Abstract
Endometrial adenocarcinoma is a common gynecological malignancy that is usually treated by surgical resection followed by radiation. However, the frequency of remote metastasis is high. The present study aimed to investigate whether patients with endometrial adenocarcinoma exhibited a positive response to treatment with a gonadotropin-releasing hormone analogue or inhibitors of neoangiogenesis, which are applied for the treatment of other malignancies. Immunohistochemical analyses were performed using 203 paraffin-embedded tissue samples of endometrial adenocarcinomas from patients who had undergone surgery at the Department of Obstetrics and Gynecology of the Ludwig Maximilians University of Munich, Germany. The tissues were incubated with antibodies against luteinizing hormone/choriogonadotropin receptor (LHCGR) and vascular endothelial growth factor receptor 2 (VEGFR2), and evaluated by bright field microscopy. The staining was categorized according to the Immune-Reactive-Score (IRS). The IRS scores were then statistically associated with various tumor traits, including tumor size, lymph node status, metastasis, grade, expression of steroid hormone receptors and patient survival. There was a significant association between VEGFR2 expression and tumor grading and estrogen receptor-α (ERα). For LHCGR, a correlation was observed with ERα and progesterone receptor (PR). No correlations were identified between VEGFR2 or LHCGR expression and the other examined tumor traits or patient survival. The associations between VEGFR2 and ERα, and between LHCGR and ERα or PR, may be explained by the interaction of these signal transduction molecules in the regulation of cellular growth and differentiation. These mechanisms also have an important role in the formation of remote metastases, which is the main cause for tumor-associated mortality. The results of the present study suggested that patients with endometrial adenocarcinoma may benefit from treatment with inhibitors of ERα, PR, VEGFR2 or LHCGR, since it could lead to a better prognosis. However, further studies are required in order to elucidate the roles of these receptors in endometrial adenocarcinoma.
Keywords: endometrial adenocarcinoma, VEGFR, LHCGR, steroid hormones
Introduction
Endometrial adenocarcinoma is the fourth-most common malignant disease in women in Germany (1); every year, ~11,300 women are diagnosed with this disease (2). In 75–90% of cases, a type I carcinoma is diagnosed, which typically occurs prior to and during the menopause, has a low grading, is minimally invasive to the myometrium, estrogen-dependent and generally has a good outcome (1). Conversely, type II carcinoma is typically diagnosed postmenopause, has a high grading, invades the myometrium deeply and has a serous or clear cell type morphology. Type II endometrial carcinoma is more aggressive and is associated with a higher risk of relapse or metastasis, and thus has a poorer prognosis, than type I endometrial carcinoma. Endometrial adenocarcinoma is characterized by peri- and postmenopausal bleeding (3); however, a reliable diagnosis is dependent on a histological analysis. Currently, endometrial adenocarcinoma is treated by surgical resection followed by radiation, which only lowers the risk of local recurrence (4). Adjuvant chemotherapy is rarely applied (5), and is also associated with a high risk of local recurrence and formation of remote metastases (25% of patients), thus demonstrating the necessity for subsequent follow-up (6).
Oncological findings in the last decade have demonstrated that enhanced tumor growth is associated with increased perfusion and metabolism of the neoplastic area (7–9). Tumorigenesis is strongly influenced by vascular endothelial growth factor (VEGF), which regulates neoangiogenesis (10). Although the clinical value of VEGF receptor (VEGFR) expression is controversial (11), previous studies of prostate, colorectal and ovarian cancer demonstrated that the expression levels of VEGF/VEGFR were associated with clinicopathological tumor data and had prognostic significance (7–9). Inhibition of VEGF/VEGFR reduces blood vessel formation by the tumor and slows tumor growth, thus improving the prognosis of patients (7–9). Furthermore, bevacizumab (Avastin®), an anti-VEGF monoclonal antibody, has been used extensively to inhibit VEGF/VEGFR in the treatment of various cancers, including colon, lung, breast, kidney and ovarian carcinoma, and has demonstrated marked anti-tumor effects when used in combination with chemotherapy.
Tumorigenesis in endometrial adenocarcinoma is influenced by the glycoprotein hormones luteinizing hormone (LH) and human choriongonadotropin (hCG), which consist of variable subunits and are differentially glycosylated (12). LH and hCG bind to the LHCG receptor (LHCGR), although hCG binds with a higher affinity than LH and has been identified as a pro-angiogenic factor (13). Previous studies have demonstrated that LHCGR is upregulated in malignant tissue, as compared with healthy tissue (14,15). In addition, the expression of LHCGR and LH/hCG has been detected in endometrial samples (16), in which they were correlated with cell proliferation (17,18) and with grading of endometrial adenocarcinomas (19). The associations between the expression of LHCGR, the addition of LH/hCG and cell invasiveness should be substantiated in primary endometrial tissue samples and in cell lines. The results of previous studies have suggested that LHCGR+ patients may benefit from a therapeutic strategy involving gonadotropin-releasing hormone (GnRH) analogues, since they were demonstrated to reduce the levels of LH (20,21). Furthermore, it has been reported that hCG regulates the expression of VEGF (22–24).
Another molecule that has a role in the angiogenesis and tumor proliferation of endometrial cancer is glycodelin, which was demonstrated to regulate the malignant growth of endometrial cancer cell lines (25). As a result of glycodelin expression, cell proliferation decreased, cells were arrested in the G1-phase of the cell cycle, and the messenger RNA expression levels of cyclin-dependent kinase inhibitors, p21, p27 and p16 were upregulated (26). Conversely, downregulation of glycodelin resulted in increased cell proliferation due to loss of progesterone-mediated cell proliferation.
All these molecules may be considered novel therapeutic options for endometrial adenocarcinoma. The present study aimed to assess this hypothesis by performing immunohistochemical staining of endometrial adenocarcinoma tissue samples, and correlating staining with tumor characteristics and outcome of the patients.
Materials and methods
Tissue samples
Tissue samples were obtained from the pathology archive of the Department of Gynecology and Obstetrics, Ludwig Maximilians University (LMU) of Munich (Munich, Germany). A total of 203 patients diagnosed with endometrial adenocarcinoma and treated by surgical resection between May 1990 and April 2001 were included in the study. However, type I and II carcinomas were not investigated separately. The present study was performed according to the Declaration of Helsinki (ethical votes 148-12 and 048-08). Follow-up of the patients was available up to 2014. Patient and tumor characteristics are shown in Table I. Staining of estrogen receptor (ER)-α and -β, as well as progesterone receptor (PR) A and B, was performed as described previously (27). The Ethics Committee of LMU of Munich approved the study (approval no., LMU-148-12). Written informed consent was obtained from the patients.
Table I.
Sample characteristics.
| Patient/tumor traits | Subgroups/no. of samples |
|---|---|
| Age at primary diagnosis, years | |
| <50 | 11 |
| 50–60 | 55 |
| 60–70 | 69 |
| 70–80 | 50 |
| >80 | 18 |
| Tumor size | |
| T1 | 166 |
| T2 | 15 |
| T3 | 17 |
| T4 | 5 |
| Lymph node status | |
| Nx | 57 |
| N0 | 135 |
| N1 | 11 |
| Metastasis status | |
| Mx | 76 |
| M0 | 124 |
| M1 | 3 |
| Grading | |
| G1 | 113 |
| G2 | 68 |
| G3 | 22 |
| ERα | |
| Positive | 92 |
| Negative | 111 |
| ERβ | |
| Positive | 28 |
| Negative | 175 |
| PRA | |
| Positive | 92 |
| Negative | 111 |
| PRB | |
| Positive | 103 |
| Negative | 100 |
ER, estrogen receptor; PR, progesterone receptor.
Preparation of tissue samples for immunohistochemistry
Paraffin-embedded tumor blocks were cut into 2–3-µm sections using a sliding microtome, mounted onto microscope slides (Menzel Gläser; Thermo Fisher Scientific, Inc., Braunschweig, Germany), covered and air-dried overnight. Subsequently, the sections were incubated in xylol (Merck Millipore, Darmstadt, Germany) at room temperature. Upon xylol removal, endogenous tissue peroxidase activity was inhibited by incubation of the tissue sections with 3% H2O2 (VWR International, Radnor, PA, USA). Next, samples were heated for 5 min in a pressure cooker in a sodium citrate buffer (Merck Millipore) at pH 6 to dissolve protein cross-links that arise during the fixation process. Finally, the tissue sections were washed in water and phosphate-buffered saline (Biochrom, Ltd., Cambridge, UK).
Staining of tissue samples
The prepared slides were initially incubated in normal goat serum (Vector Laboratories, Inc., Burlingame, CA, USA) at room temperature to prevent nonspecific binding of the primary antibody. Following removal of the blocking solution, the sections were incubated with anti-LHCGR (1:800; cat. no. SP4594P; Acris Antibodies GmbH, Herford, Germany) and anti-VEGFR2 (1:50; cat. no. AM21042PU-M; Acris Antibodies GmbH) primary antibodies for 18 h at 4°C. Subsequently, the slides were washed with phosphate-buffered saline and incubated with biotinylated secondary antibody solution (VectaStain ABC HRP kit; cat. no. PK-4001; Vector Laboratories, Inc.) for 30 min at room temperature. Upon washing to remove the secondary antibody, avidin-biotin complex reagent (Vector Laboratories, Inc.) was applied to the slides for 30 min, after which, 3,3′-diaminobenzidine (DAB; Dako North America, Inc., Carpinteria, CA, USA) was added to the slides for 1 min. DAB, which is the substrate for the biotin-coupled peroxidase, resulted in a brown precipitate that could be evaluated by bright field microscopy. Washing the slides in running tap water terminated the enzyme reaction. Nuclei were counterstained with Mayer's Hemalaun solution (PanReac AppliChem, Darmstadt, Germany) for 5 min, prior to dehydrating the sections and embedding them in Eukitt (Medite GmbH, Burgdorf, Germany). The stained samples were then evaluated under a light microscope or stored at room temperature.
Prior to performing the immunohistochemical analysis of tumor tissue samples, positive and isotype control samples were evaluated (Fig. 1). For the positive control, a sample from a mammacarcinoma tissue (collected from patients at LMU of Munich undergoing breast surgery for previous studies), which is known to overexpress LHCGR/VEGFR, was stained to assess the antibody function and to determine the optimum dilution of the antibody. The isotype control, which involved staining a sample from a mammacarcinoma tissue with control serum instead of primary antibody, was performed to reveal background staining due to the primary antibody.
Figure 1.
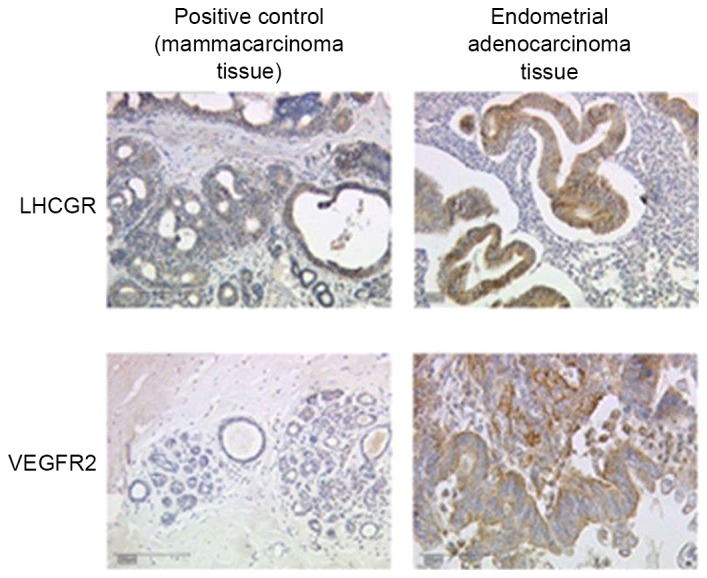
Staining of tissue samples. Left column, positive control (mammacarcinoma tissue); right column, endometrial adenocarcinoma tissue; upper row, LHCGR-staining; lower row, VEGFR2-staining. Magnification, ×10. VEGFR2, vascular endothelial growth factor receptor 2; LHCGR, luteinizing hormone/choriogonadotropin receptor.
Microscopy and evaluation of staining
Samples were visualized using the Leitz Diaplan light microscope (Ernst Leitz GmbH; Leica Camera AG, Wetzlar, Germany), with four different objectives (×6.3, ×10, ×25 and ×40 magnification). Staining was evaluated by two independent investigators according to the Immune-Reactive-Score (IRS) described by Remmele and Stegner in 1987 (28). The IRS was obtained by multiplying the staining intensity with the number of stained cells. The staining intensity was classified into groups from 0 to 3 as follows: 0, no staining reaction; 1, weak staining; 2, moderate staining; and 3, strong color reaction. The number of stained cells was similarly classified from 0 to 4 as follows: 0, 0% stained cells; 1, <10% stained cells; 2, ≤50% stained cells; 3, 51–80% stained cells; and 4, 81–100% stained cells. Therefore, the IRS is in a range from 0 to 12.
Statistical evaluation
Statistical analyses were performed using SPSS software, version 20.0 (IBM SPSS, Armonk, NY, USA). A cut-off value for the statistical evaluation of the IRS was set at a reference of the median of IRS values, which was 2 for LHCGR and 3 for VEGFR2. For single factor analysis, statistical tests were performed, as indicated in Table II. Certain tumor characteristics were pooled into subgroups and subsequently tested for statistical relevance. The subgroups, applied tests and results are shown in Table III. Survival data were evaluated using the Kaplan-Meier method, and statistical significance was examined by the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Table II.
Statistical evaluation of staining in association with tumor traits.
| Tumor trait | Statistical test applied | VEGFR2 (IRS cut-off=3) P-value | LHCGR (IRS cut-off=2) P-value |
|---|---|---|---|
| Grading | χ2 | 0.067 | 0.223 |
| Progression state | χ2 | 0.966 | 0.839 |
| Occurrence of local recurrence | χ2 | 0.335 | 0.359 |
| Tumor size | χ2 | 0.645 | 0.815 |
| FIGO | χ2 | 0.141 | 0.521 |
| ERα | χ2 | 0.025 | 0.056 |
| PRA | χ2 | 0.789 | 0.013 |
| Lymph node involvement | Mann-Whitney U | 0.373 | 0.531 |
| Occurrence of metastasis | χ2 | 0.992 | 0.733 |
| Age at diagnosis | t-testa, non-chained | 0.984 | 0.206 |
| Survival time | t-testa, non-chained | 0.738 | 0.136 |
Student's t-test. FIGO, International Federation of Gynecology and Obstetrics; ER, estrogen receptor; PR, progesterone receptor; VEGFR2, vascular endothelial growth factor receptor 2; LHCGR, luteinizing hormone/choriogonadotropin receptor; IRS, Immune-Reactive-Score.
Table III.
Statistical analysis of subgroups.
| Tumor trait | Statistical test applied | VEGFR2 (IRS cut-off=3) P-value | LHCGR (IRS cut-off=2) P-value |
|---|---|---|---|
| Grading G1, G2 vs. G3 | Kruskal-Wallis | 0.068 | 0.225 |
| Grading G1 vs. G3 | Mann-Whitney U | 0.875 | 0.113 |
| Grading G2 vs. G3 | Mann-Whitney U | 0.418 | 0.276 |
| pT <1b vs. >1b | t-test, non-chained | 0.353 | 0.423 |
| pT <2 vs. >2 | t-test, non-chained | 0.282 | 0.890 |
| Age <55 vs. >55 years | t-test, non-chained | 0.341 | 0.398 |
VEGFR2, vascular endothelial growth factor receptor 2; LHCGR, luteinizing hormone/choriogonadotropin receptor; IRS, Immune-Reactive-Score.
Results
Patient and tumor characteristics
The majority of endometrial adenocarcinoma patients were aged between 50 and 80 years (85%), exhibited no lymph node involvement (66.5% N0 vs. 5.4% N1; 28% Nx), and had no detectable evidence of metastasis formation (61.0% M0 vs. 1.4% M1; 37,4% Mx). In addition, the majority of tumors were small (81.8% pT1 vs. 18.2% pT2-4), with a low grading (89.1% G1 and G2 vs. 10.9% G3). The hormone receptor status was equally distributed (positive vs. negative), with the exception of ERβ, for which the majority of tumor tissues were negative (86.1% ERβ− vs. 13.9% ERβ+).
Immunohistochemical analysis
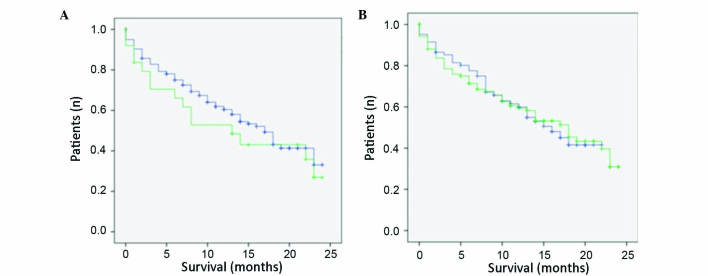
Tissue samples were stained using antibodiess against VEGFR2 and LHCGR and, by multiplying the staining intensity by the number of stained cells, IRS values were calculated and correlated with known tumor characteristics, as indicated in Table II. The correlation between VEGFR2 expression and tumor grading was not statistically significant; however, the P-value was close to be significant (P=0.067) and thus may be regarded as ‘borderline significant’. Conversely, there was no such association between LHCGR expression and tumor grading (P=0.223). Furthermore, no statistically significant correlations were observed for the two investigated receptors and the stage of progression (P=0.966 for VEGFR2; P=0.839 for LHCGR), the occurrence of local recurrence (P=0.335 for VEGFR2; P=0.359 for LHCGR), tumor size (P=0.645 for VEGFR2; P=0.815 for LHCGR), International Federation of Gynecology and Obstetrics grading (P=0.141 for VEGFR2; P=0.521 for LHCGR), lymph node involvement (P=0.373 for VEGFR2; no result for LHCGR), occurrence of remote metastasis (P=0.992 for VEGFR2; P=0.733 for LHCGR), patient age at diagnosis (P=0.984 for VEGFR2; P=0.206 for LHCGR) or time of survival (P=0.738 for VEGFR2; P=0.136 for LHCGR). However, statistically significant correlations were observed between VEGFR2 and ERα (P=0.025 for VEGFR2; P=0.056 for LHCGR) and between LHCGR and PRA (P=0.013). Conversely, there was no association between VEGFR2 expression and PRA (P=0.789). The associations between VEGFR2/LHCGR and ERβ/PRB were not analyzed, since the role and significance of these receptors is not well known. Kaplan-Meier analyses demonstrated that neither LHCGR nor VEGFR2 were associated with survival. The survival curves were similar for those patients whose tissue samples were positive for VEGFR2 or LHCGR expression, and for those patients whose tissue samples were negative for these receptors (Fig. 2). From the survival curves it was estimated that there was no statistically significant differences between the two curves (P=0.819 for VEGFR2; P=0.603 for LHCGR; Table IV).
Figure 2.
Kaplan-Meier survival curves for LHCGR and VEGFR2 expression. (A) Survival curve for LHCGR. IRS cut-off=3. Blue line, LHCGR-negative; green line, LHCGR-positive. (B) Survival curve for VEGFR2. IRS cut-off=2. Blue line, VEGFR2-negative; green line, VEGFR2-positive. Ordinate, cumulative survival; abscissa, survival in years. LHCGR, luteinizing hormone/choriogonadotropin receptor; VEGFR2, vascular endothelial growth factor receptor 2; IRS, Immune-Reactive-Score.
Table IV.
Statistical evaluation of staining and survival.
| Receptor | Cut-off IRS | Statistical test | P-value |
|---|---|---|---|
| LHCGR | 3 | Log-rank | 0.603 |
| VEGFR2 | 2 | Log-rank | 0.819 |
VEGFR2, vascular endothelial growth factor receptor 2; LHCGR, luteinizing hormone/choriogonadotropin receptor; IRS, Immune-Reactive-Score.
Subgroup analysis
Subgroups were established for the traits of tumor grading, tumor size and patient age at primary diagnosis, and were again subjected to a statistical analysis (Table III). Only a borderline significant correlation was observed for VEGFR2 expression and tumor grading (P=0.068). The other subgroups did not display a significant association with VEGFR2 or LHCGR expression.
Discussion
The present study demonstrated that there were slight correlations between VEGFR2 and tumor grading and ERα, and between LHCGR and ERα and PRA. The process of neoangiogenesis, which is involved in the formation of remote metastases, is predominantly driven by five splice variants of VEGF and the two corresponding receptors VEGFR1 and VEGFR2, whereas VEGFR2 is the key mediator of biological processes (29). An upregulation of VEGFR2 in endometrial carcinoma tissue, as compared with the normal endometrium, has been previously described (30). Furthermore, an association between VEGFR2 and tumor grading has previously been demonstrated in a number of tumor types, including epithelial dysplasia (31) and soft tissue sarcomas (32). In the latter case, an association between VEGFR2 and patient survival was also demonstrated, although this was not observed in the present study. The expression of VEGFR2 is induced by 17β-estradiol, which may explain the association between ERα and VEGFR2 in the present study. In 2006, Higgins et al (33) demonstrated that ERα, together with Sp3 and Sp4 transcription factors, interacts with VEGFR2, and that this interaction leads to the inactivation of VEGFR2 (33). Subsequently, the same research group reported a hormone-dependent downregulation of VEGFR2 by ERα, together with Sp1 and Sp3, in MCF-7 cells (34). The mechanism of interaction appeared to involve binding of the ERα-complex to the VEGFR2 promotor region (34). In addition, a previous study demonstrated an ERα-mediated increase in VEGFR2 expression in human myometrial microvascular endothelial cells (35). However, at present, it is not yet clear whether the interaction of ERα with VEGFR2 results in the activation or inactivation of VEGFR.
The present study demonstrated a preliminary association (P=0.056) between LHCGR and ERα, which has been described previously in breast cancer cell lines (19). Yuri et al (36) demonstrated that hCG, the binding partner of LHCGR, increased estrogen levels via mitochondrial signaling pathways and ovarian steroid secretion. Furthermore, the authors concluded that hCG may be considered a therapeutic option for patients with breast cancer who exhibit overexpression of LHCGR and ER (36). In addition, ER and LHCGR were demonstrated to contribute to testicular germ cell cancer development and to the formation of remote metastasis of these tumors (37). The observed association between LHCGR and PR may be explained by the induction of progesterone synthesis by LHCGR (38). A previous study added RU486, a progesterone antagonist, to luteinized human mural granulosa cells, and demonstrated inhibition of proliferation, progesterone secretion and LHCGR as a result (39). Conversely, incubation with progesterone led to an induction of LHCGR (39).
In conclusion, the present study demonstrated that there was an association between steroid hormone receptors and VEFGR and LHCGR. Steroid hormones are particularly important molecules of the human endometrium, since they regulate the composition and decomposition of the endometrium, as well as cell growth and division. VEGFR and LHCGR also participate in cell growth and neoangiogenesis, which are important features of metastasis. Therefore, the combination of these four molecules may influence the growth and metastasis of endometrial adenocarcinomas. However, it is important to remember that the use of GnRH analogues is restricted to premenopausal patients (1); thus, the formation of patient subgroups would be indispensable. Further research may identify novel therapeutic options for endometrial carcinomas that are based on existing therapies for other types of tumors. It would only be necessary to determine the hormone LHCGR and VEGFR status of a patient to administer therapy tailored to the tumor phenotype, which may have fewer side effects and a higher efficacy, thus leading to a more personalized treatment strategy for endometrial adenocarcinoma.
Acknowledgements
The authors would like to thank the ‘Förderprogramm für Forschung und Lehre’ of the LMU of Munich (grant no. 868/2014) for their financial support.
References
- 1.AGO: Empfehlungen für Diagnostik und Therapie des Endometriumkarzinoms-Aktualisierte Empfehlungen der Kommission Uterus auf Grundlage der S2k Leitlinie (V1.0, 1.6.2008) AWMF Leitlinien Register Nr 0320/342013 [Google Scholar]
- 2.GEKID: Cancer in Germany 2005/2006. Incidence and Trends. 2006 [Google Scholar]
- 3.Moodley M, Roberts C. Clinical pathway for the evaluation of postmenopausal bleeding with an emphasis on endometrial cancer detection. J Obstet Gynaecol. 2004;24:736–741. doi: 10.1080/014436104100009394. [DOI] [PubMed] [Google Scholar]
- 4.Tumorzentrum M. In: Malignome des Corpus Uteri. Christian PD Dr., Dannecker PMKaPRK, editors. W. Zuckerschwendt Verlag; München, Wien, New York: 2007. [Google Scholar]
- 5.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Treatment modalities in endometrial cancer. Curr Opin Oncol. 2007;19:479–485. doi: 10.1097/CCO.0b013e32827853c0. [DOI] [PubMed] [Google Scholar]
- 6.Beckmann K, Iosifidis P, Shorne L, Gilchrist S, Roder D. Effects of variations in hysterectomy status on population coverage by cervical screening. Aust N Z J Public Health. 2003;27:507–512. doi: 10.1111/j.1467-842X.2003.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 7.Latil A, Bièche I, Pesche S, Valéri A, Fournier G, Cussenot O, Lidereau R. VEGF overexpression in clinically localized prostate tumors and neuropilin-1 overexpression in metastatic forms. Int J Cancer. 2000;89:167–171. doi: 10.1002/(SICI)1097-0215(20000320)89:2<167::AID-IJC11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Martins SF, Garcia EA, Luz MA, Pardal F, Rodrigues M, Filho AL. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genomics Proteomics. 2013;10:55–67. [PubMed] [Google Scholar]
- 9.Raspollini MR, Amunni G, Villanucci A, Baroni G, Boddi V, Taddei GL. Prognostic significance of microvessel density and vascular endothelial growth factor expression in advanced ovarian serous carcinoma. Int J Gynecol Cancer. 2004;14:815–823. doi: 10.1111/j.1048-891X.2004.014514.x. [DOI] [PubMed] [Google Scholar]
- 10.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling-in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 11.Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, Kabbinavar F, Holden SN, Novotny WF, Frantz GD, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217–227. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 12.Pierce JG, Parsons TF. Glycoprotein hormones: Structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 13.Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Münstedt K, Rao CV, Lang U, Preissner KT. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–5296. doi: 10.1210/jc.2002-020642. [DOI] [PubMed] [Google Scholar]
- 14.Ji Q, Chen P, Aoyoma C, Liu P. Increased expression of human luteinizing hormone/human chorionic gonadotropin receptor mRNA in human endometrial cancer. Mol Cell Probes. 2002;16:269–275. doi: 10.1006/mcpr.2002.0421. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Lei ZM, Lojun S, Rao CV, Satyaswaroop PG, Day TG. Increased expression of luteinizing hormone/human chorionic gonadotropin receptor gene in human endometrial carcinomas. J Clin Endocrinol Metab. 1994;79:1483–1491. doi: 10.1210/jc.79.5.1483. [DOI] [PubMed] [Google Scholar]
- 16.Reshef E, Lei ZM, Rao CV, Pridham DD, Chegini N, Luborsky JL. The presence of gonadotropin receptors in nonpregnant human uterus, human placenta, fetal membranes, and decidua. J Clin Endocrinol Metab. 1990;70:421–430. doi: 10.1210/jcem-70-2-421. [DOI] [PubMed] [Google Scholar]
- 17.Davies S, Bax CM, Chatzaki E, Chard T, Iles RK. Regulation of endometrial cancer cell growth by luteinizing hormone (LH) and follicle stimulating hormone (FSH) Br J Cancer. 2000;83:1730–1734. doi: 10.1054/bjoc.2000.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pike MC, Peters RK, Cozen W, Probst-Hensch NM, Felix JC, Wan PC, Mack TM. Estrogen-progestin replacement therapy and endometrial cancer. J Natl Cancer Inst. 1997;89:1110–1116. doi: 10.1093/jnci/89.15.1110. [DOI] [PubMed] [Google Scholar]
- 19.Noci I, Pillozzi S, Lastraioli E, Dabizzi S, Giachi M, Borrani E, Wimalasena J, Taddei GL, Scarselli G, Arcangeli A. hLH/hCG-receptor expression correlates with in vitro invasiveness in human primary endometrial cancer. Gynecol Oncol. 2008;111:496–501. doi: 10.1016/j.ygyno.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Jankowska AG, Andrusiewicz M, Fischer N, Warchol PJ. Expression of hCG and GnRHs and their receptors in endometrial carcinoma and hyperplasia. Int J Gynecol Cancer. 2010;20:92–101. doi: 10.1111/IGC.0b013e3181bbe933. [DOI] [PubMed] [Google Scholar]
- 21.Noci I, Borri P, Bonfirraro G, Chieffi O, Arcangeli A, Cherubini A, Dabizzi S, Buccoliero AM, Paglierani M, Taddei GL. Longstanding survival without cancer progression in a patient affected by endometrial carcinoma treated primarily with leuprolide. Br J Cancer. 2001;85:333–336. doi: 10.1054/bjoc.2001.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krasnow JS, Berga SL, Guzick DS, Zeleznik AJ, Yeo KT. Vascular permeability factor and vascular endothelial growth factor in ovarian hyperstimulation syndrome: A preliminary report. Fertil Steril. 1996;65:552–555. doi: 10.1016/S0015-0282(16)58152-7. [DOI] [PubMed] [Google Scholar]
- 23.Neulen J, Yan Z, Raczek S, Weindel K, Keck C, Weich HA, Marmé D, Breckwoldt M. Human chorionic gonadotropin-dependent expression of vascular endothelial growth factor/vascular permeability factor in human granulosa cells: Importance in ovarian hyperstimulation syndrome. J Clin Endocrinol Metab. 1995;80:1967–1971. doi: 10.1210/jc.80.6.1967. [DOI] [PubMed] [Google Scholar]
- 24.Robertson D, Selleck K, Suikkari AM, Hurley V, Moohan J, Healy D. Urinary vascular endothelial growth factor concentrations in women undergoing gonadotrophin treatment. Hum Reprod. 1995;10:2478–2482. doi: 10.1093/oxfordjournals.humrep.a136327. [DOI] [PubMed] [Google Scholar]
- 25.Koistinen H, Hautala LC, Seppälä M, Stenman UH, Laakkonen P, Koistinen R. The role of glycodelin in cell differentiation and tumor growth. Scand J Clin Lab Invest. 2009;69:452–459. doi: 10.1080/00365510903056023. [DOI] [PubMed] [Google Scholar]
- 26.Ohta K, Maruyama T, Uchida H, Ono M, Nagashima T, Arase T, Kajitani T, Oda H, Morita M, Yoshimura Y. Glycodelin blocks progression to S phase and inhibits cell growth: A possible progesterone-induced regulator for endometrial epithelial cell growth. Mol Hum Reprod. 2008;14:17–22. doi: 10.1093/molehr/gam081. [DOI] [PubMed] [Google Scholar]
- 27.Shabani N, Kuhn C, Kunze S, Schulze S, Mayr D, Dian D, Gingelmaier A, Schindlbeck C, Willgeroth F, Sommer H, et al. Prognostic significance of estrogen receptor alpha (ERalpha) and beta (ERbeta), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. Eur J Cancer. 2007;43:2434–2444. doi: 10.1016/j.ejca.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. (In German) [PubMed] [Google Scholar]
- 29.Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML, Brenner RM, Giudice LC, Nayak NR. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008;22:3571–3580. doi: 10.1096/fj.08-111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Taylor A, Showeil R, Trivedi P, Horimoto Y, Bagwan I, Ewington L, Lam EW, El-Bahrawy MA. Expression profiling and significance of VEGF-A, VEGFR2, VEGFR3 and related proteins in endometrial carcinoma. Cytokine. 2014;68:94–100. doi: 10.1016/j.cyto.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 31.de Carvalho Fraga CA, Farias LC, de Oliveira MV, Domingos PL, Pereira CS, Silva TF, Roy A, Gomez RS, de Paula AM, Guimarães AL. Increased VEGFR2 and MMP9 protein levels are associated with epithelial dysplasia grading. Pathol Res Pract. 2014;210:959–964. doi: 10.1016/j.prp.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Kampmann E, Altendorf-Hofmann A, Gibis S, Lindner LH, Issels R, Kirchner T, Knösel T. VEGFR2 predicts decreased patients survival in soft tissue sarcomas. Pathol Res Pract. 2015;211:726–730. doi: 10.1016/j.prp.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Higgins KJ, Liu S, Abdelrahim M, Yoon K, Vanderlaag K, Porter W, Metz RP, Safe S. Vascular endothelial growth factor receptor-2 expression is induced by 17beta-estradiol in ZR-75 breast cancer cells by estrogen receptor alpha/Sp proteins. Endocrinology. 2006;147:3285–3295. doi: 10.1210/en.2006-0081. [DOI] [PubMed] [Google Scholar]
- 34.Higgins KJ, Liu S, Abdelrahim M, Vanderlaag K, Liu X, Porter W, Metz R, Safe S. Vascular endothelial growth factor receptor-2 expression is down-regulated by 17beta-estradiol in MCF-7 breast cancer cells by estrogen receptor alpha/Sp proteins. Mol Endocrinol. 2008;22:388–402. doi: 10.1210/me.2007-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gargett CE, Zaitseva M, Bucak K, Chu S, Fuller PJ, Rogers PA. 17Beta-estradiol up-regulates vascular endothelial growth factor receptor-2 expression in human myometrial microvascular endothelial cells: Role of estrogen receptor-alpha and- beta. J Clin Endocrinol Metab. 2002;87:4341–4349. doi: 10.1210/jc.2001-010588. [DOI] [PubMed] [Google Scholar]
- 36.Yuri T, Kinoshita Y, Emoto Y, Yoshizawa K, Tsubura A. Human chorionic gonadotropin suppresses human breast cancer cell growth directly via p53-mediated mitochondrial apoptotic pathway and indirectly via ovarian steroid secretion. Anticancer Res. 2014;34:1347–1354. [PubMed] [Google Scholar]
- 37.Brokken LJ, Lundberg-Giwercman Y, De-Meyts Rajpert E, Eberhard J, Ståhl O, Cohn-Cedermark G, Daugaard G, Arver S, Giwercman A. Association of polymorphisms in genes encoding hormone receptors ESR1, ESR2 and LHCGR with the risk and clinical features of testicular germ cell cancer. Mol Cell Endocrinol. 2012;351:279–285. doi: 10.1016/j.mce.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Kundu S, Pramanick K, Paul S, Bandyopadhyay A, Mukherjee D. Expression of LH receptor in nonpregnant mouse endometrium: LH induction of 3β-HSD and de novo synthesis of progesterone. J Endocrinol. 2012;215:151–165. doi: 10.1530/JOE-11-0486. [DOI] [PubMed] [Google Scholar]
- 39.Yung Y, Maman E, Ophir L, Rubinstein N, Barzilay E, Yerushalmi GM, Hourvitz A. Progesterone antagonist, RU486, represses LHCGR expression and LH/hCG signaling in cultured luteinized human mural granulosa cells. Gynecol Endocrinol. 2014;30:42–47. doi: 10.3109/09513590.2013.848426. [DOI] [PubMed] [Google Scholar]