Abstract
To investigate the impact of age on the clinicopathological features and survival of patients with gastric cancer (GC), and hope to better define age-specific patterns of GC and possible associated risk factors.
Using the surveillance, epidemiology, and end results (SEER) database to search the patients who diagnosed GC between 2007 and 2011 with a known age. The overall and 5-year gastric cancer specific survival (CSS) data were obtained using Kaplan–Meier plots. Multivariable Cox regression models were built for the analysis of long-term survival outcomes and risk factors.
A total of 7762 GC patients treated with surgery during the 4-year study period were included in the final study cohort. We divided into five subgroups according to the different age ranges. The overall 5-year cause-specific survival (CSS) was 60.3% in Group 1 (below 45 years), 60.3% in the Group 2 (45–55 years), 61.2% in Group 3 (56–65 years), 59.2% in Group 4 (66–75 years), and 59.2% in Group 5 (older than 76 years). Kaplan–Meier plots showed that patients older than 76 years had the worst 5-year CSS of 56.0% rate in all the subgroups. Age, tumor size, primary site, histological type, and Tumor Node Metastasis stage were identified as significant risk factors for poor survival on univariate analysis (all P < 0.001, log-rank test). Additionally, as the age increased, the risk of death for GC demonstrated a significant increase.
In conclusion, our analysis of the SEER database revealed that the prognosis of GC varies with age. Patients at age 56 to 65 group have more favorable clinicopathologic characteristics and better CSS than other groups.
Keywords: age, gastric cancer, prognosis, SEER
1. Introduction
Gastric cancer (GC) is the fifth most common malignancy and the third leading cause of cancer related death worldwide owing to its high prevalence.[1,2] Currently, the main treatment of GC consists of surgical resection plus standard D2 lymphadenectomy, adjuvant chemotherapy, and some molecular targeting therapy.[3] Despite many advances in diagnosis and treatment of this disease, the prognosis for GC remains poor.[4] In order to improve the long-term survival, it is important to better understand the mechanisms of disease progression and find new effective predictive prognostic factors as the targets of interventions.
Age plays a significant role in some cancers, such as colorectal cancer, while the notion that age is a significant prognostic factor in GC has been controversial. It mainly occurred in older population, with a peak reported incidence for patients from 50 to 70 years.[5] However, the rate of GC in young patients has increased over the past few decades, despite a reduction in the overall prevalence of the disease.[6] Age at diagnosis, a key variable, not only was used as an indispensable adjusted element in the observational studies, but also contained inestimable value for prognosis. But the cut-off of age for defining young or old patients has differed significantly among studies.[7,8] Furthermore, findings comparing prognosis between younger and older patients have been inconsistent.[9]
In this regard, we used data from the surveillance, epidemiology and end results (SEER) registries to investigate the impact of age on the clinicopathological features and survival of patients with GC, and hope to better define age-specific patterns of GC and possible associated risk factors.
2. Patients and methods
2.1. Study population and data extracted
We relied on the SEER database and used the SEER-stat software (SEER∗Stat 8.1.5) to search the patients who diagnosed GC between 2007 and 2011 with a known age. Sex, tumor site, histological type, AJCC stage, and cause-specific survival (CSS) were extracted from the SEER database. Histological types were limited to adenocarcinoma, mucinous adenocarcinoma, and signet ring cell carcinoma. Survival time was calculated from the date of diagnosis to the date of cancer-specific death. The exclusion criterions included incomplete staging information, no evaluation of histological type, no lymph nodes examined pathologically and died within 30 days after surgery. The current SEER project includes 17 population-based cancer registries, which can represent about 28% of the US population. The SEER data are available for the public so that we can deal with many issues regarding cancer-based epidemiology.
2.2. Informed consent and institutional review board approval
This study was based on a publicly available data (the SEER database) and we have got the permission to access them on purpose of research only (Reference number: 12666-Nov2014). It did not include interaction with humans or use personal identifying information. Thus, the informed consent for this part was not required.
2.3. Statistical analysis
In this study, to clarify the impact of age at diagnosis on prognosis, we first classified it into a categorical variable of five groups: below 45 years, 45 to 55 years, 56 to 65 years, 66 to 75 years, and older than 76 years. The group of patients aged 56 to 65 years was used as reference. Survival curves were generated using Kaplan–Meier analyses, differences between the curves were analyzed by log-rank test. Cox regression models were built for analysis of risk factors for survival outcomes in GC patients. Baseline characteristics were compared using the χ2 likelihood test for nominal variables, and non-parametric Wilcoxon test for continuous variables. Potential nonlinear association between age and the hazard ratios of GCSS was assessed using polynomial regression. Statistical analyses were performed using the statistical software package SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL). All P values were two-sided. P values <0.05 were considered statistically significant. All CIs were stated at the 95% confidence level.
3. Results
3.1. Characteristics and clinical features of patients
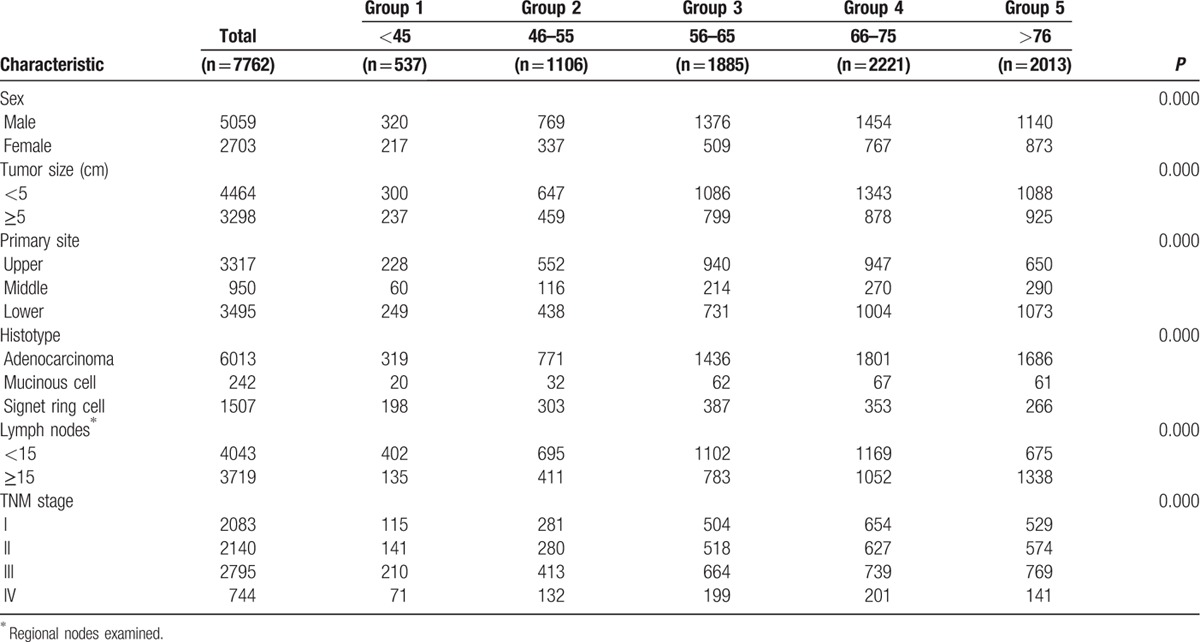
Overall, 7762 GC patients treated with surgery were included in the final study cohort. There were 5059 men (65.18%) and 2703 women (34.82%), with ages ranging from 14 to 100 years. Of these, 537 (6.92%) were in Group 1, 1106 (14.25%) were in Group 2, 1885 (24.28%) were in Group 3, 2221 (28.62%) were in Group 4, and 2013 (25.93%) were in Group 5. The median age was 36. Table 1 presents patient demographics and pathological features. Significant differences were recorded regarding patient characteristics. As shown in Table 1, it was investigated that significant differences were found among the sex, tumor size, primary site, histological type, regional nodes examined, and TNM stage. All of them had statistical difference (P = 0.000).
Table 1.
Characteristics of Patients from SEER Database by Age.
3.2. Impact of age on CSS of GC patients in the SEER database
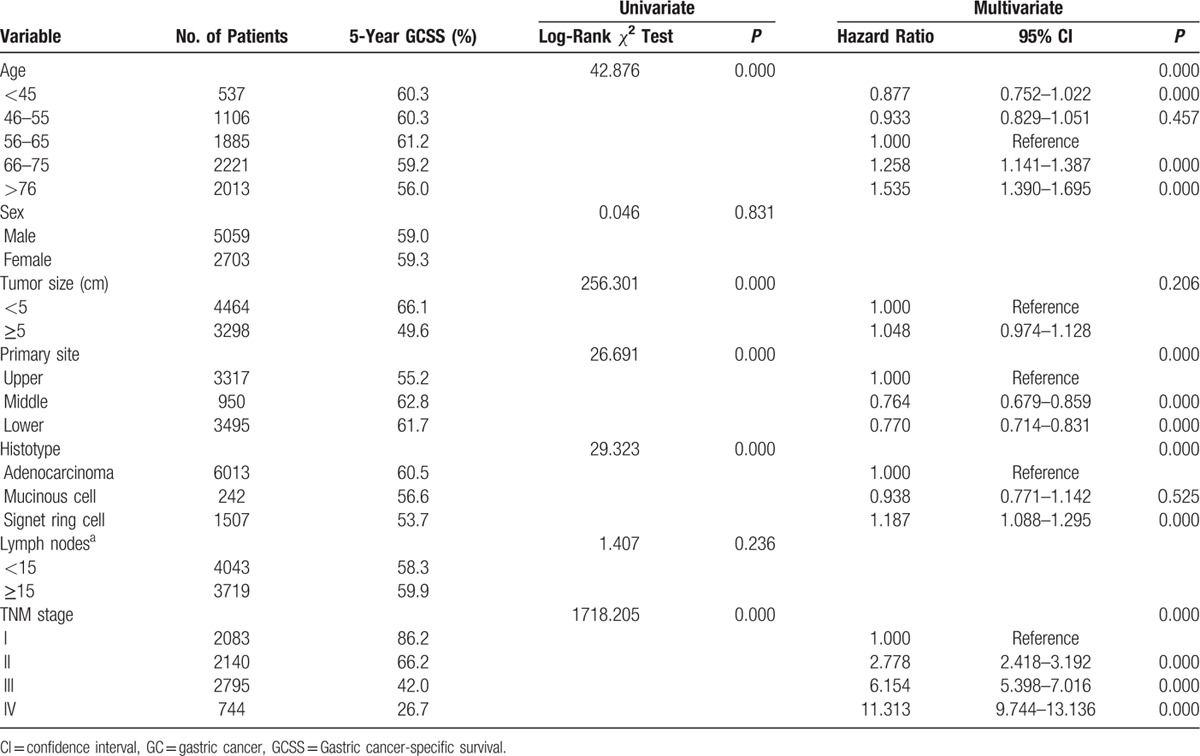
The overall 5-year CSS was 60.3% in patients below 45 years, 60.3% in patients between 45 and 55 years, 61.2% in patients between 56 and 65 years, 59.2% in patients between 66 and 75 years, and 59.2% in patients between older than 76 years. Kaplan–Meier plots showed that patients older than 76 years had the worst 5-year CSS of 56.0% rate in all the subgroups (Figure 1). Age, tumor size, primary site, histological type, and TNM stage were identified as significant risk factors for poor survival on univariate analysis (all P < 0.001, log-rank test). Additionally, we found that as the age increased, the risk of death for GC demonstrated a significant increase in a dose–response manner. The HR of CSS increased steadily with age, from 0.877 (95% CI, 0.752–1.022) in the Group 1 to 1.535 (95%, 1.390–1.695) for the Group 5. Moreover, we found a cubic polynomial regression was fitted to reflect the trend of HR changing with age differed (Figure 2). When multivariate analysis with Cox regression was performed, four variables were validated as independent prognostic factors. These included age (Group 1, HR 0.877, 95% CI 0.752–1.022, P = 0.000; Group 4, HR 1.258, 95% CI 1.141–1.387, P = 0.000; Group 5, HR 1.535, 95% CI 1.390–1.695, P = 0.000, using Group 3 as reference), while the risk between Group 2 and Group 3 was not statistical difference (P = 0.457), primary site (middle, HR 0.764, 95% CI 0.679–0.859, P = 0.000; lower, HR 0.770, 95% CI 0.714–0.831, P = 0.000, using upper as reference), histological type (signet ring cell tumor, HR 1.187, 95% CI 1.088–1.295, P = 0.000, using adenocarcinoma tumor as reference), TNM stage (Stage II, HR 2.778, 95% CI 2.418–3.192, P = 0.000; Stage III, HR 6.154, 95% CI 5.398–7.016, P = 0.000; Stage IV, HR 11.313, 95% CI 9.744–13.136, P = 0.000, using Stage I as reference) (Table 2).
Figure 1.
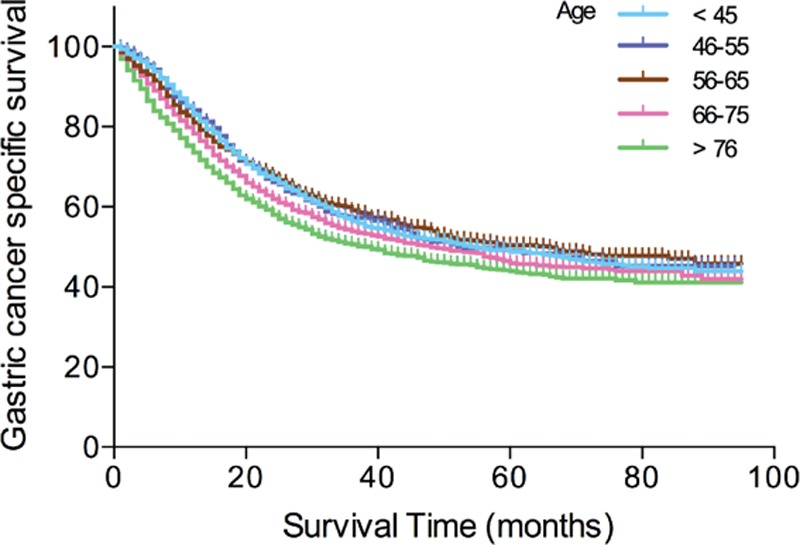
Survival curves in gastric cancer (GC) patients according to five age subgroups.
Figure 2.
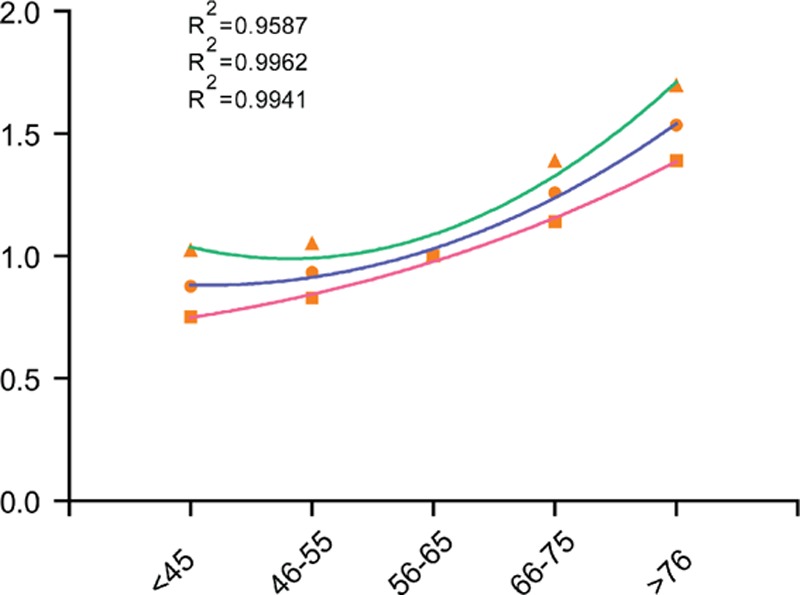
Estimates of hazard ratios of gastric cancer-specific survival changing with age using cubic polynomial regression.
Table 2.
Univariate and Multivariate Cox Analyses of GC Patients According to Various Clinicopathological Variables.
4. Discussion
Stomach adenocarcinoma, otherwise known as GC, is one of the most common cancers worldwide and is thus a global cancer burden. According to the GLOBOCAN database, GC is the fourth most common cancer and the third-leading cause of cancer-related deaths worldwide.[10] Despite many advances in the diagnosis and treatment of this disease, the prognosis of patients with GC remains poor, with a 5-year overall survival of less than 30% in most countries.[2] Knowledge of the important prognostic factors in the development of GC could help us understand this disease and make crucial therapeutic measures. Age is considered as one of the independent factors of several cancers, such as colorectal cancer and bladder cancer. Recently, some studies have investigated the prognostic outcome of GC in young patients in comparison to the elderly, but yielded inconclusive results.[11] It has been suggested that young patients suffered worse survival due to the characteristics of themselves and different tumor behavior.[12] According to this background, we used the data from SEER to evaluate the age-specific effects on the prognosis of GC with different age group.
In our study, a total of 7762 patients were included to evaluate this impact. The lowest HR of CSS was observed in patients who was diagnosed at the age of <45 years and the risk increased with age, being the highest for patients older than 76 years. Kaplan–Meier plots showed that patients in the 56 to 65 age group had a relatively good prognosis, and patients older than 76 years had the worst 5-year CSS of 56.0% rate in all the subgroups. The worse survival for the elderly might be explained by inadequate treatment partly. For example, a bad performance status can not tolerance the extensive lymphadenectomy and standardized chemotherapy.[13] While young patients have a good performance status to tolerate toxicities of standardized chemotherapy[14] and comprehensive treatment.[15] Moreover, young patients could have better physical conditions that gave them better tolerance to surgery have fewer potentially complications and a quicker return of gastrointestinal function.[16,17]
Although this was a large population-based study, it still had several potential limitations. First, the SEER database lacks several important information, such as adjuvant chemotherapy, neoadjuvant chemotherapy and the duration of chemotherapy could contribute to a favorable prognosis. Thus, our analyses could not adjust for these potential confounding factors. Second, the SEER database lacks the information of comorbidities that limits our ability to calculate the impact of comorbid conditions on CSS. Third, each patient was conducted surgery, but we did not know the surgical procedures, such as D1 or D2.
In conclusion, using the SEER database, our study revealed that the prognosis of GC varies with age. Patients between 56 and 65 years have more favorable clinicopathologic characteristics and better CSS than other groups. More well-designed and large sample of studies are required to perform to clarify this conclusion.
Acknowledgments
The authors acknowledge the efforts of the SEER Program tumor registries in the creation of the SEER database. The interpretation and reporting of these data are the sole responsibility of the authors.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CSS = cause-specific survival, GC = gastric cancer, SEER = surveillance, epidemiology and end results.
JC and JC contributed equally to this work.
This study was partially supported by grants from the National Natural Science Foundation of China (No. 81272729). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389–96. [DOI] [PubMed] [Google Scholar]
- [4].Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [5].Takatsu Y, Hiki N, Nunobe S, et al. Clinicopathological features of gastric cancer in young patients. Gastric Cancer 2016;19:472–8. [DOI] [PubMed] [Google Scholar]
- [6].Anderson WF, Camargo MC, Fraumeni JF, Jr, et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010;303:1723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saito H, Takaya S, Fukumoto Y, et al. Clinicopathologic characteristics and prognosis of gastric cancer in young patients. Yonago Acta Med 2012;55:57–61. [PMC free article] [PubMed] [Google Scholar]
- [8].Al-Refaie WB, Hu CY, Pisters PW, et al. Gastric adenocarcinoma in young patients: a population-based appraisal. Ann Surg Oncol 2011;18:2800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Park JC, Lee YC, Kim JH, et al. Clinicopathological aspects and prognostic value with respect to age: an analysis of 3,362 consecutive gastric cancer patients. J Surg Oncol 2009;99:395–401. [DOI] [PubMed] [Google Scholar]
- [10].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [11].Nakamura R, Saikawa Y, Takahashi T, et al. Retrospective analysis of prognostic outcome of gastric cancer in young patients. Int J Clin Oncol 2011;16:328–34. [DOI] [PubMed] [Google Scholar]
- [12].Smith BR, Stabile BE. Extreme aggressiveness and lethality of gastric adenocarcinoma in the very young. Arch Surg 2009;144:506–10. [DOI] [PubMed] [Google Scholar]
- [13].Goodwin RA, Asmis TR. Overview of systemic therapy for colorectal cancer. Clin Colon Rectal Surg 2009;22:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chew MH, Koh PK, Ng KH, et al. Improved survival in an Asian cohort of young colorectal cancer patients: an analysis of 523 patients from a single institution. Int J Colorectal Dis 2009;24:1075–83. [DOI] [PubMed] [Google Scholar]
- [15].Serra-Rexach JA, Jimenez AB, Garcia-Alhambra MA, et al. Differences in the therapeutic approach to colorectal cancer in young and elderly patients. Oncologist 2012;17:1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kubota T, Hiki N, Sano T, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol 2014;21:891–8. [DOI] [PubMed] [Google Scholar]
- [17].Sierzega M, Kolodziejczyk P, Kulig J, et al. Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. Br J Surg 2010;97:1035–42. [DOI] [PubMed] [Google Scholar]


