Supplemental Digital Content is available in the text
Abstract
Recent studies have observed a high level of circulating interleukin-10 (IL-10) in patients with digestive cancers, yet whether elevated IL-10 is causally associated with digestive cancers so far remained unresolved.
We therefore meta-analyzed available observational studies with Mendelian randomization method to explore this causal association by employing IL-10 gene 3 variants (-592C>A, -819C>T, and -1082A>G) as instruments.
Data were available from 52 articles encompassing 29,307 subjects. Subgroup analysis by cancer type indicated that -1082A>G was associated with increased risk of gastric cancer (odds ratio [OR] = 1.19; 95% confidence interval [CI]: 1.05–1.35; P = 0.006), and the association was reinforced for intestinal type gastric cancer (OR = 1.26; 95%CI: 1.09–1.44; P = 0.001). By ethnicity, risk estimate for -1082G allele carriers was increased by 21% for digestive cancers in East Asians (95%CI: 1.05–1.40; P = 0.009). As for the genotype–phenotype association, carriers of -1082G allele had an overall 20.21 pg/mL higher IL-10 level than those with -1082AA genotype (P = 0.023). In further Mendelian randomization analysis, the predicted OR for 10 pg/mL increment in IL-10 was 1.14 (95%CI: 1.01–16.99) in gastric cancer.
Our findings provided evidence for a causal role of genetically elevated IL-10 in the development of gastric cancer, especially in East Asians and for intestinal type gastric cancer.
INTRODUCTION
A constellation of malignancies that originate from digestive organs, such as stomach, colon, and liver, constitutes digestive cancers. In many patients with digestive system malignancies, there is a strong inherited predisposition with epidemiological data generated from human twin and family studies. For example, family members who have a mutation in a mismatch repair gene are observed to have a much higher rate of colorectal cancer than those who do not have the mutation.1 Chiba et al2 have written an excellent review on the genetic underpinnings of digestive cancers and highlighted that genes encoding inflammatory factors, interleukin family members in particular, play a contributory role in the pathogenesis of various cancers, particularly in digestive organs.
One of the most intensively evaluated members in interleukin family is interleukin-10 (IL-10), a key cytokine involved in immune response and carcinogenesis. Recent studies have observed a high level of circulating IL-10 in patients with digestive cancers and its association with poor prognosis.3 Accumulating evidence suggested that interindividual variation in circulating IL-10 may arise from common polymorphic variation in IL-10 gene. The genomic sequence of IL-10 gene is highly polymorphic and 3 promoter variants, viz -592C>A (rs1800872), -819C>T (rs1800871), and -1082A>G (rs1800896), in possible association with alterations of IL-10 function are well-defined from different populations with varying prevalence.4,5 It is reasonable to expect that if IL-10 is involved in the underlying pathological process of digestive cancers, the inherited genetic determinants that alter circulating IL-10 should affect cancer risk in the direction and magnitude predicted by its circulating level.
As available evidence regarding the association between circulating IL-10 and digestive cancers is mainly derived from observational studies, it is difficult to disentangle causation from association, especially in the presence of confounding. Mendelian randomization is considered as a viable method to obtain the causality of an exposure-disease association using genetic determinants.6,7 Bearing this in mind, we first meta-analyzed the association of 3 aforementioned variants in IL-10 gene with digestive cancer risk and changes of circulating IL-10 level. Second, we selected the variant(s) that can be simultaneously predictive of digestive cancers and circulating IL-10 as an instrument to explore their potential causal relevance by implementing Mendelian randomization method.
METHODS
According to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement, this meta-analysis was carried out (Supplementary PRISMA checklist).8 Our research (meta-analysis) is not needed to be approved by the ethics committee according to PRISMA guidelines.
Search Strategy
A literature search for observational studies that investigated the association between IL-10 gene 3 variants (-592C>A, -819C>T, and -1082A>G) and all types of digestive cancers was conducted of PubMed and Google Scholar databases covering the period from the earliest possible year to May 1, 2015. Subject terms used for the search included “interleukin 10,” “interleukin-10,” “IL 10,” “IL-10,” “gastric or stomach,” “colorectal or colon or rectal,” “esophageal,” “liver or hepatic or hepatocellular,” “pancreatic,” “gallbladder or biliary,” “cancer or carcinoma or tumor or sarcoma or leiomyoma,” together with “polymorphism or genetic or variant or mutation or allele or genotype.” Citations in retrieved articles as well as reviews on the same topic were also searched where relevant. Only articles published in English language were identified.
Trial Selection
Two investigators (KQ and WN) independently scanned the titles and abstracts to evaluate their eligibility. The full text was reviewed when an article cannot be rejected based on its title or abstract. If more than one article from the same study group or the same cohort, we only extracted the data from the most recent or complete articles.
Inclusion/Exclusion Criteria
The following criteria were used for the literature selection in this meta-analysis: clinical endpoints should be digestive cancers including esophageal cancer, gastric cancer, colorectal cancer, hepatocellular carcinoma, biliary tract cancer, and pancreatic cancer; studies should follow either a retrospective or a prospective case–control design; and the genotype/allele counts of at least 1 of the 3 variants examined and/or circulating IL-10 level across genotypes of either variant should be provided. Studies were excluded (1 point was sufficient for exclusion) if they investigated the gene function, disease progression, severity and the response to treatment, or survival. Additionally, conference abstracts or proceedings, case reports or series, editorials, narrative reviews, meta-analyses, and the non-English articles were also excluded.
Data Extraction
Two investigators (KQ and WN) independently extracted data using a standardized excel template. Disagreements were resolved by a 3rd investigator (CL). Data were collected on the 1st author, publication year, ethnicity of the study subjects, cancer type, study design, sample size, the genotype/allele counts of 3 examined variants between cases and controls, and the characteristics of the study subjects, if available, including age, gender, body mass index (BMI), smoking, drinking, family history of cancers, history of digestive diseases, and bacteria or virus infection status.
Statistics
In this meta-analysis, the association of 3 variants in IL-10 gene with digestive cancer risk was calculated under 3 genetic models of inheritance, including allelic model, homozygous genotypic model, and dominant model. Weighted odds ratios (ORs) and the corresponding 95% confidence intervals (95% CIs) were quantified by a random-effects model.9 Heterogeneity between studies was calculated by the inconsistency index (I2) statistic and I2 > 50% was designated as a threshold to indicate significant heterogeneity. Publication bias was assessed by Begg funnel plot and the corresponding Egger test. A 10% level of significance for the Egger test was considered as the presence of publication bias.10
Predefined subgroup analyses were performed a priori according to cancer type (esophageal cancer, gastric cancer, colorectal cancer, hepatocellular carcinoma, biliary tract cancer, or pancreatic cancer), ethnicity of the study subjects (Caucasian, East Asian, Latinos, or mixed), source of controls (population-based or hospital-based), study design (prospective or retrospective), and total sample size (<500 subjects or ≥500 subjects). For gastric cancer, further subgroup analyses were undertaken according to its anatomic type (gastric cardia or noncardia cancer) and histologic type (diffuse or intestinal type). Additionally, to account for the sources of heterogeneity from continuous confounders such as age, sex, BMI, smoking, drinking, family history of cancer, and Helicobacter pylori infection (only for gastric cancer studies), a set of meta-regression analyses were performed to evaluate the association of 3 examined variants with digestive cancer risk. For the variant that was simultaneously associated with the significant risk of digestive cancers or its subtypes and the significant changes of circulating IL-10 level, Mendelian randomization analysis was accordingly performed.
Data management and statistical analyses described above were completed with the STATA software (StataCorp, TX, version 11.2).
RESULTS
Eligible Articles
Figure 1 is a flow diagram that schematizes the process of article exclusion with specific reasons. Altogether 128 potentially relevant articles were identified according to the search strategy, and 52 of them were qualified after applying further the inclusion/exclusion criteria. All 52 qualified articles were written in English and published between 2003 and 2014.11–62 As data within 3 articles were separately provided by cancer type, the final analysis included a total of 56 independent studies (Table 1).
FIGURE 1.
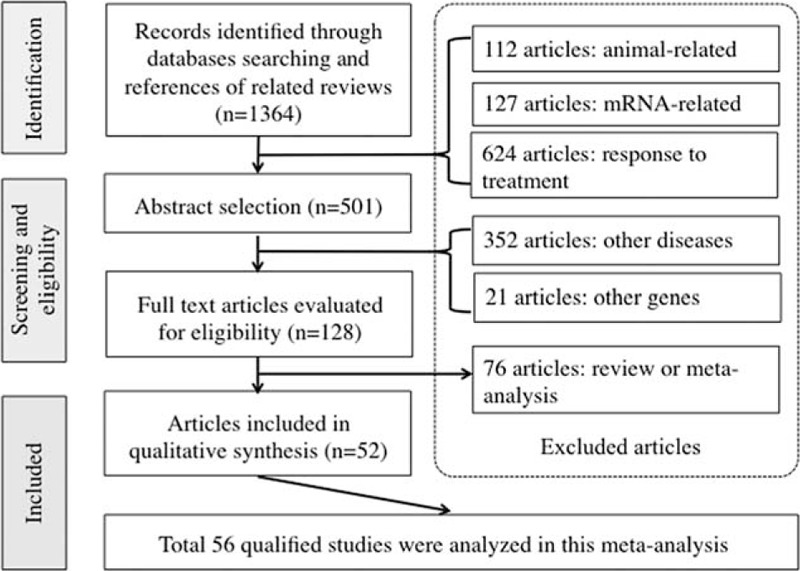
Flow diagram of search strategy and study selection.
TABLE 1.
Baseline Characteristics of Eligible Studies for Association of IL-10 Gene 3 Variants With Digestive Cancers
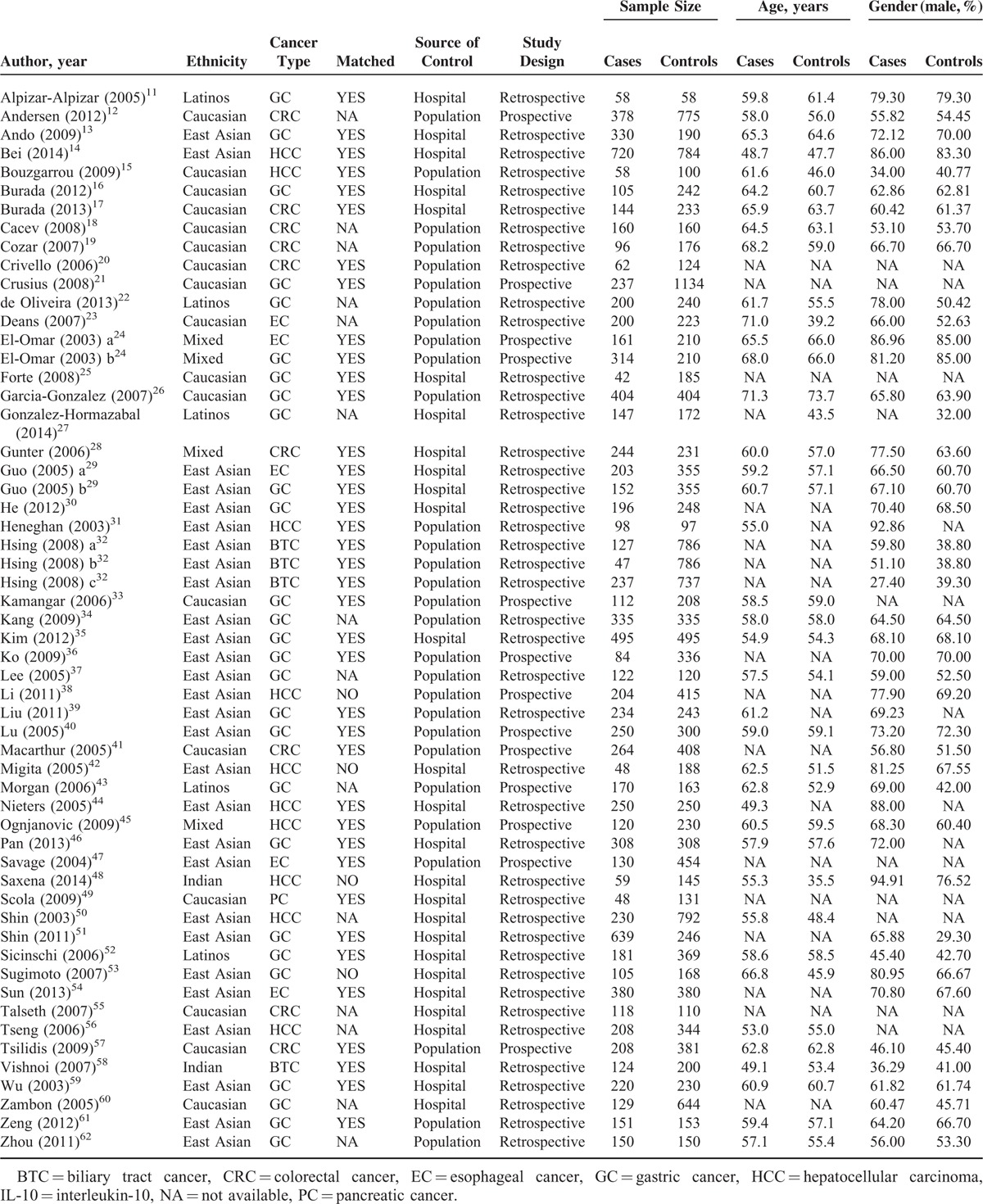
Study Characteristics
The baseline characteristics of all 56 qualified studies are shown in Table 1, and the genotype distributions and allele frequencies of 3 examined variants (-592C>A, -819C>T, and -1082A>G) in IL-10 gene between cases and controls are provided in Supplementary Table 1. In this meta-analysis, 27 studies were conducted for gastric cancer, 10 for hepatocellular carcinoma, 9 for colorectal cancer, 5 for esophageal cancer, 4 for biliary tract cancer, and 1 for pancreatic cancer. There were 30 studies involving Asians, 17 involving Caucasians, 5 involving Latinos, and 4 involving the mixed populations. Twenty-nine studies were conducted on a population-based design and 27 were on a hospital-based design. Thirty (53.57%) of 56 qualified studies had total sample size equal to or more than 500 subjects.
Association of IL-10 Gene 3 Variants With Digestive Cancers
Pooling 56 qualified studies together revealed no significant association between 3 examined variants (-592C>A, -819C>T, and -1082A>G) and digestive cancers under all 3 genetic models, yet there was evident heterogeneity. There were low probabilities of publication bias as reflected by the suggestive symmetry of Begg funnel plots (Figure 2), as well as it is associated Egger tests (P = 0.34, 0.38, and 0.07 for allelic, homozygous genotypic, and dominant models, respectively).
FIGURE 2.
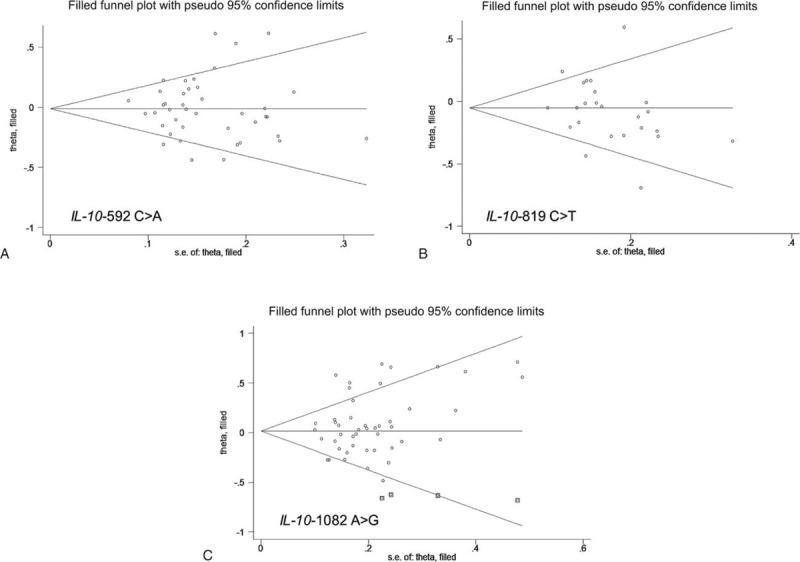
Funnel plots for studies investigating the effect of IL-10 gene 3 variants on digestive cancer risk. Vertical axis represents the log of OR; horizontal axis represents the SE of log (OR). Funnel plots are drawn with 95% confidence limits. The graphic symbols represents the data in the plot be sized proportional to the inverse variance. IL-10 = interleukin-10, OR = odds ratio, SE = standard error.
What is more, for -592C>A and -819C>T, there was no indication of significance in subgroup analyses, except for a relatively weak association between -592C>A and biliary tract cancer (OR = 1.30; 95% CI: 1.03–1.63; P = 0.028) and between -819C>T and gastric cancer (OR = 0.87; 95% CI: 0.77–0.97; P = 0.016) under allelic model (Table S2).
To account for the potential sources of between-study heterogeneity, a set of predefined subgroup analyses were conducted for -1082A>G (Table 2). By ethnicity, significant association between -1082G allele and digestive cancer risk was observed in East Asians under allelic (OR = 1.21; 95% CI: 1.05–1.40; P = 0.009; Figure 3A), homozygous genotypic (OR = 1.86; 95% CI: 1.26–2.76; P = 0.002), and dominant (OR = 1.22; 95% CI: 1.04–1.43; P = 0.013) models (Supplementary Figure 1). By cancer type, risk estimates of -1082A>G were significant for gastric cancer under allelic (OR = 1.19; 95% CI: 1.05–1.35; P = 0.006; Figure 3B), homozygous genotypic (OR = 1.48; 95% CI: 1.09–2.02; P = 0.013), and dominant (OR = 1.21; 95% CI: 1.04–1.41; P = 0.012) models (Supplementary Figure 2) for colorectal cancer under only dominant model (OR = 0.84; 95% CI: 0.72–0.99; P = 0.034). The heterogeneity between studies was relatively low for all cancer types except gastric cancer. Further subgroup analyses by anatomic types or histologic types of gastric cancer showed that risk estimates of -1082A>G were strongly reinforced for intestinal type of gastric cancer under allelic (OR = 1.26; 95% CI: 1.09–1.44; P = 0.001; Figure 3C), homozygous genotypic (OR = 1.42; 95% CI: 1.04–1.94; P = 0.028), and dominant (OR = 1.33; 95% CI: 1.11–1.60; P = 0.002) models (Supplementary Figure 3), yet without observable heterogeneity (I2 = 0.0%) and this estimate was only significant for gastric cardia cancer under dominant model (OR = 1.34; 95% CI: 1.04–1.71; P = 0.021).
TABLE 2.
Overall and Subgroup Analyses of IL-10 Gene -1082A>G With Digestive Cancer Risk
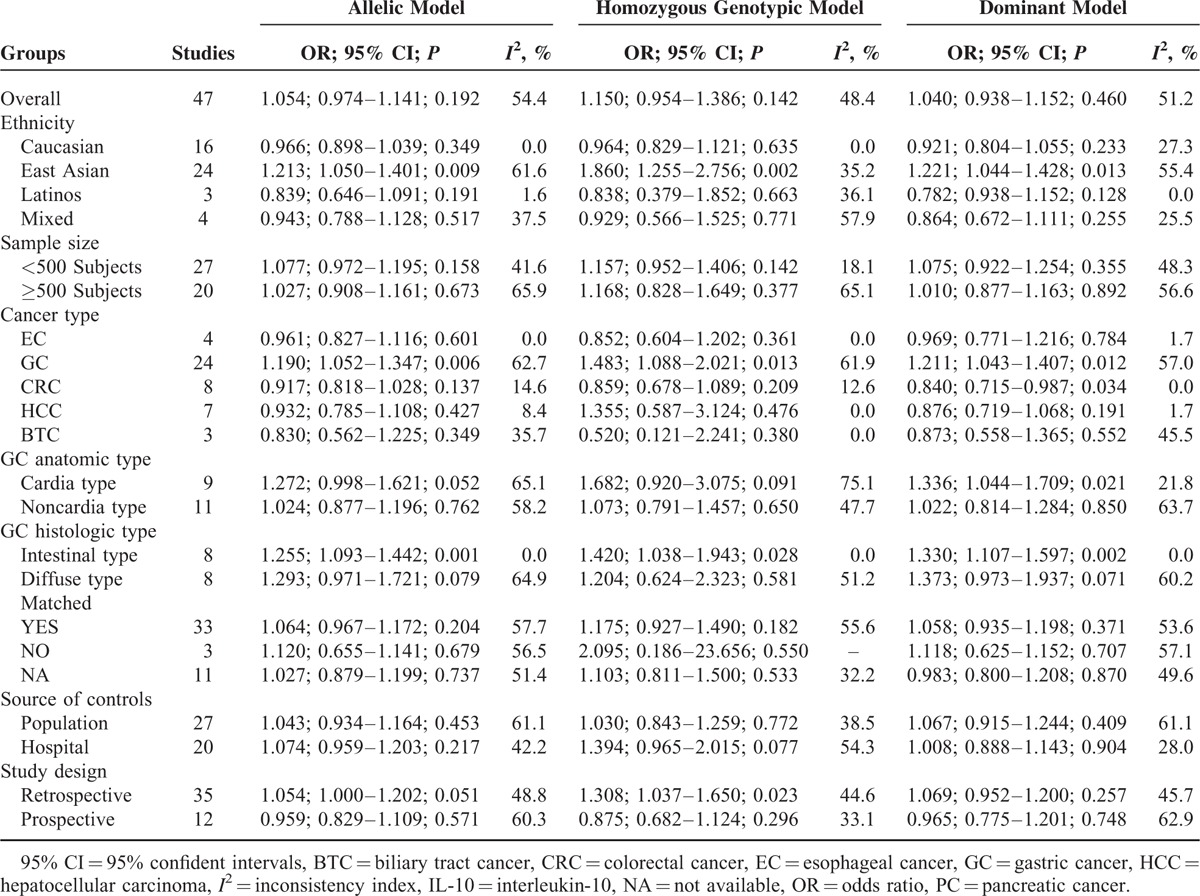
FIGURE 3.

Risk estimates of IL-10 gene -1082A>G for cancer risk under the allelic model. (A) East Asian population, (B) gastric cancer groups, and (C) intestinal type of gastric cancer. The summary treatment effect (OR) is shown by the middle of a solid diamond whose left and right extremes represent the corresponding 95% CI. Horizontal axis represents OR values, which were calculated against healthy controls. 95% CI = 95% confidence interval, IL-10 = interleukin-10, OR = odds ratio.
By study design or sample size, there were no significant differences in pooled risk estimates between the population- and hospital-based studies and between small and large studies.
Meta-Regression Analysis
To explore additional sources of between-study heterogeneity in gastric cancer, a univariate meta-regression model was constructed that included age, sex, BMI, smoking, drinking, family history of cancer, and Helicobacter pylori infection as independent variables. Age, sex, family history of cancer, and Helicobacter pylori infection were observed to significantly affect the relationship between -1082A>G and gastric cancer susceptibility (P = 0.048, 0.028, 0.014, and 0.018, respectively; Figure 4). In contrast, BMI, smoking, and drinking were not observed to affect the relationship between -1082A>G and gastric cancer susceptibility.
FIGURE 4.
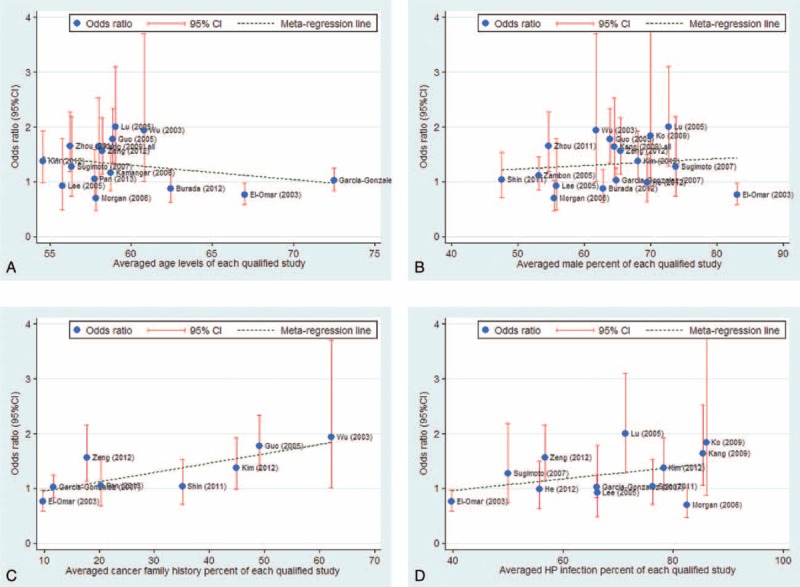
Meta-regression fitted lines by age (A), sex (B), family history of cancer percent (C), and Helicobacter pylori infection percent (D).
Association of IL-10 Variants With Circulating IL-10 Level
Genotype–phenotype association was based on 10 studies with circulating IL-10 level measured in cancer patients or healthy controls. Eight out of 10 studies were conducted -592C>A and -819C>T, and 4 for -1082A>G (Supplementary Table 3). Circulating IL-10 level was significantly elevated in -1082G allele carriers under homozygous genotypic (standard mean difference [SMD] = 29.63 pg/mL; 95% CI: 2.48–56.77; P = 0.032) and dominant (SMD = 20.21 pg/mL; 95% CI: 2.82–37.60; P = 0.023) models (Figure 5). There were low probabilities of publication bias for both models as reflected by the Begg funnel plots and the Egger tests (P = 0.31 and 0.32, respectively). As expected, there were no significant differences in the changes of circulating IL-10 level for -592C>A and -819C>T under both models (Supplementary Figure 4).
FIGURE 5.

Comparison of IL-10 gene -1082A>G genotypes for circulating IL-10 level in the allelic (left pane) and dominant (right pane) models. The summary treatment effect (SMD) is shown by the middle of a solid diamond whose left and right extremes represent the corresponding 95% CI. 95% CI = 95% confidence interval, IL-10 = interleukin-10, SMD = standard mean difference.
Prediction of Circulating IL-10 for Gastric Cancer by Mendelian Randomization
Under the assumptions required for Mendelian randomization and assuming a linear-logistic relationship between difference of circulating IL-10 level and odds of gastric cancer, the predicted ORs for 5 and 10 pg/mL IL-10 increment were 1.07 (95% CI: 1.01–4.12) and 1.14 (95% CI: 1.01–16.99), respectively. Since the 95% CIs of both predicted estimates excluded the null hypothesis value of 1, it is safe to reject the null hypothesis at a 5% significance level, and suggested a potentially causal association of circulating IL-10 level with gastric cancer.
DISCUSSION
By meta-analyzing of the data from 56 studies and on 29,307 subjects, we investigated 3 promoter variants (-592C>A, -819C>T, and -1082A>G) in IL-10 gene and its circulating level in association with the risk of digestive cancers. The most noteworthy finding of this study was that genetically elevated circulating IL-10 was significantly associated with an increased risk of gastric cancer by employing -1082A>G as an instrument. Moreover, extending previous understandings of the close relationship between IL-10 genetic variants and gastric cancer, we further pinpointed a remarkable contribution of -1082A>G to intestinal type gastric cancer. To the best of our knowledge, this is the first meta-analysis interrogating the causal relevance of circulating IL-10 and gastric cancer by implementing Mendelian randomization method.
Compared with other cancers, digestive cancers are more vulnerable to the impact of chronic inflammation, as digestive organs expose a large internal surface area to external environments. Besides exposing to chemical or biological agents of ingested foods, these organs also provide a place for many microorganisms, leading to the infiltration of many immunocytes and cytokines in pathologic conditions.2 There is growing recognition that IL-10 plays a key role in maintaining intestinal immune homeostasis,63,64 and indeed high circulating IL-10 level was observed in a large proportion of digestive cancer patients with poor prognosis, especially for gastric cancer,3 colorectal cancer,65 and hepatocellular carcinoma.66 In addition, accumulating evidence from functional investigations suggested that upregulation of IL-10 production from immunocytes exerted a great impact in tumor growth, immigration, and immune surveillance.67,68 However, whether circulating IL-10 is simply a biomarker for digestive cancers or whether elevated IL-10 level actually contributes directly to carcinogenesis is currently unclear. With this in mind, we employed the classical Mendelian randomization method in this meta-analysis and found that patients with 10 pg/mL increment in circulating IL-10 were 1.14 times more likely to develop gastric cancer. Nevertheless, a note of caution should be sounded in the interpretation of this finding, considering the unstable nature of IL-10 in the circulation according to a previous report (plasma half-life of IL-10 ranges from 2.7 to 4.5 hours),69 calling for a robust validation from well-designed, large studies with multiple measurements in circulating IL-10 to quantify this effect size reliably.
The transcriptional activation and protein production of a cytokine gene depends on the binding of regulatory factors to specific recognition sequences in the promoter. Mutations in promoter sequences of some cytokine genes may alter transcription factor recognition sites and consequently affect cytokine production.5 Previous studies have reported that a number of putative recognition sites are present in the IL-I0 promoter, such as PEA1, API, and an ETS-like element. The IL-10 variant (-1082A>G) that investigated in the present study lies within an ETS-like recognition site70 and may consequently affect the binding of this transcription factor and influence IL-10 production.
According to the Lauren classification, gastric cancer can be classified into adenocarcinomas of the diffuse and the intestinal type, and the latter is believed to arise secondary to chronic gastritis and be associated with relatively better prognosis.71 Although enormous efforts have been made to explore genetic susceptibility of IL-10 gene -1082A>G to gastric cancer risk,72–74 there has been little attention on specific subtypes of gastric cancer. Ni et al73 once performed a pilot analysis on 4 studies and found a weak association between -1082G allele and intestinal type gastric cancer. Via a comprehensive meta-analysis, we, in subgroup analyses, confirmed the contributory role of -1082A>G in the pathogenesis of intestinal type gastric cancer, and this role is less likely biased by between-study heterogeneity. Going forward, it will be of clinical importance using -1082A>G to refine risk stratification and identify high-risk individuals for cost-effective screening, surveillance, and early detection of intestinal type gastric cancer.
Despite the clear advantages of this meta-analysis including the detailed spectrum of digestive cancers, the implementation of Mendelian randomization and the large sample sizes, several possible limitations should be noted. First, we only examined 3 promoter variants in IL-10 gene, and investigation on other variants in or flanking IL-10 gene, especially some low-penetrance genes will be encouraged. It seems likely that -1082A>G by itself makes only a small or moderate contribution to risk prediction for gastric cancer patients, but whether this variant integrated with other risk factors will enhance prediction requires additional research. Second, a pleiotropic impact of the instrumental variant -1082A>G, a major drawback of Mendelian randomization, used in this meta-analysis, could not be totally excluded due to insufficient data in qualified studies. Third, nearly all involved studies in this meta-analysis had circulating IL-10 measured only once and did not reflect its long-term level in the development of digestive cancers. Therefore, the jury must refrain from drawing a conclusion until large, well-performed studies confirm or refuse our findings.
CONCLUSION
Taken together, our findings provided evidence for a causal role of genetically elevated circulating IL-10 in the development of gastric cancer by employing IL-10 gene -1082A>G as an instrument, and the risk association of this variant with digestive cancers was more evident in patients with intestinal type gastric cancer. These findings warrant further studies to investigate the exact mechanisms of circulating IL-10 level in the development of gastric cancer.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, IL-10 = interleukin-10, OR = odds ratio, PRISMA = preferred reporting items for systematic reviews and meta-analyses.
WN, QP, and TL contributed equally to this work.
This work was supported by National Natural Science Foundation of China (Grant Nos: 81201549, 81272644, and 81472247) and the Project of Innovative Research Team for Key Science and Technology in Xi’an Jiaotong University (No: 2013KCJ-23)
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Yri OE, Ekstrom PO, Hilden V, et al. Polymorphisms in genes encoding interleukin-10 and drug metabolizing enzymes GSTP1, GSTT1, GSTA1 and UGT1A1 influence risk and outcome in Hodgkin lymphoma. Leuk Lymphoma 2012; 53:1934–1944. [DOI] [PubMed] [Google Scholar]
- 2.Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology 2012; 143:550–563. [DOI] [PubMed] [Google Scholar]
- 3.Ikeguchi M, Hatada T, Yamamoto M, et al. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer 2009; 12:95–100. [DOI] [PubMed] [Google Scholar]
- 4.Suarez A, Castro P, Alonso R, et al. Interindividual variations in constitutive interleukin-10 messenger RNA and protein levels and their association with genetic polymorphisms. Transplantation 2003; 75:711–717. [DOI] [PubMed] [Google Scholar]
- 5.Turner DM, Williams DM, Sankaran D, et al. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet 1997; 24:1–8. [DOI] [PubMed] [Google Scholar]
- 6.Proitsi P, Lupton MK, Velayudhan L, et al. Genetic predisposition to increased blood cholesterol and triglyceride lipid levels and risk of Alzheimer disease: a mendelian randomization analysis. PLoS Med 2014; 11:e1001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niu W, Liu Y, Qi Y, et al. Association of interleukin-6 circulating levels with coronary artery disease: a meta-analysis implementing mendelian randomization approach. Int J Cardiol 2012; 157:243–252. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden J, Tierney JF, Copas AJ, et al. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol 2011; 11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alpizar-Alpizar W, Perez-Perez GI, Une C, et al. Association of interleukin-1B and interleukin-1RN polymorphisms with gastric cancer in a high-risk population of Costa Rica. Clin Exp Med 2005; 5:169–176. [DOI] [PubMed] [Google Scholar]
- 12.Andersen V, Egeberg R, Tjonneland A, et al. Interaction between interleukin-10 (IL-10) polymorphisms and dietary fibre in relation to risk of colorectal cancer in a Danish case-cohort study. BMC Cancer 2012; 12:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ando T, Ishikawa T, Kato H, et al. Synergistic effect of HLA class II loci and cytokine gene polymorphisms on the risk of gastric cancer in Japanese patients with Helicobacter pylori infection. Int J Cancer 2009; 125:2595–2602. [DOI] [PubMed] [Google Scholar]
- 14.Bei CH, Bai H, Yu HP, et al. Combined effects of six cytokine gene polymorphisms and SNP-SNP interactions on hepatocellular carcinoma risk in Southern Guangxi, China. Asian Pac J Cancer Prev 2014; 15:6961–6967. [DOI] [PubMed] [Google Scholar]
- 15.Bouzgarrou N, Hassen E, Farhat K, et al. Combined analysis of interferon-gamma and interleukin-10 gene polymorphisms and chronic hepatitis C severity. Hum Immunol 2009; 70:230–236. [DOI] [PubMed] [Google Scholar]
- 16.Burada F, Angelescu C, Mitrut P, et al. Interleukin-4 receptor -3223T→C polymorphism is associated with increased gastric adenocarcinoma risk. Can J Gastroenterol 2012; 26:532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burada F, Dumitrescu T, Nicoli R, et al. Cytokine promoter polymorphisms and risk of colorectal cancer. Clin Lab 2013; 59:773–779. [DOI] [PubMed] [Google Scholar]
- 18.Cacev T, Radosevic S, Krizanac S, et al. Influence of interleukin-8 and interleukin-10 on sporadic colon cancer development and progression. Carcinogenesis 2008; 29:1572–1580. [DOI] [PubMed] [Google Scholar]
- 19.Cozar JM, Romero JM, Aptsiauri N, et al. High incidence of CTLA-4 AA (CT60) polymorphism in renal cell cancer. Hum Immunol 2007; 68:698–704. [DOI] [PubMed] [Google Scholar]
- 20.Crivello A, Giacalone A, Vaglica M, et al. Regulatory cytokine gene polymorphisms and risk of colorectal carcinoma. Ann N Y Acad Sci 2006; 1089:98–103. [DOI] [PubMed] [Google Scholar]
- 21.Crusius JB, Canzian F, Capella G, et al. Cytokine gene polymorphisms and the risk of adenocarcinoma of the stomach in the European prospective investigation into cancer and nutrition (EPIC-EURGAST). Ann Oncol 2008; 19:1894–1902. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira JG, Rossi AF, Nizato DM, et al. Profiles of gene polymorphisms in cytokines and Toll-like receptors with higher risk for gastric cancer. Dig Dis Sci 2013; 58:978–988. [DOI] [PubMed] [Google Scholar]
- 23.Deans C, Rose-Zerilli M, Wigmore S, et al. Host cytokine genotype is related to adverse prognosis and systemic inflammation in gastro-oesophageal cancer. Ann Surg Oncol 2007; 14:329–339. [DOI] [PubMed] [Google Scholar]
- 24.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 2003; 124:1193–1201. [DOI] [PubMed] [Google Scholar]
- 25.Forte GI, Cala C, Scola L, et al. Role of environmental and genetic factor interaction in age-related disease development: the gastric cancer paradigm. Rejuvenation Res 2008; 11:509–512. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Gonzalez MA, Lanas A, Quintero E, et al. Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: a Nationwide Multicenter Study in Spain. Am J Gastroenterol 2007; 102:1878–1892. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Hormazabal P, Musleh M, Bustamante M, et al. Role of cytokine gene polymorphisms in gastric cancer risk in Chile. Anticancer Res 2014; 34:3523–3530. [PubMed] [Google Scholar]
- 28.Gunter MJ, Canzian F, Landi S, et al. Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev 2006; 15:1126–1131. [DOI] [PubMed] [Google Scholar]
- 29.Guo W, Wang N, Wang YM, et al. Interleukin-10-1082 promoter polymorphism is not associated with susceptibility to esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma in a population of high-incidence region of north China. World J Gastroenterol 2005; 11:858–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He B, Pan Y, Xu Y, et al. Increased risk for gastric cancer in carriers of the lymphotoxin-alpha+252G variant infected by Helicobacter pylori. Genet Test Mol Biomarkers 2012; 16:9–14. [DOI] [PubMed] [Google Scholar]
- 31.Heneghan MA, Johnson PJ, Clare M, et al. Frequency and nature of cytokine gene polymorphisms in hepatocellular carcinoma in Hong Kong Chinese. Int J Gastrointest Cancer 2003; 34:19–26. [DOI] [PubMed] [Google Scholar]
- 32.Hsing AW, Sakoda LC, Rashid A, et al. Variants in inflammation genes and the risk of biliary tract cancers and stones: a population-based study in China. Cancer Res 2008; 68:6442–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamangar F, Abnet CC, Hutchinson AA, et al. Polymorphisms in inflammation-related genes and risk of gastric cancer (Finland). Cancer Causes Control 2006; 17:117–125. [DOI] [PubMed] [Google Scholar]
- 34.Kang JM, Kim N, Lee DH, et al. The effects of genetic polymorphisms of IL-6, IL-8, and IL-10 on Helicobacter pylori-induced gastroduodenal diseases in Korea. J Clin Gastroenterol 2009; 43:420–428. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Cho YA, Choi IJ, et al. Effects of interleukin-10 polymorphisms, Helicobacter pylori infection, and smoking on the risk of noncardia gastric cancer. PloS One 2012; 7:e29643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko KP, Park SK, Cho LY, et al. Soybean product intake modifies the association between interleukin-10 genetic polymorphisms and gastric cancer risk. J Nutr 2009; 139:1008–1012. [DOI] [PubMed] [Google Scholar]
- 37.Lee JY, Kim HY, Kim KH, et al. Association of polymorphism of IL-10 and TNF-A genes with gastric cancer in Korea. Cancer Lett 2005; 225:207–214. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Chang SC, Goldstein BY, et al. Green tea consumption, inflammation and the risk of primary hepatocellular carcinoma in a Chinese population. Cancer Epidemiol 2011; 35:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Song B, Wang JL, et al. Polymorphisms of interleukin-10 promoter are not associated with prognosis of advanced gastric cancer. World J Gastroenterol 2011; 17:1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu W, Pan K, Zhang L, et al. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis 2005; 26:631–636. [DOI] [PubMed] [Google Scholar]
- 41.Macarthur M, Sharp L, Hold GL, et al. The role of cytokine gene polymorphisms in colorectal cancer and their interaction with aspirin use in the northeast of Scotland. Cancer Epidemiol Biomarkers Prevent 2005; 14:1613–1618. [DOI] [PubMed] [Google Scholar]
- 42.Migita K, Miyazoe S, Maeda Y, et al. Cytokine gene polymorphisms in Japanese patients with hepatitis B virus infection–association between TGF-beta1 polymorphisms and hepatocellular carcinoma. J Hepatol 2005; 42:505–510. [DOI] [PubMed] [Google Scholar]
- 43.Morgan DR, Dominguez RL, Keku TO, et al. Gastric cancer and the high combination prevalence of host cytokine genotypes and Helicobacter pylori in Honduras. Clin Gastroenterol Hepatol 2006; 4:1103–1111. [DOI] [PubMed] [Google Scholar]
- 44.Nieters A, Yuan JM, Sun CL, et al. Effect of cytokine genotypes on the hepatitis B virus-hepatocellular carcinoma association. Cancer 2005; 103:740–748. [DOI] [PubMed] [Google Scholar]
- 45.Ognjanovic S, Yuan JM, Chaptman AK, et al. Genetic polymorphisms in the cytokine genes and risk of hepatocellular carcinoma in low-risk non-Asians of USA. Carcinogenesis 2009; 30:758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan XF, Yang SJ, Loh M, et al. Interleukin-10 gene promoter polymorphisms and risk of gastric cancer in a Chinese population: single nucleotide and haplotype analyses. Asian Pac J Cancer Prev 2013; 14:2577–2582. [DOI] [PubMed] [Google Scholar]
- 47.Savage SA, Abnet CC, Haque K, et al. Polymorphisms in interleukin -2, -6, and -10 are not associated with gastric cardia or esophageal cancer in a high-risk chinese population. Cancer Epidemiol Biomarkers Prevent 2004; 13:1547–1549. [PubMed] [Google Scholar]
- 48.Saxena R, Chawla YK, Verma I, et al. Association of interleukin-10 with hepatitis B virus (HBV) mediated disease progression in Indian population. Indian J Med Res 2014; 139:737–745. [PMC free article] [PubMed] [Google Scholar]
- 49.Scola L, Giacalone A, Marasa L, et al. Genetic determined downregulation of both type 1 and type 2 cytokine pathways might be protective against pancreatic cancer. Ann N Y Acad Sci 2009; 1155:284–288. [DOI] [PubMed] [Google Scholar]
- 50.Shin CM, Kim N, Lee HS, et al. Intrafamilial aggregation of gastric cancer: a comprehensive approach including environmental factors, Helicobacter pylori virulence, and genetic susceptibility. Eur J Gastroenterol Hepatol 2011; 23:411–417. [DOI] [PubMed] [Google Scholar]
- 51.Shin HD, Park BL, Kim LH, et al. Interleukin 10 haplotype associated with increased risk of hepatocellular carcinoma. Hum Mol Genet 2003; 12:901–906. [DOI] [PubMed] [Google Scholar]
- 52.Sicinschi LA, Lopez-Carrillo L, Camargo MC, et al. Gastric cancer risk in a Mexican population: role of Helicobacter pylori CagA positive infection and polymorphisms in interleukin-1 and -10 genes. Int J Cancer 2006; 118:649–657. [DOI] [PubMed] [Google Scholar]
- 53.Sugimoto M, Furuta T, Shirai N, et al. Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. J Gastroenterol Hepatol 2007; 22:1443–1449. [DOI] [PubMed] [Google Scholar]
- 54.Sun JM, Li Q, Gu HY, et al. Interleukin 10 rs1800872 T>G polymorphism was associated with an increased risk of esophageal cancer in a Chinese population. Asian Pac J Cancer Prev 2013; 14:3443–3447. [DOI] [PubMed] [Google Scholar]
- 55.Talseth BA, Meldrum C, Suchy J, et al. Lack of association between genetic polymorphisms in cytokine genes and disease expression in patients with hereditary non-polyposis colorectal cancer. Scand J Gastroenterol 2007; 42:628–632. [DOI] [PubMed] [Google Scholar]
- 56.Tseng LH, Lin MT, Shau WY, et al. Correlation of interleukin-10 gene haplotype with hepatocellular carcinoma in Taiwan. Tissue Antigens 2006; 67:127–133. [DOI] [PubMed] [Google Scholar]
- 57.Tsilidis KK, Helzlsouer KJ, Smith MW, et al. Association of common polymorphisms in IL10, and in other genes related to inflammatory response and obesity with colorectal cancer. Cancer Causes Control 2009; 20:1739–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vishnoi M, Choudhuri G, Mittal B. Is IL-10-819C/T gene polymorphism modulating the risk of gallbladder disease in north Indian population? J Gastrointest Cancer 2007; 38:46–51. [DOI] [PubMed] [Google Scholar]
- 59.Wu MS, Wu CY, Chen CJ, et al. Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int J Cancer 2003; 104:617–623. [DOI] [PubMed] [Google Scholar]
- 60.Zambon CF, Basso D, Navaglia F, et al. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine 2005; 29:141–152. [DOI] [PubMed] [Google Scholar]
- 61.Zeng X, Li Y, Liu T, et al. Diverse H. pylori strains, IL-10 promoter polymorphisms with high morbidity of gastric cancer in Hexi area of Gansu Province, China. Mol Cell Biochem 2012; 362:241–248. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Y, Hu W, Zhuang W, et al. Interleukin-10-1082 promoter polymorphism and gastric cancer risk in a Chinese Han population. Mol Cell Biochem 2011; 347:89–93. [DOI] [PubMed] [Google Scholar]
- 63.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol 2008; 180:5771–5777. [DOI] [PubMed] [Google Scholar]
- 64.Kole A, Maloy KJ. Control of intestinal inflammation by interleukin-10. Curr Top Microbiol Immunol 2014; 380:19–38. [DOI] [PubMed] [Google Scholar]
- 65.Giacomelli L, Gianni W, Belfiore C, et al. Persistence of epidermal growth factor receptor and interleukin 10 in blood of colorectal cancer patients after surgery identifies patients with high risk to relapse. Clin Cancer Res 2003; 9:2678–2682. [PubMed] [Google Scholar]
- 66.Chan SL, Mo FK, Wong CS, et al. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer 2012; 118:3984–3992. [DOI] [PubMed] [Google Scholar]
- 67.Jung M, Weigert A, Tausendschon M, et al. Interleukin-10-induced neutrophil gelatinase-associated lipocalin production in macrophages with consequences for tumor growth. Mol Cell Biol 2012; 32:3938–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma YY, He XJ, Wang HJ, et al. Interaction of coagulation factors and tumor-associated macrophages mediates migration and invasion of gastric cancer. Cancer Sci 2011; 102:336–342. [DOI] [PubMed] [Google Scholar]
- 69.Huhn RD, Radwanski E, Gallo J, et al. Pharmacodynamics of subcutaneous recombinant human interleukin-10 in healthy volunteers. Clin Pharmacol Ther 1997; 62:171–180. [DOI] [PubMed] [Google Scholar]
- 70.Kube D, Platzer C, von Knethen A, et al. Isolation of the human interleukin 10 promoter. Characterization of the promoter activity in Burkitt's lymphoma cell lines. Cytokine 1995; 7:1–7. [DOI] [PubMed] [Google Scholar]
- 71.Dicken BJ, Bigam DL, Cass C, et al. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 2005; 241:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li C, Tong W, Liu B, et al. The -1082A>G polymorphism in promoter region of interleukin-10 and risk of digestive cancer: a meta-analysis. Sci Rep 2014; 4:5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ni P, Xu H, Xue H, et al. A meta-analysis of interleukin-10-1082 promoter polymorphism associated with gastric cancer risk. DNA Cell Biol 2012; 31:582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Ding Q, Shi Y, et al. The interleukin-10-1082 promoter polymorphism and cancer risk: a meta-analysis. Mutagenesis 2012; 27:305–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


