Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by an excess of cardiovascular disease (CVD) risk, estimated to be at least 50% greater when compared to the general population. Although the widespread diffusion of type 2 diabetes mellitus (T2DM) awareness, there is still a significant proportion of patients with T2DM that remain undiagnosed. Aim of this cross-sectional study was to evaluate the prevalence of undiagnosed diabetes and prediabetes in RA patients.
For the present study, 100 consecutive nondiabetic RA patients were recruited. Age- and sex-matched subjects with noninflammatory diseases (osteoarthritis or fibromyalgia) were used as controls. After overnight fasting, blood samples were obtained for laboratory evaluation including serum glucose, total cholesterol, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, triglycerides, uric acid, erythrocyte sedimentation rate (ESR), high sensitivity C-reactive protein (hs-CRP), rheumatoid factor (RF), and anti-Cyclic Citrullinated Peptide Antibodies (ACPA). A standard Oral Glucose Tolerance Test (OGTT) with 75 g of glucose was performed and blood samples were collected at time 0, 30, 60, 90, and 120 minutes, for measurement of plasma glucose concentrations.
The prevalence of impaired fasting glucose (IFG) (9/100 vs 12/100, P = 0.49), impaired glucose tolerance (IGT) (19/100 vs 12/100, P = 0.17), and concomitant IFG/IGT (5/100 vs 9/100, P = 0.27) was similar between groups, whereas the prevalence of diabetes was significantly higher in RA patients (10/100 vs 2/100, P = 0.02). In a logistic regression analysis, increasing age (OR = 1.13, 95% CI 1.028–1.245, P = 0.01) and disease duration (OR = 1.90, 95% CI 1.210–2.995, P = 0.005) were both associated with an increased likelihood of being classified as prediabetes (i.e. IFG and/or IGT) or T2DM. A ROC curve was built to evaluate the predictivity of disease duration on the likelihood of being diagnosed with T2DM. The area under the ROC curve was 0.67 (95% CI: 0.56–0.78, P = 0.004). We identified the best cut-off of 33 months that yielded a sensitivity of 61% and a specificity of 70% for classification of T2DM patients.
According to our data, RA seems to be characterized by an elevated prevalence of undiagnosed diabetes, especially in patients with longer disease duration.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by an excess of cardiovascular disease (CVD) risk, estimated to be at least 50% greater when compared to the general population.1 Cardiovascular disease has been recognized as the main cause of mortality in established RA patients,2 but recent data confirm this trend also in earlier stages of the disease.3 Several factors have been evoked as determinants of this additional risk, but the most consolidated theory attributes this phenomenon to the interplay between chronic high-grade inflammation and elevated prevalence of “classical” cardiovascular risk factors, including diabetes.4,5 Although well-identified, cardiometabolic comorbidities are still under-recognized and under-treated in clinical practice, as underlined by recent researches such as the COMOrbidities in Rheumatoid Arthritis (COMORA) study.6 Recent evidences suggest that the risk of CVD (coronary, cerebral and peripheral arterial events) could be as high as that conferred by type 2 diabetes mellitus (T2DM).7 In a large nationwide Danish study, the risk of myocardial infarction in RA was similar in magnitude to that conferred by T2DM and corresponded to the risk reported for 10 years older non-RA subjects.8 Similar results were obtained in the CARdiovascular research and RhEumatoid arthritis (CARRE) cohort, a longitudinal 3-years study that confirmed an incidence of CVD in RA at least equal to that observed for T2DM.9 For all these reasons, in 2009 the European League Against Rheumatism (EULAR) published recommendations for the management of CVD risk in RA and other inflammatory arthritides.10 This set of recommendations emphasize the need for cardiovascular monitoring in RA and the inadequacy of classic tables for CVD risk stratification when applied to RA patients, especially those with longer disease duration (> 10 years).11
In recent years, several categories of individuals at increased risk for diabetes have been identified.2,12 Specifically, a stratification according to oral glucose tolerance test (OGTT) results has been proposed by American Diabetes Association (ADA) to identify subjects with “prediabetes.”13 According to ADA criteria, patients are classified as normotolerant (NGT) if fasting plasma glucose (FPG) is < 100 mg/dL and 2-h postload glucose is < 140; as impaired fasting glucose (IFG) if FPG 100 to 125 mg/dL and 2-h postload glucose < 140 mg/dL; impaired glucose tolerance (IGT) if FPG < 100 and 2-h postload glucose 140 to 199 mg/dL and diabetic if 2-h postload glucose ≥ 200 mg/dL. Patients classified as IFG or IGT, or with a combination of both defects (IFG/IGT), are considered “prediabetic.” Prediabetes is burdened by an accelerated progression to T2DM, with an incidence rate of respectively 47.4 per 1000 person-years for IFG 45.5 per 1000 person-years for IGT and 70.4 per 1000 person-years for combined IFG/IGT.14 In addition, prediabetes is independently associated with cardiovascular mortality.15
Notwithstanding the widespread diffusion of T2DM awareness, there is still a significant proportion of patients withT2DM that remains undiagnosed in the general population 16–18 and in selected categories of individuals at high CVD risk, for example those hospitalized for acute myocardial infarction 19 or for non-ST-segment elevation acute coronary syndrome.20 In these populations, the presence of undiagnosed diabetes seems to be characterized by a worst outcome 20,21 and a higher percentage of complications 22.
Despite the evidence that the coexistence of RA and T2DM could synergistically increase CVD risk,23 neither EULAR nor ADA guidelines 10,13 recommend systematic screening for diabetes in RA patients. Therefore, the aim of the present study was to evaluate the prevalence of undiagnosed diabetes and prediabetes in RA patients.
METHODS
Patients
The study protocol was approved by the local Ethics Committee (Ethics Committee, University Hospital “Mater Domini,” Catanzaro, Italy). Informed consent was obtained from all subjects involved. For the present cross-sectional study, 100 consecutive nondiabetic RA patients were prospectively recruited at Rheumatology Outpatient Clinic, Department of Medical and Surgical Sciences, University of Catanzaro, Catanzaro, Italy. All patients satisfied the 2010 ACR/EULAR classification criteria for RA.24 According to these criteria, the classification as “definite RA” is based on the confirmed presence of synovitis in at least 1 joint, absence of an alternative diagnosis that better explains the synovitis, and achievement of a total score of 6 or greater (of a possible 10) from the individual scores in 4 domains: number and site of involved joints (score range 0–5), serologic abnormality (score range 0–3), elevated acute-phase response (score range 0–1), and symptom duration (2 levels; range 0–1).24
For comparison 100 age- and sex-matched patients with noninflammatory diseases (osteoarthritis or fibromyalgia) were used. Only adult individuals, aged > 18 years were included in the study. RA patients and control subjects were excluded if one of the following criteria was present: past or current diagnosis of diabetes or prediabetes; past or current treatment with glucose lowering drugs; fasting plasma glucose repeatedly ≥ 126 mg/dL in the absence of a definite diagnosis of T2DM made by a physician. According to these criteria, 12 RA patients and 16 controls were excluded, respectively.
Anthropometric Measurements and Disease Activity Evaluation
Height and weight were measured with patients wearing light clothing and no shoes, to the nearest 0.1 cm and 0.1 kg respectively. Body mass index (BMI) was calculated with the standard formula:
BMI= Weight/Height2
Waist circumference (WC) was assessed with a flexible tape at midpoint between the lowest rib margin and the iliac crest. Systolic (sBP) and diastolic (dBP) blood pressure were measured on the left arm with a mercury sphygmomanometer, with the patient supine and after 5 minutes of rest. For RA assessment, the Disease Activity Score including 28 joints (DAS28-CRP) was used. DAS28-CRP is a combined index assessing the number of swollen joints (SJC), number of tender joints (TJC), patients’ global assessment of health measured on a visual analogic scale (GH-VAS, range 0–100 mm), and high sensitivity C-reactive protein plasma concentration (hs-CRP, mg/L).
Laboratory Evaluation
After overnight fasting, blood samples were obtained for laboratory evaluation. Plasma glucose, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, and uric acid were measured with automated chemistry analyzer (Cobas 6000/Cobas e411, Roche Diagnostics). The erythrocyte sedimentation rate (ESR) was analyzed by capillary photometry (Test 1, Alifax). Hs-CRP was measured by immunonephelometry (CardioPhase® hsCRP, Siemens HealthCare). Rheumatoid Factor (RF) was analyzed by nephelometry (BN II system, Siemens HealthCare). Anti-Cyclic Citrullinated Peptide Antibodies (ACPA) were analyzed with chemiluminescent immunoassay (Zenit RA CCP, Menarini Diagnostics).
OGTT Case Definition and Insulin Sensitivity
A standard OGTT was performed in all patients according to the recommendations of World Health Organization.25 Briefly, after overnight fasting, the patient was invited to drink a solution with 75 g of anhydrous glucose dissolved in 200 mL of water over a time of 5 minutes; blood samples were collected at time 0, 30, 60, 90, and 120 minutes, and plasma glucose concentrations were measured.
According to OGTT results patients were classified in subgroups of glucose tolerance; according to ADA patients were classified as normotolerant if FPG< 100 mg/dL and 2-h postload glucose < 140; IFG if FPG 100–125 mg/dL and 2-h postload glucose < 140 mg/dL; IGT if FPG < 100 and 2-h postload glucose 140–199 mg/dL and diabetic if 2-h postload glucose ≥ 200 mg/dL.
Statistical Analysis
A sample size of at least 73 patients was calculated with a confidence level of 95% and a precision of ± 5%. For this calculation, the population size was set at 6600 individuals, corresponding to the theoretical number of RA patients in our Region (Calabria, Italy, 2 million inhabitants), according to previously published prevalence of RA in Italy of 0.33%; the estimated prevalence of undiagnosed diabetes was set at 5%, slightly higher of that reported for undiagnosed diabetes in the general Italian population.16
Data are expressed as mean ± standard deviation (SD), median (25th–75th percentile), or number (percentage) as appropriate. Continuous variables that were not normally distributed were ln-transformed before analysis. Student's t test was used to compare means. Fisher's exact test was used to compare prevalences. The Pearson product-moment correlation coefficient was used to evaluate correlation between variables. A logistic regression analysis was used to evaluate the contribution of selected variables on the likelihood of being classified as prediabetic or diabetic. Receiver operating characteristic (ROC) curves were built to evaluate the predictivity of selected variables on the likelihood of being classified as diabetes.
A P-value <0.05 was considered statistically significant. All tests were 2-tailed. The Statistics Package for Social Sciences (SPSS for Windows, version 17.0, SPSS Inc., Chicago, IL) was used for all analyses.
RESULTS
Characteristics of the Study Population
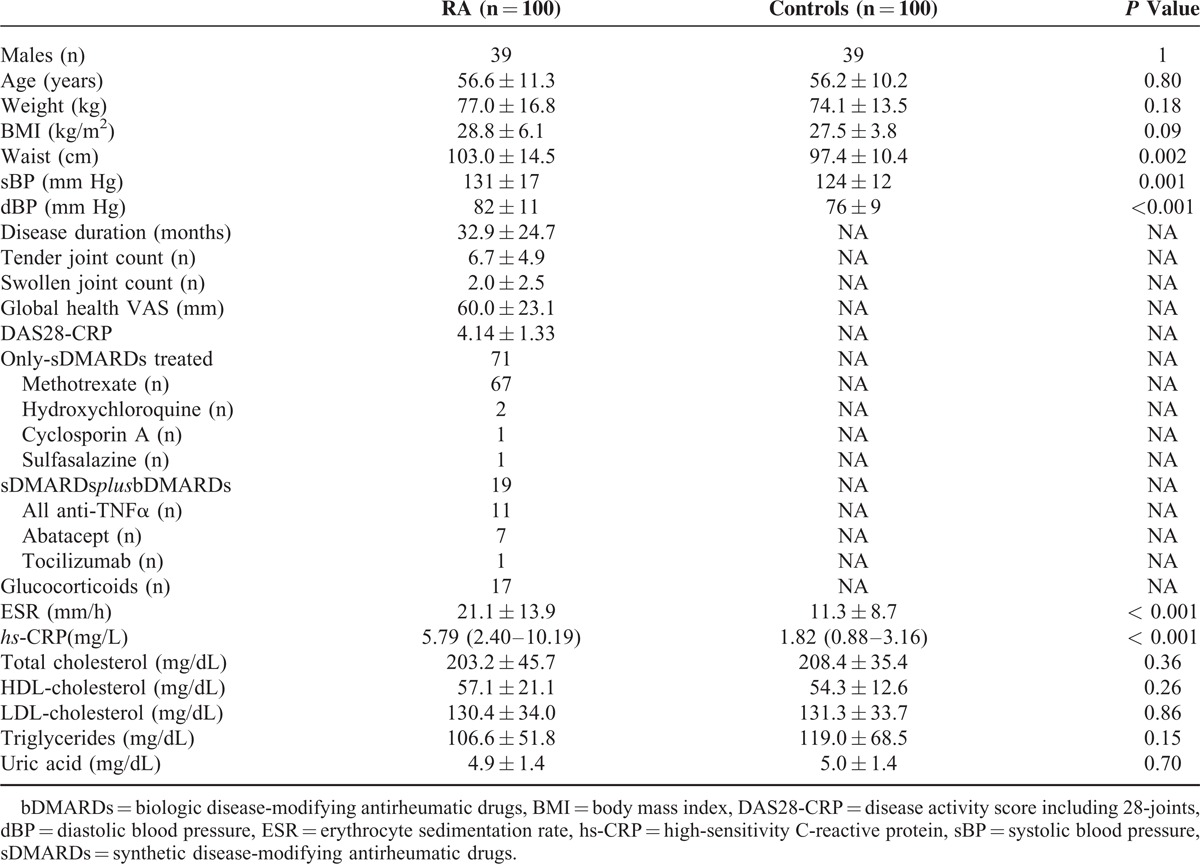
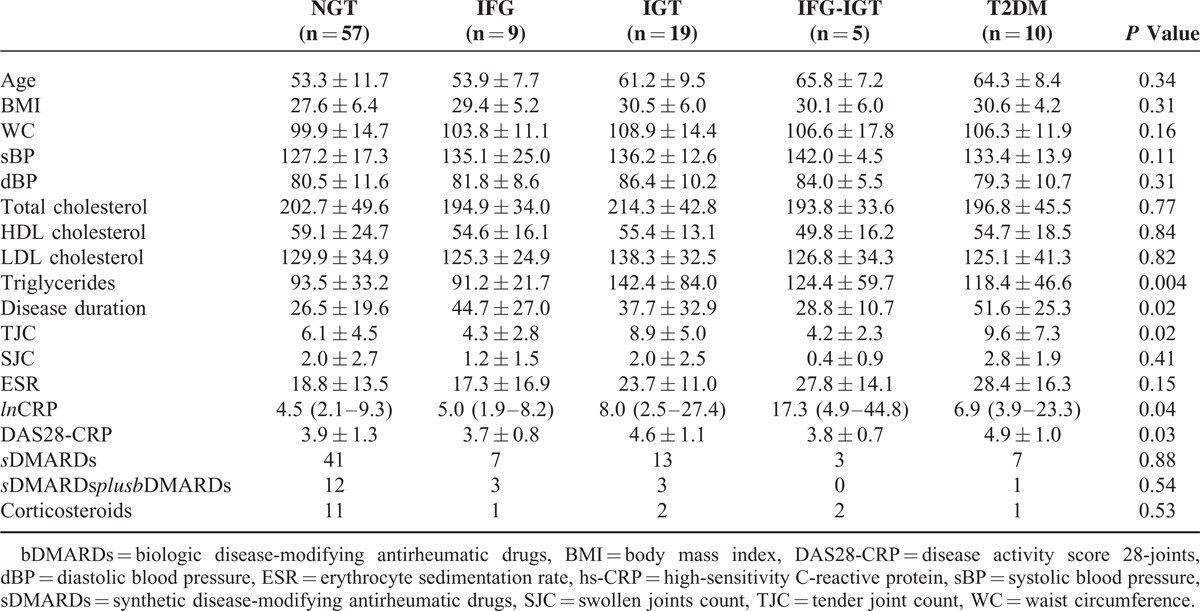
General characteristics of the study population are summarized in Table 1. In comparison to age- and sex-matched controls, RA patients had a significantly higher waist circumference (103.0 ± 14.5 cm vs 97.4 ± 10.4 cm, P = 0.002), systolic (131 ± 17 mm Hg vs 124 ± 12 mm Hg, P = 0.001) and diastolic (82 ± 11 mm Hg vs 76 ± 9 mm Hg, P = 0.001) blood pressure, ESR (21.1 ± 13.9 mm/h vs 11.3 ± 8,7 mm/h, P < 0.001), and hs-CRP (5.79[2.40–10.19] mg/L vs 1.82 [0.88–3.16] mg/L, P < 0.001) whereas no significant differences were observed for BMI, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, and uric acid.
TABLE 1.
Clinical Characteristics of the Study Population
Prevalence of Prediabetes and Diabetes
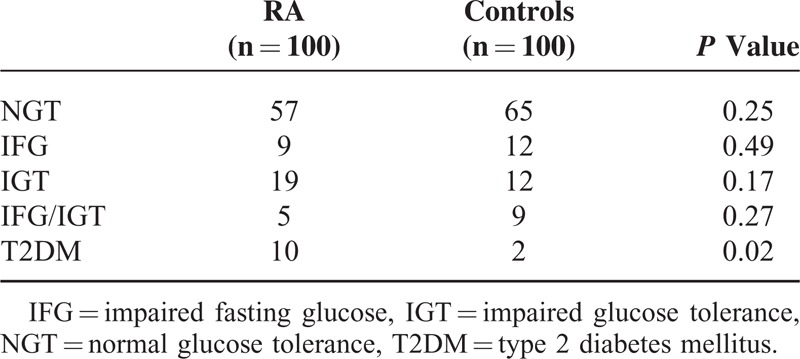
The prevalences of prediabetes and diabetes in RA patients and controls are reported in Table 2. The prevalence of IFG (9/100 vs 12/100, P = 0.49), IGT (19/100 vs 12/100, P = 0.17), and combined IFG/IGT (5/100 vs 9/100, P = 0.27) was similar between groups, whereas the prevalence of diabetes was significantly higher in RA patients (10/100 vs 2/100, P = 0.02).
TABLE 2.
Prevalence of Undiagnosed Prediabetes and Diabetes in Rheumatoid Arthritis Patients and Controls
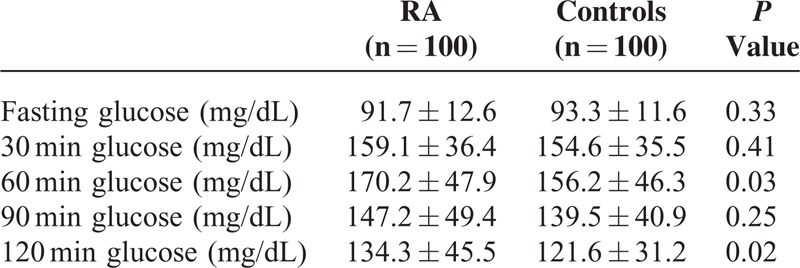
Accordingly, analysis of single time-point of plasma glucose during OGTT revealed a statistically significant difference between RA patients and controls in 60 minutes (170.2 ± 47.9 mg/dL vs 156.2 ± 46.3 mg/dL, P = 0.03) and 120 minutes (134.3 ± 45.5 mg/dL vs 121.6 ± 31.2 mg/dL, P = 0.02) afterload glucose (Table 3). However, 60 minutes plasma glucose is not considered for classification purposes in ADA criteria for diabetes and prediabetes.
TABLE 3.
Glucose Values During Oral Glucose Tolerance Test in Rheumatoid Arthritis Patients and Controls
Predictors of Prediabetes and Diabetes
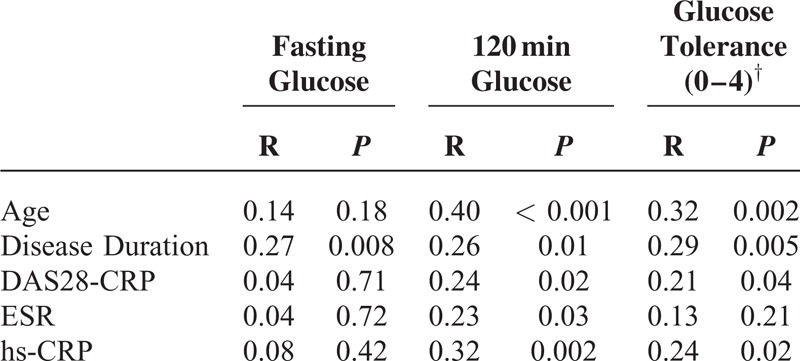
Partial correlational analysis of disease-related variables revealed a significant correlation of age (R = 0.40, P < 0.001), disease duration (R = 0.26, P = 0.01), DAS28-CRP (R = 0.24, P = 0.02), ESR (R = 0.23, P = 0.03), and hs-CRP (R = 0.32, P = 0.002) with 120 minutes plasma glucose. When glucose tolerance was set as the dependent variable, similar results were obtained except for ESR (Table 4). When fasting glucose was set as the dependent variable, only disease duration maintained its correlation (R = 0.27, P = 0.008).
TABLE 4.
Partial Correlation Analysis∗ of Disease-Related Variables Using Fasting Glucose, 120 Minutes Plasma Glucose and Glucose Tolerance as Dependent Variable
Univariate ANOVA was used to evaluate differences between glucose tolerance groups of RA patients (Table 5). The groups differed significantly for disease duration (P = 0.02), TJC (P = 0.02), hs-CRP (P = 0.04), DAS28-CRP (P = 0.03), and triglycerides (P = 0.004). No significant differences were detected in medication used between groups (Table 5).
TABLE 5.
Clinical and Laboratory Differences Between Different Groups of Glucose Tolerance
Based on the results reported above, a logistic regression model was constructed to ascertain the effects of selected variables (age, disease duration, waist circumference, hs-CRP, DAS28-CRP, and triglycerides) on the likelihood that patients have diabetes or any subtype of prediabetes. The logistic regression model was statistically significant (χ2 = 71.24, P < 0.001). The model explained 55.7% (Nagelkerke R2) of the variance in glucose metabolism classification. Increasing age and disease duration were both associated with an increased likelihood of being classified as prediabetes or T2DM. In particular, increase in 1 year of disease duration was associated with 1.90 fold higher risk of having T2DM (OR = 1.90, 95% CI 1.210–2.995, P = 0.005), whereas increase in 1 year of age was associated with a risk of 1.13 (OR = 1.13, 95% CI 1.028–1.245, P = 0.01).
Finally, we constructed an ROC curve to evaluate the predictivity of disease duration on the likelihood of being diagnosed with T2DM. The area under the ROC curve was 0.67 (95% CI: 0.56–0.78), P = 0.004). We identified the best cut-off of 33 months that yielded a sensitivity of 61% and a specificity of 70% for classification of T2DM patients.
DISCUSSION
In the present study, we reported for the first time an elevated prevalence of undiagnosed diabetes in RA patients compared to age- and sex-matched control individuals. In addition, we found significantly higher glucose values 60 and 120 minutes after the oral load. We failed however to find a significant difference in the prevalence of prediabetes in the study population. We reasoned that this apparently surprising finding could be caused by an accelerated progression of the metabolic disease in RA patients that may increase the probability to find subjects with metabolic parameters within the diabetic range rather than in the prediabetic range. On the other hand, sample size was calculated considering the outcome diabetes only; therefore the study could be underpowered to catch differences in the prevalence of prediabetes. Compared to previous studies, in which the diagnosis of T2DM was based on medical records or anamnestic data, our work has the advantage of being based on a systematic screening with OGTT, the gold-standard technique for diabetes diagnosis. In our opinion, the necessity to recognize early diabetes in RA patients is driven by the “classic” evidence that CVD risk factor tends to act in a synergistic way, thus increasing the risk conferred by a single risk factor. In support of this hypothesis, Baghdadi et al demonstrated that the risk of myocardial infarction was significantly higher in RA patients with comorbid T2DM compared to those with RA only 23. The main finding of our work is consistent with the more recent literature, showing a high prevalence of comorbid T2DM in RA patients. In particular, a recent meta-analysis of 11 case-control studies and 8 cohort studies confirmed an increased risk of T2DM in patients with RA.26 Conversely, T2DM patients, especially if females, have an elevated risk of developing RA.27 Despite this well-recognized epidemiologic relationship, the coexistence of RA and diabetes seems still to be characterized by a worse diabetes management and less frequent hemoglobin A1c testing,28 as well as by higher rates of underlying cardiovascular disease and diabetes-related complications (renal and vascular).28
The correlation between diabetes and RA, however, is a long-lasting story, hypothesized many years ago,2 and was attributed mainly to the diabetogenic effect of corticosteroid treatment, although recent evidences suggest a “neutral” effect.29 Nowadays 2 main theories have been proposed: on one hand, a privileged clustering of cardiovascular risk factors (such as obesity, alcohol consumption, or smoke) seems to characterize RA patients;30 on the other hand, the chronic high-grade inflammation has a well-established diabetogenic effect.31 Recent evidences suggest that, independently of corticosteroids and classic cardiovascular risk factor, inflammatory disease activity, per se, is a key mechanism of this increased risk. This is attributed to the interference of inflammatory cytokines, in particular TNF-α 32 and IL-6,32 on insulin signaling and consequent development of insulin resistance.33 Several studies demonstrated an elevated prevalence of insulin resistance in RA and a strict connection between visceral adiposity and inflammation.34,35
In our study, this theory is reflected once again by the evidence that both metabolic measures (such as BMI and waist circumference) and disease severity features (such as disease duration, DAS28 and hs-CRP) are significantly higher in RA patients with comorbid prediabetes or diabetes compared to NGT RA individuals and correlate with glucose tolerance classification and 2-hours postload glucose. Furthermore, postload glucose values at 60 and 120 minutes were significantly higher in RA patients compared to controls. Whereas glucose values in the first part of the curve reflect hepatic insulin sensitivity, the second part of the curve reflects more accurately peripheral insulin sensitivity, suggesting increased muscle insulin resistance in RA patients.36
In addition, controlling inflammation and disease activity with appropriate therapy seems to be beneficial in reducing insulin resistance and the risk of developing diabetes.31,37 In particular, hydroxycloroquine 37,38 and anti-TNF-alpha medications 37,39 have been demonstrated to improve insulin resistance and the risk of diabetes in inflammatory arthritis patients. In our study, probably because of a low statistical power, no significant differences were observed in treatment used between different groups of glucose tolerance.
In our study, the main disease-related feature, which correlated with the likelihood of being classified as diabetic, was disease duration. This finding seems to mirror the recent data regarding CVD risk in RA subjects that is estimated to be comparable to this of normal individuals 10 years older.8 Therefore, we can hypothesize that RA confers an “accelerated aging phenotype” to both the cardiovascular and metabolic profile of patients, as suggested by recent studies.40
Our study has a major limit, as the sample size is too small to allow us to firmly establish the true prevalence of prediabetes in RA patients. In addition, we found a significantly higher waist circumference in the RA group, but similar BMI in comparison with control individuals. This phenotype is consistent with published literature, in which lower muscle mass and increased visceral 41 and total body fat 42 were reported. A similar shift of body composition in the direction of increased visceral adiposity is independently associated with insulin resistance.43 In addition, we found higher levels of blood pressure in our patients. This is consistent with past literature on RA and it is associated with a lower percentage of individuals receiving a diagnosis of hypertension and thus appropriate treatment.44 Hypertension is associated with insulin resistance,45 and regardless of the cause that generated it (idiopathic, iatrogenic, or disease-related) could contribute to increase the risk of diabetes in RA patients.
In conclusion, our data, although limited by the relatively low number of patients and consequently the lack of subanalysis for confounding factors (such as sex, visceral adiposity, and hypertension), suggest an elevated prevalence of undiagnosed diabetes in RA patients and higher 1- and 2-hour postload glucose levels. Beside their role in the diagnosis of diabetes, these latest measures of glycemic burden are independently correlated with cardiovascular risk.46–48
If these data are confirmed on larger populations from different countries, subsequent studies will be necessary to evaluate the potential benefits on CVD outcomes of a systematic screening for T2DM in RA patients.
Footnotes
Abbreviations: ACPA = anti-Cyclic Citrullinated Peptide Antibodies, ACR = American college of rheumatology, ADA = American diabetes association, BMI = body mass index, CRP = C-reactive protein, CVD = cardiovascular disease, DAS28 = disease activity score including 28-joints, ESR = erythrocyte sedimentation rate, EULAR = European league against rheumatism, GH-VAS = global health visual analogue scale, IFG = impaired fasting glucose, IGT = impaired glucose tolerance, IL-6 = interleukin 6, NGT = normal glucose tolerance, OGTT = oral glucose tolerance test, RA = rheumatoid arthritis, RF = rheumatoid factor, ROC = receiver-operating characteristic, SJC = swollen joint count, T2DM = type 2 diabetes mellitus, TJC = tender joint count, TNF = tumor necrosis factor.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012; 71:1524–1529. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum 1994; 37:481–494. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys JH, Warner A, Chipping J, et al. Mortality trends in patients with early rheumatoid arthritis over 20 years: results from the Norfolk Arthritis Register. Arthritis Care Res 2014; 66:1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis 2011; 70:8–14. [DOI] [PubMed] [Google Scholar]
- 5.Choy E, Ganeshalingam K, Semb AG, et al. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology 2014; 53:2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 2014; 73:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE Investigation. Ann Rheum Dis 2009; 68:1395–1400. [DOI] [PubMed] [Google Scholar]
- 8.Lindhardsen J, Ahlehoff O, Gislason GH, et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 2011; 70:929–934. [DOI] [PubMed] [Google Scholar]
- 9.Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: a systematic review and meta-analysis. Clin Exp Rheumatol 2015; 33:115–121. [PubMed] [Google Scholar]
- 10.Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010; 69:325–331. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez A, Maradit Kremers H, Crowson CS, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum 2007; 56:3583–3587. [DOI] [PubMed] [Google Scholar]
- 12.Vermeer SE, Sandee W, Algra A, et al. Impaired glucose tolerance increases stroke risk in nondiabetic patients with transient ischemic attack or minor ischemic stroke. Stroke 2006; 37:1413–1417. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes A. Standards of medical care in diabetes—2014. Diabetes Care 2014; 37 suppl 1:S14–80. [DOI] [PubMed] [Google Scholar]
- 14.Morris DH, Khunti K, Achana F, et al. Progression rates from HbA1c 6.0–6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia 2013; 56:1489–1493. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Cai X, Chen P, et al. Associations of prediabetes with all-cause and cardiovascular mortality: a meta-analysis. Ann Med 2014; 46:684–692. [DOI] [PubMed] [Google Scholar]
- 16.Garancini MP, Calori G, Ruotolo G, et al. Prevalence of NIDDM and impaired glucose tolerance in Italy: an OGTT-based population study. Diabetologia 1995; 38:306–313. [DOI] [PubMed] [Google Scholar]
- 17.Muntoni S, Atzori L, Mereu R, et al. Prevalence of diagnosed and undiagnosed diabetes mellitus and impaired fasting glucose in Sardinia. Acta Diabetologica 2009; 46:227–231. [DOI] [PubMed] [Google Scholar]
- 18.Holt TA, Gunnarsson CL, Cload PA, et al. Identification of undiagnosed diabetes and quality of diabetes care in the United States: cross-sectional study of 11.5 million primary care electronic records. CMAJ Open 2014; 2:E248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meloni L, Montisci R, Sau L, et al. Admission hyperglycemia in acute myocardial infarction: possible role in unveiling patients with previously undiagnosed diabetes mellitus. J Cardiovasc Med 2013; 14:821–826. [DOI] [PubMed] [Google Scholar]
- 20.Giraldez RR, Clare RM, Lopes RD, et al. Prevalence and clinical outcomes of undiagnosed diabetes mellitus and prediabetes among patients with high-risk non-ST-segment elevation acute coronary syndrome. Am Heart J 2013; 165:918–925.e912. [DOI] [PubMed] [Google Scholar]
- 21.Flores-Le Roux JA, Comin J, Pedro-Botet J, et al. Seven-year mortality in heart failure patients with undiagnosed diabetes: an observational study. Cardiovasc Diabetol 2011; 10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Zhou C, Yu J, et al. Reduced kidney function in acute coronary syndrome patients with undiagnosed diabetes or pre-diabetes. Nephrology 2013; 18:263–268. [DOI] [PubMed] [Google Scholar]
- 23.Baghdadi LR, Woodman RJ, Shanahan EM, et al. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One 2015; 10:e0117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569–2581. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva, Switzerland:2006. [Google Scholar]
- 26.Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: a systematic review and meta-analysis. Clin Exp Rheumatol 2014. [PubMed] [Google Scholar]
- 27.Lu MC, Yan ST, Yin WY, et al. Risk of rheumatoid arthritis in patients with type 2 diabetes: a nationwide population-based case-control study. PloS One 2014; 9:e101528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartels CM, Saucier JM, Thorpe CT, et al. Monitoring diabetes in patients with and without rheumatoid arthritis: a Medicare study. Arthritis Res Ther 2012; 14:R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.den Uyl D, van Raalte DH, Nurmohamed MT, et al. Metabolic effects of high-dose prednisolone treatment in early rheumatoid arthritis: balance between diabetogenic effects and inflammation reduction. Arthritis Rheum 2012; 64:639–646. [DOI] [PubMed] [Google Scholar]
- 30.Dubreuil M, Rho YH, Man A, et al. Diabetes incidence in psoriatic arthritis, psoriasis and rheumatoid arthritis: a UK population-based cohort study. Rheumatology 2014; 53:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasko MC, Kay J, Hsia EC, et al. Diabetes mellitus and insulin resistance in patients with rheumatoid arthritis: risk reduction in a chronic inflammatory disease. Arthritis Care Res 2011; 63:512–521. [DOI] [PubMed] [Google Scholar]
- 32.Nieto-Vazquez I, Fernandez-Veledo S, Kramer DK, et al. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem 2008; 114:183–194. [DOI] [PubMed] [Google Scholar]
- 33.Ursini F, Naty S, Grembiale RD. Infliximab and insulin resistance. Autoimmun Rev 2010; 9:536–539. [DOI] [PubMed] [Google Scholar]
- 34.Kang Y, Park HJ, Kang MI, et al. Adipokines, inflammation, insulin resistance, and carotid atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther 2013; 15:R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straburzynska-Lupa A, Nowak A, Pilaczynska-Szczesniak L, et al. Visfatin, resistin, hsCRP and insulin resistance in relation to abdominal obesity in women with rheumatoid arthritis. Clin Exp Rheumatol 2010; 28:19–24. [PubMed] [Google Scholar]
- 36.Tschritter O, Fritsche A, Shirkavand F, et al. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003; 26:1026–1033. [DOI] [PubMed] [Google Scholar]
- 37.Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA 2011; 305:2525–2531. [DOI] [PubMed] [Google Scholar]
- 38.Solomon DH, Garg R, Lu B, et al. Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: a randomized, blinded crossover trial. Arthritis Care Res 2014; 66:1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antohe JL, Bili A, Sartorius JA, et al. Diabetes mellitus risk in rheumatoid arthritis: reduced incidence with anti-tumor necrosis factor alpha therapy. Arthritis Care Res 2012; 64:215–221. [DOI] [PubMed] [Google Scholar]
- 40.Crowson CS, Therneau TM, Davis JM, et al. Brief report: accelerated aging influences cardiovascular disease risk in rheumatoid arthritis. Arthritis Rheum 2013; 65:2562–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giles JT, Allison M, Blumenthal RS, et al. Abdominal adiposity in rheumatoid arthritis: association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum 2010; 62:3173–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giles JT, Ling SM, Ferrucci L, et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum 2008; 59:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita S, Nakamura T, Shimomura I, et al. Insulin resistance and body fat distribution. Diabetes Care 1996; 19:287–291. [DOI] [PubMed] [Google Scholar]
- 44.Bartels CM, Johnson H, Voelker K, et al. Impact of rheumatoid arthritis on receiving a diagnosis of hypertension among patients with regular primary care. Arthritis Care Res 2014; 66:1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. N Engl J Med 1987; 317:350–357. [DOI] [PubMed] [Google Scholar]
- 46.Lind M, Tuomilehto J, Uusitupa M, et al. The association between HbA1c, fasting glucose, 1-hour glucose and 2-hour glucose during an oral glucose tolerance test and cardiovascular disease in individuals with elevated risk for diabetes. PLoS One 2014; 9:e109506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdul-Ghani MA, Abdul-Ghani T, Ali N, et al. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008; 31:1650–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanat M, Norton L, Winnier D, et al. Impaired early- but not late-phase insulin secretion in subjects with impaired fasting glucose. Acta Diabetologica 2011; 48:209–217. [DOI] [PubMed] [Google Scholar]


