Abstract
Aberrant overexpression of C-X-C chemokine receptor type 4 (CXCR4) is a critical event during tumor metastasis. It has been previously reported that the expression of CXCR4 is linked with epithelial-mesenchymal transition (EMT) in oral squamous cell carcinoma (OSCC) tissues derived from patients. The present study addresses the role of CXCR4 in EMT in tongue squamous cell carcinoma (TSCCA) cells in vitro and in xenograft models. Small interfering (si) RNA sequences targeting the CXCR4 gene were transfected into TSCCA cells. Cell migration, invasion, apoptosis and EMT markers were determined in TSCCA cells using wound healing and Transwell assays, Annexin V/propdidum iodide double staining and western blot analysis, respectively. In vivo, tumor growth was assessed by subcutaneous inoculation of cells into BALB/c nude mice. Phenotypic EMT markers and regulatory factors were detected in the tumor tissues derived from the mice. In vitro, silencing of CXCR4 expression suppressed cell migration and invasion, and induced apoptosis. The protein expression of the EMT-associated markers N-cadherin and matrix metalloproteinases 2/9 were attenuated, while E-cadherin was increased. In vivo, CXCR4 siRNA inhibited tumor growth, and EMT-associated proteins had similar expression patterns to the experimental results observed in vitro. In conclusion, the present study demonstrated that CXCR4 silencing suppressed EMT in OSCC, thus affecting tumor metastasis.
Keywords: CXCR4, oral squamous cell carcinoma, epithelial-mesenchymal transition, knockdown, metastasis
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most common malignancies of the head and neck. It is characterized by local invasiveness and a high rate of cervical lymph node metastasis (1,2). In particular, lymph node metastasis has been revealed to directly affect the prognosis of patients (3). Despite advances in combined therapies, including radical surgery, radiotherapy and neo-adjuvant chemotherapy, the poor prognosis observed for patients with OSCC has not significantly improved during the last decade (4,5). Therefore, investigation to better understand the mechanism of metastasis in OSCC is required, in order to provide a more effective therapeutic strategy.
It has been previously demonstrated that epithelial-mesenchymal transition (EMT) is linked with OSCC progression, including cell invasion and metastasis (6,7). Tumor cells undergoing EMT aid in the dissemination of carcinoma cells, in which stationary epithelial tumor cells typically lose an epithelial phenotype, including cell polarity and adherent junctions, and acquire a narrow, fibroblast-like mesenchymal morphology and invasive properties. In addition, these cells exhibit a decrease in epithelial markers, including E-cadherin and β-catenin, and an increase in mesenchymal markers, including N-cadherin and vimentin (8,9). These alterations to the cell phenotype facilitates the cells in losing their adhesiveness, dissolving the extracellular matrix, which depends on matrix metalloproteinases (MMPs), and spreading to the surrounding tissue (10). However, little information is available regarding the type of EMT induced by chemokines.
The chemokine receptor C-X-C chemokine receptor type 4 (CXCR4), a G-protein-coupled receptor that is widely expressed on the membranes of neutrophils, lymphocytes and monocytes, and less often on epithelial cells, is the cognate receptor of stromal cell-derived factor-1α (SDF-1α; also referred to as CXCL12) (9,11). Several studies illustrate that SDF-1 and CXCR4 are associated with tumor progression and the development of lymph node and distant metastasis (12–15). In addition, CXCR4 expression has been demonstrated to be upregulated in a variety cancer types, including breast, ovarian, kidney and esophageal cancer (16–19), and elevated levels of CXCR4 in patients with cancer have been associated with a poor patient outcome (20,21).
The present authors have previously reported that an overexpression of CXCR4 is associated with lymph node metastasis in OSCC, and is significantly correlated with EMT-associated proteins (22). Further analysis concerning the regulation of CXCR4 expression accompanying EMT may be useful for the treatment of metastatic lymph nodes of OSCC. The present study used a gene silencing method to evaluate the effect of CXCR4 on EMT in vitro and in vivo in OSCC.
Materials and methods
Cell culture
Tongue squamous cell carcinoma (TSCCA) cells were purchased from the Chinese Academy of Medical Sciences Institute of Basic Medical Sciences (Beijing, China) and were grown in minimum essential medium (MEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.). Cell cultures were incubated at 37°C in a 5% CO2 atmosphere.
Small interfering (si) RNA-mediated downregulation of CXCR4 expression
The siRNAs used in the present study were chemically synthesized by GenePharma Co., Ltd. (Shanghai, China). The sequences were as follows: CXCR4 siRNA sense, 5′-CCGACCUCCUCUUUGUCAUTT-3′; negative control siRNA sense, 5′-UUCUCCGAACGUGUCACGUTT-3′. For transfection, 5×105 cells were seeded into each well of a 6-well plate and grown overnight until they reached 50–80% confluency. The cells were washed, placed in opti-MEM® reduced serum medium (Thermo Fisher Scientific, Inc.) and transfected with siRNA using Lipofectamine® 2000 (Invitrogen™; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Subsequent to a 6 h transfection, the medium was changed to MEM (Thermo Fisher Scientific, Inc.), and the cells were cultured at 37°C in 5% CO2. Three groups were generated for all experiments: Blank control group; negative siRNA control group; and CXCR4 siRNA group.
Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from the TSCCA cells using TRIzol Reagent (Invitrogen™; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. cDNA was synthesized using M-MLV Reverse Transcriptase (Invitrogen™; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. A 25 µl reaction containing 1 µl of the reverse transcription product was used for semi-quantitative PCR. The RT-qPCR cycling conditions were as follows: 30 sec at 95°C, followed by 40 cycles of 5 sec at 95°C and 34 sec at 60°C. CXCR4 was amplified using the following primers: Forward, 5′-CACTGGTGTCGGTCTCTGC-3′; and reverse, 5′-TGATTGAGTCAATGAAGTGGC-3′, which were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Human β-actin was used as an internal control, and the primer sequence was as follows: Forward, 5′-CACAGCAAGAGAGGCATCC-3′; and reverse, 5′-CTGGGGTGTTGAAGGTCTC-3′. A 2% agarose gel electrophoresis was used to analyze the amplified products.
Western blotting
Following transfection and culturing for 48 h, the cells were washed three times with ice-cold phosphate-buffered saline (PBS) and lysed in RIPA-buffer (EMD Millipore, Billerica, MA, USA). The cell lysates were cleared by centrifugation at 10,000 × g for 5 min and protein concentrations of the supernatants were determined using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). Subsequently, ~30 µg protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The gel was transferred onto polyvinylidene difluoride membranes and probed with the following primary antibodies: Anti-CXCR4 (dilution, 1:200; #ab58176), anti-N-cadherin (dilution, 1:1,500; #ab98952), anti-MMP2 (dilution, 1:5,000; #ab92536), anti-MMP9 (dilution, 1:800; #ab137867) (Abcam, Cambridge, MA, USA); and anti-E-cadherin (dilution, 1:800; #ZS-7870; Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). The membranes were incubated with primary antibodies 4°C overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (dilution, 1:15,000; #ZB-5301 and #ZB-5303; Zhongshan Golden Bridge Biotechnology Co., Ltd.) 37°C for 2 h. Immunoreactive protein bands were visualized using an enhanced chemiluminescence detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and subsequent exposure of the membrane to Hyperfilm (Thermo Fisher Scientific, Inc.). The same membrane was probed with a β-actin antibody (dilution, 1:2,000; #TA-09; Zhongshan Golden Bridge Biotechnology Co., Ltd.) as a loading control. Denistometric analysis was performed using Image J 1.47ver software (National Institutes of Health, Bethesda, MD, USA).
Wound healing assay
Cells were cultured in 6-well plates and incubated overnight. Scratches were created by scratching a straight line with a 20 µl tip vertically in the center of the dish. Cells were transfected with CXCR4 siRNA. The dishes were washed with PBS once to remove the detached cells, and a primary image of the scratch was taken under a microscope. A total of 24 and 48 h following transfection, the width of the scratches was observed and measured using Image J v1.47 software (National Institutes of Health, Bethesda, MA, USA). The relative distance was calculated as the mean width of the cell scratch following transfection / the width prior to transfection.
Transwell invasion assays
A cell invasion assay was performed using invasion chambers (BD Biosciences, Franklin Lakes, NJ, USA). In total, 50 µl Matrigel (BD Biosciences) and 200 µl cell suspension (1×105 cells/ml) was added to the inserts. MEM containing 10% FBS was added to the lower chamber. The cells were cultured in a CO2 incubator at 37°C for 48 h. The noninvasive cells in the upper chamber were cleared with cotton swabs and invasive cells were fixed with 95% ethanol for 10 min and stained with 0.5% crystal violet. Cells that had penetrated through the polyethylene terephthalate membrane were counted in 10 representative microscopic fields (magnification, ×400).
Annexin V-fluorescein isothiocyanate (FITC)/propidum iodide (PI) double-staining
An Annexin V-FITC Apoptosis Detection kit (BD Biosciences) was used to assess the apoptosis of transfected cells. TSCCA cells, which were transfected for 48 h, were collected and washed with PBS. Subsequently, the cells were resuspended in 1X Binding Buffer at a density of 1×106 cells/ml. In total, 5 µl Annexin V-FITC and 5 µl PI were added to the cells, and they were incubated at room temperature for 15 min. Subsequently, the cells were analyzed by flow cytometry, using BD FACSDiva™ software v6.1.3 and BD CellQuest™ Pro (BD Biosciences).
Nude mouse tumor xenograft model
A total of 18 BALB/c Nude 4-week-old female mice (weight, 15–18 g) were purchased from the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (Beijing, China). All animal experimental protocols were approved by the Tianjin Medical University Animal Care and Use Committee. All mice were maintained under specific pathogen-free conditions: Food and water were sterilized by high-pressure steam and feed was sterilized by Co-60 irradiation, and the light/dark cycle was 12 h, with a temperature of 24±1°C and relative humidity of 55±5%. In total, 3 mice were injected subcutaneously with 1×107 TSCCA cells, in a volume of 200 µl PBS. The mice were monitored daily and all 3 mice formed tumors subcutaneously. When the tumor size reached ~10 mm in length, the tumors were surgically removed, cut into 1 mm3 pieces and re-seeded into the inguinal region of 15 mice. Two weeks later, the mice were randomized into three groups (5 mice/group) to receive treatment: Blank control group; negative control siRNA-treated group; and CXCR4 siRNA-treated group. A mixture of 5 µl Lipofectamine 2000 and 15 µl siRNA (20 nmol/l) mixture was injected into the xenograft tumor model in a multi-site injection manner. Mice in the blank control group received 20 µl PBS only. The mice were treated every 4 days and tumors were measured with a caliper. Tumor volume was calculated as follows: Tumor volume (mm3) = [tumor length (mm) × tumor width (mm) × 2] / 2. At the end of the 22-day observation period, the mice bearing xenograft tumors were sacrificed and the tumor tissues were removed for formalin fixation and preparation of paraffin-embedded sections.
Hematoxylin and eosin (H&E) and immunohistochemistry staining
H&E staining was performed on the formalin-fixed, paraffin-embedded mice tumor tissues to observe the pathological alterations of the tumor under a microscope. For immunohistochemistry, tumor tissue sections were deparaffinized, rehydrated and incubated with primary antibodies, used previously in western blotting, overnight at 4°C. The tissues were then incubated with biotin-labeled secondary antibody (KIT9710; Maxim Biomedical, Inc., Rockville, MD, USA) for 1 h at room temperature, incubated with diaminobenzidine (Zhongshan Golden Bridge Biotechnology Co., Ltd.) and counterstained with hematoxylin. Slides were dehydrated with various concentrations of alcohol and soaked in xylene, and then mounted with neutral balsam and visualized using a light microscope.
Statistical analysis
The data are presented as the mean ± standard deviation of at least three independent experiments. One-way analysis of variance was performed using SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
CXCR4 siRNA inhibited the expression of CXCR4 in TSCCA cells
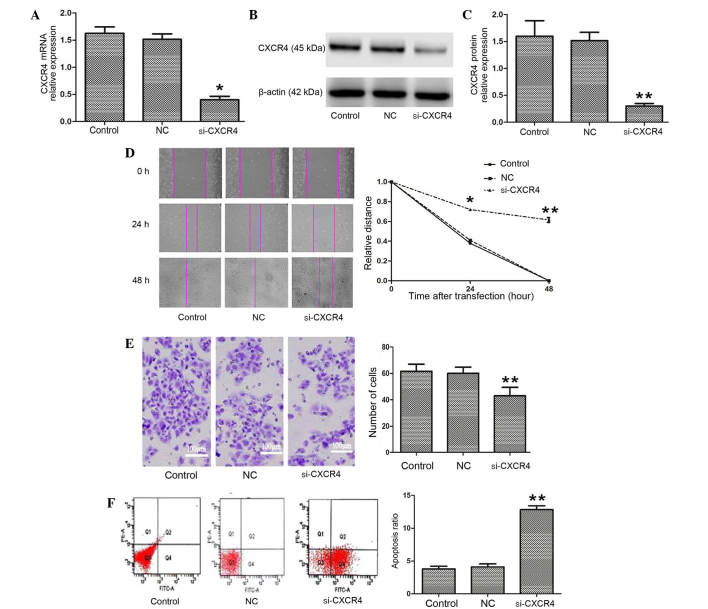
The expression of CXCR4 mRNA and protein were confirmed using semi-quantitative RT-PCR and western blotting. As shown in Fig. 1A-C, CXCR4 mRNA and protein expression in the CXCR4 siRNA group were clearly downregulated compared with the blank control and negative control groups (P=0.003). The results indicate that CXCR4 siRNA significantly silenced the expression of CXCR4 in TSCCA cells.
Figure 1.
Silencing of CXCR4 inhibits invasion and migration and promotes apoptosis in TSCCA cells. (A-C) si-CXCR4 inhibited the expression of CXCR4 mRNA and protein. (D) A wound healing assay was performed to investigate TSCCA migration following transfection with si-CXCR4. si-CXCR4 significantly suppressed cells migration. (E) A Transwell assay was performed to compare the invasive capacities of TSCCA cells. si-CXCR4 inhibited cell invasion in vitro (magnification, ×400). (F) si-CXCR4 mediated an increase in the apoptosis of TSCCA cells. *P<0.05 and **P<0.01 vs. control. CXCR4, C-X-C chemokine receptor type 4; si-CXCR4, small interfering RNA to CXCR4; TSCCA, tongue squamous cell carcinoma; control, blank control group; NC, negative control small interfering RNA group.
Silencing of CXCR4 inhibited migration and invasion and stimulated apoptosis in TSCCA cells
To better understand the biological role of CXCR4, the present study investigated the motility and invasion ability of cells using wound healing and Transwell assays. The relative distance in the wound healing assay was greater in the CXCR4 siRNA group compared with the blank control and negative control groups 24 h and 48 h following transfection (P=0.036 and P=0.008, respectively; Fig. 1D). The Transwell assay demonstrated that the number of invading cells was 61.20±5.71 and 60.50±5.58 in the blank control and negative control groups, respectively, which was significantly increased compared with the CXCR4 siRNA group (44.3±8.59; F=19.936; P<0.001; Fig. 1E). Subsequently, the effect of CXCR4 siRNA on cell apoptosis was analyzed using Annexin V and PI double staining. The Annexin V-positive early-phase apoptotic cells were significantly increased in the CXCR4 siRNA group (13.23±0.35%) compared with the blank control and negative control groups (3.30±0.72 and 3.40±0.44%, respectively; F=351.676; P=0.001 and P=0.004, respectively; Fig. 1F). The data suggest that CXCR4 is involved in the progression of OSCC.
Expression of EMT-associated proteins were regulated by silencing of CXCR4
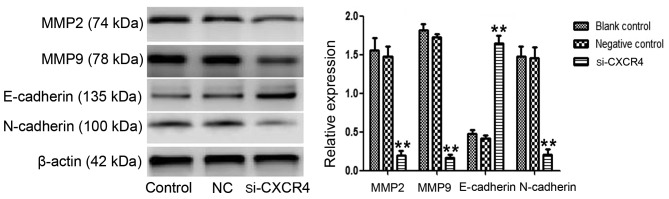
To further verify the role of CXCR4 in EMT in TSCCA cells, the protein expression levels of EMT-associated proteins, N-cadherin, E-cadherin and MMP2/9 were evaluated by western blotting. The result revealed that the expression level of the epithelial marker E-cadherin was elevated (P<0.001), while the mesenchymal marker N-cadherin was clearly attenuated (P=0.002). In addition, the expression of MMP2/9, which are assisting factors in EMT, was inhibited (P<0.001) (Fig. 2). This is consistent with the phenomenon that CXCR4 silencing suppresses cell invasion. Taken together, these results suggested that downregulation of CXCR4 facilitates cells to reverse their mesenchymal characteristic.
Figure 2.
Silencing of CXCR4 affects EMT-associated protein expression. Western blotting was used to compare the expression of EMT-associated proteins in tongue squamous cell carcinoma cells. N-cadherin and MMP2/9 expression were suppressed in the si-CXCR4 group, while E-cadherin expression was increased (**P<0.01 vs. control). CXCR4, C-X-C chemokine receptor type 4; si-CXCR4, small interfering RNA to CXCR4; EMT, epithelial-mesenchymal transition; control, blank control group; NC, negative control small interfering RNA group; MMP, matrix metalloproteinases.
Growth inhibitory effects of CXCR4 siRNA were examined in vivo in a nude mouse model
The results of the present in vitro experiments suggested that CXCR4 siRNA had an effect on tumor suppression. To further confirm this conclusion, tumor growth in a xenograft model was performed using a Lipofectamine-mediated siRNA therapy approach. At the beginning of treatment, the mean tumor volumes of the mice in the blank control, negative control and CXCR4 siRNA groups were 81.55±20.88, 89.63±46.30 and 84.74±18.44 mm3, respectively, with no statistically significant differences among these three groups. On day 13, a rapid increase in the tumor volumes in the blank control (1163.90±295.62 mm3) and negative control (1171.30±327.60 mm3) groups was observed compared to the CXCR4 siRNA group (682.33±429.69 mm3) (P=0.027). This inhibitory effect was aggravated with increasing time. At the end of the experiment, a significant decrease in tumor volume was observed in the CXCR4 siRNA group (1574.90±27.20 mm3) compared with the blank control and negative control groups (3512.12±870.05 mm3 and 3349.70±920.88 mm3, respectively) (P=0.012; Fig. 3).
Figure 3.
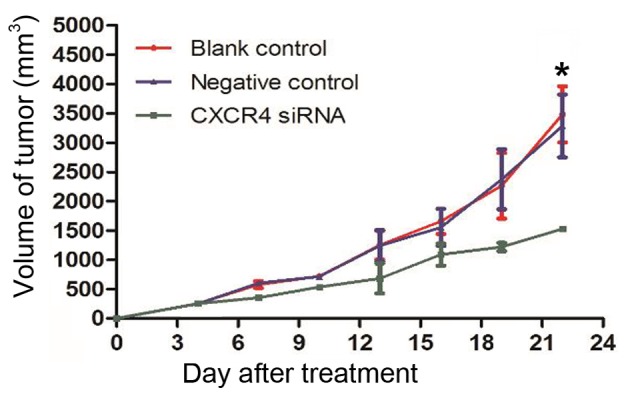
Effects of CXCR4 siRNA on xenograft tumor growth. The growth-inhibitory effect of CXCR4 siRNA began at day 13 and reached a maximum at day 22 following transfection (*P<0.05 vs. control). CXCR4, C-X-C chemokine receptor type 4; siRNA, small interfering RNA.
Silencing of CXCR4 suppressed TSCCA EMT in the xenograft model
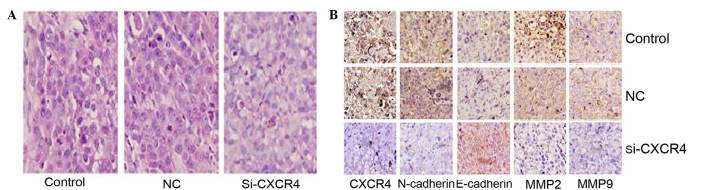
The histopathological alterations in the tumor tissues from the three treatment groups were observed using H&E staining. Cells in the control and blank groups were sheet arranged, with large nuclei. These cells exhibited multi morphology, pathological cell division and giant tumor cells, with marked atypia. By contrast, the cells in the CXCR4 siRNA group exhibited smaller nuclei, lighter staining and a reduction in the amount of neovasculature, and apoptosis and necrosis was observed (Fig. 4A). Subsequently, EMT-associated proteins were evaluated in these tissues using immunohistochemistry. Compared with the blank control and negative control groups, the expression of CXCR4, N-cadherin and MMP2/9 in the CXCR4 siRNA group were significantly reduced, and the expression of E-cadherin was increased (Fig. 4B). These results are consistent with the conclusion in vitro.
Figure 4.
H&E and immunohistochemistry analysis of TSCCA xenograft tumors. (A) Histopathological alterations of tumor tissues from nude mice models were observed using H&E staining. Compared with the negative control and blank control groups, cells in the si-CXCR4 group exhibited smaller nuclei, lighter staining, less neovasculature and more apoptosis and necrosis. (B) Immunohistochemistry analysis of CXCR4, N-cadherin, E-cadherin, MMP2/9 in TSCCA xenograft tumors. CXCR4 and N-cadherin positive staining were observed in the cytoplasm and membrane, and were suppressed in the si-CXCR4 group. E-cadherin was located in the membrane and expressed more clearly in the si-CXCR4 group. MMP2/9 was located in the cytoplasm and was inhibited in the si-CXCR4 (magnification, ×400). TSCCA, tongue squamous cell carcinoma; CXCR4, C-X-C chemokine receptor type 4; si-CXCR4, small interfering RNA to CXCR4; control, blank control group; NC, negative control small interfering RNA group; MMP, matrix metalloproteinases.
Discussion
Chemokines are a superfamily of small secreted proteins that have been identified as attractants of leukocytes to sites of infection and inflammation, and are pivotal in host defense mechanisms. They are locally produced in tissues and act on various cells through interactions with a subset of seven-transmembrane G-protein-coupled receptors (23). Among the numerous chemokines and their receptors, SDF-1, also referred to as the CXCL12/CXCR4 system, has been demonstrated to be involved in lymph node or distant metastasis in several types of cancer (17,24).
There is accumulating evidence that the CXCR4 system may facilitate lymph node metastasis in OSCC. Almofti et al (25) reported that invasion and recurrence of tumors was strongly associated with CXCR4 expression, and a CXCR4-positive group of patients had a poorer prognosis compared with a CXCR4-negative group. In addition, Uchida et al (26) examined the expression of 13 types of chemokine receptors and chemokines in OSCC cells, and revealed that upregulation of CXCR4 mRNA was detectable only in lymph node metastatic cells, including HNt and B88 cells, in comparison with nonmetastatic OSCC cells and normal gingival epithelial cells. A subsequent study confirmed that lymph node metastasis, loss in body weight and increase in tumour volumes were significantly inhibited in mice inoculated with siRNA against CXCR4 cells compared with mice inoculated with control cells (27). The present study demonstrated that CXCR4 was highly expressed in TSCCA cells. Subsequently, the present study verified that siRNA-mediated CXCR4 silencing significantly inhibited TSCCA migration and invasion using wound healing and Transwell assays. These results indicate that CXCR4 expression is a possible marker of highly-invasive OSCC.
Previous studies have revealed interactions between CXCR4 and EMT, which involves a series of events leading to tumor invasion and metastasis (28). In a study concerning hepatocellular cancer, intervention with exogenous SDF-1 induced invasion and downregulated E-cadherin expression, and upregulated vimentin expression in HepG2 cells; however, this effect was not observed in CXCR4-depleted carcinoma (29). Zhu et al (30) silenced CXCR4 in glioma U87 cells and revealed that EMT was inhibited. In addition, the authors demonstrated that the production of transforming growth factor (TGF)-β and β-catenin were decreased by CXCR4 silencing, indicating that the effect of CXCR4 on EMT may be associated with its function of affecting transcription factors. Another study revealed that SDF-1/CXCR4 signaling induced pancreatic cancer EMT through the activation of the Hedgehog pathway (31). The present study demonstrated that in the CXCR4 siRNA group of cells, the expression of N-cadherin and MMP2/9 were attenuated, while E-cadherin was upregulated, and the same results were observed in xenograft models. In addition, treatment of xenografted tumors with CXCR4 siRNA resulted in histological alterations to tumor cells in the present study. However, further studies are required to clarify the mechanism of CXCR4 regulating OSCC tumor metastasis via EMT.
In addition to supporting metastasis, CXCR4 regulates tumor growth and apoptosis (25,32). The present study demonstrated that inhibition of CXCR4 expression promoted cell apoptosis of OSCC cells in vitro and, following xenograft tumor model construction in nude mice, the tumor volume in the CXCR4 siRNA treatment group was significantly decreased compared with the blank control and negative control groups. A previous study demonstrated that CXCR4 promoted cancer cell proliferation by altering the expression of >1,500 genes involved in the cell cycle, apoptosis and multiple signaling pathways using microarray analysis technology (33). Some of these genes, including mitogen-activated protein kinases, TGF-β and MMP, are central in EMT (33). In addition, the determination of cellular fates of apoptosis or survival during tumor progression is highly affected by EMT; EMT confers cancer cells resistant to apoptosis (34,35). Therefore, growth inhibition and apoptosis promotion may be attributable to the EMT conferred by CXCR4 siRNA.
The present results demonstrate that overexpression of CXCR4 is significantly associated with EMT in OSCC cells. In view of the importance of CXCR4, the expression of CXCR4 in OSCC may be used as a molecular target of diagnosis, not only for lymph node metastatic potential, but also for EMT. In addition, blockade of CXCR4 may be investigated as a potential therapeutic target for OSCC treatment.
Acknowledgements
The present study was supported by the Tianjin Science and Technology Committee (Tianjin, China; grant no. 12JCYBJC33800).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Noguti J, De Moura CF, De Jesus GP, Da Silva VH, Hossaka TA, Oshima CT, Ribeiro DA. Metastasis from oral cancer: An overview. Cancer Genomics Proteomics. 2012;9:329–335. [PubMed] [Google Scholar]
- 3.Patel SG, Amit M, Yen TC, Liao CT, Chaturvedi P, Agarwal JP, Kowalski LP, Ebrahimi A, Clark JR, Cernea CR, et al. Lymph node density in oral cavity cancer: Results of the International consortium for outcomes research. Br J Cancer. 2013;109:2087–2095. doi: 10.1038/bjc.2013.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26:645–662. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 6.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: Parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:287–292. doi: 10.1016/j.oraloncology.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33:1755–1763. doi: 10.1038/onc.2013.128. [DOI] [PubMed] [Google Scholar]
- 9.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Murdoch C. CXCR4: Chemokine receptor extraordinaire. Immunol Rev. 2000;177:175–184. doi: 10.1034/j.1600-065X.2000.17715.x. [DOI] [PubMed] [Google Scholar]
- 12.Teng F, Tian WY, Wang YM, Zhang YF, Guo F, Zhao J, Gao C, Xue FX. Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis. J Hematol Oncol. 2016;9:8. doi: 10.1186/s13045-015-0231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang DL, Xin MM, Wang JS, Xu HY, Huo Q, Tang ZR, Wang HF. Chemokine receptor CXCR4 and its ligand CXCL12 expressions and clinical significance in bladder cancer. Genet Mol Res. 2015;14:17699–17707. doi: 10.4238/2015.December.21.43. [DOI] [PubMed] [Google Scholar]
- 14.Guo Q, Gao BL, Zhang XJ, Liu GC, Xu F, Fan QY, Zhang SJ, Yang B, Wu XH. CXCL12-CXCR4 axis promotes proliferation, migration, invasion, and metastasis of ovarian cancer. Oncol Res. 2014;22:247–258. doi: 10.3727/096504015X14343704124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu W, Qian L, Chen X, Ding B. Prognostic significance of CXCL12, CXCR4, and CXCR7 in patients with breast cancer. Int J Clin Exp Pathol. 2015;8:13217–13224. [PMC free article] [PubMed] [Google Scholar]
- 16.Gros SJ, Kurschat N, Drenckhan A, Dohrmann T, Forberich E, Effenberger K, Reichelt U, Hoffman RM, Pantel K, Kaifi JT, Izbicki JR. Involvement of CXCR4 chemokine receptor in metastastic HER2-positive esophageal cancer. PLoS One. 2012;7:e47287. doi: 10.1371/journal.pone.0047287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 18.An H, Xu L, Zhu Y, Lv T, Liu W, Liu Y, Liu H, Chen L, Xu J, Lin Z. High CXC chemokine receptor 4 expression is an adverse prognostic factor in patients with clear-cell renal cell carcinoma. Br J Cancer. 2014;110:2261–2268. doi: 10.1038/bjc.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62:5930–5938. [PubMed] [Google Scholar]
- 20.Burger JA, Kipps TJ. CXCR4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 21.Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: An emerging sensitizer for anticancer therapies? Clin Cancer Res. 2011;17:2074–2080. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, Zhang CP, Zhang S, Wang XD. Epithelial-mesenchymal transformation-mediated lymph node metastasis of oral squamous cell carcinoma and its mechanism. Chinese Journal of Clinical Oncology. 2012;39:1877–1880. [Google Scholar]
- 23.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 24.Perissinotto E, Cavalloni G, Leone F, Fonsato V, Mitola S, Grignani G, Surrenti N, Sangiolo D, Bussolino F, Piacibello W, Aglietta M. Involvement of chemokine receptor 4/stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin Cancer Res. 2005;11:490–497. [PubMed] [Google Scholar]
- 25.Almofti A, Uchida D, Begum NM, Tomizuka Y, Iga H, Yoshida H, Sato M. The clinicopathological significance of the expression of CXCR4 protein in oral squamous cell carcinoma. Int J Oncol. 2004;25:65–71. [PubMed] [Google Scholar]
- 26.Uchida D, Begum NM, Almofti A, Nakashiro K, Kawamata H, Tateishi Y, Hamakawa H, Yoshida H, Sato M. Possible role of stromal-cell-derived factor-1/CXCR4 signaling on lymph node metastasis of oral squamous cell carcinoma. Exp Cell Res. 2003;290:289–302. doi: 10.1016/S0014-4827(03)00344-6. [DOI] [PubMed] [Google Scholar]
- 27.Uchida D, Onoue T, Kuribayashi N, Tomizuka Y, Tamatani T, Nagai H, Miyamoto Y. Blockade of CXCR4 in oral squamous cell carcinoma inhibits lymph node metastases. Eur J Cancer. 2011;47:452–459. doi: 10.1016/j.ejca.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Zhang SS, Han ZP, Jing YY, Tao SF, Li TJ, Wang H, Wang Y, Li R, Yang Y, Zhao X, et al. CD133(+) CXCR4(+) colon cancer cells exhibit metastatic potential and predict poor prognosis of patients. BMC Med. 2012;10:85. doi: 10.1186/1741-7015-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Li P, Chang Y, Xu Q, Wu Z, Ma Q, Wang Z. The SDF-1/CXCR4 axis induces epithelial-mesenchymal transition in hepatocellular carcinoma. Mol Cell Biochem. 2014;392:77–84. doi: 10.1007/s11010-014-2020-8. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Yang P, Wang Q, Hu J, Xue J, Li G, Zhang G, Li X, Li W, Zhou C, et al. The effect of CXCR4 silencing on epithelial-mesenchymal transition related genes in glioma U87 cells. Anat Rec (Hoboken) 2013;296:1850–1856. doi: 10.1002/ar.22821. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Ma Q, Xu Q, Liu H, Lei J, Duan W, Bhat K, Wang F, Wu E, Wang Z. SDF-1/CXCR4 signaling induces pancreatic cancer cell invasion and epithelial-mesenchymal transition in vitro through non-canonical activation of Hedgehog pathway. Cancer Lett. 2012;322:169–176. doi: 10.1016/j.canlet.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas RM, Kim J, Revelo-Penafiel MP, Angel R, Dawson DW, Lowy AM. The chemokine receptor CXCR4 is expressed in pancreatic intraepithelial neoplasia. Gut. 2008;57:1555–1560. doi: 10.1136/gut.2007.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu T, Wu Y, Huang Y, Yan C, Liu Y, Wang Z, Wang X, Wen Y, Wang C, Li L. RNAi targeting CXCR4 inhibits tumor growth through inducing cell cycle arrest and apoptosis. Mol Ther. 2012;20:398–407. doi: 10.1038/mt.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valdés F, Alvarez AM, Locascio A, Vega S, Herrera B, Fernández M, Benito M, Nieto MA, Fabregat I. The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor Beta in fetal rat hepatocytes. Mol Cancer Res. 2002;1:68–78. [PubMed] [Google Scholar]
- 35.Robson EJ, Khaled WT, Abell K, Watson CJ. Epithelial-to-mesenchymal transition confers resistance to apoptosis in three murine mammary epithelial cell lines. Differentiation. 2006;74:254–264. doi: 10.1111/j.1432-0436.2006.00075.x. [DOI] [PubMed] [Google Scholar]