Abstract
Statins have beneficial effects on cerebral circulation and brain parenchyma during ischemic stroke and reperfusion. The primary hypothesis of this randomized parallel trial was that treatment with 80 mg/day of atorvastatin administered early at admission after acute atherosclerotic ischemic stroke could reduce serum levels of markers of immune-inflammatory activation of the acute phase and that this immune-inflammatory modulation could have a possible effect on prognosis of ischemic stroke evaluated by some outcome indicators.
We enrolled 42 patients with acute ischemic stroke classified as large arteries atherosclerosis stroke (LAAS) randomly assigned in a randomized parallel trial to the following groups: Group A, 22 patients treated with atorvastatin 80 mg (once-daily) from admission day until discharge; Group B, 20 patients not treated with atorvastatin 80 mg until discharge, and after discharge, treatment with atorvastatin has been started.
At 72 hours and at 7 days after acute ischemic stroke, subjects of group A showed significantly lower plasma levels of tumor necrosis factor-α, interleukin (IL)-6, vascular cell adhesion molecule-1, whereas no significant difference with regard to plasma levels of IL-10, E-Selectin, and P-Selectin was observed between the 2 groups.
At 72 hours and 7 days after admission, stroke patients treated with atorvastatin 80 mg in comparison with stroke subjects not treated with atorvastatin showed a significantly lower mean National Institutes of Health Stroke Scale and modified Rankin scores.
Our findings provide the first evidence that atorvastatin acutely administered immediately after an atherosclerotic ischemic stroke exerts a lowering effect on immune-inflammatory activation of the acute phase of stroke and that its early use is associated to a better functional and prognostic profile.
INTRODUCTION
Background and Objectives
In addition to their lipid-lowering effects, it has been speculated that statins may also have beneficial effects on cerebral circulation and brain parenchyma during ischemic stroke and reperfusion. Asahi et al1 reported that statins increased endothelial nitric oxide synthase (eNOS) and tissue plasminogen activator (tPA) mRNA levels, but did not change mRNA levels of plasminogen activator inhibitor-1 (PAI-1) and that in eNOS knockout mice, atorvastatin reduced the volume of ischemic tissue and improved neurologic outcomes after arterial occlusion by blood clot emboli. Aslanyan et al2 reported that statin use was associated with reduced mortality at 1 month during the follow-up after stroke, whereas in patients with recent stroke or Transient ischemic attack and without known coronary heart disease, 80 mg/day of atorvastatin reduced the overall incidence of strokes and of cardiovascular events, despite a small increase in the incidence of hemorrhagic stroke period.3
Furthermore, Pignatelli et al4 recently showed the first evidence that atorvastatin acutely and simultaneously decreases oxidative stress and platelet activation by directly inhibiting platelet Nox2 and ultimately platelet isoprostanes and thromboxane A2, providing a rationale for the use of statins to prevent or modulate thrombosis.
Inflammation plays a crucial role in mediating all the stages of the atherosclerosis process.6 Similarly, thrombosis and defective fibrinolysis may also contribute to the progression of atherosclerotic lesions.5 Interestingly, both mechanisms might have a relevant role in the pathogenesis of intracranial large artery atherosclerosis and ischemic stroke, particularly in atherosclerotic subtype.
Our group showed that patients with cardioembolic and atherothrombotic stroke subtypes showed significantly higher median plasma levels of tumor necrosis factor (TNF)-α, interleukin (IL-6), IL-1β, whereas the lacunar subtype showed significantly lower median plasma levels of TNF-α, IL-6, and IL-1β5,7,8,9 and that immune-inflammatory marker plasma levels are significantly related to ischemic lesion volume.9
Whereas recent data suggest that inflammatory reactions are involved in the pathogenesis and progression of cerebral ischaemia,10 no study has evaluated effects of atorvastatin 80 mg/day after a recent stroke on outcome and on immune-inflammatory markers to evaluate acute antithrombotic, anti-inflammatory, and neuroprotective effects of atorvastatin in acute cerebrovascular event setting.
Thus, we hypothesized that acute administration of high-dose atorvastatin early after an acute ischemic stroke could be beneficial because of its reported anti-inflammatory, antioxidant, and antithrombotic properties.
The primary objective of this study was to evaluate the effects of atorvastatin 80 mg/day in vivo on immune-inflammatory markers and on functional outcome in patients with recent acute ischemic stroke classified as atherothrombotic not treated with thrombolytic treatment.
MATERIALS AND METHODS
The trial was carried out in 2 parts. Part 1 assessed differences in serum levels of some immune-inflammatory markers as a measure of the anti-inflammatory activity of early treatment with atorvastatin; Part 2, used 2 outcome measures representing different aspects of recovery from stroke to assess whether early treatment with atorvastatin resulted in a better clinical benefit at 72 hours and 7 days (differences in National Institutes of Health Stroke Scale [NIHSS] score) and at 7 days (Rankin score).
HYPOTHESES AND DESIGN
Part 1 was designed to test whether atorvastatin had effects on lowering serum levels of some immune-inflammatory markers at 72 hours and 7 days after stroke in patients treated with atorvastatin as compared with those not treated with atorvastatin.
Part 2 was designed to test whether atorvastatin treatment is associated to a lower mean NIHSS score at 72 hours and 7 days after stroke and a lower mean Rankin score at 7 days after stroke in a greater proportion of patients treated with atorvastatin as compared with those not treated with atorvastatin.
TRIAL DESIGN
Interventions: Treatment Protocol
This randomized trial has a parallel design.
Eligibility Criteria
To be eligible for the study, patients had to have an ischemic stroke with a clearly defined time of onset, a deficit measurable on the NIHSS, and a baseline computed tomographic (CT) scan of the brain that showed no evidence of intracranial hemorrhage and classified as Large Atherosclerotic Stroke (LAAS) according TOAST classification and that have not been treated with thrombolysis.
We excluded patients treated with trombolysis owing to the fact that trombolysis treatment is the unique effective treatment in acute stroke setting with well-reported rapid effects on outcome indicators and immunoinflammatory markers that could represent a possible bias in evaluation of effects of atorvastatin treatment in early post acute phase after an acute ischemic stroke.
Patients with inflammatory or infectious diseases, cancer, hematological diseases, and severe renal or liver failure, as well as those who were under treatment with anti-inflammatory drugs, were excluded.
Stroke was defined by focal neurological signs or symptoms thought to be of vascular origin that persisted for N24 h, confirmed by brain CT and/or MRI in baseline conditions.11
Randomization
Patients were randomly assigned by the use of sequentially numbered boxes (prepared before starting the study by a computerized, non-alternating sequence) to the following groups: Group A, treatment with atorvastatin 80 mg (once-daily) from admission day and maintained after discharge as indicated by SPARCL trial3; Group B, no treatment with atorvastatin 80 mg until discharge, after discharge, treatment with atorvastatin has been started as indicated by SPARCL trial.3
In patients with disorder of consciousness or in patients with swallowing deficit, atorvastatin has been administered through a nasogastric tube after solubilitation of tablets.
We evaluated plasma levels of IL-1β, TNF-α, IL-6 and IL-10, E-selectin, P-selectin, soluble intercellular cell adhesion molecule-1 (sICAM-1), and soluble vascular cell adhesion molecule (sVCAM-1) as markers of immune-inflammatory activation, von Willebrand factor (VWF) plasma levels as a marker of endothelial dysfunction, and TPA-antigen and PAI-1 plasma levels as thrombotic/fibrinolytic markers.
These laboratory evaluations have been done at 72 h and at 7 days after symptom onset.
Outcome Measures
Two outcome measures were selected on the basis of their reliability: neurological deficit score on admission and at 72 hours and 7 days after stroke was evaluated by the NIHSS.12 The NIHSS is a tool used by healthcare providers to objectively quantify the impairment caused by a stroke. The NIHSS is composed of 11 items, each of which scores a specific ability between a 0 and 4. For each item, a score of 0 typically indicates normal function in that specific ability, whereas a higher score is indicative of some level of impairment. The individual scores from each item are summed to calculate a patient's total NIHSS score. The maximum possible score is 42, with the minimum of 0.
Disability at 7 days was evaluated by modified Rankin Scale (mRS)13 that is a commonly used scale for measuring the degree of disability or dependence in the daily activities of people who have suffered a stroke.
The scale runs from 0 to 6, running from perfect health without symptoms to death:
No symptoms.
No significant disability: able to carry out all usual activities, despite some symptoms.
Slight disability: able to look after own affairs without assistance, but unable to carry out all previous activities.
Moderate disability: requires some help, but able to walk unassisted.
Moderately severe disability: unable to attend own bodily needs without assistance, and unable to walk unassisted.
Severe disability: requires constant nursing care and attention, bedridden, incontinent.
Dead.
Study Settings
The patient and control data were collected at Internal Medicine and Cardioangiology Ward, Policlinico P. Giaccone Hospital of Palermo.
We enrolled patients with a diagnosis of acute ischemic stroke admitted to the Internal Medicine Department at the University of Palermo between December 2013 and may 2015 (date recruitment range: December 1, 2013–May 30, 2015).
All recruited patients have been evaluated after recruitment for outcome evaluation at 72 hours and 7 days after stroke onset between December 2013 and June 2015 (date follow up evaluation range: May 9, 2013–June 14, 2014).
Risk Factor Evaluation
Cardiovascular risk factors were evaluated for both subjects and controls according the following criteria: type 2 diabetes mellitus was determined using a clinical-based algorithm that considered age at onset, presenting weight and symptoms, family history, onset of insulin treatment, and history of ketoacidosis.
Atrial fibrillation was diagnosed when present on the admission ECG.
Hypertension was present when a patient had received antihypertensive treatment before admission or when hypertension was diagnosed during the hospital stay by repeated detection of blood pressure ≥160/95 mmHg. Hypercholesterolemia was defined as total serum cholesterol ≥200 mg/dL or on the basis of a clinical history of hypercholesterolemia or statin treatment.
Definition of Stroke and Stroke Subtypes
The type of acute ischemic stroke was classified according to the TOAST classification14: LAAS; CardioEmbolic Infarct; LACunar infarct; stroke of Other Determined Etiology; stroke of UnDetermined Etiology.
LAAS Patients Selection
These patients will have clinical and brain imaging findings of either significant (≥50%) stenosis or occlusion of a major brain artery or branch cortical artery, owing to atherosclerosis. Clinical findings include those of cerebral cortical impairment (aphasia, neglect, restricted motor involvement, etc) or brain stem or cerebellar dysfunction. Cortical or cerebellar lesions and brain stem or subcortical hemispheric infarcts >1.5 cm in diameter on CT or MRI are considered to be of potential large artery atherosclerotic origin. Supportive evidence by duplex imaging or arteriography of a stenosis of >50% of an appropriate intracranial or extracranial artery is needed. Diagnostic studies should exclude potential sources of cardiogenic embolism.2
Patient Evaluation and Blood Sample Collection
All the ischemic stroke patients underwent: medical history with recording of potential stroke risk factors, blood and coagulation tests, 12-lead ECG, 24-h electrocardiography monitoring, transthoracic echocardiography, carotid ultrasound, brain CT or MRI at admission (repeated between the third and the seventh day of stroke onset).
Blood samples were obtained in the nonfasting state. After 10 minutes of rest in the supine position, vital signs were recorded and blood samples were collected from the antecubital vein. Ethylediaminetetraacetic acid-anticoagulated peripheral blood was drawn from each patient within 72 hours (accepted range 72 ± 3 hours) and at 7 days from symptom onset after sample collection serum and plasma were immediately separated by centrifugation and stored in aliquots at –80°C until analysis.
We evaluated plasma levels of IL-1β, TNF-α, IL-6 and IL-10, E-selectin, P-selectin, sICAM-1, and sVCAM-1 as markers of immunoinflammatory activation, VWF plasma levels as a marker of endothelial dysfunction, TPA-antigen and PAI-1 plasma levels as thrombotic/fibrinolytic markers.
These laboratory evaluations have been done at 72 h and at 1 week after symptom onset.
IL-1β, TNF-α, IL-6 and IL-10, and VWF antigen were measured using a sandwich ELISA (Human IL-1β, TNF-α, IL-6 and IL-10 Quantikine, R&D Systems, VWF ELISA kitdurian, Instrumentation Laboratory, Milano, Italy); VCAM-1, ICAM-1, E-selectin, P-selectin, PAI-1 and TPA antigen were measured by commercial bioimmunoassay (Human sICAM-1, sVCAM-I, sE-selectin and sP-selectin Parameter, Quantikine, R&D Systems, Gentaur AssayMax Human Plasminogen Activator Inhibitor-1 [PAI-1] ELISA Kit, Gentaur AssayMax Tissue Plasminogen Activator [TPA] ELISA Kit).
The minimum detectable concentrations for the diagnostic tests were: TNF alpha, 1.6 pg/mL; IL-1β, b1 pg/mL; IL-6:b0. 0 pg/mL; IL-10, 3.9 pg/mL; ICAM-1, 0.35 ng/mL;VCAM-1, 0.6 ng/mL; E-Selectin, 0.1 ng/mL; P-Selectin, 0.5 ng/mL; vWF, 1.0%; TPA, 0.3 pg/mL; PAI-1, b50 pg/mL. Intra-assay and inter-assay coefficients of variation were: TNF alpha 4.2% and 4.6%; IL-1β 3.3% and 4.2%; IL-6 1.6% and 3.3%; IL-10 4.3% and 7.5%; ICAM-1 4.8% and 6.1%; VCAM-1 3.5% and 7.7%; E-Selectin 4.8% and 5.7%; P-Selectin 4.9% and 8.8%; vWF 5% and 10%; TPA 4.8% and 5%; PAI-1 5.7% and 8.3%.
Primary Outcome
Primary outcome is the differences in mean immuno-inflammatory marker serum levels between group A and B at 72 hours and 7 days after stroke.
Secondary Outcomes
Secondary outcomes are as follows:
Differences in mean NIHSS at 72 hours after stroke between group A and B.
Differences in mean NIHSS at 7 days after stroke between group A and B.
Difference in mean Rankin score at 7 days after stroke between group A and B.
For trial design and manuscript preparation we followed CONSORT reporting guidelines.
Sample Size and Statistical Analysis
For power calculations, we assumed a decrease of 30% of chosen serum cytokines (IL-6, TNF-α, IL-1β) if at least 20 patients were assigned to each group; this would give a power of >80% to detect differences of these magnitudes among groups15 and to detect prognostic differences among the 2 groups of enrolled subjects because of changes of immunoinflammatory markers and of outcome indicators.
Categorical variables are reported as counts (percentage) and continuous variables as mean andSD unless otherwise indicated. Differences between percentages were assessed by χ2 test or Fisher exact test. A Student unpaired t test and Pearson correlation analysis were used for normally distributed continuous variables. Appropriate nonparametric tests (Mann-Whitney U test or Spearman rank correlation test) were used for all the other variables. Interventional study data were analyzed for the assessment of treatment effect on immunoinflammatory variables plasma levels and outcome indicators (NIHSS and mRS) and 1 within-subject factor (time at 2 levels: after 72 hours and 7 days after the beginning of the treatment). As covariates, we considered the possible random differences in age, sex, body mass index, blood pressure, and blood lipid profile between the 2 groups. Values of P < 0.05 were regarded as statistically significant. All calculations were made with Statistical 7 software for Windows (StatSoft, Tulsa, OK). Statistical informations of our manuscript have been evaluated by an expert.
RESULTS
Among 113 patients admitted to our ward with a diagnosis of acute ischemic stroke in the enrolment period, we enrolled 99 patients (53 males and 39 females) with acute ischemic stroke.
Fourteen patients were excluded for the presence of exclusion criteria (5 for previous inflammatory or infectious diseases, 4 for cancer, 2 for hematological diseases 3 for undertreatment with anti-inflammatory drugs).
Among stroke patients were randomized to treatment protocol only subjects classified as LAAS a TOAST Classification. Forty-two patients were classified as LAAS.
Randomization
Among subjects classified as LAAS, 22 patients (Group A) were randomized to treatment with atorvastatin 80 mg/day and 20 patients (Group B) were randomized to no treatment with atorvastatin in the acute phase of stroke (see Figure 1: flow diagram of participants).
FIGURE 1.
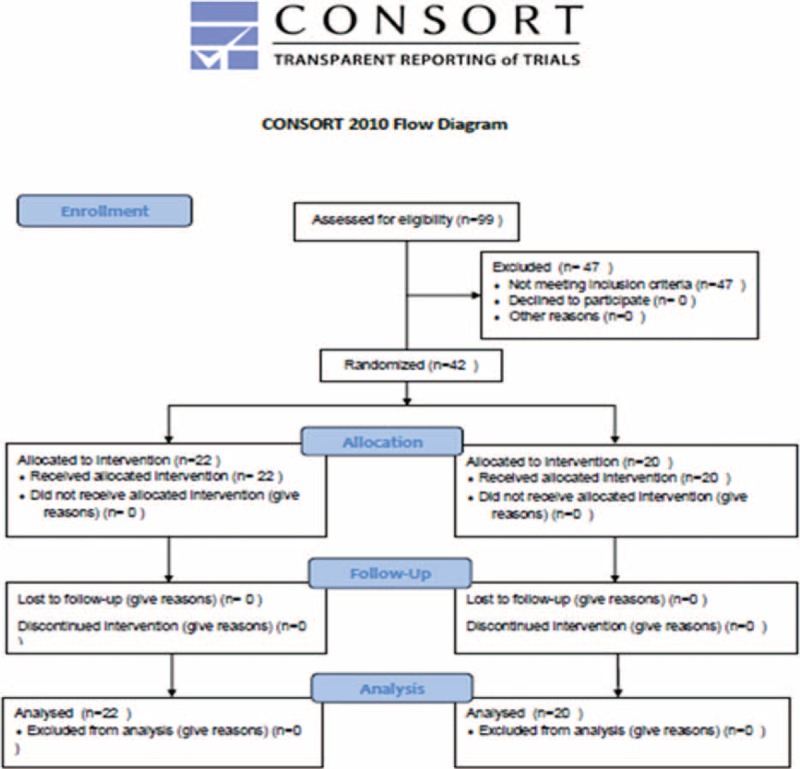
Consort flow diagram.
No significant difference was observed between the 2 groups with regard to cardiovascular risk factor prevalence (hypertension, diabetes, dyslipidemia, previous stroke), WBC and platelet count, serum lipid profile, and with regard to cardiovascular therapy before stroke (antiplatelets, antihypertensive drugs, statins).
No patient of both group interrupted in drug administration because of muscular or hepatic toxicity or other collateral effects. Among stroke patients treated with atorvastatin 80 mg, no significant differences have been observed either at 72 hours or 7 days after admissions with regard to serum levels of alanine transaminase, aspartate transaminase, gamma-glutamyltransferase, and alkaline phosphatase.
Characteristics of Enrolled Patients
Demographic, laboratory, and clinical variables of stroke patients with LAAS TOAST subtype are listed in Table 1.
TABLE 1.
Baseline Characteristics of Patients With Acute Ischemic Stroke Treated With Atorvastatin (Group A) and Not Treated With Atorvastatin (Group B)

Mean time from stroke onset to statins administration was 12 ± 4.8 hours in group A.
No significant difference with regard to low-density lipoprotein cholesterol (136 ± 21.1 vs 134 ± 1.8, P = 0.23) and high-density lipoprotein cholesterol (39 ± 11.2 vs 40.02 ± 1.1; P = 0.32) values was observed in subjects of group A after 7 days of atorvastatin treatment.
Inflammatory Marker Changes at 72 hours After Stroke
With regard to inflammatory marker plasma levels at 72 hours after acute ischemic stroke, subjects of group A showed in comparison with subjects of group B significantly lower plasma levels of CRP (2.4 [0.8–2.9] mg/dL vs 3.5 [1.2–4.6] mg/dL, P = 0.035; TNF-α (24.3 [7.75–38] pg/mL vs 33.5 (10.25–44) pg/mL; P = 0.023), IL-1β (8.4 [4–9.8] pg/mL vs 11 [5–14] pg/mL, P = 0.038), IL-6 (10.8 [7.2–19) pg/mL vs 12.6 [8.5–18] pg/mL, P = 0.010), VCAM-1 (19 [12.5–27] pg/mL vs 23 [14.6–36] pg/mL, P = 0.025), and ICAM-1 (13 [7.7–19] pg/mL vs 18.7 [10.2–29] pg/mL, P < 0.0001) (see Table 2 and Figure 2 A–G).
TABLE 2.
Outcome Indicators and Immunoinflammatory Markers at 72 Hours After Acute Event in Subjects With Acute Ischemic Stroke Early Treated With Atorvastatin 80 mg and Not Treated With Atorvastatin 80 mg
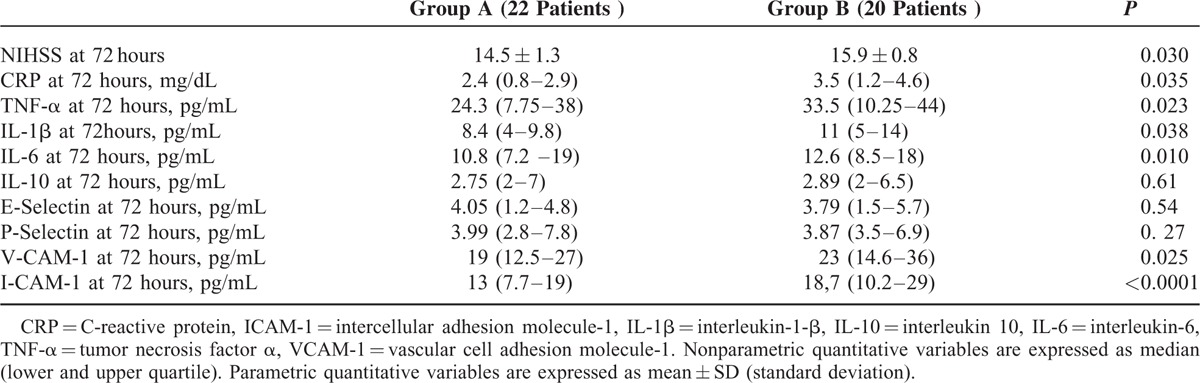
FIGURE 2.
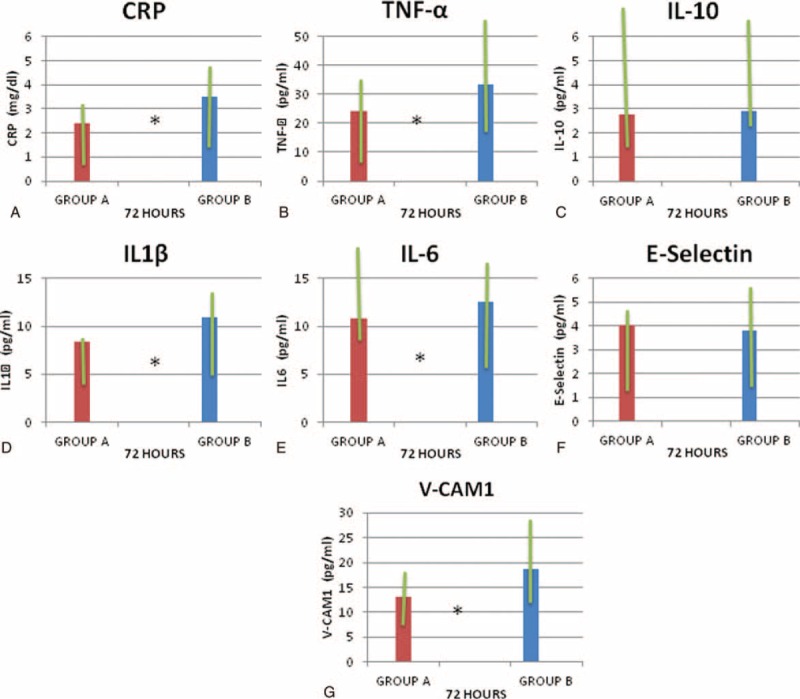
Median (lower and upper quartile) of serum levels of C-reactive protein (CRP) (A); tumor necrosis factor (TNF-α) (B); interleukin-10 (IL-10) (C); IL-1β (D); IL-6 (E); E-selectin (F); and vascular cell adhesion molecule-1 (VCAM-1) (G) at 72 hours after acute event in subjects with acute ischemic stroke early treated with atorvastatin 80 mg (Group A) and not treated with atorvastatin 80 mg (Group B). ∗P < 0.05.
Inflammatory Marker Changes at 7 Days After Stroke
With regard to inflammatory marker plasma levels at 7 days after acute ischemic stroke, subjects of group A in comparison with subjects of group B showed significantly lower plasma levels of CRP (1.9 [0.7–2.0] mg/dL vs 2.7 [1.5–3.3] mg/dL, P = 0.043), TNF-α (16.3 [6.75–26] pg/mL vs 22.3 [8.75–38] pg/mL, P = 0.031), IL-1β (6.6 [3–11] pg/mL vs 9.5 [6.8–12] pg/mL, P = 0.022), IL-6 (8.7 [5.5–14] pg/mL vs 10.4 [6.7–17] pg/mL, P = 0.023), VCAM-1 (13 [7.7–29] pg/mL vs 18.7 [7.8–34] pg/mL, P = 0.025), ICAM-1 (13.5 [11.5–26] pg/mL vs 18.5 [16.5–31] pg/mL P = 0.039) (see Table 3 and Figure 3 A–H).
TABLE 3.
Outcome Indicators and Immunoinflammatory Markers at 7 Days After Acute Event in Subjects With Acute Ischemic Stroke Early Treated With Atorvastatin 80 mg and Not Treated With Atorvastatin 80 mg
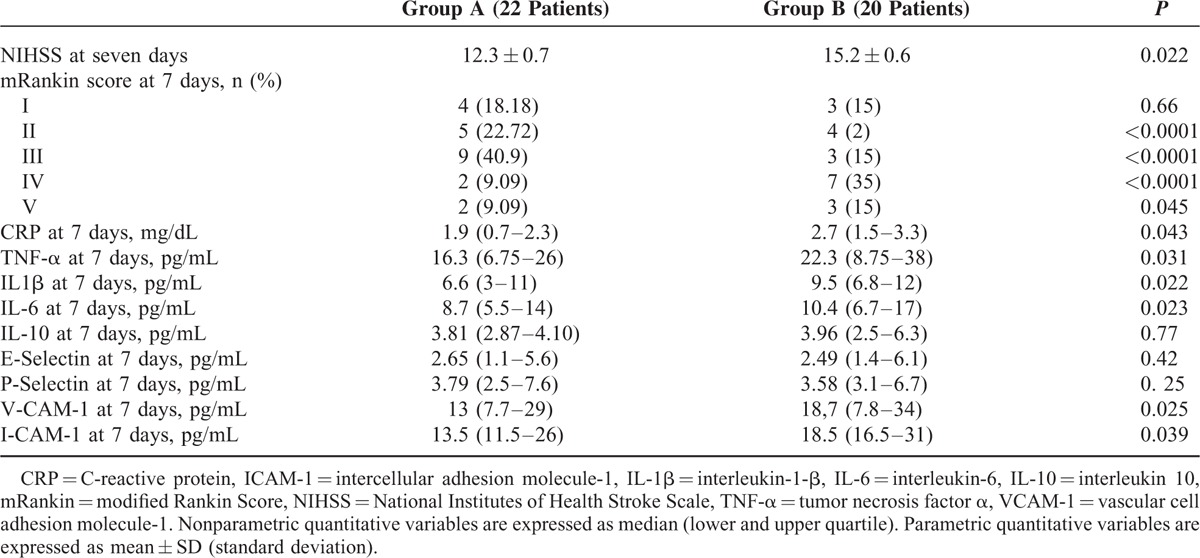
FIGURE 3.
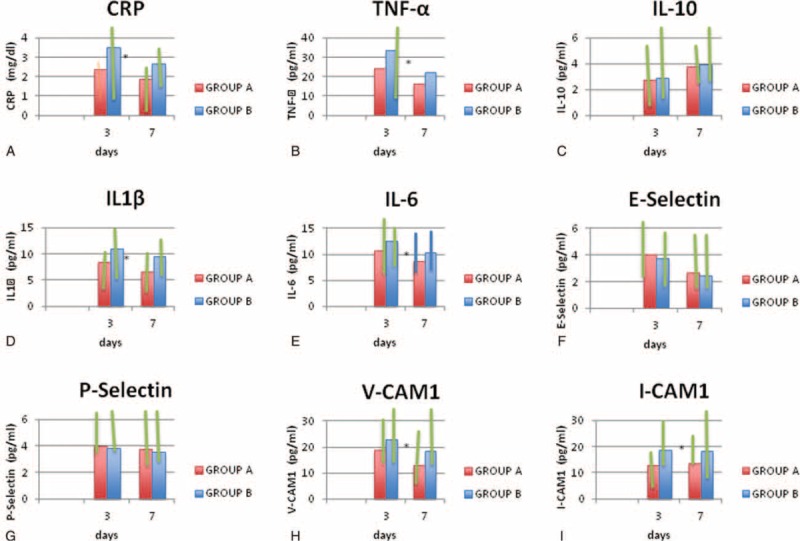
Serum median (lower and upper quartile) levels of C-reactive protein (CRP) (A); tumor necrosis factor (TNF-α) (B); interleukin-10 (IL-10) (C), IL-1β (D); IL-6 (E); E-selectin (F); P-selectin (G); vascular cell adhesion molecule-1 (VCAM-1) (H); and intercellular adhesion molecule-1 (ICAM-1) (I) at 72 hours and 7 days after acute event in subjects with acute ischemic stroke early treated with atorvastatin 80 mg (Group A) and not treated with atorvastatin 80 mg (Group B). ∗P < 0.05.
No significant difference with regard to plasma levels of IL-10, E-selectin, and P-selectin was observed between subjects treated with atorvastatin and those not treated with atorvastatin both at 72 h and at 7 days after acute ischemic stroke (see Tables 2 and 3).
Outcome Indicators Change at 72 Hour After Stroke
With regard to chosen outcome indicators, stroke patients treated with atorvastatin 80 mg at 72 hours after admission showed a significantly lower mean NIHSS score in comparison with stroke subjects not treated with atorvastatin (14.5 ± 1.3 vs 15.9 ± 0.8; P = 0.030); analogously at 7 days after admission, stroke patients treated with atorvastatin showed a significantly lower mean NIHSS score 80 mg in comparison with stroke subjects not treated with atorvastatin (12.3 ± 0.7 vs 15.2 ± 0.6; P = 0.022) (see Table 3).
Outcome Indicators Change at 7 Days After Stroke
With regard to mRS, at 7 days after admission, stroke patients treated with atorvastatin 80 mg in comparison with stroke subjects not treated with atorvastatin showed a higher percentage of patients were classified as II class (22.72% vs 2%; P < 0.0001) and as III class (40.9% vs 15%; P < 0.0001), whereas a lower percentage of patients were classified as IV class (9.09% vs 35%; P < 0.0001) and V (9.09% vs 15 %; P = 0.045) (see Table 3).
Patients with highest reduction of immune-inflammatory markers at 7 days are also patients with a better change of outcome indicators at 7 days. Stroke subjects who at 7 days after admission showed mean serum levels of TNF-α >20 pg/mL showed a mean NIHSS of 17.5 ± 1.0, those with mean serum levels of IL-1β >7 pg/mL showed a mean NIHSS of 17.8 ± 1.8, and those with mean serum levels of Il-6 >9 pg/mL showed a mean NIHSS of 18.0 ± 1.4, whereas patients who at 7 days after admission showed mean serum levels of TNF-α <20 pg/mL showed a mean NIHSS of 12.8 ± 1.7, those with mean serum levels of IL-1β <7 pg/mL showed a mean NIHSS of 10.1 ± 1.5 and those with mean serum levels of IL-6 <9 pg/mL showed a mean NIHSS of 11.3 ± 1.6. Furthermore, stroke subjects who at 7 days after admission showed mean serum levels of TNF-α >20 pg/mL showed a mean Rankin score of 3.8 ± 0.9, those with mean serum levels of IL-1β >7 pg/mL showed a mean Rankin score of 4 ± 0.5, and those with mean serum levels of Il-6 >9 pg/mL showed a mean Rankin score of 3.9 ± 0.4, whereas patients who at 7 days after admission showed mean serum levels of TNF-α <20 pg/mL showed a mean Rankin score of 2 ± 0.8, those with mean serum levels of IL-1β <7 pg/mL showed a mean Rankin score of 2.9 ± 0.3, and those with mean serum levels of IL-6 <9 pg/mL showed a mean Ramkin score of 2.8 ± 0.3.
DISCUSSION
Our findings provide the first evidence that atorvastatin acutely administered early after an atherosclerotic ischemic stroke exerts a lowering effect on serum markers immune-inflammatory activation of the acute phase of stroke and that its use is associated to a better outcome profile in terms of acute neurological deficit and disability grade.
We cannot affirm that cytokine serum level reduction in our enrolled patients with acute ischemic stroke is only because of atorvastin treatment owing to the fact that there exists a progressive lowering of cytokines after brain ischemic events. Thus, our interpretation of this finding is related to a possible overlapping anti-inflammatory efficacy of atorvastatin to the normal dynamics of inflammatory markers after acute ischemic stroke. Nevertheless, the aim of our study was not to show a reduction of serum levels of cytokines compared with baseline levels, but to evaluate differences in serum levels of cytokines at 72 hours after stroke and a more significant reduction of these levels at 7 days after stroke and their relationship with outcome differences in subjects treated with early oral atorvastatin and in subjects not treated with atorvastatin.
No significant changes in lipid blood profile were observed at 7 days after stroke onset; thus, our findings appear independent from blood lipid changes, perhaps owing to the fact that 7 days of treatment is a too short observation period to detect significant change in lipid blood profile.
Experimental and clinical studies demonstrated that statins exert an antioxidant effect with a mechanism potentially involving downregulation of Nox2 and upregulation of nitric oxide synthase coupling.16,17
Previous studies using several markers of platelet activation in patients with stable or unstable cardiovascular disease have already shown that statins exert an antiplatelet effect that was early and apparently independent from the cholesterol-lowering property of statins.18,19,20
Moreover, recently, Pignatelli et al4 provided the first evidence that atorvastatin acutely and simultaneously decreases oxidative stress and platelet activation by directly inhibiting platelet Nox2 and ultimately platelet isoprostanes and thromboxane A2, whereas clinical21–30 and experimental22–26 evidence suggest that part of the beneficial effects of statins are independent of their lipid-lowering properties, their so-called pleiotropic effects.31
A recent study32 showed that in 129 patients receiving statins, 123 initiated statins within 4 weeks, and in 600 patients not on statins, multivariate logistic regression analysis demonstrated that poststroke statins were associated with a significant probability of a favorable outcome at 12 weeks and mRS, whereas prestroke statins demonstrated a trend toward significance.
These findings could possibly explain our results showing that high-dose atorvastatin administered early after an atherosclerotic stroke is significantly associated with a lower degree of serum immune-inflammatory markers both at 72 hours and 7 days after stroke and that this lower immune-inflammatory degree is possibly related to a better prognosis evaluated by means outcome indicators such as NIHSS and mRS scores.
Several explanations could be considered to explain our findings concerning significant effects of atorvastatin on immunoinflammatory activation of the acute phase and on functional outcome indicators at 72 hours and 7 days after stroke onset.
In addition to their activities as cholesterol-lowering drugs, statins are known to have complex pleiotropic effects, including anti-inflammatory, immunomodulatory, and endothelial function-enhancing effects both in vitro and in human studies.33 Statins inhibit the mevalonate pathway, which involves the synthesis of isoprenoids and the intracellular trafficking of membrane-associated proteins such as G-proteins. GTP-binding proteins have crucial roles in intracellular inflammatory signaling by acting as molecular switches for various protein kinases. Specific targets, including Ras, Rho, and Rac subfamilies, are thought to be important in systemic inflammatory syndromes, such as sepsis, because of their key roles in intracellular signaling.34–35
Furthermore, a very recent study reported how therapy with statins, either previous or early initiation, after an ischemic stroke, could improve the survival and readmission rates by lowering both cholesterol and high-sensitivity C-reactive protein levels.36
In vitro and animal studies have shown that statins reduce the release of TNF-α and IL-1b.35,37 In a murine model of cerebral malaria,37 treatment with lovastatin intravital examination of pial vessels of infected animals demonstrated a reverse of rolling and adhesion of leukocytes to inflamed endothelium and reduction of ICAM-1 and CD11b mRNA levels and a reduction of IL-1b, TNF-a, and IL-12 levels that were elevated in the brains of mice on post infection period.
Statins could also impair immunoinflammatory cell trafficking. Lovastatin changed the expression of specific cytokines, promoting a shift from a Th1 to a Th2 response.38–42 Thus, statins would thus modulate the immune system through coordinated activity in various arms of the immune reaction, including terminal differentiation of immune cells, as well as the regulation of the molecules that regulate these cells to the sites at which they exert their immunomodulatory activity.
Furthermore, statins in brain may be both neuroprotective and regenerative, and could lead to a new direction in understanding the potential therapeutic efficacy of statins in AD and in particular atorvastatin treatment has been reported as neuroprotective against cell degeneration induced by amyloid-beta,43 reducing inflammatory and oxidative responses and increasing the expression of glutamatergic transporters.44,45
Recent experimental data from a murine model of ischemic stroke demonstrate that prophylactic statin therapy augments cerebral blood flow, reduces infarct size by 30%, and improves neurological outcome in normocholesterolemic animals.43 Statins have been shown to reduce infarct size in experimental animal models of stroke by means a summa of neuroprotective action such as upergulation of endothelia eNOS and inhibition of inducible inducible nitric oxide synthase (iNOS), lowering neuronal inflammation and amelioration of ischemic oxidative stress in brain.
Furthermore, statins may also exert antithrombotic activities mediated by changes of the coagulation system.44,45,46
A previous pilot, double-blind, randomized, multicenter clinical trial47 has been conducted to study for the first time safety and efficacy of simvastatin in the acute phase of ischemic stroke in terms of the evolution of several inflammation markers (IL-6, IL-8, IL-10, monocyte chemoattractant protein-1, ICAM-1, VCAM-1, C-reactive protein, TNF-α, E-selectin, L-selectin, and nitrites + nitrates) and neurological outcome was evaluated at baseline, day 1, 3, 5, 7, and 90. Authors used simvastatin at an initial dose of 40 mg/day for the first week followed by a dose of 20 mg/day until day 90. No differences were found among the biomarkers studied regarding treatment allocation, although simvastatin patients improved significantly by the third day. These findings contrast with our results probably owing to differences in statin dosage used in the trial and owing to the fact that we used high dosage of atorvastatin, a statin with well-proven anti-inflammatory and neuroprotective antinflammatory properties.
Limitations
A limitation of our study is the small sample size suggesting us a certain caution when interpreting our results.
Among possible limitations of our study, we acknowledge the potential weakness of the open design of the study; however, randomization and blind analysis of laboratory variables were likely to limit this bias.
Another possible limitation is offered by high atorvastatin dosage that we used, but we chose to use high dosage of atorvastatin owing to the fact that other experimental studies4 that evaluated the role of atorvastatin as antioxidant and antithrombotic agents and clinical studies that reported the beneficial effects of atorvastatin in secondary prevention, such as Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study3 used high dosage (80 mg) of atorvastatin, thus making plausible to test only this high dosage in our study.
CONCLUSIONS
Interpretation and Generalizability
In conclusion, our findings provide a rationale for generalizability of the early use of high-dosage atorvastatin in patients with acute ischemic stroke as a possible neuroprotective treatment. Further study is necessary to see whether a similar effect can be achieved and maintained in a longer follow-up examination without toxicity effects that we did not observe at a short observation period. A Possible external validity or generalizability to other circumstances could be related to the possibility of evaluation of a possible neuroprotective role of statins such as atorvastatin in other circumstances of acute neurological disorders such as thraumatic brain insult or cerebral hemorrhages, but further study is needed to evaluate atorvastatin role in this clinical setting. Furthermore, although the biological plausibility of our findings, since the sample size was assessed for primary end point our conclusion regarding the associated improvement of clinical outcome should be only considered as an interest hypothesis-generating result that should encourage the development of a further larger, multicenter, and controlled randomized trials.
Footnotes
Abbreviations: CD11b = 11 b cluster differentiation, CT = computerized tomography, eNOS = endothelial nitric oxide synthase, GTP-binding proteins = Guanosine-5’-triphosphate binding proteins, ICAM1 = Intercellular Adhesion Molecule 1, IL-1β = Interleukin-1 beta, IL-10 = Interleukin-10, IL-6 = Interleukin-6, iNOS = inducible nitric oxide synthase, LAAS = Large Artery Atherosclerotic Stroke, MRI = Magnetic Resonance Imaging, mRNA = messenger RNA levels, mRS = modified Rankin score, NIHSS = National Institutes of Health Stroke Scale, Nox2 = NADPH isoform 2, ODE = stroke of Other Determined Etiology, PAI-1 = plasminogen activator inhibitor-1, Ras = Ras protein, Rho = Ras homologue protein, SPARCL = tSroke Prevention with Aggressive Reductions in Cholesterol Levels, Th1 = T helper 1, Th2 = T helper 2, TNF-α = tumor necrosis alfa, TOAST = Trial Org 10172 in Acute Stroke Treatment, tPA = tissue plasminogen activator, UDE = stroke of UnDetermined Etiology, VCAM-1 = Vascular cell adhesion molecule 1, VWF = Von Willebrand factor.
AT and DDR contributed equally to the manuscript.
Authors received a research grant by Pfizer.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
A professional medical statistician (Dr Danilo Di Bona, MD) conducted our statistical analysis.
The study protocol was approved by the Policlinico P. Giaccone Hospital of Palermo Ethic Committee and it was in accordance to the Declaration of Helsinki.
Policlinico P. Giaccone Hospital of Palermo Ethic Committee approved trial design, consent procedure and format for written consent by all partecipants (date approval 10 novembre 2013).
All recruited patients gave a written consent to participate to the study.
All documents were conserved by the principal investigator Prof Antonino Tuttolomondo.
Trial registration on Clinical Trials.gov has begun in November 2013 but because of the technical problems registration process has been completed in August 2014 when the researchers have yet started the recruitment.(www.ClinicalTrials.gov; identifier, NCT02225834).
The full trial protocol can be accessed on www.ClinicalTrials.gov.
The authors confirm that all ongoing and related trials for this drug/intervention are registered.
Complete date range for patient recruitment and follow-up has been reported has been indicated on Study Settings section (see page 7).
Our manuscript has been prepared according CONSORT 2010 Checklist.
The authors report no conflicts of interest.
REFERENCES
- 1.Asahi M, Huang Z, Thomas S, et al. Protective effects of statins involving both eNOS and tPA in focal cerebral ischemia. J Cereb Blood Flow Metab 2005; 25:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslanyan S, Weir CJ, McInnes GT, et al. Statin administration prior to ischaemic stroke onset and survival: exploratory evidence from matched treatment-control study. Eur J Neurol 2005; 12:493–498. [DOI] [PubMed] [Google Scholar]
- 3.Amarenco P, Bogousslavsky J, Callahan A, 3rd, et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–559. [DOI] [PubMed] [Google Scholar]
- 4.Pignatelli P, Carnevale R, Pastori D, et al. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 2012; 126:92–103. [DOI] [PubMed] [Google Scholar]
- 5.Tuttolomondo A, Pecoraro R, Casuccio A, et al. Peripheral frequency of CD4+ CD28- cells in acute ischemic stroke: Relationship with stroke subtype and severity markers. Med (Baltimore) 2015; 94:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider DJ, Ricci MA, Taatjes DJ, et al. Changes in arterial expression of fibrinolytic system proteins inatherogenesis. Arterioscler Thromb Vasc Biol 1997; 17:3294–3301. [DOI] [PubMed] [Google Scholar]
- 7.Tuttolomondo A, Di Raimondo D, Di Sciacca R, et al. Arterial stiffness and ischemic stroke in subjects with and without metabolic syndrome. Atherosclerosis 2012; 225:216–219. [DOI] [PubMed] [Google Scholar]
- 8.Tuttolomondo A, Pecoraro R, Di Raimondo D, et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke with and without metabolic syndrome. Diabetol Metab Syndr 2014; 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuttolomondo A, Pecoraro R, Pinto A. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: a review of the evidence to date. Drug Des Devel Ther 2014; 8:2221–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakase T, Yamazaki T, Ogura N, et al. The impact of inflammation on the pathogenesis and prognosis of ischemic stroke. J Neurol Sci 2008; 271:104–109. [DOI] [PubMed] [Google Scholar]
- 11.Hatano S. Experience from a multicenter Stroke register; a preliminary report. Bull World Health Organ 1976; 54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 12.Lyden P, Shuaib A, Ng K, et al. Clomethiazole acute stroke study in ischemic stroke (CLASS-I): Final results. Stroke 2002; 33:122–128. [DOI] [PubMed] [Google Scholar]
- 13.Rankin J. Cerebral vascular accidents in patients over the age of 60: Prognosis. Scott Med J 1957; 2:200–215. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Ramirez V, Macias-Islas MA, Ortiz GG, et al. Efficacy of fish oil on serum of TNF (, IL-1 (, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid Med Cell Longev 2013; 2013:709493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antoniades C, Bakogiannis C, Leeson P, et al. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation 2011; 124:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos MT, Fuset MP, Ruano M, et al. Effect of atorvastatin on platelet thromboxane A (2) synthesis in aspirin-treated patients with acute myocardial infarction. Am J Cardiol 2009; 104:1618–1623. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Masuda H, Kariyazono H, et al. Effects of atorvastatin and aspirin combined therapy on inflammatory responses in patients undergoing coronary artery bypass grafting. Cytokine 2006; 36:201–210. [DOI] [PubMed] [Google Scholar]
- 19.No authors listed. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383. [PubMed] [Google Scholar]
- 20.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients withaverage cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med 1996; 335:1001–1009. [DOI] [PubMed] [Google Scholar]
- 21.Hebert PR, Gaziano JM, Chan KS, et al. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA 1997; 278:313. [PubMed] [Google Scholar]
- 22.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) study group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357. [DOI] [PubMed] [Google Scholar]
- 23.Byington RP, Davis BR, Plehn JF, et al. Reduction of stroke events with pravastatin: The Prospective Pravastatin Pooling (PPP) project. Circulation 2001; 103:387–392. [DOI] [PubMed] [Google Scholar]
- 24.The Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebocontrolled trial. Lancet 2002; 360:7–22.12114036 [Google Scholar]
- 25.Stroke Council, American Heart Association, American Stroke Association. Statins after ischemic stroke and transient ischemic attack: an advisory statement from the Stroke Council, American Heart Association and American Stroke Association. Stroke 2005; 35:1023. [DOI] [PubMed] [Google Scholar]
- 26.Laufs U, La Fata V, Plutzky J, et al. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998; 97:1129–1135. [DOI] [PubMed] [Google Scholar]
- 27.Endres M, Laufs U, Huang Z, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA 1998; 95:8880–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMGcoenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 1997; 95:1126–1131. [DOI] [PubMed] [Google Scholar]
- 29.Amin-Hanjani S, Stagliano NE, Yamada M, et al. Mevastatin, an HMG-CoA reductase inhibitor,reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke 2001; 32:980–986. [DOI] [PubMed] [Google Scholar]
- 30.Kawashima S, Yamashita T, Miwa Y, et al. HMG-CoA reductase inhibitor has protective effects against stroke events in stroke-prone spontaneously hypertensive rats. Stroke 2003; 34:157–163. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan CJ, Murphy MB, Buckley BM. Statins do more than just lower cholesterol. Lancet 1996; 348:1079–1082. [DOI] [PubMed] [Google Scholar]
- 32.Moonis M, Kane K, Schwiderski U, et al. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke 2005; 36:1298–1300. [DOI] [PubMed] [Google Scholar]
- 33.Terblanche M, Almog Y, Rosenson RS, et al. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis 2007; 7:358–368. [DOI] [PubMed] [Google Scholar]
- 34.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol 2006; 6:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niessner A, Steiner S, Speidl WS, et al. Simvastatin suppresses endotoxin-induced upregulation of toll-like receptors 4 and 2 in vivo. Atherosclerosis 2006; 189:408–413. [DOI] [PubMed] [Google Scholar]
- 36.Arévalo-Lorido JC, Carretero-Gómez J, Fernández-Recio JM, et al. Lowering C-reactive protein with statins after an ischemic stroke avoids mortality and readmissions. A prospective cohort study. Ann Med 2015; 47:226–232. [DOI] [PubMed] [Google Scholar]
- 37.Reis PA, Estato V, da Silva TI, et al. Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria. PLoS Pathog 2012; 8:e1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mira E, León B, Barber DF, et al. Statins induce regulatory T cell recruitment via a CCL1 dependent pathway. J Immunol 2008; 181:3524–3534. [DOI] [PubMed] [Google Scholar]
- 39.Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends Immunol 2007; 28:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehrenstein MR, Jury EC, Mauri C. Statins for atherosclerosis: as good as it gets? N Engl J Med 2005; 352:73–75. [DOI] [PubMed] [Google Scholar]
- 41.Jury EC, Isenberg DA, Mauri CMR. Atorvastatin restores Lck expression and lipid raft-associated signaling in T cells from patients with systemic lupus erythematosus. J Immunol 2006; 177:7416–7422. [DOI] [PubMed] [Google Scholar]
- 42.Annunziato FL, Cosmi F, Liotta E, et al. Phenotype, localization, and mechanism of suppression of CD4-CD25- human thymocytes. J Exp Med 2002; 196:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piermartiri TC, Figueiredo CP, Rial D, et al. Atorvastatin prevents hippocampal cell death, neuroinflammation and oxidative stress following amyloid-beta (1-40) administration in mice: evidence for dissociation between cognitive deficits and neuronal damage. Exp Neurol 2010; 226:274–284. [DOI] [PubMed] [Google Scholar]
- 44.Endres M, Laufs U, Huang Z, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 1998; 95:8880–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferro D, Basili S, Alessandri C, et al. Inhibition of tissuefactor-mediated thrombin generation by simvastatin. Atherosclerosis 2000; 149:111–116. [DOI] [PubMed] [Google Scholar]
- 46.Colli S, Eligini S, Lalli M, et al. Vastatins inhibit tissue factor in cultured human macrophages: a novel mechanism of protection against atherothrombosis. Arterioscler Thromb Vasc Biol 1997; 17:265–267. [DOI] [PubMed] [Google Scholar]
- 47.Montaner J, Chacón P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J Neurol 2008; 15:82–90. [DOI] [PubMed] [Google Scholar]


