Abstract
Currently, the association between sarcopenia and long-term prognosis after gastric cancer surgery has not been investigated. Moreover, the association between sarcopenia and postoperative complications remains controversial. This large-scale retrospective study aims to ascertain the prevalence of sarcopenia and assess its impact on postoperative complications and long-term survival in patients undergoing radical gastrectomy for gastric cancer.
From December 2008 to April 2013, the clinical data of all patients who underwent elective radical gastrectomy for gastric cancer were collected prospectively. Only patients with available preoperative abdominal CT scan within 30 days of surgery were considered for analysis. Skeletal muscle mass was determined by abdominal (computed tomography) CT scan, and sarcopenia was diagnosed by the cut-off values obtained by means of optimum stratification. Univariate and multivariate analyses evaluating risk factors of postoperative complications and long-term survival were performed.
A total of 937 patients were included in this study, and 389 (41.5%) patients were sarcopenic based on the diagnostic cut-off values (34.9 cm2/m2 for women and 40.8 cm2/m2 for men). Sarcopenia was an independent risk factor for severe postoperative complications (OR = 3.010, P < 0.001), but not for total complications. However, sarcopenia did not show significant association with operative mortality. Moreover, sarcopenia was an independent predictor for poorer overall survival (HR = 1.653, P < 0.001) and disease-free survival (HR = 1.620, P < 0.001). Under the adjusted tumor-node-metastasis (TNM) stage, sarcopenia remained an independent risk factor for overall survival and disease-free survival in patients with TNM stage II and III, but not in patients with TNM stage I.
Sarcopenia is an independent predictive factor of severe postoperative complications after radical gastrectomy for gastric cancer. Moreover, sarcopenia is independently associated with overall and disease-free survival in patients with TNM stage II and III, but not in patients with TNM stage I.
INTRODUCTION
Gastric cancer is the fifth most common cancer and the third leading cause of cancer death globally.1 In 2012, an estimated 951,600 new stomach cancer cases and 723,100 deaths occurred.1 Despite potential improvements in treatment, the prognosis of gastric cancer remains poor, particularly in China and Western countries.2–4 Surgical resection remains the most effective therapy for potentially curable gastric cancer.5 However, gastrectomy is associated with high complications rates and operative mortality.3,6 In addition, a large proportion of patients died within 5 years after a potentially curative (R0) resection.2–4 Therefore, the prognostic assessment of patients with gastric cancer after radical surgery is critical for guiding therapeutic schedule and follow-up strategies.
Sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength.7 It is a condition with many causes, such as malnutrition, aging, inactivity, inflammatory disease, and cancer.7 Most recently, 3 studies have investigated the association between sarcopenia and short-term outcomes after gastric cancer surgery, including postoperative mortality and complication, but came up with inconsistent results.8–10 Moreover, up to date, no study has reported the impact of sarcopenia on long-term prognosis after radical gastrectomy for gastric cancer.
The purpose of this retrospective study was to ascertain the impact of sarcopenia on postoperative complications and long-term prognosis in patients undergoing radical gastrectomy for gastric cancer, using the prospectively maintained clinical database with a large sample size.
METHODS
Patients
This retrospective study was performed using data from a prospectively maintained database of patients undergoing gastric cancer surgery at the Gastrointestinal Surgical Department, the First Affiliated Hospital of Wenzhou Medical University. Only patients underwent radical gastrectomy and had abdominal computed tomography (CT) image within 1 month before surgery were included in this study. The treatment for gastric cancer was based on the Japanese Gastric Cancer Treatment Guideline.11,12 All participants provided their written informed consent and this study was approved by the ethics committee of The First Affiliated Hospital of Wenzhou Medical University.
Follow-Up
All patients were followed up within the first month after surgery. After that, patients were followed up every 3 months for 2 years, every 6 months thereafter for up to 5 years, and every 1 year thereafter. Patients were contacted by phone and were scheduled to come back to the hospital to fulfill the follow-up program in the above time points. The follow-up program was consisted of a physical examination, laboratory tests, and ultrasonography and/or CT and/or endoscopy. The last follow-up date was December 2015.
Assessment of Skeletal Muscle Mass
A cross-sectional CT image of the third lumbar vertebra (L3) in the inferior direction was selected for estimating muscle mass as described previously.13 Skeletal muscles were separated from other tissues by a Hounsfield units threshold range of −29 to +15014 and tissue boundaries were manually outlined as needed. The muscles in the L3 region contain psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis. To minimize the measurement bias, 1 investigator (S.-L.W.) who was blinded for the patient and surgical characteristics was trained to identify and measure the muscle area, using a professional imaging software (INFINITT PACS software version 3.0.11.3 BN17 32 bit, INFINITT Healthcare Co., Ltd, Seoul, Korea). L3 muscle cross-sectional areas computed from each image were normalized for height (m2) to obtain the L3 skeletal muscle index (L3 SMI, cm2/m2).15 The median time between the date of the CT scan and the surgical date was 3 days (interquartile range [IQR] = 3 days).
Data Collection
Referring to our prospectively maintained computer database, the following data were collected and analyzed retrospectively: the patient demographic and clinicopathological features, including age, gender, body mass index (BMI), hemoglobin concentration, plasma albumin concentration (plasma albumin <35 g/L is defined as hypoproteinemia), American Society of Anesthesiologists (ASA) grade, comorbidity, previous abdominal surgery, histologic type, tumor location, tumor size, tumor-node-metastasis (TNM) stage of tumor, lymphovascular invasion; operative and treatment characteristic, including operative time, estimated blood loss, transfusion, adjuvant chemotherapy, type of resection, type of reconstruction, extent of lymph node dissection and combined resection; postoperative outcomes, including operative mortality, length of postoperative hospital stays, postoperative complications, overall survival and disease-free survival. Postoperative complications were classified according to the Clavien–Dindo classification.16 Total complications were defined as complications classified as Grade II or above. Severe complications were defined as those classified as Grade III or above and the operative mortality was defined as death within 30 days of the operation. Overall survival was calculated from the date of surgery to the date of death from any cause and was censored at the last follow-up. Disease-free survival was calculated as the time from the date of surgery to the date of relapse or death from any cause and was censored at the last verifiable disease-free date.
Statistical Analysis
The normally distributed continuous data were presented as mean and standard deviation (SD). The nonnormally distributed continuous distributed data were presented as median and IQR. Categorical variables were presented as numbers and percentages. Clinical variables were compared using Student t test (normally distributed data), Pearson Chi-square test or Fisher exact test (categorical data), and Mann–Whitney U test (nonnormally distributed continuous data and ranked data) as appropriate. Overall and disease-free survival rates were estimated by Kaplan–Meier method, and the difference of overall and disease-free survival between the subgroups was compared with the log-rank test. For the test of potential risk factors associated with the outcomes, univariate analyses with clinically relevant parameters were performed. Variables with a P-value of <0.10 were included into subsequent multivariate (logistic regression or Cox proportional hazards regression) analysis.
To determine the sex-specific cut-off values for the L3 SMI at which the survival difference was most significant, we used optimum stratification to find the most significant P-value by means of log-rank Chi-square statistics. This method has been previously described in literature to solve the threshold value of the continuous covariable (L3 SMI) at which patients with sarcopenia and patients without sarcopenia are best separated with respect to time to mortality.15 The cut-off values obtained by this method were used to classify patients into sarcopenic and nonsarcopenic.
All tests were 2-sided and a P-value <0.05 was considered statistically significant. The statistical analyses were performed using SPSS statistics version 22.0 (IBM, Armonk, NY) and SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Patients
From December 2008 to April 2013, a total of 937 patients met our inclusion criteria and were included for analysis. The median follow-up time for these patients was 62.33 months. Patient demographic and clinical characteristics are listed in Table 1.
TABLE 1.
Patient Demographic and Clinical Characteristics
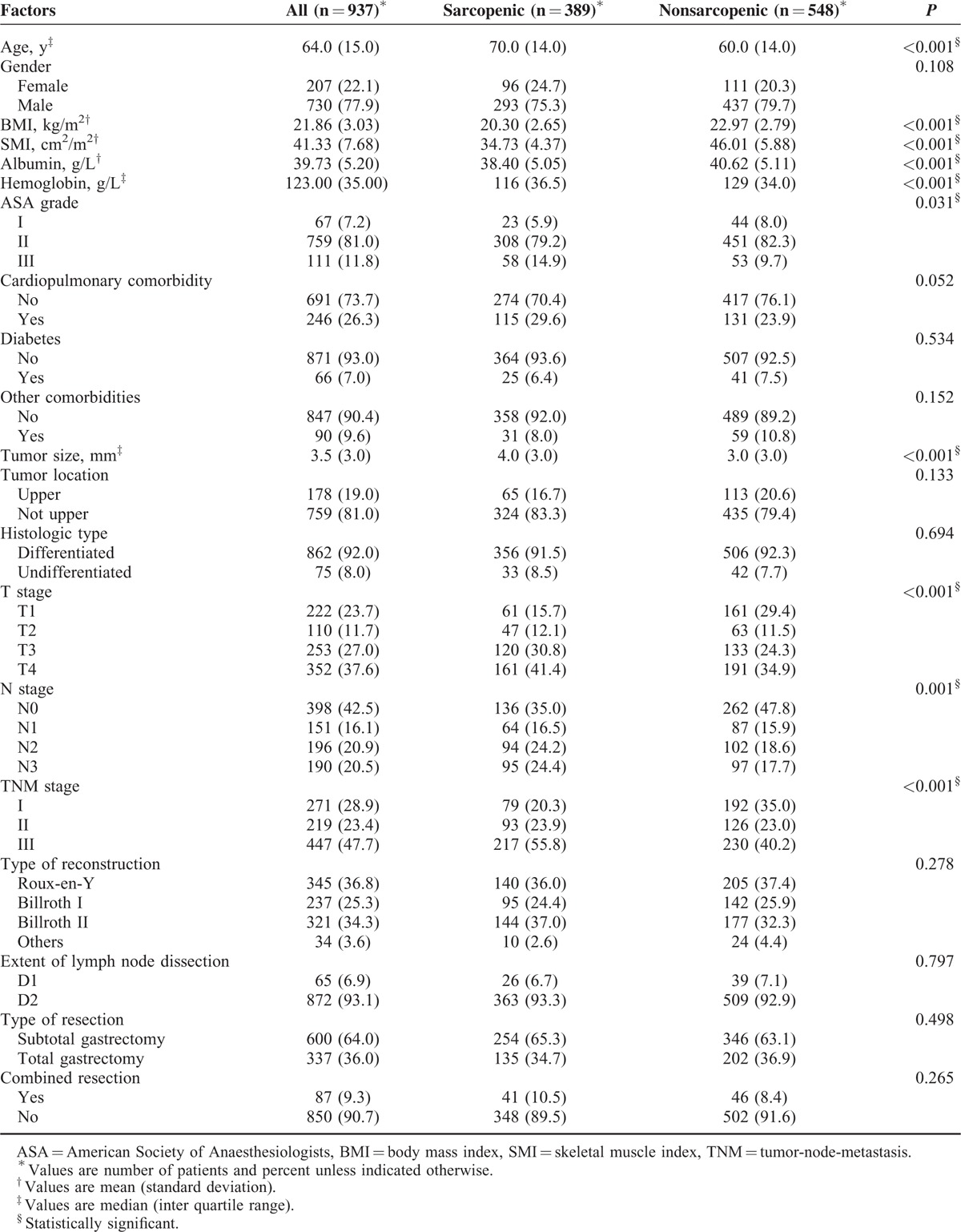
Cut-Off Values for L3 Skeletal Muscle Mass Index
Sex-specific cut-off values for L3 SMI associated with overall mortality were 34.9 cm2/m2 for women and 40.8 cm2/m2 for men, obtained by means of optimum stratification. Using these cut-off values, 41.5% patients were found to be sarcopenic (Table 1). Demographic and clinical characteristics of patients with and without sarcopenia are shown in Table 1. Patients with sarcopenia had an older age, a lower BMI, a lower albumin and hemoglobin concentration, and a higher ASA grade than those without. As for tumor characteristics, patients with sarcopenia had a larger tumor size, a more advanced T stage, N stage, and TNM stage than those without. Other host-related factors such as gender, cardiopulmonary comorbidity, and diabetes were not related to the presence of sarcopenia. Operative details (type of reconstruction, extent of lymph node dissection, combined resection and type of resection) were similar between the 2 groups (Table 1).
Sarcopenia and Short-Term Postoperative Outcomes
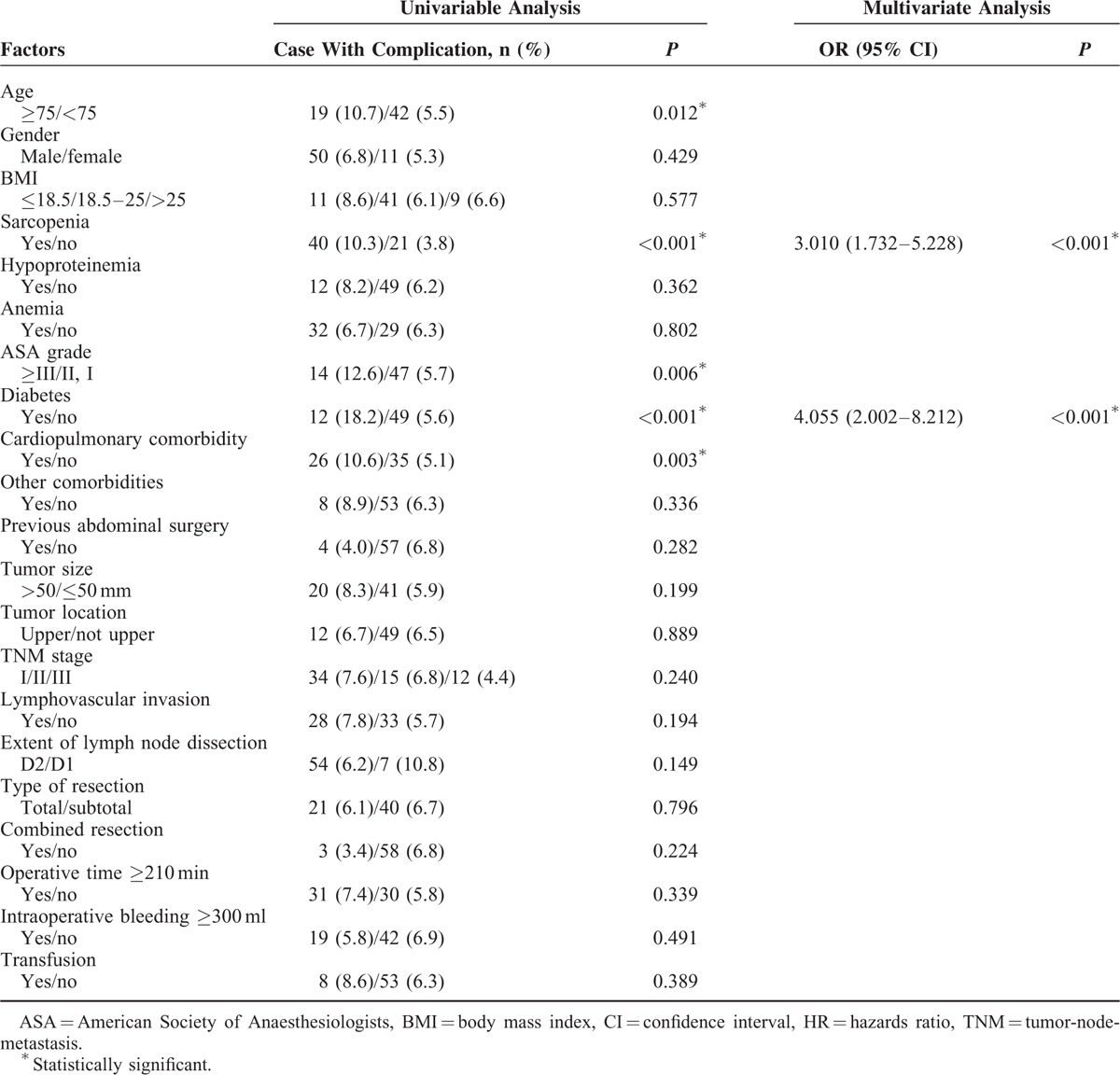
Median postoperative hospital stay was 11 days (IQR = 5) for patients with sarcopenia compared with 10 days (IQR = 4) for patients without sarcopenia (P = 0.027). Operative mortality was similar between sarcopenic and nonsarcopenic patients (1.8% vs 0.5%; P = 0.130). Total postoperative complications occurred in 227 (24.2%) patients. The distribution of postoperative complications according to the Clavien–Dindo classification is listed in Table 2. Patients with sarcopenia had a significant higher incidence of total complications and severe complications compared with those without sarcopenia (28.5% vs 21.2%, P = 0.004; 10.3% vs 3.8%, P < 0.001, respectively). Multivariate logistic analysis showed that sarcopenia was an independent risk factor for severe complications (Table 3), but not for total complications (data were not shown).
TABLE 2.
Details of Postoperative Complications According to the Clavien–Dindo Classification
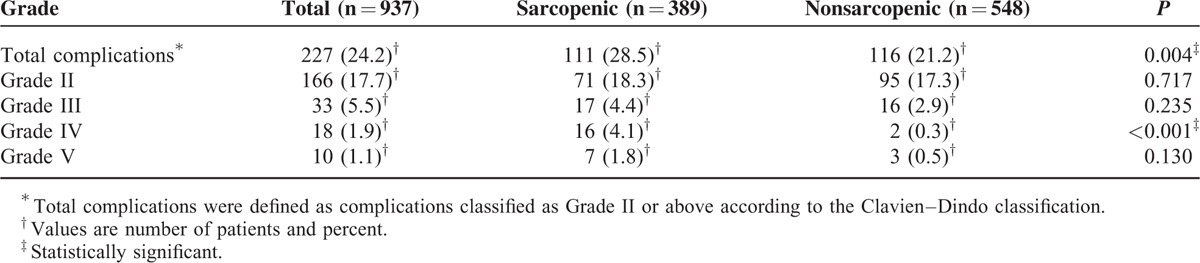
TABLE 3.
Univariate and Multivariate Logistic Regression Analysis of Factors Associated With Severe Complications
Sarcopenia and Overall Survival
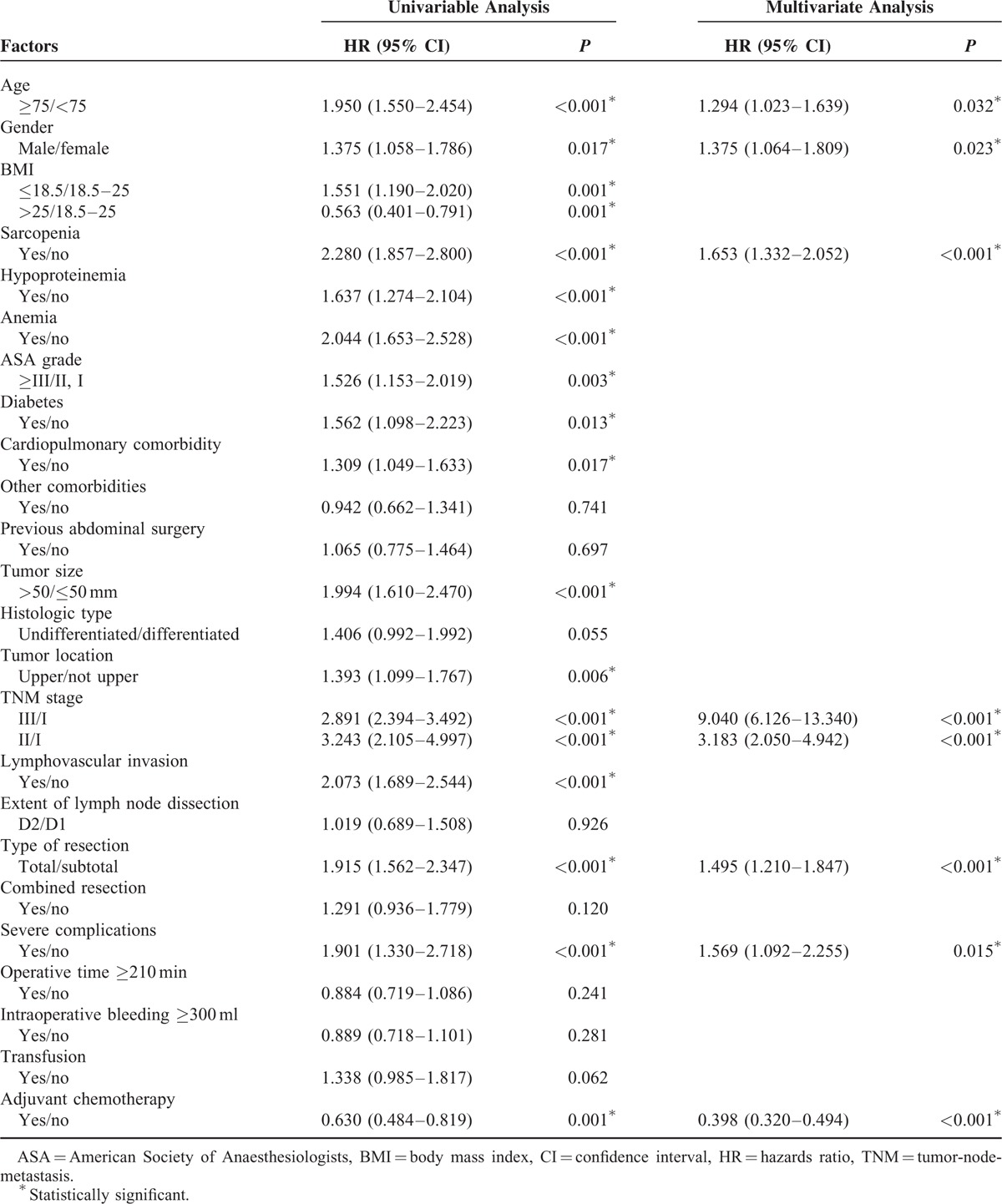
As shown in Figure 1, patients with sarcopenia had a poorer overall survival than patients without sarcopenia (P < 0.001). The 1-, 3-, and 5-year overall survival rates were 78.9, 53.8, and 42.6%, respectively, for patients with sarcopenia, and were 91.4, 73.6, and 69.4%, respectively, for those without sarcopenia. The results of univariate and multivariate analysis of factors associated with overall survival are shown in Table 4. Age ≥75, male gender, sarcopenia, advanced TNM stage, severe complications and total gastrectomy were independent risk factors for a poorer overall survival rate, whereas adjuvant chemotherapy was a protective factor.
FIGURE 1.
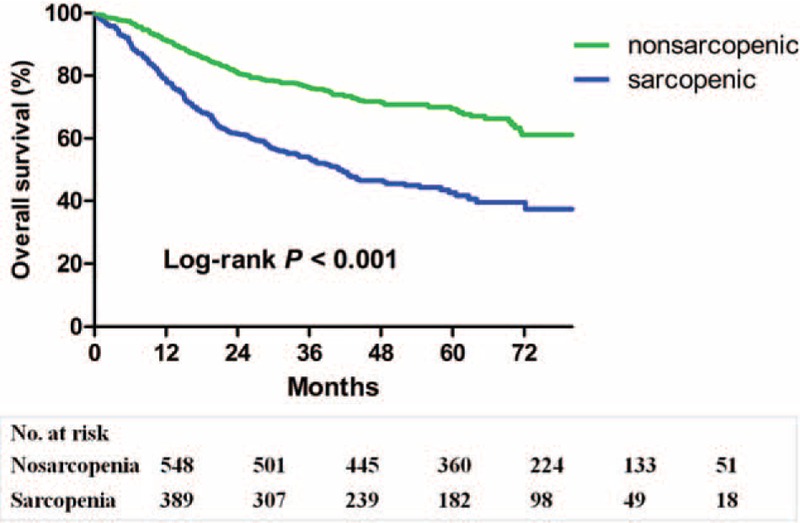
Kaplan–Meier survival curves for overall survival in patients with and in those without sarcopenia.
TABLE 4.
Univariate and Multivariate Analysis of Factors Associated With Overall Survival
Sarcopenia and Disease-Free Survival
As shown in Figure 2, patients with sarcopenia had a poorer disease-free survival than patients without sarcopenia (P < 0.001). The 1-, 3-, and 5-year disease-free survival rates were 74.7, 54.7, and 47.2%, respectively, for the sarcopenic patients, and were 88.8, 73.5, and 69.7%, respectively for the nonsarcopenic patients. Table 5 shows the results of univariate and multivariate analysis of factors associated with disease-free survival. Sarcopenia, undifferentiated histologic type, operative time ≥210 minutes, advanced TNM stage and total gastrectomy were independently associated with a lower disease-free survival rate, whereas adjuvant chemotherapy was a protective factor.
FIGURE 2.
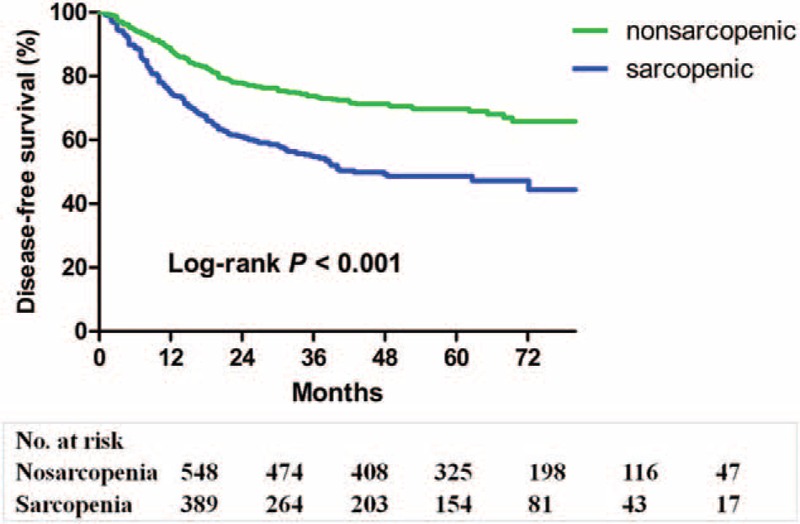
Kaplan–Meier survival curves for disease-free survival in patients with and in those without sarcopenia.
TABLE 5.
Univariate and Multivariate Analysis of Factors Associated With Disease-Free Survival
Impact of Sarcopenia on the Long-Term Prognosis Under Adjusted TNM Stage
Sarcopenic patients had a significantly poorer overall survival (P < 0.001) and disease-free survival (P < 0.001) than nonsarcopenic patients under TNM stage III. Similarly, the overall survival (P < 0.001) and disease-free survival (P = 0.014) were lower in the sarcopenic than nonsarcopenic group under TNM stage II (Figure 3). However, for patients with TNM stage I, no significant differences were shown between the sarcopenic and nonsarcopenic groups for overall survival (P = 0.201) or disease-free survival (P = 0.344).
FIGURE 3.
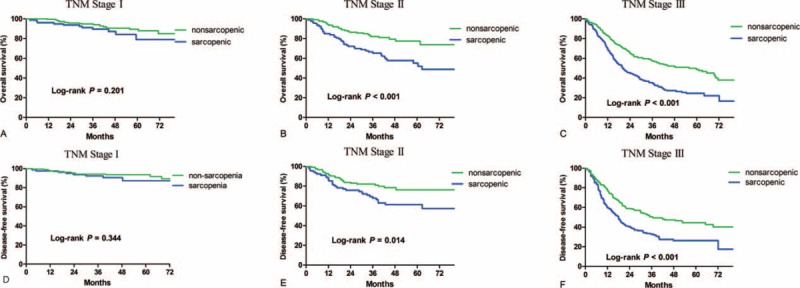
Kaplan–Meier survival curves for overall survival and disease-free survival in patients with and in those without sarcopenia under adjusted TNM stage.
DISCUSSION
Sarcopenia is characterized by progressive and generalized loss of skeletal muscle mass and strength with an increased risk of adverse outcomes.7 In the present study, we reported an incidence of sarcopenia of 41.5% in patients with gastric cancer. Sarcopenia was identified as an independent risk factor for severe complications. Moreover, sarcopenia was independently associated with a worse overall and disease-free survival.
According to consensus of European Working Group on Sarcopenia in Older People (EWGSOP) in 2010,7 sarcopenia is categorized into primary sarcopenia and secondary sarcopenia by its cause. Age-related sarcopenia without other evident causes is defined as “primary” sarcopenia, whereas “secondary” sarcopenia is considered when 1 or more other causes are evident, such as inflammatory disease, malignancy, or malnutrition. In the present study, we enrolled patients with gastric cancer, therefore, both advanced age and malignancy can be the main causes of sarcopenia in our patient cohort. Previous studies have investigated sarcopenia in patients with different types of malignancy.14,17–22 It is noteworthy that most previous studies adopted the cut-off values proposed by Prado et al15 or van Vledder et al,23 both of which were based on the characteristics of the Western population. However, it is well known that the Western people generally have a larger physique and a higher BMI than the Eastern people, which may make these values unapplicable to the Eastern population. Therefore, we propose the cut-off values obtained from our study to be applicable for the diagnosis of sarcopenia in patients with gastric cancer in Eastern population and identified the cut-off values of 34.9 cm2/m2 for women and 40.8 cm2/m2 for men.
Three previous studies have investigated the association of sarcopenia with postoperative complications after gastric cancer surgery and have obtained inconsistent results.8–10 In the present study, we identified sarcopenia as an independent risk factor for severe complications after gastrectomy for gastric cancer, which is consistent with a previous study in patients over 65 years of age who underwent gastrectomy for gastric cancer.10 Severe complications are of vital clinical significance since surgical or endoscopical interventions are required for these complications.16 Moreover, severe complications are associated with a higher operative mortality24 and a poorer long-term survival.25 Sarcopenic patients have an increased complication rate for the following possible reasons. First, sarcopenia was associated with a lower BMI (P < 0.001) and lower serum albumin level in the present study, both of which are common index to evaluate nutritional status. Moreover, skeletal muscle mass has been reported to be a new index for nutritional assessment.26 It is well known that poor nutritional status is associated with an increased postoperative complication rate.27 Thus, sarcopenia serves as a reflection of poor nutritional status, associated with an increased postoperative complication rate. Second, the clinical relevance of sarcopenia is due to the value of muscle mass and strength as critical components in maintaining physical function, mobility, and vitality.26 Low muscle quality would lead to physical disability and frailty, and subsequently results in an impaired postoperative recovery process.26
In the present study, we firstly reported that sarcopenia has a negative impact on long-term survival after gastric cancer surgery. The mechanisms by which sarcopenia confers increased risk of tumor relapse and mortality are still unclear, but the following reasons can be hypothesized. First, sarcopenia may be a reflection of the increased metabolic activity of a more aggressive tumor biology, and the increased metabolic activity lead to a more severe systemic inflammation and subsequently result in muscle wasting.28 Second, it has been reported that myokines secreted from muscle cells can inhibit the cancer cell growth.29 Therefore, we speculate that reduced muscle mass can lead to an impaired myokine response and an increased risk of cancer relapse.29 However, there is no evidence available to support this conclusion. Third, in this study, we found that sarcopenic patients have a lower tolerance for adjuvant chemotherapy (P < 0.001, detailed data were not shown). Since adjuvant chemotherapy is a strong independent protective factor for overall survival and disease-free survival, lower tolerance for chemotherapy can partially explain the negative impact of sarcopenia on long-term survival. Fourth, our study and a previous study both showed that patients with sarcopenia are susceptible to severe complications after radical gastrectomy.8,10 Moreover, severe postoperative complications were associated with lower survival rate after gastrectomy, revealed by our and previous study.25 Therefore, the higher complication rate in the sarcopenic patients can also explain the worse long-term prognosis after gastrectomy for gastric cancer.
It is well known that advanced tumor stage is associated with a poor long-term prognosis of malignancy. Similarly, in our study, we found that TNM stage was an independent risk factor for the worse overall and disease-free survival. It was noteworthy that sarcopenic patients had a more advanced TNM stage in this study. This is not surprising since malignancy is a significant cause of sarcopenia.7 To objectively evaluate the impact of sarcopenia on the long-term prognosis, we stratified the patients according to their TNM stage, and compared the long-term prognosis between sarcopenic and nonsarcopenic patients. The results showed that sarcopenic patients have a significantly poorer overall survival and disease-free survival than nonsarcpenic patients under TNM stage II and III. However, for patients with TNM stage I, the difference was not significant, although there was a trend toward worse long-term prognosis in the sarcopenic patients. Since patients with TNM stage I generally have a long postoperative survival time. We propose a longer follow-up period is needed to further demonstrate the impact of sarcopenia on long-term postoperative survival in patients with TNM stage I.
BMI is another common measurement of patient general condition. In this study, lower BMI was correlated with sarcopenia but not with the postoperative outcomes. The Eastern population has a significant lower BMI compared with the Western population, as shown by our patient cohorts with a mean BMI of 21.86. Our study demonstrated a better predictive capacity of sarcopenia over BMI for postoperative outcomes, even in Eastern patients who generally have a low BMI. This result strengthens the notion that skeletal muscle should be considered the most clinically relevant body composition.23,30
This study identified sarcopenia as a potent risk assessment parameter for postoperative outcomes after gastric cancer surgery. Compared with the widely used measurements such as ASA grade, weight loss or BMI, sarcopenia is a more subjective and precise parameter. CT scan is an accurate approach for the quantification of skeletal muscle mass with a reported measurement error of about 1.4%.31 In addition, abdominal CT scan is a noninvasive, inexpensive and convenient examination, routinely available for most patients with gastric cancer before surgery, and is generally used to assess tumor location, size, and to look for abdominal metastases. Therefore, we propose that sarcopenia, as determined by abdominal CT scan should be included in the risk stratification of patients undergoing radical gastrectomy for gastric cancer. In addition to CT scans, magnetic resonance imaging (MRI) is another gold standard for estimating muscle mass.7 X-ray absorptiometry (DXA) is an alternative method for research and clinical use. The main drawback of these methods is that these equipments are not portable.7 Bioimpedance analysis (BIA) is a portable, inexpensive, easy to use, and readily reproducible method to estimate the volume of lean body mass.7
However, according to the EWGSOP,7 sarcopenia should be defined as both low muscle mass and low muscle function (strength or performance). In this study, we included only muscle mass for the definition of sarcopenia due to the retrospective study design and this is a limitation of this study. There are several other limitations in the present study. First, this was a retrospective single-center study. However, the clinical data were prospectively collected and the follow-up strategy was strictly implemented. We therefore believe the results of this study were reliable. Second, for 304 patients, preoperative CT images were unavailable within 30 days of surgery, and therefore these patients were excluded, which may introduce selection bias to the study. To assess for possible bias introduced by these missing data, we compared the characteristics of the 304 excluded patients with that of the patients in the analytic cohort. No substantial differences were observed between the 2 groups with regard to clinicopathological features, postoperative complications, or long-term survival between the 2 groups (data not shown).
The present study indicated that sarcopenia could be a potential therapeutic target for improving the treatment of gastric cancer in the future. Resistance training has been recognized as a highly effective strategy to offset sarcopenia.32 Moreover, adequate nutritional intake and certain nutritional supplements, such as leucine and omega-3 polyunsaturated fatty acids, could have a synergistic effect with resistance training in maintaining muscle mass.32
This study was strengthened by its large sample size, prospectively data collection, and strict follow-up strategy. Moreover, the duration between the date of the CT scan and the date of surgery was short and similar among the patients, with a median of 3 days (IQR = 3 days). Since skeletal muscle mass reduces with the progression of tumor and cancer cachexia,7,33,34 the muscle mass may have reduced from the date of the CT scan to the date of surgery. We minimized such bias by reducing this time duration. In addition, all patients underwent a standard radical gastrectomy for gastric cancer according to Japanese gastric cancer treatment guidelines, and the long-term survival of our study were comparable to that reported by previous large-scale studies,4,35 both of which make the study feasible to generalize to patients outside of our department.
In conclusion, we firstly identified the diagnostic cut-off values for sarcopenia in patients with gastric cancer. Using these criteria, the incidence of sarcopenia was 41.5% in the present patient cohort. Sarcopenia is an independent predictive factor of severe postoperative complications after radical gastrectomy for gastric cancer. Moreover, sarcopenia is independently associated with overall and disease-free survival in patients with TNM stage II and III, but not in patients with TNM stage I. Sarcopenia, as determined by abdominal CT scan, could be included in the preoperative risk assessments of patients undergoing radical gastrectomy for gastric cancer.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BIA = bioimpedance analysis, BMI = body mass index, CT = computed tomography, DXA = X-ray absorptiometry, EWGSOP = European Working Group on Sarcopenia in Older People, IQR = interquartile range, MRI = magnetic resonance imaging, SD = standard deviation, SMI = skeletal muscle index, TNM = tumor-node-metastasis.
This work was supported by the National Natural Science Foundation of China (no. 81171857) and the clinical nutriology of medical supporting discipline of Zhejiang Province (11-ZC24).
C-LZ, D-DH, and W-YP contributed equally to this work.
H-YS, Department of Public Health, Wenzhou Medical University, contributed to the optimization of the statistical methods of this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y, Ejaz A, Spolverato G, et al. Conditional survival after surgical resection of gastric cancer: a multi-institutional analysis of the us gastric cancer collaborative. Ann Surg Oncol 2015; 22:557–564. [DOI] [PubMed] [Google Scholar]
- 3.Lepage C, Sant M, Verdecchia A, et al. Operative mortality after gastric cancer resection and long-term survival differences across Europe. Br J Surg 2010; 97:235–239. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Zheng C, Fang C, et al. Time trends of clinicopathologic features and surgical treatment for gastric cancer: results from 2 high-volume institutions in southern China. Surgery 2015; 158:1590–1597. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Guidelines for Treatment of Gastric Cancer. Version 3. 2015. Available at: http://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf [Assessed May 7, 2015]. [Google Scholar]
- 6.Papenfuss WA, Kukar M, Oxenberg J, et al. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol 2014; 21:3008–3014. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SL, Zhuang CL, Huang DD, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: a prospective study. Ann Surg Oncol 2016; 23:556–564. [DOI] [PubMed] [Google Scholar]
- 9.Tegels JJ, van Vugt JL, Reisinger KW, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol 2015; 112:403–407. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda Y, Yamamoto K, Hirao M, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer 2015; 8. [DOI] [PubMed] [Google Scholar]
- 11.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Guidelines for diagnosis and treatment of carcinoma of the stomach. April 2004. edition. Available at: http://www.jgca.jp/pdf/Guidelines2004_eng.pdf [Accessed March 12, 2008]. [Google Scholar]
- 13.Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008; 33:997–1006. [DOI] [PubMed] [Google Scholar]
- 14.Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012; 107:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008; 9:629–635. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ida S, Watanabe M, Yoshida N, et al. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol 2015; 22:4432–4437. [DOI] [PubMed] [Google Scholar]
- 18.Joglekar S, Asghar A, Mott SL, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol 2015; 111:771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol 2015; 22:2663–2668. [DOI] [PubMed] [Google Scholar]
- 20.Voron T, Tselikas L, Pietrasz D, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg 2015; 261:1173–1183. [DOI] [PubMed] [Google Scholar]
- 21.Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012; 16:1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reisinger KW, van Vugt JL, Tegels JJ, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 2015; 261:345–352. [DOI] [PubMed] [Google Scholar]
- 23.van Vledder MG, Levolger S, Ayez N, et al. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012; 99:550–557. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Yoshikawa T, Aoyama T, et al. Severity of complications after gastrectomy in elderly patients with gastric cancer. World J Surg 2012; 36:2139–2145. [DOI] [PubMed] [Google Scholar]
- 25.Jiang N, Deng JY, Ding XW, et al. Effect of complication grade on survival following curative gastrectomy for carcinoma. World J Gastroenterol 2014; 20:8244–8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr 2014; 38:940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall JC. Nutritional assessment of surgery patients. J Am Coll Surg 2006; 202:837–843. [DOI] [PubMed] [Google Scholar]
- 28.Dodson S, Baracos VE, Jatoi A, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 2011; 62:265–279. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012; 8:457–465. [DOI] [PubMed] [Google Scholar]
- 30.Muscaritoli M, Anker SD, Argiles J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010; 29:154–159. [DOI] [PubMed] [Google Scholar]
- 31.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998; 85:115–122. [DOI] [PubMed] [Google Scholar]
- 32.Phillips SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr 2015; 6:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12:489–495. [DOI] [PubMed] [Google Scholar]
- 34.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013; 31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 35.Strong VE, Wu AW, Selby LV, et al. Differences in gastric cancer survival between the U.S. and China. J Surg Oncol 2015; 112:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


