Abstract
To examine racial/ethnic and gender disparities in antiretroviral (ART) use and viral suppression among HIV-infected persons in care and identify factors that might account for observed disparities.
The Medical Monitoring Project (MMP) is a complex sample survey of HIV-infected adults receiving medical care in the United States.
We used weighted interview and medical record data collected 06/2009 to 05/2012 to estimate the prevalence of ART use and viral suppression among gender-stratified racial/ethnic groups. We used χ2 tests to identify significant differences in outcomes between white men versus other groups, and logistic regression models to identify the most parsimonious set of factors that could account for each observed difference.
We found no significant disparity in ART use between white and Hispanic men, and no disparities between white men and white and Hispanic women after adjustment for disease stage, age, and poverty. Disparities in ART use between white men and black persons persisted after adjusting for other factors, but the observed differences were relatively small. Differences in ART use and adherence, demographic characteristics, and social determinants of health such as poverty, education, and insurance accounted for the observed disparities in viral suppression between white men and all groups except black men. In our model, accounting for these factors reduced the prevalence difference in viral suppression between white and black men by almost half.
We found that factors associated with disparities differed among men and women of the same race/ethnicity, lending support to the assertion that gender affects access to care and health status among HIV-infected patients. In addition to supporting efforts to increase ART use and adherence among persons living with HIV, our analysis provides evidence for the importance of social determinants of health in understanding racial/ethnic and gender differences in ART use and viral suppression.
INTRODUCTION
Racial, ethnic, and gender disparities in care, treatment, and health outcomes have long been observed among the human immunodeficiency virus (HIV)-infected population in the United States,1–3 and reducing these disparities is a key objective of the National HIV/AIDS Strategy.4 Disparities in viral suppression are of paramount concern, as viral suppression is essential for reducing morbidity, mortality, and onward HIV transmission.5,6
However, studies of racial/ethnic and gender disparities among persons receiving HIV care have, in most cases, been limited by small sample sizes that do not allow for examination of gender-stratified racial/ethnic groups; restricted study populations (e.g., persons initiating antiretroviral therapy[(ART], persons recently incarcerated, veterans, and university clinic-based cohorts); and incomplete assessment of potential confounders and mediators. These limitations have important implications. First, consideration of both gender and race/ethnicity is needed because the distribution of racial/ethnic groups varies among HIV-infected men and women in the United States. In 2012, among HIV-infected men, the proportion of black, Hispanic/Latino (hereafter referred to as “Hispanic”), and white persons was 36%, 23%, and 36%, respectively, compared to 59%, 19%, and 17% among HIV-infected women.7 Most1,2,8–10 studies adjust for gender and race/ethnicity in multivariable models; only a few have examined gender-stratified racial/ethnic groups.3,11,12 However, analysis of racial/ethnic disparities without stratification by gender may obscure important race/ethnicity by gender disparities, and may help elucidate the sometimes contradictory findings of studies of gender differences in ART initiation3,10 and virologic failure.11,12 Further, identifying confounders and mediators of disparities among gender-stratified racial/ethnic groups across diverse care settings can inform the development of effective, customized strategies for disparity-reduction among the most affected groups.
To address these gaps, we examined the prevalence of ART use and viral suppression among HIV-infected persons receiving medical care by gender-stratified racial/ethnic group using population-based estimates of HIV-infected persons receiving care in the United States and Puerto Rico. In addition, we conducted analyses to identify potential confounders and mediators of the observed disparities between white men and the other groups.
METHODS
Medical Monitoring Project (MMP)
We analyzed data from the 2009 to 2011 cycles of the MMP, an HIV surveillance system designed to produce annual nationally representative estimates of behavioral and clinical characteristics of HIV-infected adults receiving medical care in the United States and Puerto Rico. MMP methods, including weighting procedures and response rates, have been described in detail elsewhere.13–15 Briefly, MMP uses a 3-stage, probability-proportional-to-size sampling method. First, U.S. states and 1 territory were sampled, then facilities in those areas providing outpatient HIV care, and finally, eligible HIV-infected patients. All sampled states and territories participated in every cycle. The facility response rate was 76% in 2009, 81% in 2010, and 83% in 2011. The patient response rate was 51% in 2009, 50% in 2010, and 49% in 2011. Eligible persons were HIV-infected, age 18 years or older, and had received medical care in participating facilities between January and April in the cycle year for which they were sampled. Interview and medical record abstraction data were collected June 2009 to May 2012. Data were weighted on the basis of known probabilities of selection at state or territory, facility, and patient levels.16 In addition, predictors of nonresponse were determined from analysis of data from sampled facilities and patients, and data were then weighted to adjust for nonresponse, following established methods.17,18 Factors associated with nonresponse varied by cycle year and project area, but in general we found that facility size, private practice, younger age, black and Hispanic race, and shorter time since HIV diagnosis were associated with nonresponse and informed the weighting classes for the data.
In accordance with the federal human subjects protection regulations19 and guidelines for defining public health research,20 MMP was determined to be a nonresearch, public health surveillance activity used for disease control program or policy purposes. Participating states or territories and facilities obtained local institutional review board approval to conduct MMP if required locally. Informed consent was obtained from all interviewed participants.
Analytic Methods
In this analysis, we included 12,394 male and female MMP participants who self-identified as black, non-Hispanic (hereafter referred to as black); Hispanic; or white, non-Hispanic (hereafter referred to as white). We examined 2 outcomes: self-reported current ART use and viral suppression, defined as all viral load results over the past 12 months documented in the medical record as undetectable or <200 copies/mL. We examined viral suppression among all persons instead of among only those taking ART (as is common in many clinical studies) because understanding the factors that influence suppression among all persons is important for providing information that can be used to improve population-level health among HIV-infected persons.
For our examination of disparities in outcomes, white men were chosen as the reference group because we expected them to have the highest levels of ART use and viral suppression. Although all measures of disparities have strengths and weaknesses, use of “best” group comparisons is useful for this analysis because attainment of levels of ART use and viral suppression equivalent to that seen among white men is theoretically achievable by the other groups, and thus is a measure of disparity that meets its common definition: a difference that is inequitable.21 To identify confounders and mediators of the difference in ART use and viral suppression between white men and the other subgroups, we performed multivariable analysis for each subgroup pair (e.g., white men compared to Hispanic men, white men compared to black women, etc.) to determine which factors might account for the observed disparity. We used logistic regression models with predicted marginals to calculate the unadjusted and adjusted predicted prevalence of each outcome, after adjusting for the other variables in the model. We then estimated the prevalence difference (PD) between compared subgroups for each outcome to assess how the racial disparity changed with the inclusion of the other variables in the model.
Candidate variables for inclusion in the models predicting ART use and viral suppression were informed by the scientific literature on factors associated with the outcome and gender and race/ethnicity. Variables assessed for confounding (not hypothesized to be on the causal pathway between gender-stratified racial/ethnicity and the outcome) were age and self-reported sexual orientation. Self-reported variables assessed as mediators (hypothesized to be on the causal pathway between gender-stratified racial/ethnicity and the outcome) were education (less than high school, high school diploma or equivalent, and more than high school), homelessness, health insurance/coverage (any private insurance, only public insurance/coverage, and Ryan White HIV/AIDS Program coverage only or uninsured), household poverty,22 incarceration, drug use, binge drinking in the 30 days prior to interview (≥5 drinks in 1 sitting for men and ≥4 drinks in 1 sitting for women), years since HIV diagnosis (<5, 5–9, and 10+), and depression in the 2 weeks prior to interview (none, other depression, and major depression, as measured by the Patient Health Questionnaire [PHQ-8] algorithm).23 These variables were measured in the 12 months prior to interview, unless otherwise specified. Two additional variables derived from medical record review were also assessed as mediators: current HIV disease stage (AIDS or nadir CD4+ 0–199, no AIDS and nadir CD4+ 200–499, and no AIDS and nadir CD4+ >=500)24 and care utilization (at least 1 viral load test every 6 months in the year prior to interview). In the models estimating ART use, we included the HIV disease stage variable as mediating variable to capture the potential effect of U.S. clinical guidelines at the time, which did not recommend ART prescription for persons with no history of AIDS and nadir CD4+ >=500.25 In the models estimating viral suppression, we assessed a 3-level variable measuring self-reported ART use and 100% adherence to all ART doses in past 3 days (no ART use, ART use and nonadherent, and ART use and adherent) as a potential mediator.
We ran 1 set of models for ART use and another for viral suppression because ART use is key for viral suppression, and viral suppression is a primary indicator of treatment success. To assess factors that confounded or mediated the relationship between the compared subgroups and each outcome, we began by identifying variables that met the following criteria: associated with both the outcome and gender-stratified racial group at P < 0.10 according to Rao–Scott Chi-square tests; and inclusion in the model changed the prevalence ratio (PR) between the subgroup comparison and the outcome by at least 1 percentage point for the ART use models and at least 2 percentage points for the viral suppression models. A lower threshold was set for the ART use models because the unadjusted PRs were very high and thus variation was restricted by ceiling effects. We then built forward stepwise models, starting with variables that we expected to mediate the relationship between gender-stratified racial/ethnic group and the outcome (i.e., HIV disease stage for ART use and ART use/adherence for viral suppression). We then added additional variables to the models, starting with those that reduced the PR by the largest amount, until the PD between the compared subgroups was either no longer significant or not reduced further by adding additional candidate variables to the model. The intent of our analytic strategy was to identify the most parsimonious set of predictors that could account for the observed difference in the outcome among each compared subgroup; our measures of association are not meant to be compared across the models.
As our measure of adherence to ART was self-reported and only captured the past 3 days, to further explore the persisting lower level of viral suppression among black compared to white men we examined differences in factors that can affect adherence among white and black men taking ART. Variables examined were: being bothered by side effects, medication beliefs (sure one can take ART as directed, sure ART has positive effect, and sure of resistance if nonadherent), satisfaction with social support, use of and need for professional adherence support services, ART nonpersistence (intentionally not taking ART for 2 days or more), and need for help filling out hospital forms (a proxy for inadequate health literacy).26
Finally, to facilitate efforts to increase viral suppression among black men, we examined differences in the variables described above between black men with detectable and undetectable viral load to determine whether the factors associated with viral suppression among black men were the same as those influencing the differences in suppression between white men and the other gender-stratified racial/ethnic groups.
All analyses accounted for the complex sample design and unequal selection probabilities by using the survey procedures in SAS 9.3 (SAS Institute Inc., Cary, NC) and SUDAAN 10.0.1 (RTI International, Research Triangle Park, NC) and all percentages reported are weighted.
RESULTS
Disparities in ART Use and Viral Suppression
Among HIV-infected white, Hispanic, and black persons in care, we estimated that 31% were white men, 5% white women, 15% Hispanic men, 5% Hispanic women, 26% black men, and 17% black women (Table 1). In general, white men were older, more educated, more likely to have private health insurance, and less likely to be homeless, living in household poverty, or recently incarcerated compared to other subgroups (see Table 1, Supplemental Digital Content for frequencies by gender-stratified racial/ethnic groups). White men had the highest prevalence of ART use; all subgroups except for Hispanic men were significantly less likely than white men to use ART (Figure 1). Among those not taking ART, proportionally fewer white men reported not taking ART based on a doctor's advice (54% [95% confidence interval (CI) 47–60] compared to 65% [CI 53–77] for white women, 66% for Hispanic men [CI 55–76], Hispanic women [CI 48–84] and black men [CI 60–72], and 72% [CI 65–78] for black women); tests for significance were not done due to small cell sizes. White men also had the highest prevalence of viral suppression; all subgroups were significantly less likely than white men to have viral suppression (Figure 1).
TABLE 1.
Comparison of Antiretroviral Use and Viral Suppression Prevalence Between White Men and Other Race/Ethnicity by Gender Groups, Adjusted for Selected Characteristics–Medical Monitoring Project, United States, 2009 to 2011 (N = 12,394)
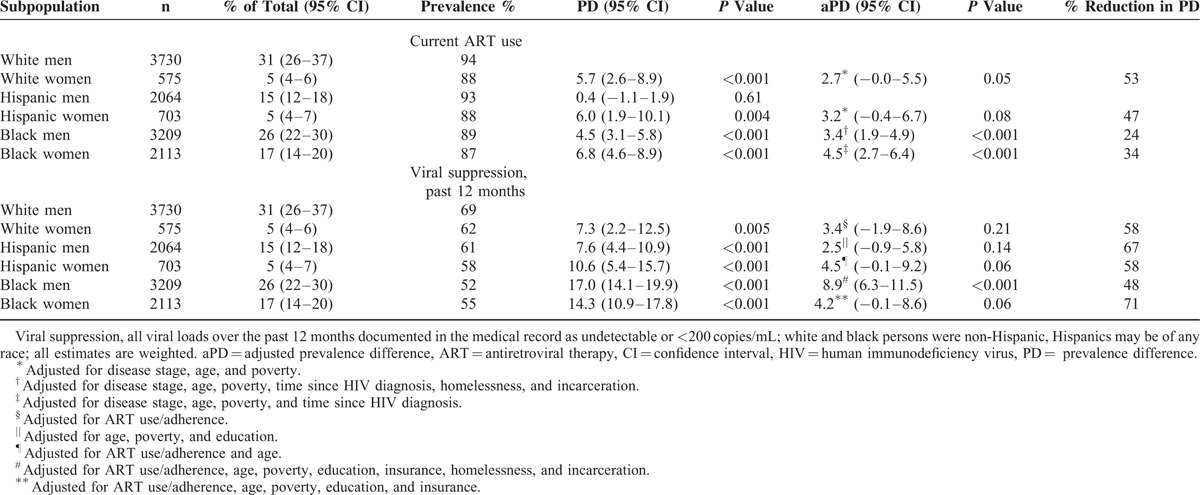
FIGURE 1.
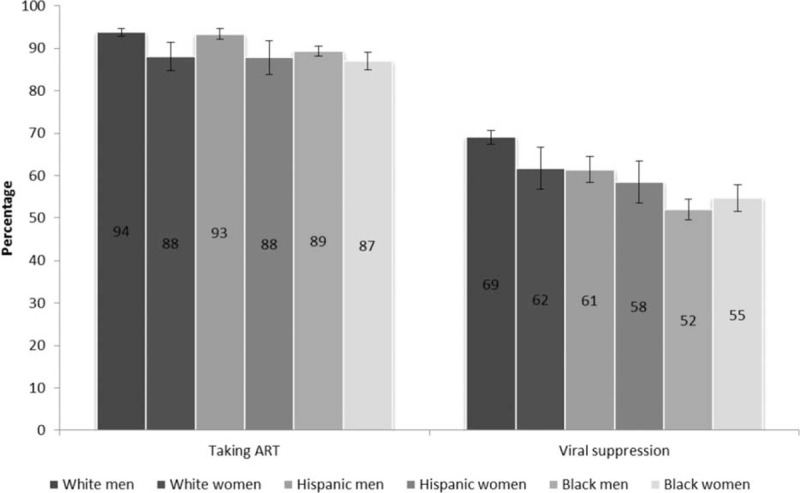
Antiretroviral therapy use and viral suppression among HIV-infected white, Hispanic, and black men and women – Medical Monitoring Project, United States, 2009 to 2011 (N = 12,394), viral suppression, all viral loads over the past 12 months documented in the medical record as undetectable or <200 copies/mL among all persons; white and black persons were non-Hispanic, Hispanics may be of any race. ART = antiretroviral therapy, HIV = human immunodeficiency virus.
Had we not stratified the racial/ethnic estimates by gender, important differences between groups would have been less evident. Overall prevalence of ART use was 88% [CI 87–89] among blacks, 92% [90–94] among Hispanics, and 93% [92–94] among whites. Table 1 illustrates that white and Hispanic women actually had levels of ART use comparable to black men and women. Similarly, the overall prevalence of viral suppression was 53% [CI 51–55] among blacks, 61% [CI 58–64] among Hispanics, and 68% [CI 66–70] among whites, but suppression among white women was actually closer to that of Hispanic men, and Hispanic women had levels of suppression somewhere between white and black women (Table 1).
Multivariable Analysis of Disparities in ART Use and Viral Suppression
The final multivariable models designed to identify mediators of the difference in ART use and viral suppression between white men and all subgroups except for Hispanic men are presented in Table 1 (see Table 2a and b, Supplemental Digital Content for incremental models). The PD in ART use between white men and black men and women decreased but remained statistically significant after accounting for other factors. The PD in ART use between white men and white and Hispanic women were rendered insignificant after accounting for disease stage, age, and poverty, and was no longer significant (P = 0.05 and 0.08, respectively).
Regarding the PD in viral suppression, the difference between white men and all groups except for black men were no longer statistically significant after accounting for other factors. The PD in viral suppression between white and black men decreased from 17.0 to 8.9 after accounting for ART use, adherence, age, poverty, education, insurance, homelessness, and incarceration (P < 0.001). Although the difference remained significant, accounting for these variables reduced the PD between white and black men by 48%.
Differences Between White and Black Men
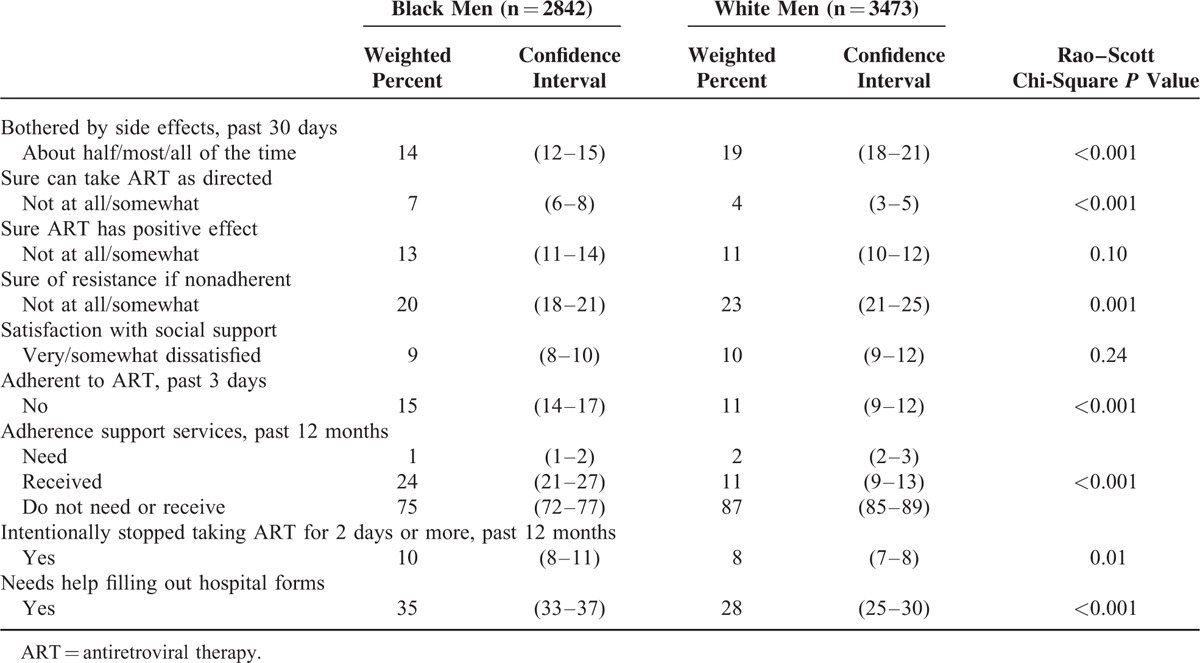
As our measure of adherence to ART was self-reported and only captured the past 3 days, to explore the lower level of viral suppression among black compared to white men, we examined differences among these subgroups in factors that can affect adherence (Table 2). Among the significant differences between the groups that are associated with worse adherence, compared to white men, black men reported being less sure they could take ART as prescribed, more likely to need help filling out hospital forms (an indicator of health literacy),26 and were more likely to purposefully not take ART for longer than 2 days in the past 12 months. However, black men were more likely to report some factors associated with better adherence, such as being less bothered by side effects, being more certain of becoming resistant to ART if they were nonadherent, and using professional adherence support.
TABLE 2.
Selected Characteristics of Non-Hispanic Black and White Men Taking Antiretroviral therapy – Medical Monitoring Project, United States, 2009 to 2011
Differences Between Black Men by Viral Suppression
To facilitate efforts to increase levels of viral suppression among black men, we examined selected characteristics of black men with detectable and undetectable viral load (Table 3 ). In general, black men with detectable viral load were more likely to be younger, less educated, un- or under-insured, poorer, substance-users, more recently diagnosed, not taking ART, depressed, and less engaged in care compared to black men with undetectable viral load. Among those taking ART, black men with detectable viral load were more likely to be bothered by side effects, unsure if they could take ART as prescribed, unsure if ART would have a positive effect on their health, and nonadherent. They were also more likely to have received professional adherence support and to have purposefully not taken ART for longer than 2 days in past 12 months.
TABLE 3.
Selected Characteristics of Non-Hispanic Black Men, by Viral Suppression – Medical Monitoring Project, United States, 2009 to 2011
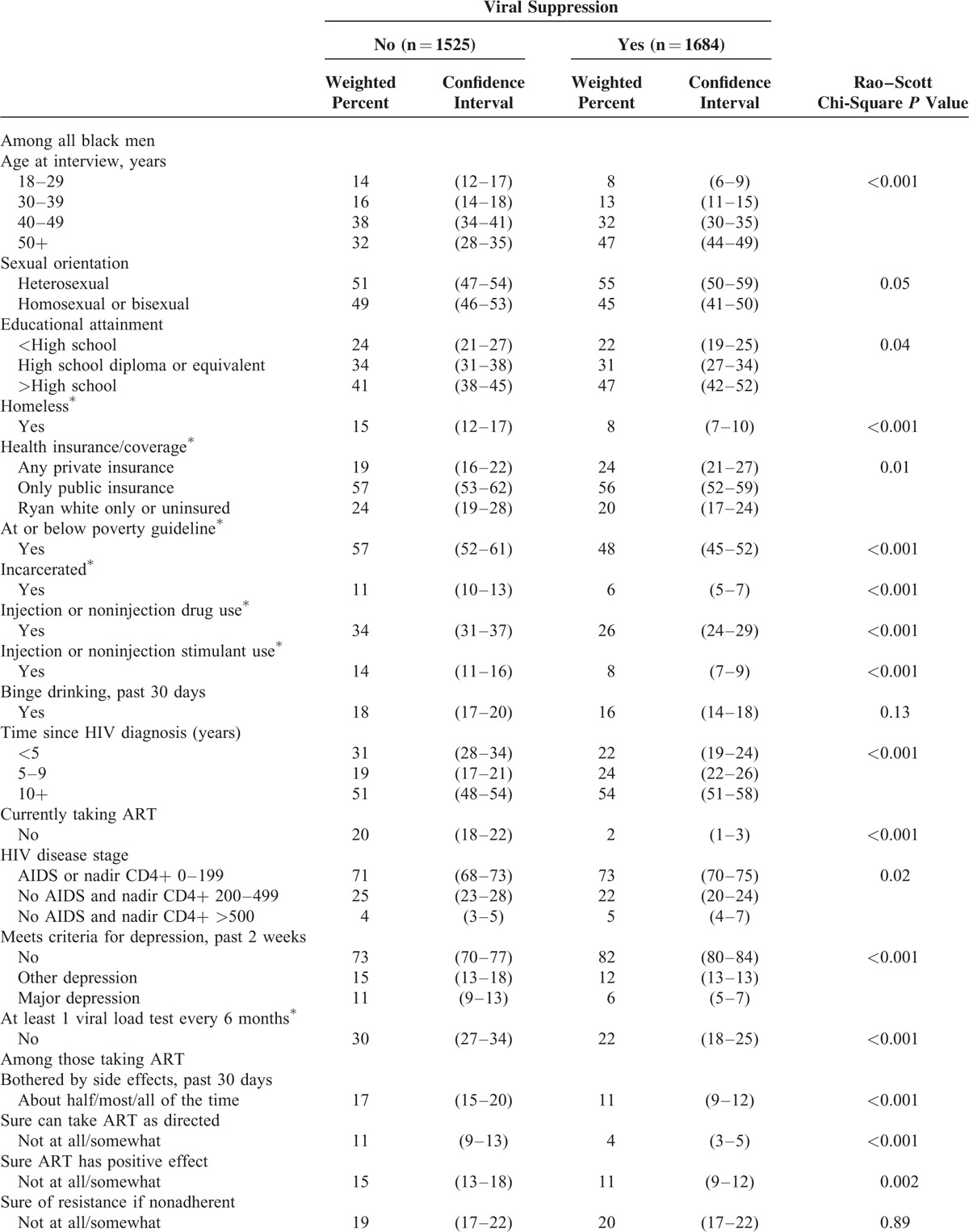
TABLE 3 (Continued).
Selected Characteristics of Non-Hispanic Black Men, by Viral Suppression – Medical Monitoring Project, United States, 2009 to 2011

DISCUSSION
This analysis illustrates the benefits of examining gender-stratified racial/ethnic groups, providing more detailed estimates of ART use and viral suppression for specific groups than would be gained by examination of race/ethnicity or gender alone. That the factors associated with disparities in these outcomes differed among men and women of the same race/ethnicity is evidence of the important effect of gender on access to care and health status.3,11 We identified factors that accounted for the differences in viral suppression between white men and most other groups, which can be used to inform the development of customized strategies to reduce gender and racial/ethnic disparities. For example, our findings suggest that focusing on improving ART use and adherence among white women may be effective to achieve equity in levels of viral suppression vis-à-vis white men, but among black women additional complementary strategies to address the effects of poverty, education, and insurance status may be needed.
We found no disparities in ART use between white and Hispanic men and that disparities in ART use between white men and white and Hispanic women were due to differences between these groups in disease stage, age, and poverty. As U.S. clinical guidelines began recommending ART prescription regardless of CD4 count in 2012, it is possible that disparities in ART use for these groups will further decline. However, disparities between white men and black persons of both genders were reduced but could not be fully explained by accounting for differences in disease stage, age, poverty, time since HIV diagnosis, homelessness, and incarceration. We also assessed potential confounders such as sexual orientation, and mediators such as education, insurance status, drug use, depression, and care utilization, but these did not account for the disparities. However, ART use among the population in care is high among all groups, and the observed differences were relatively small (adjusted prevalence difference range: 3.3–4.5). Nevertheless, increasing ART use among black persons is important for improving viral suppression; for all groups ART use was key in accounting for disparities in viral suppression compared to white men.
Although there are several efficacious interventions to improve adherence,27 information is lacking on how to increase initiation and maintenance of ART use among black persons. Although we examined the effects of a wide range of possible confounders and mediators in our models, there were factors we were unable to include that may contribute to the disparities in ART use among these groups. Multiple studies have found that medical mistrust negatively impacts adherence to care among black persons.28,29 Although there is great enthusiasm about the safety, tolerability, and efficacy of newer antiretrovirals among the medical community, this information may not reach, be accepted, or be understood by all HIV-infected persons, especially those with limited education and health literacy.
Differences in ART use and adherence, demographics, and social determinants of health such as poverty, education, and insurance accounted for the disparities in viral suppression between white men and all groups except black men; although in our model, accounting for these factors reduced the PD in viral suppression between white and black men by almost half. However, the persistent disparity in suppression among black and white men is troubling, especially considering that black men had the lowest proportion of viral suppression among all examined subgroups; we estimate that half of black men in care are not achieving durable viral suppression. Black men with detectable viral load were more likely to be young, a group that has seen increases in HIV incidence in recent years.30 Factors we were unable to include in our models may help explain disparities in viral suppression between black and white men. Examining differences in prescribed regimen and HIV care setting may be promising areas for future studies.
For all groups, age, poverty, and education emerged as important factors accounting for some of the disparities in ART use and viral suppression. Youth-focused programs may need to be prioritized. A better understanding of the ways in which poverty affects suppression among persons in care may be needed. The negative health effects of chronic stress brought on by poverty may be one area of inquiry.31–33 Food insecurity may also play a role in linking poverty to lower levels of viral suppression among those in care.34 Education is a distal factor that may need further exploration, since the downstream effects of low education are potentially accounted for in the model through the inclusion of factors such as poverty, lack of adequate insurance, and nonadherence. Education may be a significant predictor of later viral suppression because it is capturing the effect of childhood/young adulthood resource disparities, which may have long lasting health consequences.35
In addition, other factors such as health coverage for black men and women, and homelessness and incarceration for black men, were important mediators accounting for some of the disparity in viral suppression between white men and other subgroups. The implementation of health care reform in the United States may help decrease racial disparities in viral suppression by increasing access to adequate health coverage among HIV-infected persons of color. Programs to prevent homelessness and incarceration, as well as housing services and community re-entry programs, among black men may be needed.
Although we found some factors that promote adherence were more likely among black men (such as fewer side effects), others factors that are associated with lower adherence were also more common among black men (such as lower health literacy). Further, we found that the same factors that affect suppression among all groups also affect likelihood of suppression among black men. More work may be warranted to understand the persistent disparities between black and white men so they may be addressed. One area of exploration may be how medical mistrust affects the patient–provider relationship.28,29 Gwadz et al36 found that readiness for ART (which in turn affects later adherence and hence viral suppression) was associated with physician trust among black patients.
Our analysis is subject to several limitations. First, our patient response rates ranged from 49% to 51%. However, our population-based sampling method is a strength that allowed us to make adjustments to minimize nonresponse bias based on known characteristics of nonresponders. However, the possibility of residual nonresponse bias exists. Second, many of our measures were self-reported, and thus may be subject to recall and/or desirability biases. However, we have no reason to believe these biases would systematically vary according to gender or race/ethnicity, and therefore whatever measurement error exists should not substantially affect our findings regarding disparities.
Examining differences in ART use and viral suppression among gender-stratified racial/ethnic groups receiving HIV medical care allows for identification of disparities and their mediators at the last stage of the HIV care continuum, which is needed to complement efforts to increase testing and linkage to and engagement in care. As these efforts, along with a relatively stable number of new infections and decreasing mortality, increase the number of HIV-infected persons receiving HIV medical care, understanding what it takes to achieve equitable levels of viral suppression among all racial/ethnic and gender groups will be even more crucial.
Taken as a whole, our analysis of confounders and mediators of disparities in ART use and viral suppression provides evidence for the importance of social determinants of health in understanding these differences. Our findings identified differences between groups in the factors associated with disparities, which may be used to inform tailored, multifaceted strategies to improve health equity among these groups, and provide a clear call to action regarding the need to address social determinants of health in order to reduce racial/ethnic and gender disparities in health among HIV-infected patients.
Supplementary Material
Acknowledgements
The authors thank participating MMP patients, facilities, project areas, and Provider and Community Advisory Board members. The authors also thank the Clinical Outcomes Team and Behavioral and Clinical Surveillance Branch at CDC and the MMP 2009 Study Group Members (http://www.cdc.gov/hiv/statistics/systems/mmp/resources.html#StudyGroupMembers); Funding for the Medical Monitoring Project is provided by the Centers for Disease Control and Prevention.
Footnotes
Abbreviations: ART = antiretroviral therapy, MMP = Medical Monitoring Project, PD = prevalence difference, PR = prevalence ratio.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Portions of the analysis were presented at the 9th International Conference on HIV Treatment and Prevention Adherence in Miami, FL, USA in June 2014.
This study is funded by the Medical Monitoring Project is provided by the Centers for Disease Control and Prevention.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Althoff KN, Rebeiro P, Brooks JT, et al. Disparities in the quality of HIV care when using US Department of Health and Human Services indicators. Clin Infect Dis 2014; 58:1185–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr 2005; 38:96–103. [DOI] [PubMed] [Google Scholar]
- 3.Meditz AL, MaWhinney S, Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis 2011; 203:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. Washington, DC: White House Office of National AIDS Policy; 2010. [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS (Lond, Engl) 2014; 28:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Epidemiology of HIV Infection through 2013. http://www.cdc.gov/hiv/pdf/g-l/cdc-hiv-genepislideseries-2013.pdf (accessed April 6, 2014). [Google Scholar]
- 8.Fleishman JA, Yehia BR, Moore RD, et al. Disparities in receipt of antiretroviral therapy among HIV-infected adults (2002–2008). Med Care 2012; 50:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer JP, Zelenev A, Wickersham JA, et al. Gender disparities in HIV treatment outcomes following release from jail: results from a multicenter study. Am J Public Health 2014; 104:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak RM, Hart RL, Chmiel JS, et al. Disparities in initiation of combination antiretroviral treatment and in virologic suppression among patients in the HIV Outpatient Study (HOPS), 2000–2013. J Acquir Immune Defic Syndr (1999) 2015; 70:23–32. [DOI] [PubMed] [Google Scholar]
- 11.Lesko CR, Cole SR, Miller WC, et al. Ten-year survival by race/ethnicity and sex among treated, HIV-infected adults in the United States. Clin Infect Dis 2015; 60:1700–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribaudo HJ, Smith KY, Robbins GK, et al. Racial differences in response to antiretroviral therapy for HIV infection: an AIDS clinical trials group (ACTG) study analysis. Clin Infect Dis 2013; 57:1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair JM, Fagan JL, Frazier EL, et al. Behavioral and clinical characteristics of persons receiving medical care for HIV infection – Medical Monitoring Project, United States, 2009. Morbidity and mortality weekly report. Surveill Summ (Washington, DC: 2002) 2014; 63 Suppl 5:1–22. [PubMed] [Google Scholar]
- 14.Bradley H, Frazier E, Huang P, et al. Behavioral and Clinical Characteristics of Persons Receiving Medical Care for HIV Infection Medical Monitoring Project United States, 2010. Atlanta, GA, October 2014. [PubMed] [Google Scholar]
- 15.Bradley H, Frazier E, Huang P, et al. Behavioral and Clinical Characteristics of Persons Receiving Medical Care for HIV Infection Medical Monitoring Project United States, 2011. Atlanta, GA, January, 2015. [PubMed] [Google Scholar]
- 16.Harding L, Iachan R, Johnson C, et al. Weighting Methods for the 2010 Data Collection Cycle of the Medical Monitoring Project. Paper presented at: 2013 Joint Statistical Meeting; August 3–8, 2013; Montréal, QC, H2Z 1H2, Canada. [Google Scholar]
- 17.Särndal C-E, Lundström S. Estimation in Surveys with Nonresponse. Chichester: John Wiley & Sons; 2005. [Google Scholar]
- 18.Heeringa S, West BT, Berglund PA. Applied Survey Data Analysis. Boca Raton, FL: Taylor & Francis; 2010. [Google Scholar]
- 19.Protection of Human Subjects, US Federal Code Title 45 Part 46. 2009; Available at http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html (accessed February 4, 2014). [Google Scholar]
- 20.Centers for Disease Control and Prevention. Distinguishing Public Health Research and Public Health Nonresearch. 2010; Available at http://www.cdc.gov/od/science/integrity/docs/cdc-policy-distinguishing-public-health-research-nonresearch.pdf (accessed February 4, 2014). [Google Scholar]
- 21.Keppel KG, Pearcy JN, Klein RJ. Measuring progress in Healthy People 2010. Healthy People 2010 statistical notes from the Centers for Disease Control and Prevention/National Center for Health Statistics 2004; 25:1–16. [PubMed] [Google Scholar]
- 22.U.S. Department of Health & Human Services. Poverty Guidelines, Research, and Measurement. http://aspe.hhs.gov/poverty/index.cfm (accessed July 17, 2015). [Google Scholar]
- 23.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009; 114:163–173. [DOI] [PubMed] [Google Scholar]
- 24.Schneider E, Whitmore S, Glynn KM, et al. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged 18 months and for HIV infection and AIDS among children aged 18 months to <13 years. Morbidity and Mortality Weekly Report Recommendations and reports/Centers for Disease Control 2008; 57:1–12. [PubMed] [Google Scholar]
- 25.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents Department of Health and Human Services. 2012; 1-161. [Google Scholar]
- 26.Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008; 23:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charania MR, Marshall KJ, Lyles CM, et al. Identification of evidence-based interventions for promoting HIV Medication adherence: findings from a systematic review of U.S.-based studies, 1996–2011. AIDS Behav 2013; 18:646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogart LM, Wagner G, Galvan FH, et al. Conspiracy beliefs about HIV are related to antiretroviral treatment nonadherence among African American men with HIV. J Acquir Immune Defic Syndr 2010; 53:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaston GB, Alleyne-Green B. The impact of African Americans’ beliefs about HIV medical care on treatment adherence: a systematic review and recommendations for interventions. AIDS Behav 2013; 17:31–40. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012; 17: [Google Scholar]
- 31.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med 1991; 325:606–612. [DOI] [PubMed] [Google Scholar]
- 32.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998; 338:171–179. [DOI] [PubMed] [Google Scholar]
- 33.Kalichman SC, Grebler T. Stress and poverty predictors of treatment adherence among people with low-literacy living with HIV/AIDS. Psychosom Med 2010; 72:810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang EA, McGinnis KA, Fiellin DA, et al. Food insecurity is associated with poor virologic response among HIV-infected patients receiving antiretroviral medications. J Gen Intern Med 2011; 26:1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology (Cambridge, Mass) 2014; 25:473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gwadz M, Applegate E, Cleland C, et al. HIV-infected individuals who delay, decline, or discontinue antiretroviral therapy: comparing clinic- and peer-recruited cohorts. Front Pub health 2014; 2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


