Supplemental Digital Content is available in the text
Abstract
Use of Chinese herbal medicines (CHM) in symptom management for cancer palliative care is very common in Chinese populations but clinical evidence on their effectiveness is yet to be synthesized.
To conduct a systematic review with meta-analysis to summarize results from CHM randomized controlled trials (RCTs) focusing on symptoms that are undertreated in conventional cancer palliative care.
Five international and 3 Chinese databases were searched. RCTs evaluating CHM, either in combination with conventional treatments or used alone, in managing cancer-related symptoms were considered eligible. Effectiveness was quantified by using weighted mean difference (WMD) using random effect model meta-analysis.
Fourteen RCTs were included. Compared with conventional intervention alone, meta-analysis showed that combined CHM and conventional treatment significantly reduced pain (3 studies, pooled WMD: −0.90, 95% CI: −1.69 to −0.11). Six trials comparing CHM with conventional medications demonstrated similar effect in reducing constipation. One RCT showed significant positive effect of CHM plus chemotherapy for managing fatigue, but not in the remaining 3 RCTs. The additional use of CHM to chemotherapy does not improve anorexia when compared to chemotherapy alone, but the result was concluded from 2 small trials only. Adverse events were infrequent and mild.
CHM may be considered as an add-on to conventional care in the management of pain in cancer patients. CHM could also be considered as an alternative to conventional care for reducing constipation. Evidence on the use of CHM for treating anorexia and fatigue in cancer patients is uncertain, warranting further research.
INTRODUCTION
Cancer has been considered as a global public health issue.1 With continuing improvement in cancer treatment, more individuals diagnosed with cancer are surviving with the disease, indicating that a large number of patients will live with cancer and cancer treatment-related symptoms.2,3 Symptoms that are frequently experienced by cancer patients include fatigue, paresthesias and dysesthesias, chronic pain, anorexia, insomnia, limbs edema, and constipation.4 Studies have found that among cancer patients, the prevalence was 60% to 90% for fatigue,5 around 66% for paresthesias and dysesthesias,6 50% to 70% for chronic pain,7 around 85% for anorexia,8 30% to 50% for insomnia,4 31% for limbs edema,6 and 30% to 80% for constipation.9 Quality of life among cancer patients are affected when they experience 1 or more of these symptoms.3
Despite the high prevalence of these symptoms reported in cancer patients, treatments from conventional medicine are far from satisfactory. Currently, treatment options for managing fatigue are very limited. Within these few choices, adverse effects have further restricted their clinical use,10 leaving this symptom widely under-treated.10 For paresthesias and dysesthesias, although co-analgesics and antidepressants are available for controlling these symptoms, their effectiveness is not satisfactory. A substantial number of patients are not sufficiently relieved, with 10% to 15% of patients being refractory to pharmacotherapy.11,12 For the management of cancer-related pain, the World Health Organization analgesics ladder (non-opioids, adjuvants, and opioids analgesics) provides a stepwise relief approach.13 However, about 40% would continue to have poorly controlled pain despite the treatment.14 Progestational agents and corticosteroids may be effective for anorexia, but both of them cause considerable adverse effects without improving survival.15,16,17 For insomnia, although benzodiazepines and nonbenzodiazepine hypnotics are often prescribed, evidence on their effectiveness among cancer patients is lacking.18 Other study has suggested that the use of sleeping pills may worsen symptoms severity and quality of life among cancer patients.18 Finally, evidence on the effect of antidepressants on improving sleep quality is mixed for cancer patients.4
In view of these evidence gaps in conventional medicine, the role of Chinese herbal medicine (CHM) in symptom management can be explored. There are systematic reviews (SRs) demonstrating the effectiveness of CHM as an adjuvant treatment for improving quality of life,19 increasing survival rate,20 and reducing chemotherapy-induced toxicity21 among cancer patients. Another SR indicated mixed results for reducing pain.22 However, there are several shortcomings with regard to existing SRs. For instance, 1 SR did not reporting details on treatment prescription used in control groups as well as baseline treatment, limiting the usefulness of evidence reported.22 Another SR21 did not report the herbal compositions prescribed in the included trials. Although results from this SR indicated that the adjuvant use of CHM significantly reduced chemotherapy-induced toxicity,21 clinical usefulness of such evidence is restricted by poor reporting.
More importantly, there are no existing SRs synthesizing evidence on the effectiveness of CHM for managing common cancer symptoms of fatigue, paresthesias and dysesthesias, chronic pain, anorexia, insomnia, limbs edema, and constipation. In view of this research gap, this SR aims to summarize results from CHM randomized controlled trials (RCTs) focusing on these outcomes.
METHODS
This systematic review and meta-analysis was strictly reported according to the PRISMA checklist. Ethical approval was not necessary for this study because all the analyses were conducted based on the data retrieved from published trials.
Inclusion Criteria
We included studies according to the following criteria:
RCTs comparing effect of CHM, either in combination with other treatments or used alone, in managing cancer or cancer treatment-related symptoms. There is no restriction of the type of cancer diagnosis.
The RCT has to report the effectiveness of CHM on at least 1 of the following outcomes measured with validated instruments: fatigue, paresthesias and dysesthesias, chronic pain, anorexia, insomnia, limbs edema, and constipation. For measurement of pain, 3 validated scales (Visual Analogue Scales, Numerical Rating Scales, and Verbal Rating Scales) recommended by the Research Network of the European Association of Palliative Care23 should be used.
The RCT included at least 1 CHM indexed in the 2010 China Pharmacopeia Chinese herbal medicine index.24 We did not impose any restriction on the forms of CHM, with single herbs, herbal formulations, and Chinese proprietary medicines included.
Control group included conventional treatment, chemotherapy, radiotherapy, placebo, or no treatment.
RCT reported detailed information on the regimens prescribed in both treatment and control groups. Follow-up duration should also be clearly reported where applicable.
Literature Search
Five international databases and 3 Chinese databases were searched without any language restriction. International databases included the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL Plus, the Allied and Complementary Medicine Database (AMED). When searching MEDLINE25 and EMBASE,26 specialized search filter for clinical trials were used. Chinese databases include Chinese Biomedical Databases, Wan Fang Digital Journals, and Taiwan Periodical Literature Databases. Detailed search strategies and related results were shown in Appendix 1.
Literature Selection, Data Extraction, and Risk of Bias Assessment
Eligibility of the retrieved studies were screened and assessed according to inclusion criteria. The following data were extracted from included RCTs: 1) Basic characteristics of the RCT, name of first author, year of publication, country where the trial was conducted, eligibility criteria for participants, diagnostic criteria of 2) Information related to patients’ characteristics, CHM, control interventions, and outcomes. 3) Effect size for each interested outcome and adverse effects related to CHM treatment.
The Cochrane risk of bias tool27 was used to assess risk of bias of included RCTs. Risk of bias in 6 domains were assessed for each included RCT, including sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, and selective reporting. Based on the information provided by the trial authors, each domain was judged to have low, high, or unclear risk of bias.
Two reviewers independently selected the literature (JZ, MF), extracted the data, and assessed the risk of bias of included studies (XW, PL), with disagreement resolved by discussion and consensus. A third reviewer (VC) was consulted if disagreement cannot be resolved between the two reviewers.
Data Analysis
Relative risk (RR) and 95% confidence interval (CI) was used to express effectiveness of CHM when the outcome was dichotomous. Weighted mean difference and 95% CI was used when the outcome was continuous. Level of heterogeneity across trials was measured with I2 statistic, with I2 < 25% considered as low level of heterogeneity, 25% to 50% as moderate level, and higher than 50% as high level.28 Random effects model was used to account for variations across trials29 during data synthesis.
Publication bias would be assessed using funnel plot if more than 10 trials were available for a single outcome.30 The symmetry of the funnel plot would be assessed with Egger test, with a P < 0.1 indicating presence of publication bias. Data analyses were conducted with STATA Version 13.0 (STATA Corporation, College Station, TX), with a 2-tailed significance level of 0.05 except for test for publication bias (P = 0.10). The protocol of this SR has been registered in PROSPERO (http://www.crd.york.ac.uk/PROSPERO/DisplayPDF.php?ID=CRD42015023931).
RESULTS
Study Selection
Electronic databases search yielded 4767 records, of which 797 duplications were excluded, and 3970 records remained for citation screening. Three thousand seven hundred eighteen citations were excluded after the screening of titles and abstracts, and 252 full texts were retrieved for eligibility assessment. Among them, 238 publications were excluded because of the following reasons: CHM was used in the control group (n = 26); did not report prespecified outcomes (n = 34); did not use a validated instrument for outcome assessment (n = 111); no control group in the study (n = 25); did not include cancer patients (n = 3); did not evaluate CHM (n = 24); time points for outcome measurement were unspecified (n = 12); did not report details on the control interventions (n = 1), not an RCT (n = 1), and did not report number of patients in each group (n = 1). As a result, 14 RCTs were included in this SR. Detailed flow chart for literature selection can be found in Figure 1.
FIGURE 1.
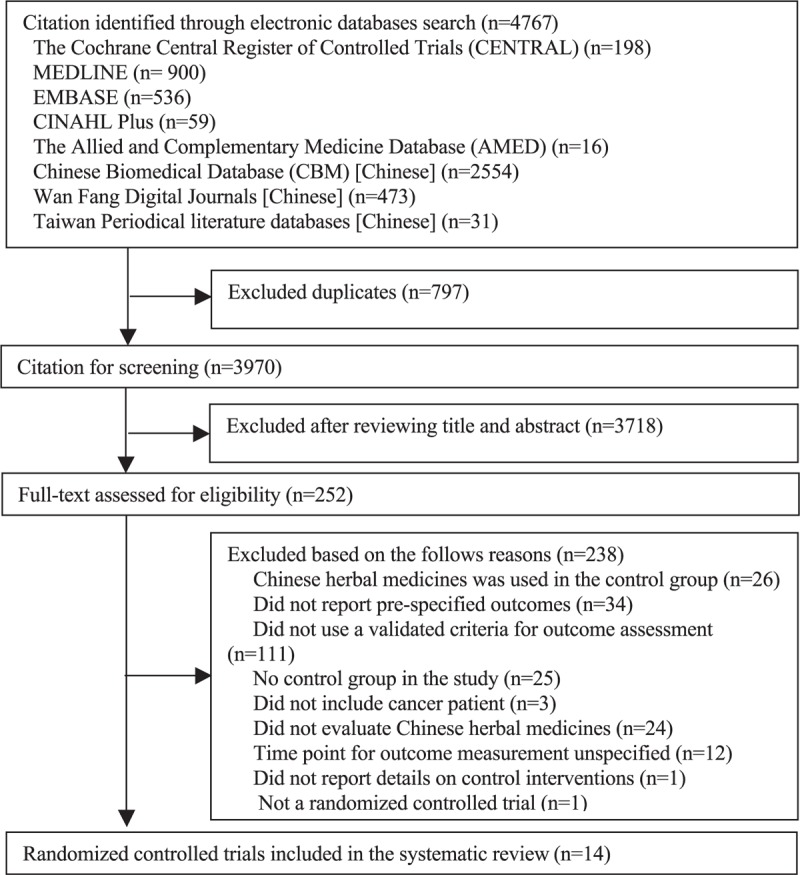
Flow chart for literature selection of randomized controlled trial on Chinese herbal medicine for symptoms management in cancer palliative care.
Characteristics of Included Studies
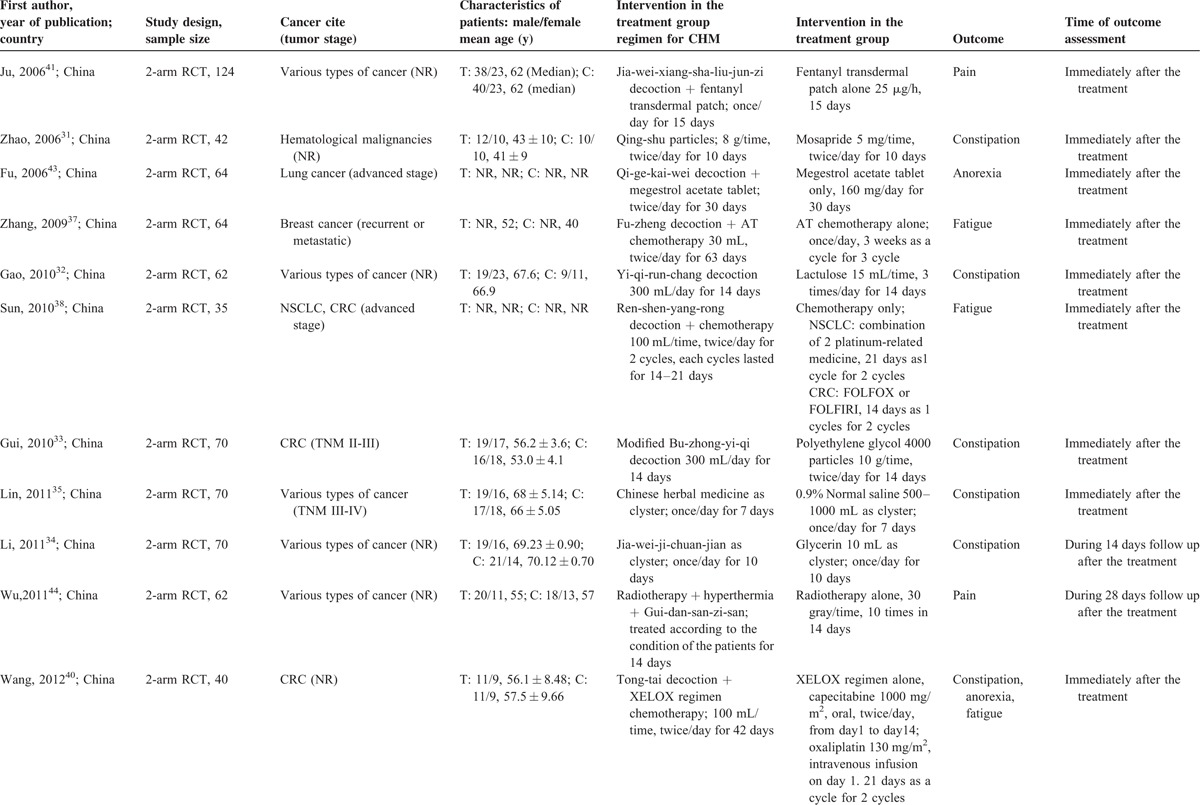
The 14 RCTs were published between 2006 and 2013, and all were conducted in mainland China. Majority of the included RCTs did not have any restrictions on the site of tumors (n = 8). Three only included colorectal cancer patients, the remaining 3 focused on patients with hematological malignancies, lung cancer, and breast cancer respectively. Forms of CHM included oral medications, external applications, and clyster. Details of each CHM prescription were shown in Appendix 2.
Six RCTs31–36 assessed the effectiveness of CHM for constipation by directly comparing it with conventional medicine; 7 RCTs investigated the add-on effects of CHM on top of conventional medicine or chemotherapy for managing fatigue,37–40 pain,41,42 anorexia,40,43 and constipation40 in cancer patients; the remaining RCT44 compared the combination of Gui-dan-san-zi-san, hyperthermia and radiotherapy with radiotherapy alone in reducing pain among cancer patients. Treatment durations range from 7 to 60 days, with the majority of them assessed outcome immediately after completion of CHM treatment, with only 2 RCTs35,44 followed up the patients after the treatment ended. Details were seen in Table 1 .
TABLE 1.
Basic Characteristics of Included Randomized Controlled Trials on Chinese Herbal Medicine for Symptoms Management in Cancer Palliative Care
Risk of Bias of Included RCTs
All the included RCTs were published in Chinese and provided limited information regarding risk of bias. Although all RCTs stated that patients were randomly allocated to treatment and control groups, only 4 of them described how the random sequences were generated and were judged to have low risk of bias for sequence generation. One of the 14 RCTs mentioned the use of sequentially numbered, opaque, and sealed envelope for allocation concealment and hence judged to have low risk of bias. The remaining 13 were judged to have unclear risk of bias as no information about allocation concealment were provided. Thirteen and 8 RCTs were judged to have high risk of bias for blinding of participants and personnel, and blinding of outcome assessments, respectively. The remaining 1 and 4 RCTs were judged to have unclear risk of bias for blinding of participants and personnel, and blinding of outcome assessments, respectively. On the other hand, all the included RCTs had low risk of bias for incomplete outcome data and selective reporting for our interested outcomes. Details are presented in Table 2.
TABLE 1 (Continued).
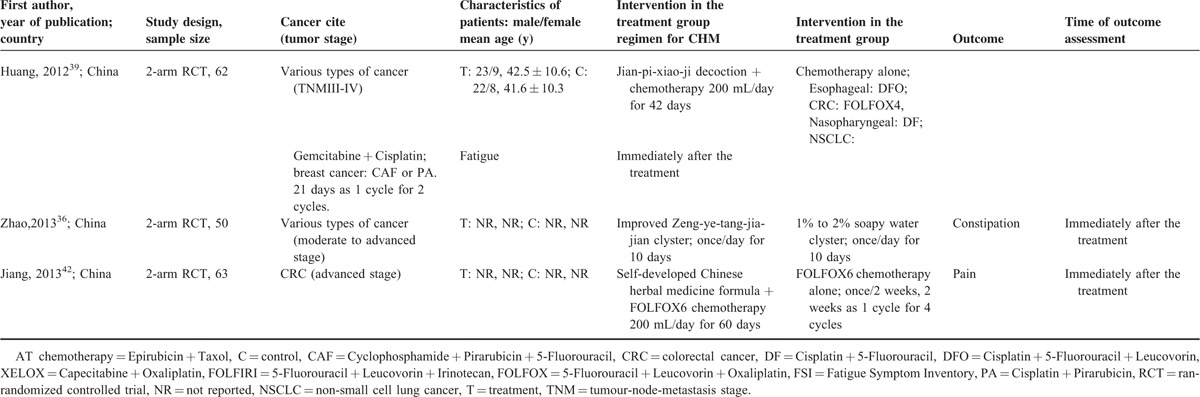
Basic Characteristics of Included Randomized Controlled Trials on Chinese Herbal Medicine for Symptoms Management in Cancer Palliative Care
Effectiveness of CHM for Symptom Management for Cancer Palliative Care
Pain
Three RCTs reported results on pain relief (Figure 2). Changes in pain severity were measured with Numeric Rating Scale in these 3 studies. One RCT42 only included advance colorectal cancer patients while the remaining 2 RCTs41,44 included patients with various types of advanced cancer. Meta-analysis of these trials showed that, combined CHM and conventional treatment significantly reduced pain score as compared with conventional medicine alone (pooled WMD:−0.90, 95% CI: −1.69 to −0.11, I2 = 87.7%). Significant heterogeneity was observed, which may account for by the differences in CHM formulae, treatment duration, cancer types, and baseline treatment. Subgroup analysis was not conducted because of the limited number of studies. Sensitivity analysis based on rigor was not conducted as all the 3 RCTs were judged to have unclear risk of bias for sequence generation.
FIGURE 2.
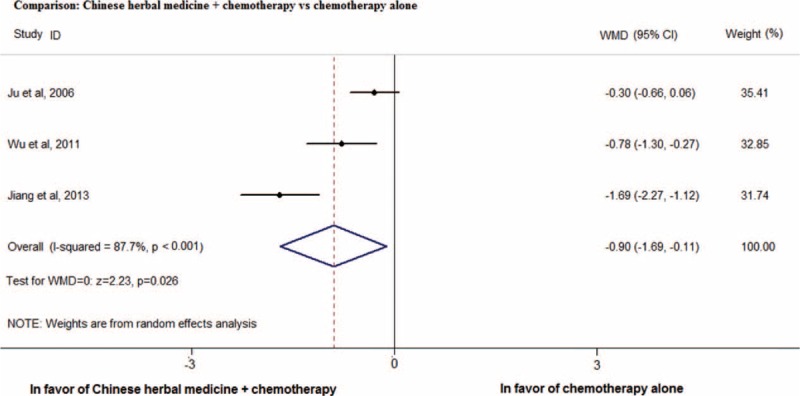
Meta-analysis on the additional use of Chinese herbal medicine for reducing pain score in cancer patients (Comparison: Chinese herbal medicine + chemotherapy vs chemotherapy alone).
One41 of the 3 RCTs reported adverse events. As compared to fentanyl transdermal patch alone, patients who used additional Jia-wei-xiang-sha-liu-jun-zi decoction had similar occurrence in vomiting, dizziness, drowsiness, abdominal discomfort, and skin allergy.
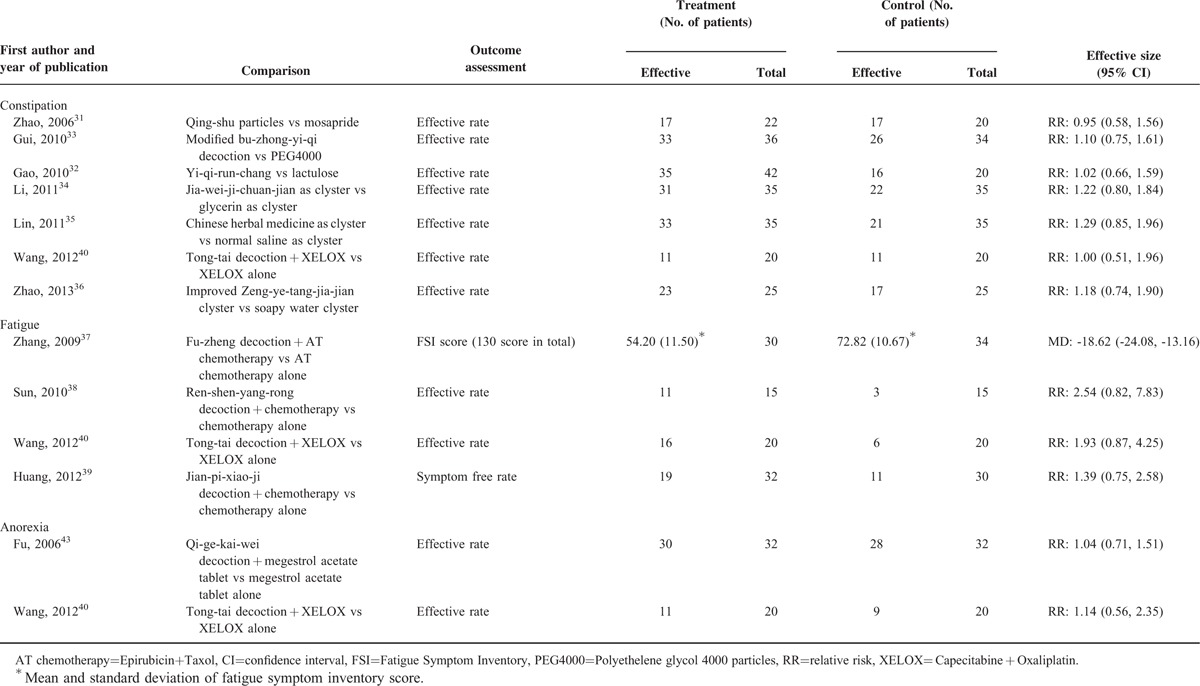
Constipation
Seven RCTs investigated the effect of CHM in managing constipation (Table 3). One RCT40 compared Tong-Tai decoction plus chemotherapy to chemotherapy alone. No difference was found in terms of the proportion of patient achieving satisfactory relief between the 2 groups. The remaining 6 RCTs31–36 compared CHM to conventional medications in managing constipation. Results showed that CHM and conventional medications had similar effect in reducing constipation. Data were not pooled because of the differences in the definition of satisfactory relief among included studies.
TABLE 2.
Risk of Bias of Included Randomized Controlled Trials on Chinese Herbal Medicine for Symptoms Management in Cancer Palliative Care
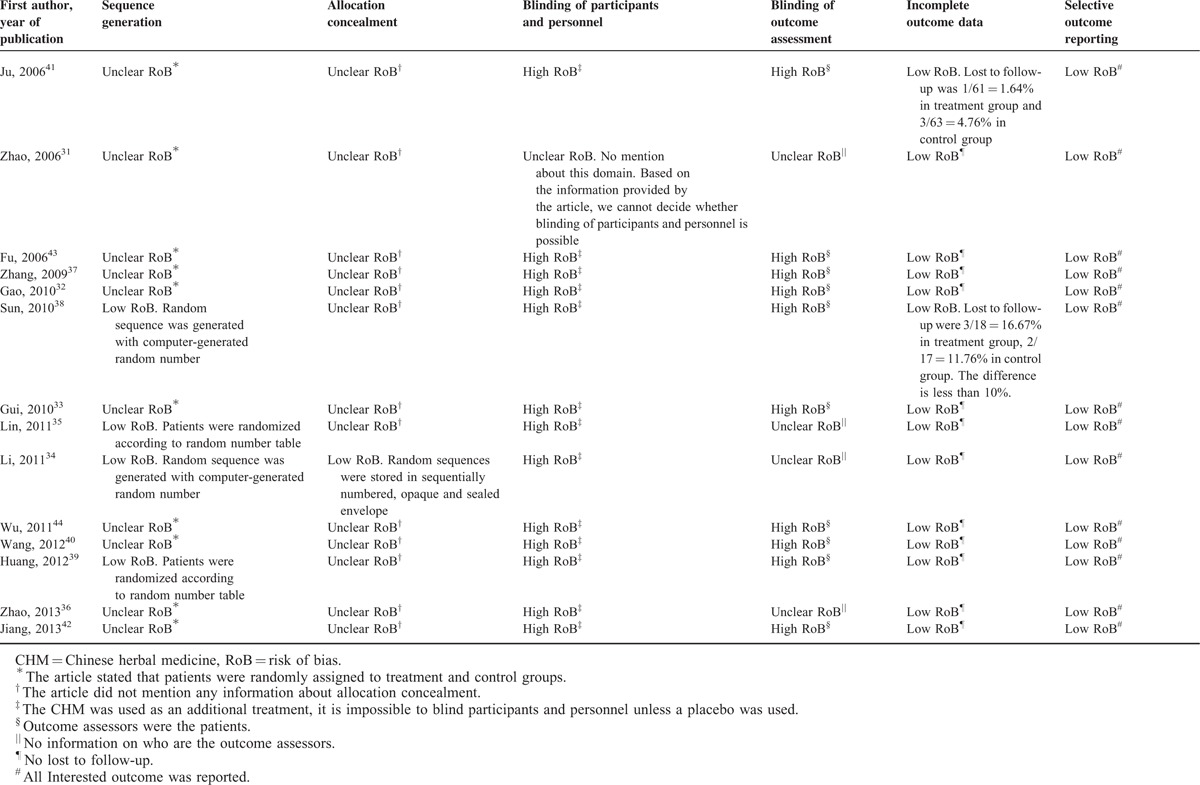
TABLE 3.
Effectiveness of Chinese Herbal Medicine Symptoms Management in Cancer Palliative Care
Three of the 7 RCTs mentioned adverse events. Two31,33 of them reported no adverse events in both treatment and control groups except for mild abdominal discomfort. No additional treatment was required to manage this event. The remaining one40 reported that no significant difference between Tong-tai decoction plus XELOX (capecitabine + oxaliplatin) and XELOX alone group with respect to the incidence of chemotherapy-induced toxicity among colorectal cancer patients.
Fatigue
Four RCTs reported reduction of fatigue (Table 3). Three RCTs38–40 reported no significant difference between combined use of CHM and chemotherapy versus chemotherapy alone for reducing fatigue. The remaining RCT37 compared the combination of chemotherapy (Epirubicin plus Taxol) and Fu-zheng decoction to chemotherapy alone in breast cancer patients. After 9 weeks of treatment, the combined treatment group showed significantly lower Fatigue Symptom Inventory score than the chemotherapy alone group (MD: −18.62, 95% CI: −24.08 to −13.16). Because of the heterogeneity in outcome measurement approach, data were not pooled among included RCTs.
Three of the 4 RCTs provided information on adverse effect. Two studies37,39 reported that the CHM and chemotherapy-combined treatment group had significantly lower incidence in chemotherapy-induced toxicity including leucopenia, neurotoxicity, and nausea and vomiting. One38 reported that there was no adverse event observed.
Anorexia
Two RCTs reported evidence on CHM for treating anorexia in cancer patients (Table 3). One43 compared Qi-ge-kai-wei decoction plus megestrol acetate versus megestrol acetate alone in advanced lung cancer patients. Although a higher proportion of patients in the combined treatment group reported improvement (93.8% versus 87.5%), the difference was not of statistical significance. The other RCT40 found that the combined use of Tong-tai decoction and chemotherapy showed more improvement than chemotherapy alone in advanced colorectal cancer patients (55.0% versus 45.0%), but again no significant difference was found. Considering the clinical heterogeneity of these 2 studies, and different criteria used for defining satisfactory relief, meta-analysis was not conducted.
One study43 reported adverse effects. Although the CHM and conventional medicine combined treatment group showed fewer incidence in alanine aminotransferase increment, for thrombotic vasculitis, edema, high blood pressure or blood glucose, constipation, heart failure, and difficulty in breathing, the difference was not significant.
DISCUSSION
This SR summarized evidence on the effectiveness of CHM for the management of pain, constipation, fatigue, and anorexia among cancer patients. Fourteen RCTs with a total of 878 cancer patients were included in this SR. Our results showed that the combined use of CHM and conventional medicine slightly relieved pain when compared with conventional treatment alone. CHM alone showed similar effectiveness with conventional medicine in managing constipation. The additional use of Fu-zheng decoction can reduce fatigue in breast cancer patients who are receiving chemotherapy. Current evidence did not show any superior effect on combined use of CHM and conventional medicine for anorexia when compared to conventional medicine alone. On safety, patients in the combined group generally showed lower or similar occurrence of adverse events when compared with patients in conventional medicine only group. We did not identify any eligible RCT providing evidence on CHM for managing paresthesias and dysesthesias, insomnia and limbs edema in cancer patients.
The role of CHM in symptom management of cancer patients has gained increasing attention. Based on previous SRs, no firm conclusion has been reached on using CHM for the managing pain45,46 and fatigue,45 because of the lack of “rigorous clinical trials.” In current SR, we only included RCTs which reported detailed methodological details. Results from our study suggested that clinicians may consider additional use of CHM on top of conventional care for better management of pain and fatigue, of which both are under-treated in clinical practice.47,48 CHM could be considered as an alternative choice for treating constipation in cancer patients as it has similar effectiveness as conventional medicine.
All included RCTs generally had short treatment duration, with 2 of them shortly followed up the patients (14–28 days) after treatment. That raises the question of whether treatment and follow-up durations were long enough for CHM to demonstrate its beneficial effects.22 Future RCTs on this area should consider appropriate treatment and follow-up duration based on expert consensus.
All the included RCTs were published in Chinese and we have observed a lack of compliance to the Chinese version COSORT statement.49 Poor reporting is the major contributor to uncertainties in our risk of bias assessment. For example, although all the studies stated themselves as RCTs, more than half of them (10/14) did not provide information on how random sequences were generated. Also, only 1 RCT mentioned about allocation concealment. Blinding is another key limitation to the evidence base as all the included outcomes were measured in a subjective manner.50 As a result, we cannot exclude the possibility of overestimating or underestimating the effectiveness of CHM.51 That said, all included studies had good performance in preventing bias related to incomplete outcome data and selective outcome reporting.
Among RCTs that reported adverse event outcomes, results were consistent with other reviews,22,45 which indicated that CHM is generally safe with low risk of incurring serious adverse effect. However, safety issue did not receive attention in 6 of the 14 RCTs, which indicates the need of paying more attention on safety surveillance in future trials.22
During literature selection, more than 100 RCTs were excluded due to not using validated instrument in outcome assessment. This phenomenon is consistent with findings from other researchers.22 Self-developed outcome assessment criteria were often used in these studies without prior validation, and some of them did not even provide information on the criteria they used. That could be considered as a huge waste of research resources as results from these studies cannot generate any useable or meaningful clinical evidence. Future RCTs are strongly suggested to use internationally recognized and validated scales to measure outcomes, so as to facilitate the comparison with similar studies, and enhance usefulness of the research findings.
Readers should be noted that all the included RCTs were conducted in Chinese population under Chinese health care settings, which may limit the generalizability of results. Because of the limited number of included studies, we did not assess the publication bias in this SR, so we cannot determine whether such bias exist or not.
In conclusion, CHM may be considered as an add-on to conventional medicine in the management of pain in cancer patients. CHM could also be considered as an alternative to conventional medicine for reducing constipation. Evidence on the use of CHM for treating anorexia and fatigue in cancer patients is uncertain, warranting further research. Future RCTs should improve in the following areas: 1) choosing an internationally recognized and validated instruments for outcome measurement; 2) reporting detailed safety outcomes; and 3) implementing strict adherence to the CONSORT reporting statements.49,52
Supplementary Material
Acknowledgment
We are grateful to Ms Wong Hoi Lam Charlene for the administrative support.
Footnotes
Abbreviations: AMED = Allied and Complementary Medicine Database, CBM = Chinese Biomedical Databases, CENTRAL = Cochrane Central Register of Controlled Trials, CHM = Chinese herbal medicines, CI = confidence interval, NRS = Numeric Rating Scale, RCTs = randomized controlled trials, RR = relative risk, SR = systematic review, WMD = weighted mean difference, XELOX = capecitabine + oxaliplatin.
YZ and ALZ contributed equally to the article.
This systematic review was supported by Hospital Authority of Hong Kong (Reference number: 8110016609).
VCHC is Honorary Research Fellow of the Australian Research Centre in Complementary and Integrative Medicine (ARCCIM), Faculty of Health, University of Technology Sydney, Sydney, Australia. The remaining authors declare no conflict of interest.
REFERENCES
- 1.The Lancet. Moving cancer up the global health agenda. The Lancet 2010; 375:2051. [DOI] [PubMed] [Google Scholar]
- 2.Stark L, Tofthagen C, Visovsky C, et al. The symptom experience of patients with cancer. J Hosp Palliat Nurs 2012; 14:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshields TL, Potter P, Olsen S, et al. Documenting the symptom experience of cancer patients. J Support Oncol 2011; 9:216–223. [DOI] [PubMed] [Google Scholar]
- 4.Pachman DR, Barton DL, Swetz KM, et al. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol 2012; 30:3687–3696. [DOI] [PubMed] [Google Scholar]
- 5.Del Fabbro E, Dalal S, Bruera E. Symptom control in palliative care—Part II: cachexia/anorexia and fatigue. J Palliat Med 2006; 9:409–421. [DOI] [PubMed] [Google Scholar]
- 6.Queiroz G, Santos A, Pereira R, et al. Prevalence of paresthesia, fatigue, edema and pain after treatment for breast cancer. Appl Cancer Res 2009; 29:173–178. [Google Scholar]
- 7.Pujol LA, Monti DA. Managing cancer pain with nonpharmacologic and complementary therapies. J Am Osteopath Assoc 2007; 107 (12 suppl 7):Es15–Es21. [PubMed] [Google Scholar]
- 8.Bruera E. Clinical management of anorexia and cachexia in patients with advanced cancer. Oncology 1992; 49 suppl 2:35–42. [DOI] [PubMed] [Google Scholar]
- 9.Perdue C. Managing constipation in advanced cancer care. Nursing Times 2005; 101:36–40. [PubMed] [Google Scholar]
- 10.Bruera E, Yennurajalingam S. Palliative care: Overview of fatigue, weakness, and asthenia. 2015; http://www.uptodate.com/contents/palliative-care-overview-of-fatigue-weakness-and-asthenia?source=machineLearning&search=fatigue&selectedTitle=2∼150§ionRank=4&anchor=H29611194#H29611194 Accessed 12 March, 2015. [Google Scholar]
- 11.Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005; 118:289–305. [DOI] [PubMed] [Google Scholar]
- 12.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain 2010; 150:573–581. [DOI] [PubMed] [Google Scholar]
- 13.Jadad AR, Browman GP. The WHO analgesic ladder for cancer pain management. Stepping up the quality of its evaluation. JAMA 1995; 274:1870–1873. [PubMed] [Google Scholar]
- 14.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. New Engl J Med 1994; 330:592–596. [DOI] [PubMed] [Google Scholar]
- 15.Loprinzi CL, Ellison NM, Schaid DJ, et al. Controlled trial of megestrol-acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer I 1990; 82:1127–1132. [DOI] [PubMed] [Google Scholar]
- 16.Willox JC, Corr J, Shaw J, et al. Prednisolone as an appetite stimulant in patients with cancer. Brit Med J 1984; 288:27–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jatoi A, Kumar S, Sloan JA, et al. On appetite and its loss. J Clin Oncol 2000; 18:2930–2932. [DOI] [PubMed] [Google Scholar]
- 18.Paltiel O, Marzec-Boguslawska A, Soskolne V, et al. Use of tranquilizers and sleeping pills among cancer patients is associated with a poorer quality of life. Qual Life Res 2004; 13:1699–1706. [DOI] [PubMed] [Google Scholar]
- 19.Jin X, Julieta RB, Sze DM, et al. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst Rev 2012; 6: CD007731. [DOI] [PubMed] [Google Scholar]
- 20.Su R, Li L, Xu H-B, et al. A systematic review of therapeutic efficacy and safety of adjuvant therapy of compound sophora flavescens injection in the treatment of tumor [Chinese]. China Pharma 2013; 24:4154–4163. [Google Scholar]
- 21.Fu J, Yu J, Xu H-B, et al. Systematic evaluation about efficiency detoxification of Chinese traditional medicine adjuvant chemotherapy for solid tumors [Chinese]. Guiding J Tradit Chin Med Pharma 2010; 16:108–112. [Google Scholar]
- 22.Molassiotis A, Potrata B, Cheng KKF. A systematic review of the effectiveness of Chinese herbal medication in symptom management and improvement of quality of life in adult cancer patients. Complement Ther Med 2009; 17:92–120. [DOI] [PubMed] [Google Scholar]
- 23.Caraceni A, Cherny N, Fainsinger R, et al. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage 2002; 23:239–255. [DOI] [PubMed] [Google Scholar]
- 24.Yang D, Zhong-zhi Q, Yan-ze L, et al. New Collection of Crude Drugs in Chinese Pharmacopoeia 2010 I. Callicarpa Linn. and Related Items. Chin Herb Med 2010; 02:272–288. [Google Scholar]
- 25.Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. Vol. 3302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong SSL, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Lib Assoc 2006; 94:41–47. [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Vol. 3432011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Vol. 3272003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. Vol. 3422011. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. www.cochrane-handbook.org. [Google Scholar]
- 31.Zhao WH, Su ZX, Cao XM, et al. The effect of Qing-Shu Particles in the Treatment on Constipation resulted from Chemotherapy to the Lymphoma Patients [Article in Chinese]. Modern Oncol 2006; 14:1286–1287. [Google Scholar]
- 32.Gao YL, Li LY, Li PW. Clinical study on the effectiveness of Yi-Qi-Run-Chang Method for opioids induced constipation [Article in Chinese]. J Trad Chin Med Emergency 2010; 19:585–586. [Google Scholar]
- 33.Gui L, Liu YX, Ma HR, et al. Effictiveness of modified Bu-Zhong-Yi-Qi decoction for chemotherapy induced constipation among colorectal cancer patients [Article in Chinese]. China Pharma 2010; 21:2574–2575. [Google Scholar]
- 34.Li ZM, Dong QH, Chen GF, et al. Clinical observation on the effectiveness of Jia-Wei-Ji-Chuan-Jian Clyster for constipation on cancer patients [Ariticle in Chinese]. J New Chin Med 2011; 43:100–101. [Google Scholar]
- 35.Lin HF, Wu X, Lin XM. Chinese herbal medicine retention clyster for constipation in 35 patients with advanced cancer [Ariticle in Chinese]. Fujian J TCM 2011; 42:41. [Google Scholar]
- 36.Zhao JY, Sun MF. The obserbation of improved increasing liquid Tong-ga Minus Clyster in the treatment of opioids induced constipation [Ariticle in Chinese]. China Modern Doctor 2013; 51:119–121. [Google Scholar]
- 37.Zhang LL, Chen L, Jia YT, et al. The effect of Fu-zheng-he-ji to the quality of life on patients with recurrent or metastatic breast cancer. J Pract Trad Chin Int Med 2009; 23:35–36. [Google Scholar]
- 38.Sun H, Li ZD, Wang W, et al. A randomized controlled trial on Ren-shen-yang-rong decoction for improving fatigue in cancer patients who are receiving chemotherapy [Article in Chinese]. Chin J Basic Med in Trad Chin Med 2010; 16:155–157. [Google Scholar]
- 39.Huang ZF, Wei JS, Yuan Y, et al. Effect of Jian-pi-xiao-ji decoction on quality of life in patients with cancer related fatigue [Article in Chinese]. World Chin Med 2012; 7:481–483. [Google Scholar]
- 40.SW Wang. Clinical Observation of the Efficacy of Tong-tai Decoction in Combination with XELOX Regimen Chemotherapy for Treating Advanced Colorectal Cancer [Article in Chinese]. Nanjing, China: Nanjing University of Chinese Medicine, Nanjing University of Chinese Medicine; 2012. [Google Scholar]
- 41.Ju SB, Zou YH. Effectiveness on Jia-Wei-Xiang-Sha-Liu-Jun-Zi decoction plus Fentanyl Transdermal Patch for cancer related pain in 60 advance cancer patients [Ariticle in Chinese]. New J Trad Chin Med 2006; 38:55–56. [Google Scholar]
- 42.Jiang GS, Zhang G, Ren WD. The combination of traditional Chinese medicine and Western medicine for advanced colorectal cancer. Chin J Experiment Trad Med Formulae 2013; 19:323–325. [Google Scholar]
- 43.Fu DZ. Improvement of anorexia and weight reducing in patients with lung cancer by integrated Chinese and Western medicine [Article in Chinese]. Zhejiang Journal of Intergrative Traditional Chinese and Western Medicine 2006; 16:471–472. [Google Scholar]
- 44.Wu XD, Zheng NY, Xu LQ, et al. Clinical observation on the combination of Gui-Dan-San-Zi-San-Re-Wei and Hyperthermia, radiotherapy for treating pain in cancer patients with bone metastases [Article in Chinese]. J New Chin Med 2011; 43:102–103. [Google Scholar]
- 45.Qi F, Li A, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends 2010; 4:297–307. [PubMed] [Google Scholar]
- 46.Xu L, Lao LX, Ge A, et al. Chinese herbal medicine for cancer pain. Integr Cancer Therap 2007; 6:208–234. [DOI] [PubMed] [Google Scholar]
- 47.Schaffer D, Florin T, Eagle C, et al. Risk of serious NSAID-related gastrointestinal events during long-term exposure: a systematic review. Med J Aust 2006; 185:501–506. [DOI] [PubMed] [Google Scholar]
- 48.Koornstra RHT, Peters M, Donofrio S, et al. Management of fatigue in patients with cancer—A practical overview. Cancer Treat Rev 2014; 40:791–799. [DOI] [PubMed] [Google Scholar]
- 49.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials (Chinese version). J Chin Integr Med 2010; 8:701–741. [Google Scholar]
- 50.Wood L, Egger M, Gluud LL, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008; 336:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savović J, Jones HE, Altman DG, et al. Influence of reported study design characteristics on intervention effect estimates from Randomized, controlled trials. Ann Intern Med 2012; 157:429–438. [DOI] [PubMed] [Google Scholar]
- 52.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010; 152:726–732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


