Abstract
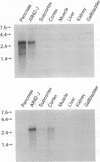
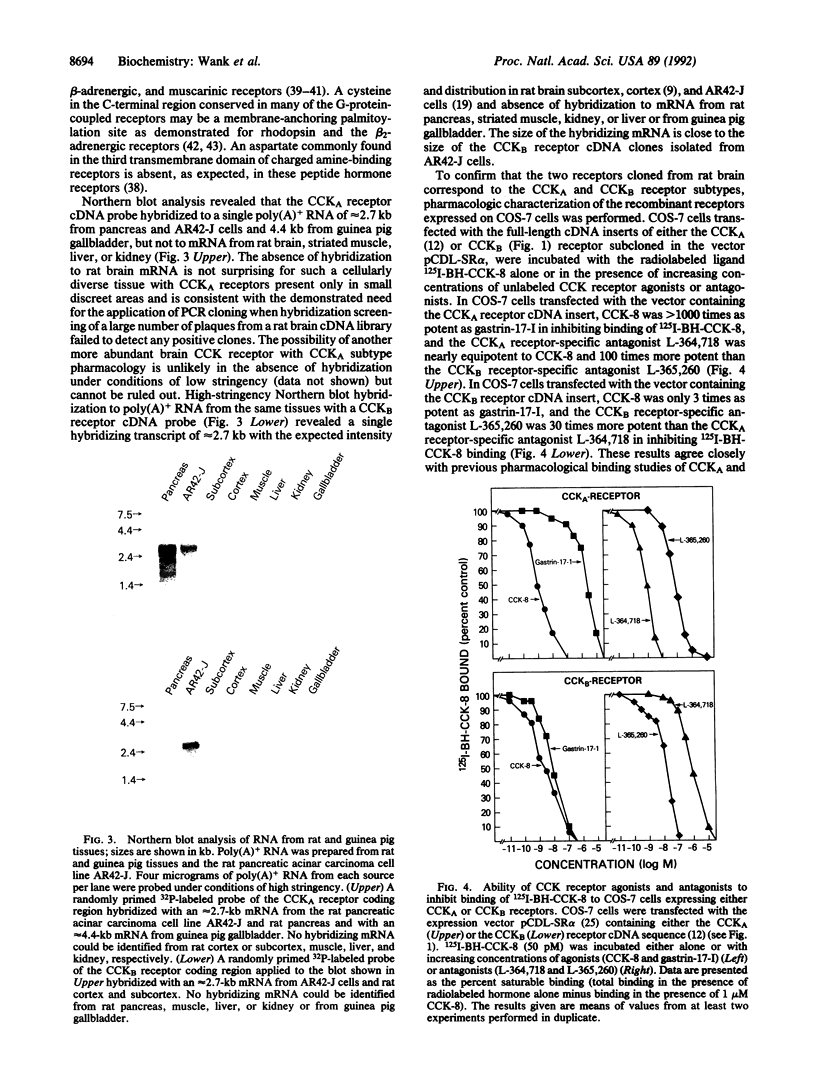
Cholecystokinin was one of the first gastrointestinal peptides discovered in the mammalian brain. In the central nervous system there is evidence for CCKA and CCKB receptor subtypes. The CCKA receptors occur in a few localized areas of the central and peripheral nervous systems where they modulate feeding and dopamine-induced behavior. CCKB receptors occur throughout the central nervous system where they modulate anxiety, analgesia, arousal, and neuroleptic activity. We have recently purified and cloned a CCKA receptor cDNA from rat pancreas that allowed isolation of an identical cDNA from rat brain by using the polymerase chain reaction. Using low-stringency hybridization screening of cDNA libraries from rat brain and AR42-J cells, which possess large numbers of CCKB receptors, we identified previously unreported cDNAs, the sequence of which were identical in both tissues. The cDNA sequence encodes a 452-amino acid protein that is 48% identical to the CCKA receptor and contains seven transmembrane domains characteristics of guanine nucleotide-binding regulatory protein-coupled receptors. COS-7 cells transfected with this cDNA expressed binding sites for agonists and antagonists characteristic of a CCKB receptor subtype. We conclude that this cDNA isolated from rat brain and AR42-J cells is a receptor of the CCKB subtype and that the respective cDNAs for both CCKA and CCKB are identical in the brain and gastrointestinal system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battey J. F., Way J. M., Corjay M. H., Shapira H., Kusano K., Harkins R., Wu J. M., Slattery T., Mann E., Feldman R. I. Molecular cloning of the bombesin/gastrin-releasing peptide receptor from Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):395–399. doi: 10.1073/pnas.88.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar K. N., Makhlouf G. M. Receptors on smooth muscle cells: characterization by contraction and specific antagonists. Am J Physiol. 1982 Apr;242(4):G400–G407. doi: 10.1152/ajpgi.1982.242.4.G400. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J. N. Cholecystokinin-dopamine interactions. Trends Pharmacol Sci. 1991 Jun;12(6):232–236. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Candelore M. R., Register R. B., Scattergood W., Rands E., Strader C. D. Structural features required for ligand binding to the beta-adrenergic receptor. EMBO J. 1987 Nov;6(11):3269–3275. doi: 10.1002/j.1460-2075.1987.tb02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Dourish C. T., O'Neill M. F., Coughlan J., Kitchener S. J., Hawley D., Iversen S. D. The selective CCK-B receptor antagonist L-365,260 enhances morphine analgesia and prevents morphine tolerance in the rat. Eur J Pharmacol. 1990 Jan 25;176(1):35–44. doi: 10.1016/0014-2999(90)90129-t. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fourmy D., Zahidi A., Fabre R., Guidet M., Pradayrol L., Ribet A. Receptors for cholecystokinin and gastrin peptides display specific binding properties and are structurally different in guinea-pig and dog pancreas. Eur J Biochem. 1987 Jun 15;165(3):683–692. doi: 10.1111/j.1432-1033.1987.tb11495.x. [DOI] [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Campbell N. J., Shaw T. M., Woodruff G. N. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci. 1987 Sep;7(9):2967–2976. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. R., Shaw T. M., Graham W., Woodruff G. N. Autoradiographical detection of cholecystokinin-A receptors in primate brain using 125I-Bolton Hunter CCK-8 and 3H-MK-329. J Neurosci. 1990 Apr;10(4):1070–1081. doi: 10.1523/JNEUROSCI.10-04-01070.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Snyder S. H. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorpes E., Mutt V. Cholecystokinin and pancreozymin, one single hormone? Acta Physiol Scand. 1966 Jan-Feb;66(1):196–202. doi: 10.1111/j.1748-1716.1966.tb03185.x. [DOI] [PubMed] [Google Scholar]
- Karnik S. S., Sakmar T. P., Chen H. B., Khorana H. G. Cysteine residues 110 and 187 are essential for the formation of correct structure in bovine rhodopsin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8459–8463. doi: 10.1073/pnas.85.22.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A., Nishiyama K., Nakanishi H., Uratsuji Y., Nomura H., Takeyama Y., Nishizuka Y. Studies on the phosphorylation of myelin basic protein by protein kinase C and adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1985 Oct 15;260(23):12492–12499. [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lambert M., Diem Bui N., Christophe J. Functional and molecular characterization of CCK receptors in the rat pancreatic acinar cell line AR 4-2J. Regul Pept. 1991 Feb 1;32(2):151–167. doi: 10.1016/0167-0115(91)90043-g. [DOI] [PubMed] [Google Scholar]
- Lignon M. F., Bernad N., Martinez J. Pharmacological characterization of type B cholecystokinin binding sites on the human JURKAT T lymphocyte cell line. Mol Pharmacol. 1991 May;39(5):615–620. [PubMed] [Google Scholar]
- Lotti V. J., Chang R. S. A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365,260. Eur J Pharmacol. 1989 Mar 21;162(2):273–280. doi: 10.1016/0014-2999(89)90290-2. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran T. H., Robinson P. H., Goldrich M. S., McHugh P. R. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986 Jan 1;362(1):175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989 May 5;264(13):7564–7569. [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Probst W. C., Snyder L. A., Schuster D. I., Brosius J., Sealfon S. C. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992 Jan-Feb;11(1):1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Hansen H. F., Marley P. D., Stengaard-Pedersen K. Molecular forms of cholecystokinin in the brain and the relationship to neuronal gastrins. Ann N Y Acad Sci. 1985;448:11–23. doi: 10.1111/j.1749-6632.1985.tb29902.x. [DOI] [PubMed] [Google Scholar]
- Sacerdote P., Wiedermann C. J., Wahl L. M., Pert C. B., Ruff M. R. Visualization of cholecystokinin receptors on a subset of human monocytes and in rat spleen. Peptides. 1991 Jan-Feb;12(1):167–176. doi: 10.1016/0196-9781(91)90184-q. [DOI] [PubMed] [Google Scholar]
- Saito A., Sankaran H., Goldfine I. D., Williams J. A. Cholecystokinin receptors in the brain: characterization and distribution. Science. 1980 Jun 6;208(4448):1155–1156. doi: 10.1126/science.6246582. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y., Nakanishi S. Molecular characterization of rat substance K receptor and its mRNAs. Biochem Biophys Res Commun. 1989 Dec 15;165(2):695–702. doi: 10.1016/s0006-291x(89)80022-1. [DOI] [PubMed] [Google Scholar]
- Shigemoto R., Yokota Y., Tsuchida K., Nakanishi S. Cloning and expression of a rat neuromedin K receptor cDNA. J Biol Chem. 1990 Jan 15;265(2):623–628. [PubMed] [Google Scholar]
- Singh P., Rae-Venter B., Townsend C. M., Jr, Khalil T., Thompson J. C. Gastrin receptors in normal and malignant gastrointestinal mucosa: age-associated changes. Am J Physiol. 1985 Dec;249(6 Pt 1):G761–G769. doi: 10.1152/ajpgi.1985.249.6.G761. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]
- Wada E., Way J., Shapira H., Kusano K., Lebacq-Verheyden A. M., Coy D., Jensen R., Battery J. cDNA cloning, characterization, and brain region-specific expression of a neuromedin-B-preferring bombesin receptor. Neuron. 1991 Mar;6(3):421–430. doi: 10.1016/0896-6273(91)90250-4. [DOI] [PubMed] [Google Scholar]
- Wank S. A., Harkins R., Jensen R. T., Shapira H., de Weerth A., Slattery T. Purification, molecular cloning, and functional expression of the cholecystokinin receptor from rat pancreas. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3125–3129. doi: 10.1073/pnas.89.7.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder D. G., Moody T. W. High affinity binding of cholecystokinin to small cell lung cancer cells. Peptides. 1987 Jan-Feb;8(1):103–107. doi: 10.1016/0196-9781(87)90171-9. [DOI] [PubMed] [Google Scholar]
- Yokota Y., Sasai Y., Tanaka K., Fujiwara T., Tsuchida K., Shigemoto R., Kakizuka A., Ohkubo H., Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989 Oct 25;264(30):17649–17652. [PubMed] [Google Scholar]
- Yu D. H., Noguchi M., Zhou Z. C., Villanueva M. L., Gardner J. D., Jensen R. T. Characterization of gastrin receptors on guinea pig pancreatic acini. Am J Physiol. 1987 Dec;253(6 Pt 1):G793–G801. doi: 10.1152/ajpgi.1987.253.6.G793. [DOI] [PubMed] [Google Scholar]