Abstract
Although icodextrin solution has been highlighted in the fluid management compared to glucose-based solutions, proof of a beneficial effect of icodextrin solution on residual renal function (RRF) is lacking. We conducted a multicenter prospective randomized controlled open-label trial to investigate whether icodextrin solution can preserve RRF.
One hundred patients with urine volume ≥750 mL/day from 8 centers in Korea were randomly assigned to receive 1 exchange of icodextrin solution for a ≥8 hour-dwell time and 2 exchanges of 1.5% glucose-based biocompatible neutral pH solution or 1 exchange of ≥2.5% and 2 exchanges of 1.5% glucose-based biocompatible solutions. Using mixed-effects general linear models, we analyzed changes in residual glomerular filtration rate (GFR) and daily urine volume at 1 year.
Forty-nine patients were assigned to the icodextrin group and 51 to the glucose solution group. During follow-up, the slope of the decline in residual GFR was −0.170 mL/min/month/1.73 m2 in the icodextrin group, while it was −0.155 mL/min/month/1.73 m2 in the glucose solution group (95% confidence interval [CI], −0.06 to 0.10; P = 0.701). Daily urine volume decreased faster in the glucose solution group than in the icodextrin group (−31.02 vs −11.88 mL per month; 95% CI, −35.85 to −2.44; P = 0.025). Results were consistent when we analyzed using intention-to-treat and per protocol principles. There were no differences in fluid status, peritoneal ultrafiltration, and peritoneal transport between groups during follow-up.
This study clearly showed that icodextrin solution preserves residual urine volume better than glucose solution.
INTRODUCTION
Peritoneal dialysis (PD) has been established as an effective dialysis treatment in end-stage renal disease (ESRD), and approximately 200,000 patients worldwide are maintained on PD.1 However, many researchers have much concern about the deleterious effects of the high glucose content of PD solutions on the peritoneal membrane overtime.2 Therefore, there was growing need for the development of new solutions designed to minimize glucose-induced toxicity, and thus PD solutions containing low glucose degradation products or an alternative osmotic agent to glucose, such as icodextrin and amino-acids, have been developed. Icodextrin is a mixture of high molecular weight, water-soluble glucose polymers isolated by the fractionation of hydrolyzed cornstarch.3 Although diffusion across the peritoneal capillary is the principal mechanism for glucose absorption from the peritoneal cavity, icodextrin mainly is absorbed by convective fluid movement out of the peritoneal cavity via the lymphatic system.4 This results in relatively constant osmotic pressure, which can provide sustained ultrafiltration during the long dwell time.
A number of studies have reported that icodextrin-based solution provides various clinical benefits compared to conventional glucose-based solutions.5,6 Indeed, icodextrin solution is particularly helpful and has been widely used to treat fluid overload in PD patients.7–14 However, whether icodextrin can preserve residual renal function (RRF) remains controversial. Icodetextrin solution has the merit of sustained ultrafiltration, but this can have harmful impact on RRF as excessive ultrafiltration may induce underhydration, leading to a faster decline in RRF. Konings et al15 first raised this concern and found a greater reduction in residual glomerular filtration rate (GFR) in patients using icodextrin than in those using glucose solutions. In addition, Paniagua et al11 demonstrated similar effects of icodextrin on residual GFR in high and high-average transport PD patients with diabetes. In contrast, favorable effects of icodextrin on RRF have also been reported. A previous study by Davies et al7 observed that icodextrin better preserved urine volume compared to a glucose-based solution during 6 months, and another recent study found that a combination of 3 biocompatible PD solutions including icodextrin better preserved urine volume during 12 months.16 These all findings indicate that RRF can be preserved by icodextrin. On the other hand, several randomized controlled trials reported neutral effects of icodextrin on RRF evidenced by a similar decline in renal creatinine clearance or urine volume by icodextrin and glucose-based solution.12,17–19 These conflicting results regarding the effect of icodextrin on RRF can be attributable to differences in study design, baseline volume status, and other unknown factors that can affect RRF during the study period. Most studies have limitations in RRF being defined as a secondary outcome, insufficient statistical power, relatively short observation period, or small sample size. Above all, we speculate that treatment-associated changes in volume status might differ depending on different concentrations of comparative glucose solution, thus resulting in conflicting findings. In fact, in the study by Konings et al,15 the glucose solution had a concentration of 1.36%, whereas the solution used by Davies et al7 had a concentration of 2.27%.
However, given the strong relationship between adequate ultrafiltration or RRF and clinical outcomes in PD patients, we believe that a more judicious approach is warranted, with particularly strict attention to fluid status. With this background in mind, we conducted this study to investigate whether icodextrin solution can better preserve RRF in PD patients compared to conventional glucose-based solutions.
METHODS
Patients and Study Design
This multicenter prospective randomized controlled open-label trial was undertaken from October 2010 to June 2014 at 8 centers in Korea (Clinicaltrials. gov registration NCT01170858). This study was carried out in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of all participating hospitals. Written informed consent was obtained from all participants before enrollment.
Adult PD patients (aged ≥20 years) with ESRD who were maintained on PD with the Baxter ultrabag system and had a measured urine volume ≥750 mL/day at enrollment were eligible for the study. Patients who had a life expectancy of <12 months, had been on hemodialysis or received a kidney transplant before the initiation of PD, had been on automated PD, had been treated with icodextrin solution before enrollment, had poor medical conditions that interfered with their ability to comply with the study protocol, and had known or suspected allergies to the trial product were excluded from the study. Patients who were suspected of having uncontrolled volume status requiring the repeated use of 4.25% glucose PD solutions in addition to 2.5% glucose solution or icodextrin, or volume depletion or hypotension caused by 2.5% glucose PD solution or icodextrin solution was also excluded from the study. During the recruitment period of October 2010 to May 2013, 205 patients fulfilled the inclusion criteria; 100 patients consented to participate in the study.
After a 4-week screening period, 100 patients were randomly assigned to receive either 1 exchange of icodextrin solution (Extraneal, Baxter Healthcare Ltd., Singapore; icodextrin group) for ≥8 hour-dwell time and 2 exchanges of 1.5% glucose-based biocompatible neutral pH solution (Physioneal, Baxter Healthcare Ltd., Singapore) or 1 exchange of ≥2.5% and 2 exchanges of 1.5% glucose-based biocompatible solutions (Physioneal, Baxter Healthcare Ltd., Singapore; control group). To ensure adequate allocation concealment, the randomization was performed centrally using a web-based system and stratified according to diabetic nephropathy and center. The subsequent escalation in the number of continuous ambulatory PD exchanges and the additional use of glucose solution were permitted in both groups to achieve adequate control of small solute clearance or ultrafiltration. However, patients were excluded if they had to use 2 or more exchanges of 4.25% high concentration glucose solutions per day to control volume overload.
Clinical Follow-Up and Data Collection
Demographic and clinical data were collected at enrollment and included age, gender, cause of ESRD, comorbidities, and center size. The Charlson comorbidity index was used to compare the comorbid conditions at baseline.20 Two centers that had fewer than 40 PD patients were considered “less experienced center.” The clinic visits were scheduled every 3 months, and clinical and laboratory data were obtained at each visit. These included body weight, blood pressure, serum hemoglobin, albumin, osmolality, sodium, fasting glucose, HbA1c, total cholesterol, low-density lipoprotein, triglycerides, high-sensitive C-reactive protein, prescribed dialysate volume and dialysate glucose exposure, and medications. During each visit, all adverse reactions were also recorded.
Residual GFR and fluid status were assessed at baseline and at 6 and 12 months. Residual GFR was calculated as the average urea and creatinine clearance from a 24-hour urine collection.21 To assess fluid status, we used 3 different assessment tools: echocardiography for left atrial volume index, left ventricular end diastolic diameter, and inferior vena cava (IVC) collapsibility index; measurement of plasma atrial natriuretic peptide (ANP) levels; and whole-body multifrequency bioelectrical impedance analysis (Fresenius Medical Care, Germany). Plasma ANP level was measured using an enzyme-linked immunosorbent assay kit (Abcam, Cambridge, UK) according to the manufacturer's protocol. IVC collapsibility index was calculated as (maximal diameter on expiration − minimal diameter on deep inspiration)/maximal diameter on expiration × 100. All patients were followed for 12 months. Each assessment was performed by a single observer who was blinded to patient information and treatment allocations.
Study Outcomes
The primary outcome was the change in RRF including the slope of the decline in residual GFR and daily urine volume over 12 months. The secondary outcome was the change in fluid status during the study period.
Power Calculation
At least 50 subjects were required for each group in order to detect a 50% difference in residual GFR and urine volume over 12 months between groups, if the type I error rate was 5% and the type II error was 20% given a 30% drop-out rate during the study period.
Statistical Analysis
All values are expressed as mean ± standard deviations or percentages. Statistical analyses were performed using SAS version 9.2.3 (SAS, Institute Inc., Cary, NC) and R version 3.0.2 (http://cran.r-project.org/). The data were compared using Student's t-test and the Chi-square test. The Kolmogorov–Smirnov test was used to determine the normality of the parameters distributions. If the data did not show a normal distribution, they were expressed as the median and interquartile range (or after log-transformation) and were compared using the Mann–Whitney U-test or the Kruskal–Wallis test.
We primarily analyzed data on an intention-to-treat (ITT) basis. Additional per-protocol analysis was also performed on patients who completed the entire trial according to the protocol. For the primary outcome of change in RRF, the slope of the decline in residual GFR (mL/min/month/1.73 m2) and daily urine volume (mL per month) over 12 months were calculated and compared using generalized linear mixed models for repeated measures, and expressed as the estimate coefficient and 95% confidence interval (CI). No confounding baseline variables were found, and no additional covariates were therefore added to the model. For the secondary efficacy endpoints, mean changes in fluid status parameters from baseline to 6 and 12 months were summarized for each treatment group, and comparisons between groups were also made based on the generalized linear mixed model. In each case, the generalized linear mixed model incorporated time (corresponding to those visits when the endpoint of interest was measured), treatment group (icodextrin vs glucose-based solution), and interaction (time-by-treatment group) as the primary independent class variables. A P-value of ≤0.05 was considered statistically significant.
RESULTS
Patient Characteristics
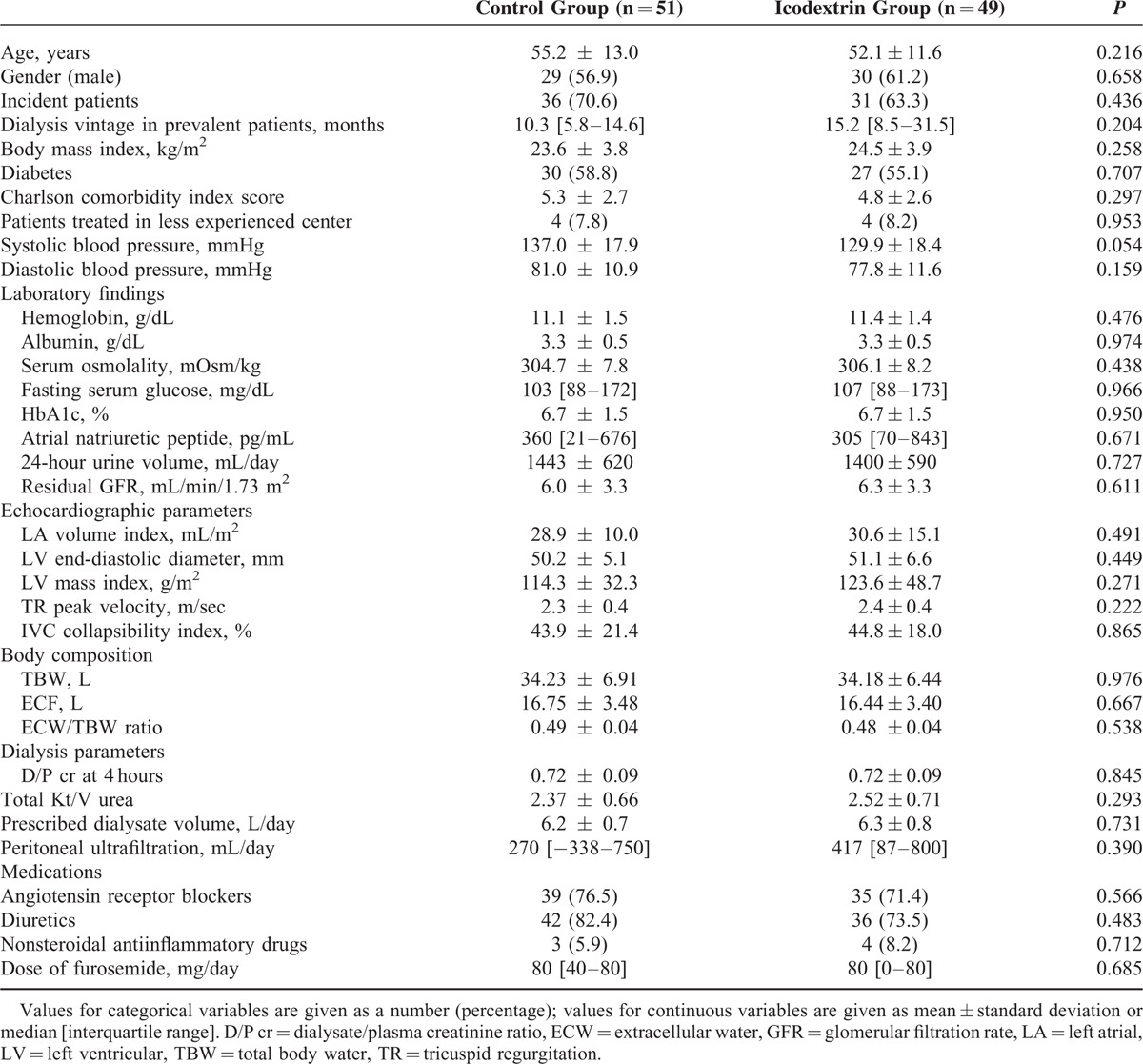
Between September 2010 and May 2013, a total of 100 patients were randomized to receive either icodextrin-containing (n = 49) or glucose (n = 51, control) solution (Figure 1). The 2 groups were well balanced for all baseline characteristics (Table 1).
FIGURE 1.
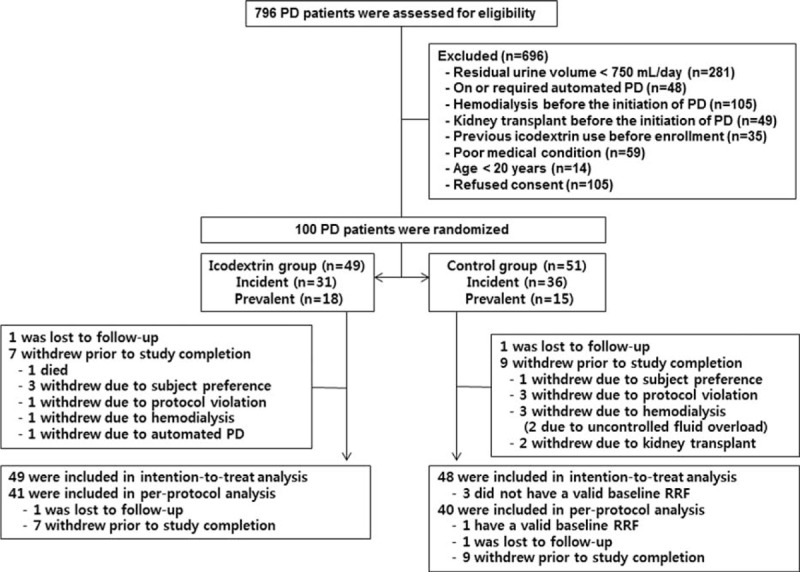
Enrollment, randomization, and follow-up of study participants. PD = peritoneal dialysis, RRF = residual renal function.
TABLE 1.
Baseline Characteristics of the Study Participants
Decrease in Residual Renal Function
We performed both ITT primary efficacy analysis (n = 97) and per-protocol analysis (n = 81) to evaluate the decrease in RRF between groups in terms of the slope of residual GFR decline (Figure 2) and daily urine volume (Figure 3) at 1 year. During the 1 year of therapy, in the ITT population, the mean slope of decrease in residual GFR was −0.170 mL/min/month/1.73 m2 in the icodextrin group and −0.155 mL/min/month/1.73 m2 in the control group (95% CI, −0.06 to 0.10; P = 0.701). When the curvature of the GFR decline was modeled by analyzing log-transformed data, the log scale trends in the icodextrin and control groups were −0.042 and −0.042 mL/min/month/1.73 m2, respectively (95% CI, −0.02 to 0.02; P = 0.994). In the per-protocol population, the differences in decreases of residual GFR between treatments over the entire study period were not statistically significant (−0.149 in the icodextrin group vs −0.153 mL/min/1.73 m2 per month in the control group, respectively; 95% CI, −0.08 to 0.08; P = 0.920).
FIGURE 2.
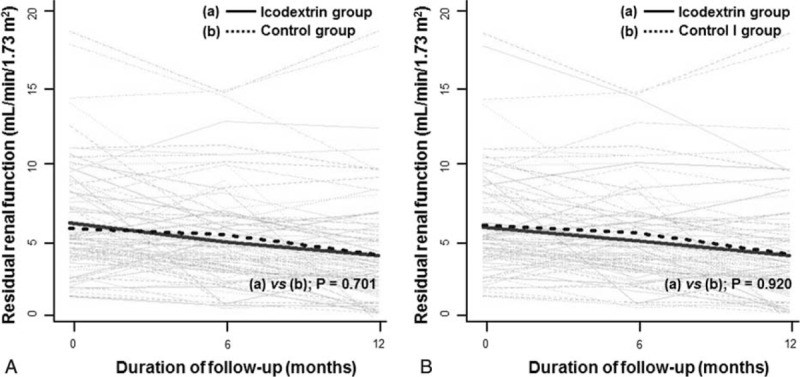
Changes in residual glomerular filtration rates overtime between groups. (A) intention-to-treat analysis, (B) per-protocol analysis. Gray lines represent individual patient measurements, and solid and dash lines represent predicted slopes.
FIGURE 3.
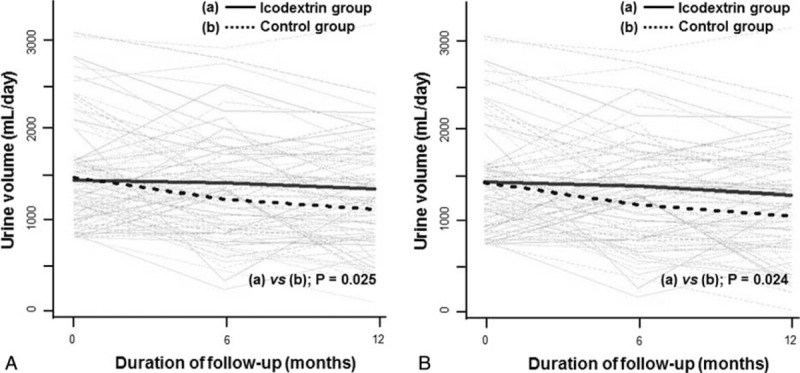
Changes in daily urine volume over time between groups. (A) intention-to-treat analysis, (B) per-protocol analysis. Gray lines represent individual patient measurements, and solid and dash lines represent predicted slopes.
In contrast, daily urine volume declined faster in the control group than in the icodextrin group (−31.02 vs −11.88 mL per month [95% CI, −35.85 to −2.44; P = 0.025] in the ITT population and −31.96 vs −12.12 mL per month [95% CI, −32.67 to −2.61; P = 0.024] in the per-protocol population, respectively). Notably, these significant differences in the slope of daily urine volume decline between groups were more profound in the models using log-transformed data (P = 0.010 in the ITT and P = 0.007 in the per-protocol population, respectively). Furthermore, we created several multivariable models adjusted for clinical factors, underlying kidney diseases, high-sensitive C-reactive protein, volume parameters, and medications such as angiotensin receptor blockers, nonsteroidal antiinflammatory drugs, and diuretics. These rigorous adjustment models consistently revealed significantly faster decreases in residual urine volume in the control group than in the icodextrin group (data not shown). In a post-hoc analysis, no relationship between these treatment differences with respect to urine volume and the use of diuretics including furosemide dosage was found.
Secondary Outcomes
Linear mixed models with repeated measures revealed no differences between treatment groups for most of the secondary endpoints. Not surprizingly, the use of 2.5% glucose solution resulted in higher exposure to glucose than icodextrin solution throughout the study (Table 2). Fluid status was thoroughly evaluated using 3 different methods: echocardiography, body composition measured by bioelectrical impedance analysis, and plasma ANP concentrations. Overall, these parameters in the 2 groups were comparable at all-time points (Figure 4). Similarly, peritoneal ultrafiltration and peritoneal membrane transport did not differ between groups during follow-up. Other clinical and laboratory findings and the use of medications such as angiotensin receptor blockers and diuretics were also similar between groups throughout the study.
TABLE 2.
Secondary Outcomes by Treatment Group
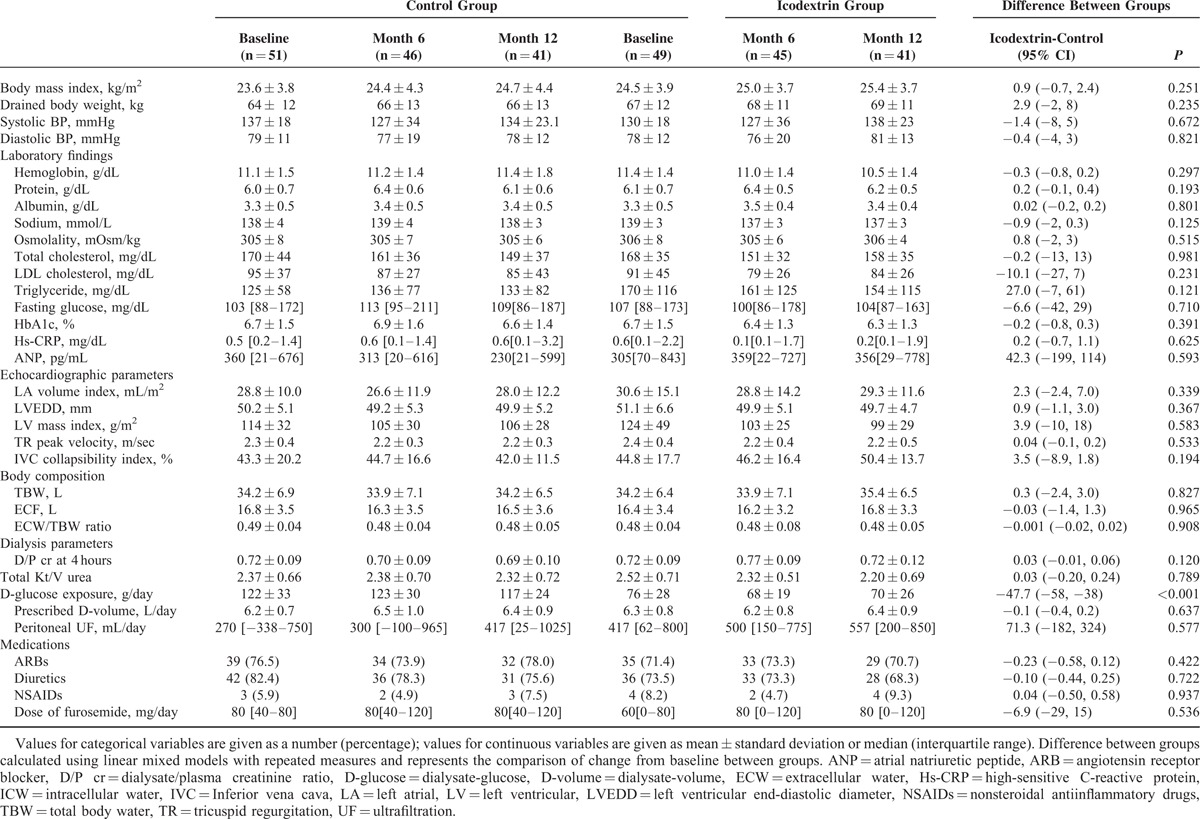
FIGURE 4.
Changes in fluid status such as left ventricular end-diastolic diameter (A), inferior vena cava collapsibility index (B), extracellular water/total body water ratio (C), and plasma atrial natriuretic peptide concentrations (D) overtime between groups. ECW = extracellular water, LVEDD = left ventricular end-diastolic diameter, IVC = inferior vena cava, TBW = total body water.
Adverse Events
Seventy-nine percent (n = 79) of patients experienced at least 1 adverse events. The number of patients who experienced an adverse event and the overall number of adverse events was similar between the 2 groups. All adverse events are shown in Table 3, and the rates were not significantly different between groups. Peripheral edema occurred in 28 (19%) and 20 (13%) patients in the control and icodextrin group, respectively (P = NS), which disappeared after replacing 1.5% glucose solution with 1 exchange of 2.5% or 4.25% glucose solution for several days. Two patients in the control group dropped out during the study because they used 2 or more exchanges of 4.25% glucose solution per day to manage volume overload.
TABLE 3.
Adverse Events by Treatment Group
DISCUSSION
In this study, we aimed to evaluate the effect of icodextrin solution on the preservation of RRF in PD patients compared to glucose solutions. We showed that icodextrin solution attenuated the rate of decline in daily urine volume compared to the controls, but did not affect residual GFR. These findings were consistently reproduced in the ITT and per-protocol populations, and even when using rigorously adjusted multivariable models. Our robust findings suggest that icodextrin is a promising dialysis solution for achieving important therapeutic goals, such as the preservation of residual urine volume and fluid management in PD patients.
Fluid overload has long been considered a therapeutic target in patients undergoing dialysis22 because it is associated with adverse outcomes such as hypertension, left ventricular hypertrophy, congestive heart failure,23–27 and even malnutrition–inflammation complex syndrome.28,29 For these reasons, there has been a tremendous demand to adequately maintain euvolemia to improve clinical outcomes in these patients. To this end, multidisciplinary approach has been suggested, including sodium and water restriction, preservation of RRF, diuretic use, and preservation of peritoneal membrane function.30 In particular, the preservation of RRF is of paramount importance in achieving this goal because loss of kidney function can predict morbidity and mortality.31,32 Interestingly, among the many methods introduced to date, recent trials have shown that the use of biocompatible PD solution itself can preserve RRF.33–35 However, these studies had limitations, including less peritoneal ultrafiltration induced by biocompatible solutions than by glucose-based solutions, and this raised concerns about fluid management. Icodextrin solution has been highlighted as an alternative solution to improve fluid status by increasing peritoneal ultrafiltation.8,11,36–39 Nevertheless, there are many controversial observations regarding whether RRF can be preserved by icodextrin.7,11,12,15,17,19 Most of these studies analyzed RRF as a secondary outcome and were limited by insufficient assessment of fluid status and a lack of statistical power. Moreover, there is a growing concern about faster decline of RRF by icodextrin-induced higher ultrafiltration compared to glucose solution.11,15,40 Our randomized controlled study addressed this issue and clearly demonstrated that icodextrin solution preserves RRF better than glucose solutions. This finding is unlikely to be explained by escalation of high concentration glucose solution or the use of diuretics because glucose exposure did not change in the control group throughout the study and the use of medications that could potentially affect urine volume was comparable between groups.
RRF is generally evaluated by assessing both residual GFR and urine volume. We showed that urine volume was preserved by icodextrin, while residual GFR was not. However, residual GFR is a numerical assessment of small solute clearance and may not represent true kidney function. In fact, Bargman et al41 reanalyzed the Canada–USA (CANUSA) study and found that residual urine volume was more important than residual GFR in predicting adverse outcomes. In accordance with our findings, previous studies have shown that treatment regimens including icodextrin preserved urine volume but not residual GFR.7,16,19 These findings suggest that residual urine volume has important implications beyond GFR from a clinical viewpoint.
Daily use of long-dwell of icodextrin has been reported to improve fluid status.7,11 However, our study did not demonstrate benefits of icodextrin on overall fluid status despite a significant preservation of urine volume in the icodextrin group. Of note, the control group had median urine volume >1000 mL/day at enrollment, and this was maintained relatively well until the end of the study although residual urine volume declined faster than in the icodextrin group. This can be partly explained by the use of biocompatible solutions in both the control and icodextrin groups. Several randomized controlled trials have shown that biocompatible solutions can preserve RRF better than conventional glucose solutions.33–35 In addition, it is possible that reciprocal increased peritoneal ultrafiltration caused by high concentration glucose solutions might compensate for a decrease in urine volume to maintain fluid status.42 In fact, there have been discrepant findings in residual urine volume depending on peritoneal ultrafiltration as seen in the studies by Davies et al7 and Konings et al.15 To overcome this issue, in our study, the control group used 2.5% glucose solution to induce peritoneal ultrafiltration similar to icodextrin solution. It should be noted that icodextrin preserved urine volume better than glucose solution, while maintaining peritoneal ultrafiltration comparable to the high concentration glucose solution. When we calculated total ultrafiltration volume as a sum of urine volume and peritoneal ultrafiltration, there was a slightly higher total ultrafiltration in the icodextrin group than in the control group by approximately 200 mL/day (data not shown). This small difference may not be adequately reflected in overall changes of fluid status measured by the current methods used in this study. Nevertheless, we thoroughly evaluated fluid status using 3 different assessment tools and found that overall fluid balance was similar between groups. If icodextrin solution preserves residual urine volume and maintains adequate peritoneal ultrafiltration for a longer period, we should expect more favorable volume control in patients using icodextrin. Further long-term investigation is required to detect difference in fluid status parameters in patients receiving icodextrin compared to glucose solution.
The mechanism through which icodextrin preserves RRF is unclear. Davies et al43 showed that plasma ANP levels were not significantly changed in the icodextrin group, whereas ANP levels decreased much more in the control group. This finding raised the potential possibility that icodextrin metabolites can increase oncotic pressure and hence maintain intravascular volume by shifting water from other compartments, while significantly reducing extracellular fluid volume. Such assumption was partly supported by our findings that ANP levels and IVC collapsibility index were relatively well maintained in the icodextrin group compared to the glucose solution group although the differences in the changes of these parameters were not statistically significant. However, ANP is excreted by the kidney and ANP levels are not correlated with acute changes in fluid status in patients with impaired kidney function. Measurement of IVC collapsibility index is an operator-dependent technique and also limited by interpatient variability. Unfortunately, there is no good single parameter to represent intravascular volume. Given the wide variability in the measurement of ANP levels and IVC collapsibility index and a lack of evidence for these parameters as valid biomarkers to assess intravascular volume in dialysis patients, other reliable markers should be developed in the future and used for the assessment of fluid status in clinical practice.
Our study has several limitations. First, fluid status was not directly measured by dilutional methods using deuterium or sodium bromide. Although the use of deuterium and sodium bromide is highly reproducible and accurate, these methods are very costly and cumbersome, and therefore not commonly used in clinical practice. Instead, we thoroughly evaluated fluid status using 3 different assessment tools besides clinical assessment and collected longitudinal data. This made our findings more reliable and provided better interpretation of the changes in fluid status. Second, the decline in residual GFR, a major primary endpoint, was not significantly different between the 2 groups. We calculated the sample size based on both residual GFR and urine volume to detect a 50% difference in these parameters over 12 months in the icodextrin group versus the glucose solution group. Contrary to our expectation, residual GFR did not differ by 50% between groups, whereas urine volume did at the end of the study. It should be mentioned that residual GFR is simply calculated based on creatinine and blood urea nitrogen levels and such small solute clearance may not tell true RRF as aforementioned. Third, we did not measure sodium concentration in the dialysate fluids, thus sodium removal by icodextrin was not evaluated. Adding icodextrin solution in patients undergoing automated PD can result in sustained sodium loss and ultrafiltration via the periteoneum17,44 although sodium removal capacity is similar between icodextrin and conventional glucose solutions.7,45 Nevertheless, RRF contributes to sodium removal and fluid control more than the peritoneum, thus it is very inspiring that the use of icodextrin can maximize sodium removal by enhancing urinary and peritoneal sodium loss. Finally, as this is an open-label trial, treatment solutions were not blinded to physicians and patients. However, randomization was successful and there were no significant differences in baseline characteristics between groups. To minimize biased results caused by the study design, RRF, peritoneal ultrafiltration, fluid status, and other laboratory parameters were evaluated by observers who were blinded to detailed information regarding treatment allocation.
In conclusion, this prospective randomized trial showed that icodextrin solution preserves residual urine volume better than glucose solution. However, overall fluid status was not significantly changed during the 1-year study period. Further long-term studies are required to evaluate whether icodextrin solution improves fluid status, and therefore, provides a beneficial clinical effect in patients undergoing PD.
Acknowledgements
The authors thank Baxter International Inc. Baxter for the support. The authors also thank Jung-Hwa Ryu, Hyang Sook Yoon, Jung Eun Lee, Eunhee Kim, Younhee Lee, Sanghee Lee, and Sun Wook Ahn for sincere contribution to this work.
Footnotes
Abbreviations: ANP = atrial natriuretic peptide, CI = confidence interval, ESRD = end-stage renal disease, GFR = glomerular filtration rate, ITT = intention-to-treat, IVC = inferior vena cava, PD = peritoneal dialysis, RRF = residual renal function.
This work was supported by Baxter International Inc. Baxter.
The sponsor had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lameire N, Van Biesen W. Epidemiology of peritoneal dialysis: a story of believers and nonbelievers. Nat Rev Nephrol 2010; 6:75–82. [DOI] [PubMed] [Google Scholar]
- 2.Krediet RT. The peritoneal membrane in chronic peritoneal dialysis. Kidney Int 1999; 55:341–356. [DOI] [PubMed] [Google Scholar]
- 3.Moberly JB, Mujais S, Gehr T, et al. Pharmacokinetics of icodextrin in peritoneal dialysis patients. Kidney Int 2002; 62 Suppl 81:S23–S33. [DOI] [PubMed] [Google Scholar]
- 4.Paniagua R, Orihuela O, Ventura MD, et al. Echocardiographic, electrocardiographic and blood pressure changes induced by icodextrin solution in diabetic patients on peritoneal dialysis. Kidney Int 2008; 73 Suppl 108:S125–S130. [DOI] [PubMed] [Google Scholar]
- 5.Cho Y, Johnson DW, Badve S, et al. Impact of icodextrin on clinical outcomes in peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2013; 28:1899–1907. [DOI] [PubMed] [Google Scholar]
- 6.Qi H1, Xu C, Yan H, et al. Comparison of icodextrin and glucose solutions for long dwell exchange in peritoneal dialysis: a meta-analysis of randomized controlled trials. Perit Dial Int 2011; 31:179–188. [DOI] [PubMed] [Google Scholar]
- 7.Davies SJ, Woodrow G, Donovan K, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol 2003; 14:2338–2344. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein F, Healy H, Abu-Alfa A, et al. Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol 2005; 16:546–554. [DOI] [PubMed] [Google Scholar]
- 9.Furuya R, Odamaki M, Kumagai H, et al. Beneficial effects of icodextrin on plasma level of adipocytokines in peritoneal dialysis patients. Nephrol Dial Transplant 2006; 21:494–498. [DOI] [PubMed] [Google Scholar]
- 10.Li PK, Culleton BF, Ariza A, et al. Randomized, controlled trial of glucose-sparing peritoneal dialysis in diabetic patients. J Am Soc Nephrol 2013; 24:1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paniagua R, Ventura MD, Avila-Diaz M, et al. Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int 2009; 29:422–432. [PubMed] [Google Scholar]
- 12.Takatori Y, Akagi S, Sugiyama H, et al. Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic nephropathy by improving body fluid management: a randomized controlled trial. Clin J Am Soc Nephrol 2011; 6:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfson M, Piraino B, Hamburger RJ, et al. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis 2002; 40:1055–1065. [DOI] [PubMed] [Google Scholar]
- 14.Han SH, Ahn SV, Yun JY, et al. Effects of icodextrin on patient survival and technique success in patients undergoing peritoneal dialysis. Nephrol Dial Transplant 2012; 27:2044–2050. [DOI] [PubMed] [Google Scholar]
- 15.Konings CJ, Kooman JP, Schonck M, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int 2003; 63:1556–1563. [DOI] [PubMed] [Google Scholar]
- 16.Lui SL, Yung S, Yim A, et al. A combination of biocompatible peritoneal dialysis solutions and residual renal function, peritoneal transport, and inflammation markers: a randomized clinical trial. Am J Kidney Dis 2012; 60:966–975. [DOI] [PubMed] [Google Scholar]
- 17.Plum J, Gentile S, Verger C, et al. Efficacy and safety of a 7.5% icodextrin peritoneal dialysis solution in patients treated with automated peritoneal dialysis. Am J Kidney Dis 2002; 39:862–871. [DOI] [PubMed] [Google Scholar]
- 18.Posthuma N, ter Wee PM, Donker AJ, et al. Assessment of the effectiveness, safety, and biocompatibility of icodextrin in automated peritoneal dialysis. The Dextrin in APD in Amsterdam (DIANA) Group. Perit Dial Int 2000; 20 Suppl 2:S106–S113. [PubMed] [Google Scholar]
- 19.Yoon HE, Chang YK, Shin SJ, et al. Benefits of a continuous ambulatory peritoneal dialysis (CAPD) technique with one icodextrin-containing and two biocompatible glucose-containing dialysates for preservation of residual renal function and biocompatibility in incident CAPD patients. J Korean Med Sci 2014; 29:1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 21.Nolph KD, Moore HL, Prowant B, et al. Cross sectional assessment of weekly urea and creatinine clearances and indices of nutrition in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 1993; 13:178–183. [PubMed] [Google Scholar]
- 22.Peritoneal Dialysis Adequacy Work Group, Burkart JM, Piraino B, et al. Clinical practice recommendations for peritoneal dialysis adequacy. Am J Kidney Dis 2006; 48 Suppl 1:S130–S158. [DOI] [PubMed] [Google Scholar]
- 23.Lameire N, Van Biesen W. Importance of blood pressure and volume control in peritoneal dialysis patients. Perit Dial Int 2001; 21:206–211. [PubMed] [Google Scholar]
- 24.Gunal AI, Duman S, Ozkahya M, et al. Strict volume control normalizes hypertension in peritoneal dialysis patients. Am J Kidney Dis 2001; 37:588–593. [PubMed] [Google Scholar]
- 25.Wang X, Axelsson J, Lindholm B, et al. Volume status and blood pressure in continuous ambulatory peritoneal dialysis patients. Blood Purif 2005; 23:373–378. [DOI] [PubMed] [Google Scholar]
- 26.Holtta T, Happonen JM, Ronnholm K, et al. Hypertension, cardiac state, and the role of volume overload during peritoneal dialysis. Pediatr Nephrol 2001; 16:324–331. [DOI] [PubMed] [Google Scholar]
- 27.Lameire N, Van Biesen W. Hypervolemia in peritoneal dialysis patients. J Nephrol 2004; 17 Suppl 8:S58–S66. [PubMed] [Google Scholar]
- 28.Cheng LT, Tang W, Wang T. Strong association between volume status and nutritional status in peritoneal dialysis patients. Am J Kidney Dis 2005; 45:891–902. [DOI] [PubMed] [Google Scholar]
- 29.Vicente-Martinez M, Martinez-Ramirez L, Munoz R, et al. Inflammation in patients on peritoneal dialysis is associated with increased extracellular fluid volume. Arch Med Res 2004; 35:220–224. [DOI] [PubMed] [Google Scholar]
- 30.Clinical practice guidelines for peritoneal adequacy, update 2006. Am J Kidney Dis 2006; 48 Suppl 1:S91–S97. [DOI] [PubMed] [Google Scholar]
- 31.Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int 2006; 69:1726–1732. [DOI] [PubMed] [Google Scholar]
- 32.Marron B, Remon C, Perez-Fontan M, et al. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int 2008; 73 Suppl 108:S42–S51. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Oh J, Chung W, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant 2009; 24:2899–2908. [DOI] [PubMed] [Google Scholar]
- 34.Haag-Weber M, Kramer R, Haake R, et al. Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant 2010; 25:2288–2296. [DOI] [PubMed] [Google Scholar]
- 35.Johnson DW, Brown FG, Clarke M, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23:1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad M, Jeloka T, Pliakogiannis T, et al. Icodextrin produces higher ultrafiltration in diabetic than in non-diabetic patients on continuous cyclic peritoneal dialysis. Int Urol Nephrol 2008; 40:219–223. [DOI] [PubMed] [Google Scholar]
- 37.Krediet R, Mujais S. Use of icodextrin in high transport ultrafiltration failure. Kidney Int 2002; 62 Suppl 81:S53–S61. [DOI] [PubMed] [Google Scholar]
- 38.Johnson DW, Arndt M, O'Shea A, et al. Icodextrin as salvage therapy in peritoneal dialysis patients with refractory fluid overload. BMC Nephrol 2001; 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkie ME, Plant MJ, Edwards L, et al. Icodextrin 7.5% dialysate solution (glucose polymer) in patients with ultrafiltration failure: extension of CAPD technique survival. Perit Dial Int 1997; 17:84–87. [PubMed] [Google Scholar]
- 40.Ha IS, Yap HK, Munarriz RL, et al. Risk factors for loss of residual renal function in children treated with chronic peritoneal dialysis. Kidney Int 2015; 88:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bargman JM, Thorpe KE, Churchill DN, et al. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12:2158–2162. [DOI] [PubMed] [Google Scholar]
- 42.Kooman JP, Cnossen N, Konings CJ, et al. Is there a competition between urine volume and peritoneal ultrafiltration in peritoneal dialysis patients? Contrib Nephrol 2006; 150:111–118. [DOI] [PubMed] [Google Scholar]
- 43.Davies SJ, Garcia Lopez E, Woodrow G, et al. Longitudinal relationships between fluid status, inflammation, urine volume and plasma metabolites of icodextrin in patients randomized to glucose or icodextrin for the long exchange. Nephrol Dial Transplant 2008; 23:2982–2988. [DOI] [PubMed] [Google Scholar]
- 44.Akonur A, Guest S, Sloand JA, et al. Automated peritoneal dialysis prescriptions for enhancing sodium and fluid removal: a predictive analysis of optimized, patient-specific dwell times for the day period. Perit Dial Int 2013; 33:646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodríguez-Carmona A, Pérez Fontán M. García López E, García Falcón T, Díaz Cambre H. Use of icodextrin during nocturnal automated peritoneal dialysis allows sustained ultrafiltration while reducing the peritoneal glucose load: a randomized crossover study. Perit Dial Int 2007; 27:260–266. [PubMed] [Google Scholar]


