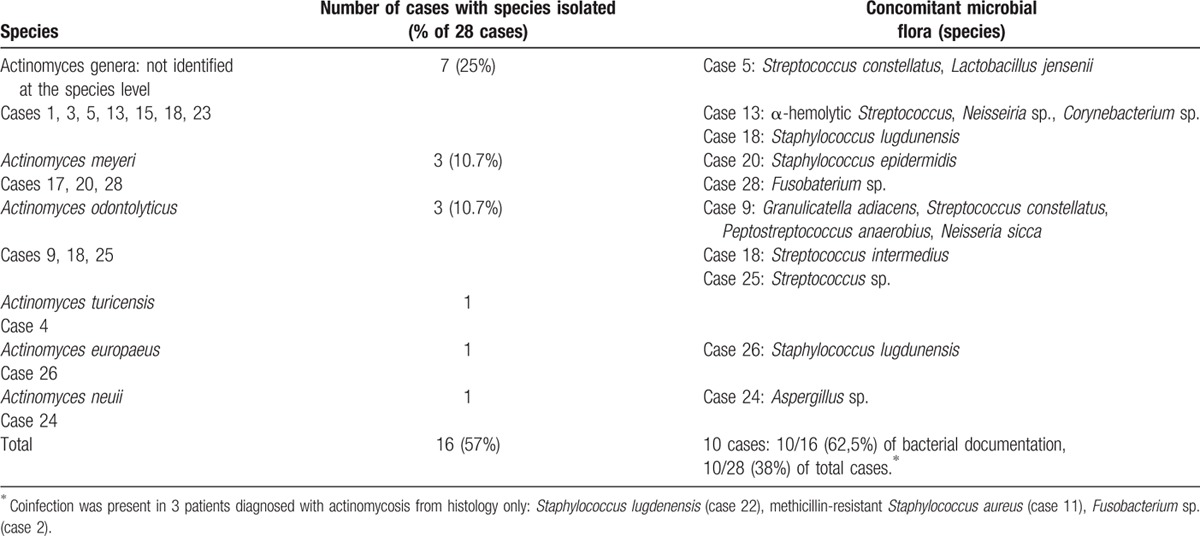
Diagnosis was obtained from either microbiology (50%, n = 14) or histopathology (42%, n = 12), or both methods (7%, n = 2). Positive specimens were surgical biopsy (71%), blood culture (18%), bronchoalveolar lavage, urine culture, IUD culture, or vaginal sample. The incubation period needed to recover the bacteria ranged from 3 days to 3 weeks. Actinomycosis genus identification and species were obtained in 16 patients (57%) and 9 patients (32%), respectively (Table 2). Coinfection was found in 13 patients (46%), among which 3 patients were diagnosed from histology criteria only. Antibiotic susceptibility was evaluated in 13 patients (among 16): almost all strains were susceptible to amoxicillin, rifampicin, clindamycin, and cotrimoxazole.
Table 2.
Microbiological identification and coinfection in 28 cases of actinomycosis.
Median follow-up time was 12 months (5.25–18), with 6 patients lost to follow-up. All patients were treated with antibiotics, mainly beta-lactams and especially amoxicillin, which was used in two-thirds of patients (Table 1). The median duration of treatment was 120 days (60–180): 90 days (77–135) in abdominopelvic forms, 180 days (90–180) in orocervicofacial forms, and 150 days (90–180) in cardiothoracic forms. Comparing the duration of treatment between 2 groups of patients, diagnosed before and after 2009, respectively (n = 13 each), showed no difference. Among the 4 patients who were treated for less than 30 days, 1 patient died, 2 were lost to follow-up, and 1 had positive outcome. Eight patients (28%) underwent surgery. Surgery did not significantly change antibiotic treatment duration in comparison with patients not undergoing surgery (P = 0.43). The outcome was favorable in 19/28 (67%) and poor in 3 (10.7%) patients. In regards to poor outcome, 1 recurrence occurred, leading to hysterectomy. It was the only case where medical treatment failure led to surgery as a rescue therapy. Two patients died from pathologic conditions that were not related to actinomycosis: carcinomatoses (patient 4) and chronic myelomonocytic leukemia (patient 24).
4. Discussion
In our study, we have described 28 cases of miscellaneous forms of actinomycosis, a rare life-threatening infectious disease with nonspecific features whose prognosis is excellent if treated early. Thus, this infection represents a great diagnostic challenge.
Actinomycosis is certainly under-reported as a consequence of diagnostic errors, difficulties in confirming the disease, and the empirical utilization of antibiotics. Data regarding incidence estimates are lacking from developing countries. The incidence in Cleveland, USA, was reported to be 1 per 300,000 in 1970s, whereas the Department of Health in the United Kingdom reported 71 cases of actinomycosis in England between 2002 and 2003 (0.0006% of hospital consultations).[1] Largest series focused on single anatomical forms,[2,13–17] whereas some case reports of unusual presentations have been published.[18,19] To date, there is only 1 published study composed of multiple forms, limited to immunocompromised patients.[20] Most of our patients were immunocompetent. The design of our study does not enable us to calculate the incidence of the disease, but the results indicate its rarity with less than 30 cases identified in 6 different hospital centers over 14 years.
The onset of the disease is usually chronic with indolent course, but may be acute as described in patient 23, which is, to date, the first described actinomycosis-related septic shock complicated with cardiac arrest. Slow-growing abscesses were present in most cases, resulting in mass-like features, but the presence of fever was variable, which was only 57% in our study. Actinomycosis was not considered in the differential diagnosis of most patients on admission and the median duration from first symptoms to diagnosis was 1 month. The clinical picture was misinterpreted as malignancies or other infectious diseases, such as tuberculosis, pyogenic abscesses, and nocardiosis. For the latter, Actinomyces is indistinguishable from Nocardia on Gram stain. However, Nocardia is rather encountered in immunosuppressed people, grows aerobically, and is acid-fast.[21] Our study highlights the miscellaneous clinical patterns of actinomycosis, and the essential differential diagnoses are discussed below according to main anatomical forms.
Presence of Actinomyces in culture of bronchopulmonary secretions can be misleading because coexistence of actinomycosis with tuberculosis or neoplasm is possible, leading to a more challenging diagnosis and treatment of thoracic actinomycosis.[22] In our study, 1 patient presented history of tuberculosis that misled diagnosis of actinomycosis, whereas intracellular organism pneumonia was initially diagnosed in another patient. In a series of 94 patients with pulmonary actinomycosis confirmed pathologically, only 6 patients (6.4%) were initially diagnosed as having pulmonary actinomycosis on the basis of clinical and radiological findings.[13] In another study, diagnosis was reported to be suspected on admission in only 7% to 18% of patients with thoracic involvement who later turned out to have pulmonary actinomycosis infection.[15]
It has been estimated that fewer than 10% of patients are diagnosed preoperatively in abdominal forms, because of a similar presentation to other common conditions such as malignancies, tuberculosis, or Crohn disease.[8,23] In our study, no patient was diagnosed preoperatively. In abdominal forms, the lack of improvement with conventional therapy for a presumptive diagnosis of Crohn disease frequently leads to surgery, correcting the diagnosis. Cases of actinomycosis mimicking digestive neoplasm have been reported,[24] and also PET scan use in cases mimicking unresponsive or relapsing cancer, later diagnosed from surgical biopsy as actinomycosis.[25]
In our study, failure to isolate the organism was 42%, which is consistent with other series.[1,20] Twenty patients (71%) were diagnosed from surgical biopsy. As shown in our study, clinical symptoms and imaging lack of specificity to definite the disease, thus biopsy should be performed in cases when actinomycosis is considered as differential diagnosis. Some species have been associated to peculiar body sites, such as Actinomyces meyeri and central nervous system (patient 28), and Actinomyces europaeus and soft tissue (patient 26). Little is known regarding the virulent determinants involved in such associations.[3]
The mouth is an important source of Actinomyces bacteriemia.[3] However, patients 23 and 25 had no recent history of dental procedures or periodontitis and the point of entry was not found. The presumed point of entry was the sinus for patient 24, since he had sinus aspergillosis removed 3 months earlier.
The proportion (46%) and composition of concomitant microbial flora were consistent with the literature. Since isolation of Actinomyces species from polymicrobial infections is frequent, they must be assumed to contribute to the pathogenic processes.[3] Concomitant organisms are considered to synergistically enhance the infectious process.[4]
Treatment recommendations are based on small case series and in vivo studies, as no randomized controlled studies have evaluated antibiotic regimens. Two-thirds of patients were treated with amoxicillin. There is no consensus over the utilization of a lactamase inhibitor, which offers additional cover against potential coisolates. Principle of long-term antibiotic therapy has been traditionally recommended based on clinical experiences and has been justified by relapses occurring in this disease.[15] The duration of antibiotic therapy was extremely variable in our study, ranging from 3 weeks to 4 years. Trend towards a shorter course of antibiotics, as described in recent years, was not observed in our study.[1,26] This indicates that long-term treatment still prevails in clinical practice, although it may not be needed. Thus, the length of treatment is controversial and further studies are needed.
Owing to the misleading nature of this disease, many patients received empiric therapy, which may contribute to underdiagnose actinomycosis cases via bacterial decapitation for cultures. However, most of the empirically treated patients with actinomycosis may present a good outcome because of the usual susceptibility of Actinomyces to many antibiotics used as first choice in those empirical settings. Moreover, whereas prognosis is excellent in early-treated patients—mortality range from 0% to 28% depending on site of infection—delay in time to diagnose the disease may lead to increased mortality.[27]
This study has several limitations. Owing to the retrospective design, cases of actinomycosis could be missing as those diagnosed after discharge. Five patients were lost to follow-up; however, the other patients were followed for 1 year in average, which can be considered as satisfactory in the setting of an infectious disease. We cannot ascertain the significance of some specimens obtained from nonsterile site. However, considering Actinomyces spp. was the only organism recovered from several specimens in this situation, we assumed that they should be involved in the pathogenesis. Considering the rarity of the disease, studies with larger scale should be performed to include more patients. Forty-two per cent of our patients were diagnosed from histological criteria, but are those criteria specific enough to ascertain actinomycosis? More precise bacterial identification methods are increasingly used, including 16SrRNA gene sequence analysis or matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry.[28–30] These methods were not performed in our study, although they should be encouraged to accurate the diagnosis and taxonomy of Actinomyces. Among microbiologically positive cases, 25% were identified at the genus but not species level: altogether 75% of our patients were unidentified Actinomyces species. Noteworthy, novel species have been recognized in recent years from these methods, highlighting a high number of currently unnamed species-level Actinomyces taxa.[3]
5. Conclusions
Actinomycosis is an insidious disease wearing many masks. We described 28 cases of miscellaneous clinical pictures. Although misleading forms are not unusual, abdominopelvic, orocervicofacial, and thoracic cases were predominant in our multicenter study and still stand as the classic forms. Therefore, clinicians should be aware of those 3 groups to avoid long diagnostic delay and rely on typical clinical clues: IUDs and pelvic forms, surgery and abdominal forms, poor oral hygiene and orocervicofacial forms, tuberculosis or pseudotumoral forms, and pulmonary forms. Other forms are more anecdotal, and the scarcity of the infection justifies that other differential diagnoses will be first mentioned. Only then should biopsy procedure be performed while raising histologist's and microbiologist's awareness of possible actinomycosis to enhance the chance of correct diagnosis. Earlier diagnosis should then be obtained, preventing complicated forms and improving the outcome of actinomycosis while decreasing costly unnecessary investigations.
Acknowledgments
The authors thank the following persons: N. Jourde, J. Constans, M. Dupon (for their help in the management of clinical data); P. Gasque, R. Mylvaganam (manuscript review).
Footnotes
Abbreviations: CT = computed tomography, HIV = human immunodeficiency virus, IQR = interquartile range, IUD = intrauterine contraceptive device, MALDI-TOF = matrix-assisted laser desorption/ionization time-of-flight, MALT = mucosa-associated lymphoid tissue, MRI = magnetic resonance imaging, PET = positron emission tomography.
The authors state that they have no conflict of interest concerning this article.
References
- [1].Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ 2011;343:d6099. [DOI] [PubMed] [Google Scholar]
- [2].Pulverer G, Schütt-Gerowitt H, Schaal KP. Human cervicofacial actinomycoses: microbiological data for 1997 cases. Clin Infect Dis 2003;37:490–7. [DOI] [PubMed] [Google Scholar]
- [3].Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev 2015;28:419–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Peters BM, Jabra-Rizk MA, Graeme AO, et al. Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev 2012;25:193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jordan HV, Kelly DM, Heeley JD. Enhancement of experimental actinomycosis in mice by Eikenella corrodens. Infect Immun 1984;46:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Smego RA, Foglia G. Actinomycosis. Clin Infect Dis 1998;26:1255–61. [DOI] [PubMed] [Google Scholar]
- [7].Oostman O, Smego RA. Cervicofacial actinomycosis: diagnosis and management. Curr Infect Dis Rep 2005;7:170–4. [DOI] [PubMed] [Google Scholar]
- [8].Garner JP, Macdonald M, Kumar PK. Abdominal actinomycosis. Int J Surg Lond Engl 2007;5:441–8. [DOI] [PubMed] [Google Scholar]
- [9].Steininger C, Willinger B. Resistance patterns in clinical isolates of pathogenic Actinomyces species. J Antimicrob Chemother 2016;71:422–7. [DOI] [PubMed] [Google Scholar]
- [10].Duvignaud A, Ribeiro E, Moynet D, et al. Cervical spondylitis and spinal abscess due to Actinomyces meyeri. Braz J Infect Dis 2014;18:106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Constans J, Barcat D, Skopinski S, et al. Thoracic actinomycosis sensitive to amoxicillin. Rev Médecine Interne Fondée Par Société Natl Francaise Médecine Interne 2002;23:1030–1. [DOI] [PubMed] [Google Scholar]
- [12].Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–55. [DOI] [PubMed] [Google Scholar]
- [13].Kim SR, Jung LY, Oh I-J, et al. Pulmonary actinomycosis during the first decade of 21st century: cases of 94 patients. BMC Infect Dis 2013;13:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Song J-U, Park HY, Jeon K, et al. Treatment of thoracic actinomycosis: a retrospective analysis of 40 patients. Ann Thorac Med 2010;5:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park JY, Lee T, Lee H, et al. Multivariate analysis of prognostic factors in patients with pulmonary actinomycosis. BMC Infect Dis 2014;14:10.doi:10.1186/1471-2334-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Choi M-M, Baek JH, Beak JH, et al. Clinical features of abdominopelvic actinomycosis: report of twenty cases and literature review. Yonsei Med J 2009;50:555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Smego RA. Actinomycosis of the central nervous system. Rev Infect Dis 1987;9:855–65. [DOI] [PubMed] [Google Scholar]
- [18].Acevedo F, Baudrand R, Letelier LM, et al. Actinomycosis: a great pretender. Case reports of unusual presentations and a review of the literature. Int J Infect Dis 2008;12:358–62. [DOI] [PubMed] [Google Scholar]
- [19].Cabot RC, Rosenberg ES, Harris NL, et al. Case 25-2015: an 8-year-old girl with a chest-wall mass and a pleural effusion. N Engl J Med 2015;373:657–67. [DOI] [PubMed] [Google Scholar]
- [20].Pierre I, Zarrouk V, Noussair L, et al. Invasive actinomycosis: surrogate marker of a poor prognosis in immunocompromised patients. Int J Infect Dis 2014;29:74–9. [DOI] [PubMed] [Google Scholar]
- [21].Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc 2012;87:403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cheung MK, Lam WY, Fung WYW, et al. Sputum Microbiota in Tuberculosis as Revealed by 16S rRNA Pyrosequencing. PLoS One 2013;8:e54574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cintron JR, Del Pino A, Duarte B, et al. Abdominal actinomycosis. Dis Colon Rectum 1996;39:105–8. [DOI] [PubMed] [Google Scholar]
- [24].Fichte S, Brodhun M, Göttinger S, et al. Vertebral and pulmonary actinomycosis mimicking metastatic lung cancer. J Neurol Surg Part Cent Eur Neurosurg 2013;74suppl 1:e188–92. [DOI] [PubMed] [Google Scholar]
- [25].Weisshaupt C, Hitz F, Albrich WC, et al. Pulmonary actinomycosis and Hodgkin's disease: when FDG-PET may be misleading. BMJ Case Rep 2014;2014: pii: bcr2014206034. doi: 10.1136/bcr-2014-206034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sudhakar SS, Ross JJ. Short-term treatment of actinomycosis: two cases and a review. Clin Infect Dis 2004;38:444–7. [DOI] [PubMed] [Google Scholar]
- [27].Pézier TF, Kastrinidis N, Widmer G-M, et al. Fatally invasive actinomycosis masquerading as a tonsillar carcinoma. Head Neck 2014;36:E129–30. [DOI] [PubMed] [Google Scholar]
- [28].Kolbert CP, Persing DH. Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Curr Opin Microbiol 1999;2:299–305. [DOI] [PubMed] [Google Scholar]
- [29].Ng LSY, Sim JHC, Eng LC, et al. Comparison of phenotypic methods and matrix-assisted laser desorption ionisation time-of-flight mass spectrometry for the identification of aero-tolerant Actinomyces spp. isolated from soft-tissue infections. Eur J Clin Microbiol Infect Dis 2012;31:1749–52. [DOI] [PubMed] [Google Scholar]
- [30].Seng P, Drancourt M, Gouriet F, et al. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis 2009;49:543–51. [DOI] [PubMed] [Google Scholar]


