Abstract
Bupropion is widely used for treating bipolar disorder (BD), and especially those with depressive mood, based on its good treatment effect, safety profile, and lower risk of phase shifting. However, increasing evidence indicates that the safety of bupropion in BD patients may not be as good as previously thought.
The aim of this study was to summarize data on the treatment effect and safety profile of bupropion in the treatment of BD via a meta-analysis.
Electronic search through PubMed and ClinicalTrials.gov was performed.
The inclusion criteria were: (i) studies comparing changes in disease severity before and after bupropion treatment or articles comparing the treatment effect of bupropion in BD patients with those receiving other standard treatments; (ii) articles on clinical trials in humans. The exclusion criteria were (i) case reports/series, and (ii) nonclinical trials.
All effect sizes from 10 clinical trials were pooled using a random effects model. We examined the possible confounding variables using meta-regression and subgroup analysis.
Bupropion significantly improved the severity of disease in BD patients (P < 0.001), and the treatment effect was similar to other antidepressants/standard treatments (P = 0.220). There were no significant differences in the dropout rate (P = 0.285) and rate of phase shifting (P = 0.952) between BD patients who received bupropion and those who received other antidepressants.
We could not perform a detailed meta-analysis of every category of antidepressant, nor could we rule out the possible confounding effect of concurrent psychotropics or include all drug side effects. Furthermore, the number of studies recruited in the meta-analysis was relatively small.
Our findings reconfirm the benefits of bupropion for the treatment of bipolar depression, which are similar to those of other antidepressants. However, the rate of phase shifting with bupropion usage was not as low compared to other antidepressants as previously thought, which should serve to remind clinicians of the risk of phase shifting when prescribing bupropion to BD patients regardless of the suggestions of current clinical practice guidelines.
INTRODUCTION
Bipolar disorder (BD) is one of the most complicated psychiatric illnesses worldwide. In patients with BD, bipolar depression is one of the most problematic mood states, resulting in a higher risk of suicide, more frequent episodes, and longer duration of illness than manic/hypomanic episodes.1 The management of bipolar depression is complicated by the risk of phase shifting. Although several risk factors for phase shifting such as a history of previous mood swings, earlier age at onset, and poorer response to antidepressants are known, phase shifting in patients with bipolar depression still remains unpredictable.2
Even though the prescription of antidepressants for patients with BD is known to be effective, their use is currently under debate because of the possible risk of phase shifting. In previous studies, treatment with antidepressants such as serotonin-norepinephrine reuptake inhibitors (SNRIs) 3 and selective serotonin reuptake inhibitors (SSRIs) 4 has been reported to increase the risk of phase shifting in BD patients. Current case-controlled and randomized-controlled trials have revealed inconsistent findings on the benefit/harm of antidepressant treatment in BD patients.5,6 The results of 1 recent meta-analysis suggested that antidepressants are not statistically superior to a placebo or other current standard treatments for bipolar depression.7
Bupropion has unique pharmacokinetics as a norepinephrine-dopamine reuptake inhibitor (NDRI), and it is has been approved by the Food and Drug Administration (FDA) for the treatment of major depression since 1989. In addition, increasing evidence suggests that bupropion has unique benefits in the treatment of BD because of its ability to ameliorate depressive symptoms with a lower risk of phase shifting than other antidepressants.4,8,9 Furthermore, it has been recommended as first-line treatment, either as monotherapy or combination therapy, in recent clinical guidelines based on its safety for BD patients and lower risk of phase shifting.10,11 However, with increasing clinical experience of the usage of bupropion, an increasing number of clinical studies have reported phase shifting in BD patients related to the use of bupropion.12–18 The safety of bupropion as monotherapy or combination therapy in the treatment of BD patients has thus attracted the attention of clinicians19,20 and prompted further research to evaluate the role of bupropion in the treatment of BD. Therefore, the aim of the present study was to summarize the current data about the role of bupropion, including the treatment effect and safety profile, in the treatment of BD via a thorough meta-analysis.
METHODS AND MATERIALS
Literature Search and Screening
We performed a systematic literature search through PubMed and ClinicalTrials.gov using the search term (bipolar disorder) AND (bupropion) for all articles written in English up to January 12th, 2016. The search was conducted by 2 independent authors (P-TT and Y-WC). At the initial stage of screening, both authors screened all articles via the titles and abstracts. Inconsistent selections and disagreements were resolved by consensus. In the subsequent stage of screening for eligibility, we used the following inclusion criteria: (i) (a) studies comparing changes in disease severity before and after bupropion treatment, either as monotherapy or combination therapy, or (b) articles comparing the treatment effect of bupropion in BD patients to other standard treatments; and (ii) articles on clinical trials in humans. The exclusion criteria were: (i) case reports/series; and (ii) nonclinical trials. The selection protocol is illustrated in Figure 1. We also assessed the quality of the clinical trials using Jadad scores (Supplementary Table 1).21
FIGURE 1.
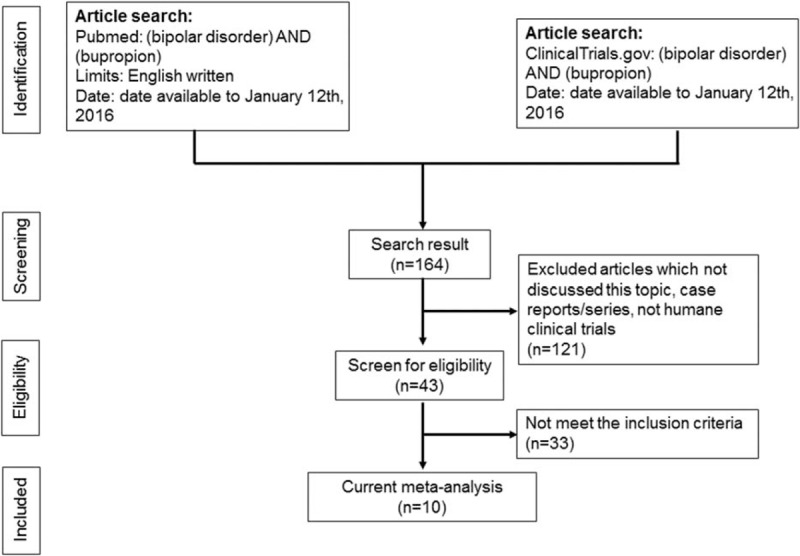
Flowchart of the selection strategy and inclusion/exclusion criteria for current meta-analysis.
Data Extraction
The primary outcome was changes in disease severity rating scale, which included the Hamilton Depression Rating Scale (HAM-D),22 Inventory of Depressive Symptomatology (IDS),23 Montgomery-Åsberg Depression Rating Scale (MADRS),24 Schedule for Affective Disorders and Schizophrenia, version for measuring Changes in symptomology (SADS-C),25 and the Global Assessment of Functioning (GAF).26 We extracted all primary outcomes and clinical variables from all of the studies selected in the final stage. When data were not available in the articles, we attempted to contact the authors to ask for the original data. Furthermore, if multiple rating scales were used in one study, we gave preference to the HAM-D, MADRS, or IDS over the SADS-C or GAF because they are more specific to depressive symptoms. In addition, as most of the studies used the HAM-D, we gave the HAM-D first priority.
Meta-Analytic Methods and Data Extraction
In the present study, the meta-analysis consisted of 3 parts, including (a) studies comparing changes in disease severity before and after bupropion treatment, (b) studies comparing the treatment effect of bupropion in BD patients to other standard treatments, and (c) differences in drop-out or phase-shifting rates. All of the effect sizes (ESs) expressing changes in disease severity and phase-shifting rate in each of the recruited studies were defined as the standardized mean difference, based on Hedges’ adjusted g.27 In addition, we defined the ESs expressing differences in the drop-out rate in each of the recruited studies as the odds ratio. ESs > 0 indicated (a) significantly worse disease severity with bupropion treatment, (b) a better response with the use of bupropion, and (c) higher drop-out or phase-shifting rates with the use of bupropion. When no rating scales were available in the study or the original data could not be obtained from the authors, we chose other statistical parameters such as the t or P value with the sample size or the “response rate” or “remission rate” according to the specific definition in each study. All of the ESs were synthesized using a random effects model for each meta-analysis.
All of the meta-analytic procedures in the present study were performed using Comprehensive Meta-Analysis software, version 2 (Biostat, Englewood, NJ). Two-tailed P values < 0.05 were considered to be statistically significant. We investigate heterogeneity using Q statistics, their related P value, and the I2 statistic. Furthermore, in order to investigate the possible confounding effect of clinical variables, we used meta-regression to examine these variables using the unrestricted maximum likelihood method. The clinical variables entered in the meta-regression included mean age, female gender, chlorpromazine equivalence, duration of illness, dose of bupropion, body mass index (BMI), level of education, mean duration of current treatment, age at onset, and disease severity (HAM-D score). In addition, we performed subgroup meta-analysis to investigate the possible confounding effect of “bupropion monotherapy versus combination therapy,” “drug-free or not,” “specific category of antidepressant,” and “first episode or not.” Through funnel plots and the statistical evaluation of Egger's regression,28 we investigated the possibility of publication bias. The present study fulfilled the criteria of Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) (Supplementary Figure 1 and Supplementary Table 2).29
RESULTS
Studies Included in the Meta-Analysis
Initially, 43 articles were screened for eligibility, of which 33 were excluded for the following reasons: 11 were excluded because they were review articles,10,30–39 9 because they were case reports/series,9,12,14,18,40–44 11 which did not compare changes in disease severity after bupropion treatment or differences in the treatment effect or drop-out or phase-shifting rates with bupropion and other treatments,15,20,45–53 and 1 which was a meta-analysis.54 The remaining 10 articles were then entered into the current meta-analysis (Table 1).3,4,13,16,17,55–59 The average Jadad score was 0.9.
TABLE 1.
Summary of Characteristics of Studies in Current Meta-Analysis
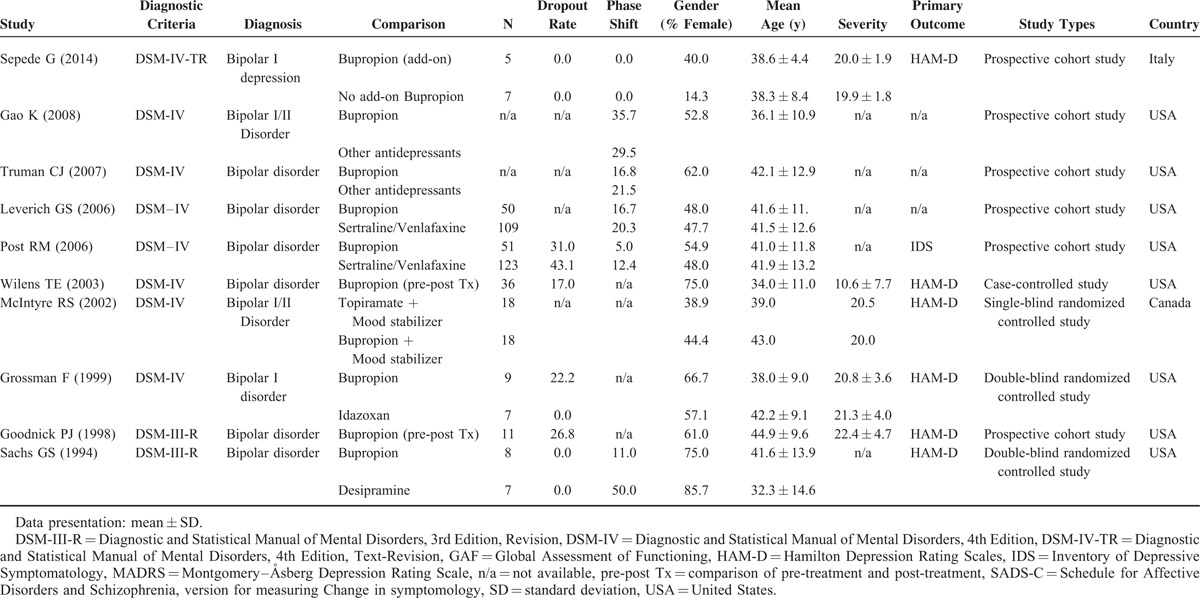
The Main Results of the Current Meta-Analysis of the Treatment Effect of Bupropion in Patients With BD and Depressive Episodes
We first performed a meta-analysis of studies that compared the treatment effect of bupropion in BD patients with depressive episodes. A total of 109 BD patients who received bupropion were extracted from 5 studies (Table 1).55–59 The treatment effect of bupropion in these BD patients significantly decreased disease severity (ESs = −0.021, 95% confidence interval [CI]: −0.033 to −0.010, P < 0.001) (Figure 2A). There was significant heterogeneity within these studies (Q = 6429.82, df = 4, I2 = 99.94%, P < .001). Furthermore, no significant publication bias was detected using Egger's test (t = 1.58, df = 3, 2-tailed P = 0.211) and visual examination of the funnel plot.
FIGURE 2.
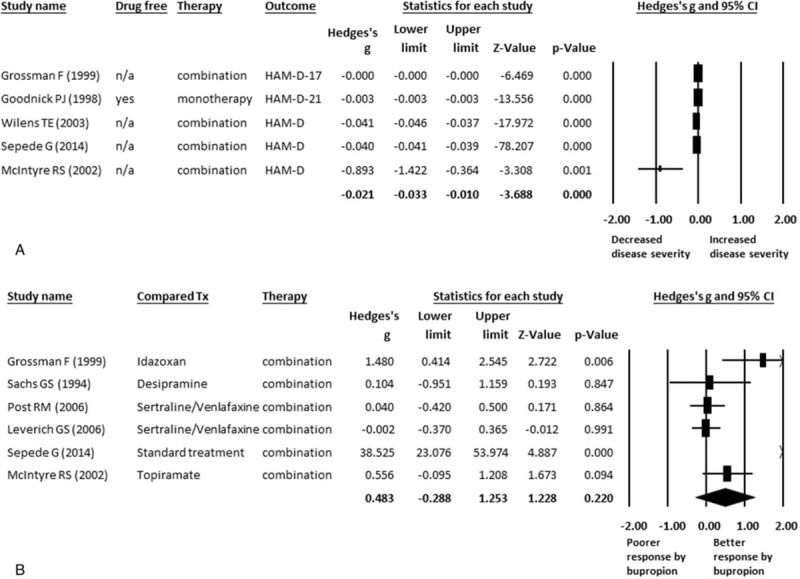
(A) MA of treatment effect of bupropion in BD. Significantly decreased disease severity after treatment of bupropion in BD patients (P < 0.001). (B) MA of treatment effect of bupropion and other treatment in BD. No any significant difference between the treatment effect of bupropion and other treatment in BD patients (P = 0.220). BD = bipolar disorder; CI = confidence interval; HAM-D = Hamilton Depression Rating Scales; MA = meta-analysis; n/a = not available; Tx = treatment.
Among these 5 studies, we could find out 1 outlier study by McIntyre et al 2002 57 (Figure 2A), which might influence the results of current meta-analysis. When we excluded the data of this study from the pooled analysis, the result remained significantly decreased disease severity after bupropion treatment (ESs = −0.021, 95% CI: −0.032 to −0.010, P < 0.001) with significant heterogeneity (Q = 6418.89, df = 3, I2 = 99.95%, P < .001). Hence the results of the study by McIntyre et al (2002) did not influence the pooled ES significantly.
We could only perform meta-regression analysis of clinical variables including mean age, female gender, duration of current treatment, and dosage of bupropion because of the limited available data. Among them, we only found significant associations between changes in disease severity and mean age (slope = 0.004, P = .017) but not in female gender, duration of current treatment, and dosage of bupropion (slope = 0.0003, P = 0.505; slope = 0.009, P = 0.097; slope = 0.00009, P = 0.218, respectively).
We could not perform further subgroup analysis for “bupropion monotherapy versus combination therapy,” “drug-free or not,” and “first episode or not” because only a few studies used monotherapy,59 included subjects who were drug-free,59 or those with a first episode.
The Main Results of the Meta-Analysis Comparing Different Effects of Bupropion and Other Treatments in BD Patients
We then compared the different effects of bupropion and other treatments through meta-analysis. A total of 141 BD patients who received bupropion and 271 who received other treatments including idazoxan, desipramine, sertraline, venlafaxine, and topiramate, were extracted from 6 articles.3,4,13,55,57,58 We did not find any statistically significant differences in the treatment effect between the BD patients receiving bupropion and those receiving other treatments (ESs = 0.483, 95% CI: −0.288 to 1.253, P = 0.220) (Figure 2B). In addition, there was significant heterogeneity within these studies (Q = 31.98, df = 5, I2 = 84.37%, P < 0.001), and significant publication bias was detected using Egger's test (t = 4.01, df = 4, 2-tailed P = 0.016).
The Main Results of the Meta-Analysis of Comparisons of Safety Profiles
With regard to the safety of bupropion, we focused on 2 specific areas: the drop-out rate and phase-shifting rate, either to a manic or hypomanic state. We did not find any significantly differences in the rate of phase shifting in the BD patients treated with bupropion or other treatments, including desipramine, SSRIs, SNRIs, or other antidepressants (ESs = −0.130, 95% CI: −0.367 to 0.108, P = 0.285) (Figure 3A), and there was no significant heterogeneity (Q = 9.69, df = 6, I2 = 38.08%, P = 0.138) or publication bias (t = 1.14, df = 5, 2-tailed P = 0.306). Furthermore, subgroup meta-analysis of the phase-shifting rate in specific categories of antidepressants revealed the same results, and there were no significant differences in the rates of phase shifting in the BD patients receiving bupropion compared to those receiving SSRIs (ESs = −0.147, 95% CI: −0.538 to 0.243, P = 0.460) or SNRIs (ESs = −0.119, 95% CI: −0.420 to 0.183, P = 0.440).
FIGURE 3.
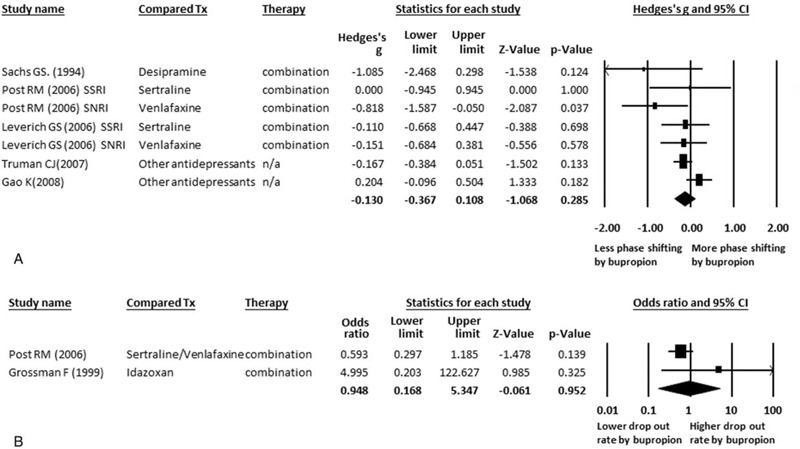
(A) MA of comparison of phase shifting by bupropion and other treatment in BD No any significant difference between the rate of phase shifting in bupropion and other treatment in BD patients (P = 0.285). (B) MA of comparison of drop-out rate of bupropion and other treatment in BD. No any significant difference between the rate of drop-out in bupropion and other treatment in BD patients (P = 0.952). BD = bipolar disorder; CI = confidence interval; MA = meta-analysis; n/a = not available; SNRI = serotonin-norepinephreine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; Tx = treatment.
There were also no significant differences in the drop-out rate between the BD patients treated with bupropion or those treated with other antidepressants (odds ratio ESs = 0.948, 95% CI: 0.168 to 5.347, P = 0.952) (Figure 3B).
DISCUSSION
The main findings of the current meta-analysis are the significant improvements in disease severity after bupropion treatment in BD patients. In addition, bupropion was found to exert a similar treatment effect to other treatments in BD patients. Furthermore, in addition to the safety of bupropion being similar to other antidepressants in terms of drop-out rate, the phase-shifting rate in the patients receiving bupropion was not lower than that in the patients receiving other antidepressants, as previously assumed.
Our results are consistent with previous reports about the significant benefits of bupropion for the treatment of depressive symptoms in patients with BD.10,54 Furthermore, in our meta-regression analysis, the treatment effect of bupropion in BD patients was significantly positively associated, albeit only slightly, with mean age rather than the duration of treatment or dosage of bupropion. We could not rule out the possible confounding effects of concurrently prescribed psychotropics on the treatment effect, which may have had beneficial effects on disease severity, led to unnecessary drug–drug interactions, or prevented phase shifting. In addition, our results may be affected by the small number of studies included and thus the limited amount of data available. Therefore, caution should be taken in applying our results to clinical practice.
Bipolar depression remains a troublesome affective disorder which attracts a large amount of attention due to the high risk of phase shifting to a manic/hypomanic state during the treatment of BD. At present, many review articles and clinical guidelines suggest the usage of bupropion, either as monotherapy or combination therapy, in the treatment of bipolar depression,10,11,38,54,60,61 and evidence suggests the significant benefits of bupropion treatment in such patients. Our results also support that the treatment effect of bupropion is similar to that of other antidepressants/standard treatments.
Furthermore, in order to ameliorate the disease severity of bipolar depression, some clinical guidelines suggest that bupropion be considered as the first-line of treatment in such patients.10,11 In these studies, bupropion has been reported to be safe and to carry the least risk of phase shifting among other antidepressants based on current evidence.11,38 However, an increasing number of studies have reported their clinical experience of bupropion usage in clinical practice 4,12,15,20,43,46,55 in recent years, and thus the safety of bupropion with regard to phase shifting needs to be seriously reconsidered. The present study provides up-to-date key information about the safety of bupropion in BD patients. The rate of phase shifting with bupropion use seems to be similar to other antidepressants such as desipramine, sertraline, venlafaxine, or other categories of antidepressants. This suggests that bupropion may not be as safe as previously presumed with regard to phase shifting, and there therefore clinicians should pay special attention to the risk of phase shifting when prescribing bupropion for patients with bipolar disorder.
LIMITATION
There are several limitations to this study. First, we did not perform detailed subgroup meta-analysis for every category of antidepressant used in each study because of the limited available data. Second, significant publication bias was detected in the current meta-analysis. This may implicate the clinical importance of the present study. Third, we could not rule out the possible confounding effect of concurrent psychotropics, which may have benefitted disease severity, prevented phase shifting, or induced drug–drug interactions. Fourth, with regard to drug safety, we could only perform analysis on the drop-out and phase-shifting rates because of the limited available data. However, many side effects related to the usage of bupropion have been reported, including seizures and headache.62 Fifth, the number of studies recruited in the current meta-analysis was relatively small, especially in the meta-analysis of phase-shifting rate, which may limit the applicability of the results. However, the forest plot of meta-analysis of phase-shifting rate showed that the direction of most studies was in the same direction, namely “favor less phase shifting with bupropion usage.” Sixth, in the meta-analysis of treatment effect of bupropion on the disease severity, there was 1 outlier study.57 Although the results of meta-analysis remained similar after removing the outlier, there might be some confounding effects on the results of current meta-analysis. We found that the treatment duration of bupropion was higher in the study by McIntyre et al (2002) than others. In addition, the female proportion in this study was also lower than that in other studies. Although there was insignificantly positive association, the female gender proportion and the treatment duration of bupropion had some positive association with the treatment effect of bupropion. Therefore, this might be, at least partly, the cause of the discrepancy of these studies. Finally, the total duration of current treatment among the studies recruited in the current meta-analysis was relatively short, ranging from 4 to 8 weeks only.
CONCLUSION
The current meta-analysis reconfirms the benefits of bupropion in the treatment of bipolar depression and that the benefits are similar to other antidepressants. However, the phase-shifting rate with bupropion usage was not lower than that with the use of other antidepressants as previously thought despite a similar drop-out rate between the use of bupropion and other antidepressants. The current findings have important clinical implications, in that they should remind clinicians about the risk of phase shifting when prescribing bupropion in BD patients regardless of the suggestions of the current clinical practice guidelines.
Supplementary Material
Footnotes
Abbreviations: BD = bipolar disorder, BMI = body mass index, CI = confidence interval, ES = effect size, FDA = Food and Drug Administration, GAF = Global Assessment of Functioning, HAM-D = Hamilton Depression Rating Scales, IDS = Inventory of Depressive Symptomatology, MA = meta-analysis, MADRS = Montgomery–Åsberg Depression Rating Scale, n/a = not available, NDRI = norepinephrine-dopamine reuptake inhibitor, PRISMA = Preferred Reporting Items for Systematic reviews and Meta-Analyses, SADS-C = Schedule for Affective Disorders and Schizophrenia, version for measuring Change in symptomology, SNRI = serotonin-norepinephreine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor, Tx = treatment.
Author contribution: D-JL, one of the first authors, takes the responsibility of data summarization and writing down the first edition of draft of current literature. P-TT, one of the first authors, takes the responsibility of literature searching and first-step of revision of the draft written by DJL. Y-WC, the other one of the first authors, takes the responsibility of literatures searching, the procedure of meta-analysis, and part of revision of method/result. CKW, one of the senior psychiatrists, raises the important comment of the comparison of drug side effect profiles and rate of phase shifting in treatment of BD patients. PYL, the other one of the senior psychiatrists and the corresponding authors, takes the responsibility of collecting all the manuscript from the other authors and final revision of current literature.
All authors reviewed and approved the manuscript.
D-JL, P-TT, and Y-WC equally contributed to this study.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Thase ME. Bipolar depression: issues in diagnosis and treatment. Harv Rev Psychiatry 2005; 13:257–271. [DOI] [PubMed] [Google Scholar]
- 2.Valenti M, Pacchiarotti I, Bonnin CM, et al. Risk factors for antidepressant-related switch to mania. J Clin Psychiatry 2012; 73:e271–e276. [DOI] [PubMed] [Google Scholar]
- 3.Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 2006; 189:124–131. [DOI] [PubMed] [Google Scholar]
- 4.Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patients with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006; 163:232–239. [DOI] [PubMed] [Google Scholar]
- 5.Bauer M, Ritter P, Grunze H, et al. Treatment options for acute depression in bipolar disorder. Bipolar Disord 2012; 14 Suppl 2:37–50. [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam JD, Lorenzo-Luaces L, Soeller I, et al. Safety and effectiveness of continuation antidepressant versus mood stabilizer monotherapy for relapse-prevention of bipolar II depression: a randomized, double-blind, parallel-group, prospective study. J Affect Disord 2015; 185:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry 2011; 72:156–167. [DOI] [PubMed] [Google Scholar]
- 8.Shopsin B. Bupropion's prophylactic efficacy in bipolar affective illness. J Clin Psychiatry 1983; 44 (5 Pt 2):163–169. [PubMed] [Google Scholar]
- 9.Wright G, Galloway L, Kim J, et al. Bupropion in the long-term treatment of cyclic mood disorders: mood stabilizing effects. J Clin Psychiatry 1985; 46:22–25. [PubMed] [Google Scholar]
- 10.Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 2013; 15:1–44. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric A. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002; 159 (4 Suppl):1–50. [PubMed] [Google Scholar]
- 12.Aggarwal A, Sharma RC. Bupropion-induced mania and hypomania: a report of two cases. J Neuropsychiatry Clin Neurosci 2011; 23:E51–52. [DOI] [PubMed] [Google Scholar]
- 13.Sachs GS, Lafer B, Stoll AL, et al. A double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry 1994; 55:391–393. [PubMed] [Google Scholar]
- 14.Brown ES, Dilsaver SC, Shoaib AM, et al. Depressive mania: response of residual depression to bupropion. Biol Psychiatry 1994; 35:493–494. [DOI] [PubMed] [Google Scholar]
- 15.Joffe RT, MacQueen GM, Marriott M, et al. Induction of mania and cycle acceleration in bipolar disorder: effect of different classes of antidepressant. Acta Psychiatr Scand 2002; 105:427–430. [DOI] [PubMed] [Google Scholar]
- 16.Gao K, Kemp DE, Ganocy SJ, et al. Treatment-emergent mania/hypomania during antidepressant monotherapy in patients with rapid cycling bipolar disorder. Bipolar Disord 2008; 10:907–915. [DOI] [PubMed] [Google Scholar]
- 17.Truman CJ, Goldberg JF, Ghaemi SN, et al. Self-reported history of manic/hypomanic switch associated with antidepressant use: data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). J Clin Psychiatry 2007; 68:1472–1479. [DOI] [PubMed] [Google Scholar]
- 18.Fogelson DL, Bystritsky A, Pasnau R. Bupropion in the treatment of bipolar disorders: the same old story? J Clin Psychiatry 1992; 53:443–446. [PubMed] [Google Scholar]
- 19.Hui Poon S, Sim K, Baldessarini RJ. Pharmacological approaches for treatment-resistant bipolar disorder. Curr Neuropharmacol 2015; 13:592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song HR, Kwon YJ, Bahk WM, et al. Current prescription pattern of maintenance treatments for bipolar patients in Korea: A focus on the transition from acute treatments. Psychiatry Clin Neurosci 2016; 70:42–50. [DOI] [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996; 26:477–486. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389. [DOI] [PubMed] [Google Scholar]
- 25.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 1978; 35:837–844. [DOI] [PubMed] [Google Scholar]
- 26.Hall RC. Global assessment of functioning. A modified scale. Psychosomatics 1995; 36:267–275. [DOI] [PubMed] [Google Scholar]
- 27.Hedges LV, Olkin I. Statistical ,Methods for Meta-Analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Post RM, Leverich GS, Nolen WA, et al. A re-evaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Foundation Bipolar Network. Bipolar Disord 2003; 5:396–406. [DOI] [PubMed] [Google Scholar]
- 31.Parikh SV, LeBlanc SR, Ovanessian MM. Advancing bipolar disorder: key lessons from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Can J Psychiatry 2010; 55:136–143. [DOI] [PubMed] [Google Scholar]
- 32.Yatham LN, Goldstein JM, Vieta E, et al. Atypical antipsychotics in bipolar depression: potential mechanisms of action. J Clin Psychiatry 2005; 66 Suppl 5:40–48. [PubMed] [Google Scholar]
- 33.Calabrese JR, Shelton MD, Rapport DJ, et al. Current research on rapid cycling bipolar disorder and its treatment. J Affect Disord 2001; 67:241–255. [DOI] [PubMed] [Google Scholar]
- 34.Clayton AH. Extended-release bupropion: an antidepressant with a broad spectrum of therapeutic activity? Expert Opin Pharmacother 2007; 8:457–466. [DOI] [PubMed] [Google Scholar]
- 35.Tundo A, de Filippis R, Proietti L. Pharmacologic approaches to treatment resistant depression: evidences and personal experience. World J Psychiatry 2015; 5:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieta E, Valenti M. Pharmacological management of bipolar depression: acute treatment, maintenance, and prophylaxis. CNS Drugs 2013; 27:515–529. [DOI] [PubMed] [Google Scholar]
- 37.Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ 2015; 351:h4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry 2013; 170:1249–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evins AE, Cather C, Laffer A. Treatment of tobacco use disorders in smokers with serious mental illness: toward clinical best practices. Harv Rev Psychiatry 2015; 23:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall RD, Johannet CM, Collins PY, et al. Bupropion and sertraline combination treatment in refractory depression. J Psychopharmacol 1995; 9:284–286. [DOI] [PubMed] [Google Scholar]
- 41.Haykal RF, Akiskal HS. Bupropion as a promising approach to rapid cycling bipolar II patients. J Clin Psychiatry 1990; 51:450–455. [PubMed] [Google Scholar]
- 42.Erfurth A, Michael N, Stadtland C, et al. Bupropion as add-on strategy in difficult-to-treat bipolar depressive patients. Neuropsychobiology 2002; 45 Suppl 1:33–36. [DOI] [PubMed] [Google Scholar]
- 43.Hussain H, Butt MA. Bupropion-induced hypomania in a patient with unipolar depression. Aust N Z J Psychiatry 2008; 42:746. [DOI] [PubMed] [Google Scholar]
- 44.Nierenberg AA. Low-dose buspirone, melatonin and low-dose bupropion added to mood stabilizers for severe treatment-resistant bipolar depression. Psychother Psychosom 2009; 78:391–393. [DOI] [PubMed] [Google Scholar]
- 45.Stoll AL, Mayer PV, Kolbrener M, et al. Antidepressant-associated mania: a controlled comparison with spontaneous mania. Am J Psychiatry 1994; 151:1642–1645. [DOI] [PubMed] [Google Scholar]
- 46.Frye MA, Helleman G, McElroy SL, et al. Correlates of treatment-emergent mania associated with antidepressant treatment in bipolar depression. Am J Psychiatry 2009; 166:164–172. [DOI] [PubMed] [Google Scholar]
- 47.Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007; 356:1711–1722. [DOI] [PubMed] [Google Scholar]
- 48.Altshuler LL, Post RM, Hellemann G, et al. Impact of antidepressant continuation after acute positive or partial treatment response for bipolar depression: a blinded, randomized study. J Clin Psychiatry 2009; 70:450–457. [DOI] [PubMed] [Google Scholar]
- 49.Gardner EA. Long-term preventive care in depression: the use of bupropion in patients intolerant of other antidepressants. J Clin Psychiatry 1983; 44 (5 Pt 2):157–162. [PubMed] [Google Scholar]
- 50.Haeberle A, Greil W, Russmann S, et al. Mono- and combination drug therapies in hospitalized patients with bipolar depression. Data from the European drug surveillance program AMSP. BMC Psychiatry 2012; 12:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarate CA, Jr, Tohen M, Baraibar G, et al. Prescribing trends of antidepressants in bipolar depression. J Clin Psychiatry 1995; 56:260–264. [PubMed] [Google Scholar]
- 52.Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001; 3:259–265. [PubMed] [Google Scholar]
- 53.Manwani SG, Pardo TB, Albanese MJ, et al. Substance use disorder and other predictors of antidepressant-induced mania: a retrospective chart review. J Clin Psychiatry 2006; 67:1341–1345. [DOI] [PubMed] [Google Scholar]
- 54.Bond DJ, Noronha MM, Kauer-Sant’Anna M, et al. Antidepressant-associated mood elevations in bipolar II disorder compared with bipolar I disorder and major depressive disorder: a systematic review and meta-analysis. J Clin Psychiatry 2008; 69:1589–1601. [DOI] [PubMed] [Google Scholar]
- 55.Sepede G, Di lorio G, Lupi M, et al. Bupropion as an add-on therapy in depressed bipolar disorder type I patients with comorbid cocaine dependence. Clin Neuropharmacol 2014; 37:17–21. [DOI] [PubMed] [Google Scholar]
- 56.Wilens TE, Prince JB, Spencer T, et al. An open trial of bupropion for the treatment of adults with attention-deficit/hyperactivity disorder and bipolar disorder. Biol Psychiatry 2003; 54:9–16. [DOI] [PubMed] [Google Scholar]
- 57.McIntyre RS, Mancini DA, McCann S, et al. Topiramate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002; 4:207–213. [DOI] [PubMed] [Google Scholar]
- 58.Grossman F, Potter WZ, Brown EA, et al. A double-blind study comparing idazoxan and bupropion in bipolar depressed patients. J Affect Disord 1999; 56:237–243. [DOI] [PubMed] [Google Scholar]
- 59.Goodnick PJ, Dominguez RA, DeVane CL, et al. Bupropion slow-release response in depression: diagnosis and biochemistry. Biol Psychiatry 1998; 44:629–632. [DOI] [PubMed] [Google Scholar]
- 60.Connolly KR, Thase ME. The clinical management of bipolar disorder: a review of evidence-based guidelines. Prim Care Companion CNS Disord 2011; 13: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goodwin GM. Consensus Group of the British Association for P. Evidence-based guidelines for treating bipolar disorder: revised second edition—recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2009; 23:346–388. [DOI] [PubMed] [Google Scholar]
- 62.Dunner DL, Zisook S, Billow AA, et al. A prospective safety surveillance study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 1998; 59:366–373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


