Supplemental Digital Content is available in the text
Abstract
To evaluate the influences of using intracranial pressure (ICP) monitoring on the prognosis of patients with severe traumatic brain injury.
Systematic search were conducted in PubMed, Embase, Cochrane Library, Wanfang, and CNKI. The eligible studies were identified for pooling analysis under fixed- or random effects model. Hospital mortality, functional outcomes, length of hospital stay, and the related complications in patients were extracted.
Six randomized controlled trials with 880 cases and 12 cohort studies with 12,606 cases were included. Combined analysis found that ICP monitoring was effective for reducing the risk rate of electrolyte disturbances (RR = 0.47, 95% confidence interval (CI): 0.63–0.90), rate of renal failure (RR = 0.50, 95% CI: 0.30–0.83), and for improving favorable prognosis (RR = 1.15, 95% CI: 1.00–1.35). However, ICP monitoring was not significant for hospital mortality (RR = 0.91, 95% CI: 0.77–0.1.06), decreasing rate of pulmonary infection (RR = 0.93, 95% CI: 0.76–1.14), rate of mechanical ventilation (RR = 1.02, 95% CI: 0.86–1.09), and duration of hospital stays (weighted mean difference (WMD) = 0.06, 95% CI: −0.03, 0.16).
ICP monitoring may not reduce the risk of hospital mortality, but plays a role in decreasing the rate of electrolyte disturbances, rate of renal failure, and increasing favorable functional outcome. However, effect of other outcomes need to be further confirmed in the future randomized controlled trials (RCTs) with larger sample size.
INTRODUCTION
Despite the fact that the morbidity and mortality have declined every 10 years, traumatic brain injury (TBI) is still a significant cause of death and disability during emergency room visits and hospital stays.1,2 One severe complications of moderate or severe TBI is the acute increased intracranial pressure (ICP). Approximately 50% of TBI comatose patients with abnormal computed tomography (CT) scan also suffer from high ICP.3 The ICP elevation could cause interference of blood circulation, decline of perfusion pressure, obstruction of venous reflux, delay of intracranial blood flow, and even brain damage, brain shift, and cerebral hernia.4 Finally, patients usually died from the secondary brain stem injury.5 Therefore, early diagnosis and treatment of ICP could significantly improve the prognosis of TBI patients. The ICP monitoring is a method that records the dynamic change of ICP by a pressure monitor or sensor and play it out through a digital signal and image.6 As ICP cannot be predicted through routine clinical examinations and its dynamic change after severe TBI, ICP becomes an effective tool for its diagnosis and treatment.7 Several lines of evidences reported that the ICP monitoring could improve the prognosis after severe TBI. A study with 2134 patients found that ICP monitoring could reduce the mortality of severe TBI compared with patients without ICP monitoring.8 Another study involving 10,628 severe TBI patients confirms that ICP monitoring is associated with lower mortality.9 A prospective study also suggests that patients managed according to the Brain Trauma Foundation ICP guidelines experienced significantly improved survival.10 However, some studies report negative reports. A study with a sample size of 1646 shows a higher mortality and worse neurological dysfunctions occurred after ICP monitoring.11 A retrospective analysis did not find significant difference between the ICP monitoring group and the control group.12 It is clear that the effectiveness and safety of ICP monitoring remains controversial.
A recent meta-analysis gave a negative finding that TBI patients did not benefit from ICP monitoring.13 We read this meta-analysis with great interest and found it did not include several important studies, which may reduce the convincingness of this conclusion. Thus, we retrieved the relevant databases, made stricter inclusion and exclusion and conducted a more comprehensive analysis with the aim to provide stronger evidence for clinical practice.
MATERIALS AND METHODS
The ethical approval is not needed because this is a systematic review and meta-analysis of published studies.
Search Strategy
We systematically searched the following electronic databases from initial to May 31, 2015: PubMed, Web of Science, and 2 Chinese databases (China National Knowledge Internet and Wangfang). The following keywords were used: “ICP monitoring” OR “intracranial pressure monitoring” OR “brain injury” OR “head injury” OR “severe traumatic brain injury.” We also retrieved the references in the publications so as not to miss any relevant studies. The search language was limited to English and Chinese.
Inclusion and Exclusion Criteria
All included studies should meet the following criteria: the study design is a randomized controlled trial (RCT), case–control study or cohort study; subject are these patients diagnosed with severe TBI within Glasgow Coma Scale (GCS) < 8 within 24 hours after injury, or in case of no GCS scores, the Abbreviated Injury Scale (AIS) ≧ 3; ICP monitoring was used for severe TBI, and other clinical intervention was used for the control group. One of the following outcome indices for pooled analysis: hospital mortality, functional outcomes, length of hospital stay, and the related complications in patients. These patients (the ICP monitoring group) with GCS > 8 or AIS > 3 or who died within 24 hours were excluded. These studies without enough information were also excluded.
Data Extraction and Quality Assessment
From the studies included in this meta-analysis, we extracted the following information: the first author, publication years, study design, number of patients, age range, sex ratio, in-hospital mortality, favorable outcomes (GOS ≥ 4 or GOS-E ≥ 5), length of hospital stay and related complications. Two researchers collected the above information, and cross-check was conducted to guarantee its accuracy. Any disagreement between them was resolved via judgment from the third researcher. The chance-adjusted interrater agreement for data extraction was substantial (kappa statistic = 0.82; 95% confidence interval (CI): 0.67–0.93).
We used the Newcastle-Ottawa scale (NOS) to evaluate the quality of included cohort study according to Cochrane bias assessment criterion.14 The NOS scale includes selection (3 questions), comparability (1 question), and outcome (3 question) with 10 cores. We used to Cochrane Collaboration tool to evaluate the quality of RCT. The evaluation tool consisted of the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Study with or without 1 or more items was considered to have high-risk bias, low risk and unclear, respectively.
Statistical Analysis
The whole analysis was conducted on Stata 11.0 (College Station, TX). The indices included here was in-hospital mortality, functional outcomes, length of hospital stay, and related complications. The heterogeneity among the studies was evaluated by Cochran Q test and I2 statistic. I2 < 25% is considered low, 25% to 50% is moderate, and >50% is treated as high-level heterogeneity, respectively.15–17 A random-effect model was used if heterogeneity existed and otherwise, a fixed-effect model was applied. For continuous data, pooled estimation was calculated using the weighted mean difference (WMD) or standardized mean difference (SMD). Odds ratio (OR) was used for categorical variable. Both of them were followed by 95% CI. Sensitivity analysis was also conducted to explore the potential factors of heterogeneity and pooled estimated stability. Publication bias was evaluated by testing funnel plot asymmetry, Begg test and Egger test. Significance was set at a P-value of less than 0.05. This is a meta-analysis and do not apply the ethic stamens.
RESULTS
Study Selection Flow
Our initial search returned 930 records, of which 595 articles went through abstract screening after exclusion of repetitive publications and reviews, and 62 studies went through full-text review. We got 2 studies from the cited records. Finally, 18 studies, including 6 RCTs18–23 and 12 cohort studies8,11,24–33 met the inclusion criteria. The flow chart of screening literatures is presented in Figure 1 and checklist is given in Table S2.
FIGURE 1.
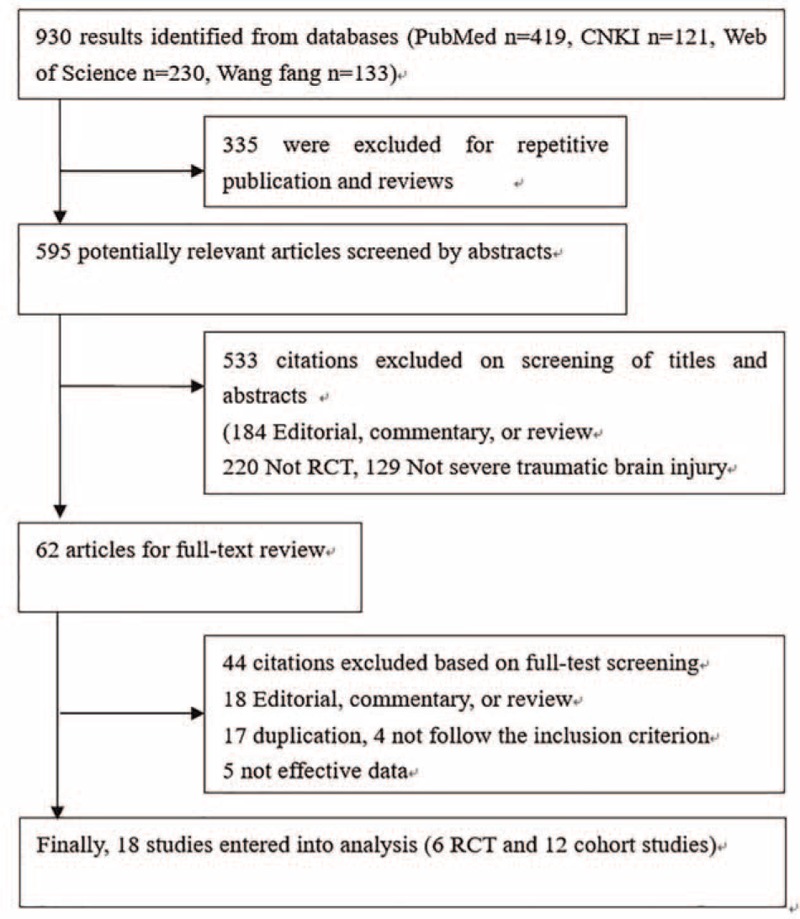
Flow diagram of the study selection process.
General Characteristics of Included Studies
All included studies were published from 2000 to 2013. The 6 RCTs included 880 TBI patients (ICP+: 435 and ICP−: 445), 12 cohort studies consisted of 12606 TBI patients (ICP+: 4303 and ICP−: 8303). The rate of men from the included studies ranged from 61% to 95.8%. Although some studies used different criteria for TBI patients, they all used GCS ≦ 8 as the ICP monitoring criteria. All studies report in-hospital mortality. Favorable outcome was presented in 9 studies,18,21–23,27,30–33 occurrence of renal failure was recorded in 4 studies,19,21–23 electrolyte disturbance was reported in 3 studies,19,21,22 pulmonary infection was found in 4 studies,18,19,21,23 2 cohort studies gave hospital stays,11,30 and 2 cohort studies shown the use of mechanical ventilation.24,25 The general characteristics of the included studies are provided as supplementary materials (Table S1).
Quality of Assessment
Different evaluation criteria were used as the different design. The cohort studies refer to the NOS (see Supplementary materials 3). The score of 12 cohort studies ranged from 6 to 8, and the mean score is more than 7. One study is lack of outcome of ascertainment of exposure, 7 study were lack of length of follow-up, and none have adequacy of follow-up. We used to Cochrane Collaboration tool to evaluate the quality of RCT. The risk bias of 6 RCTs is shown in Figure 2. Two trials were considered to have high-risk bias, and 4 studies were judged to be at unclear risk of bias. Among 6 RCTs, none were double-blinded. But, it is quite difficult to conduct a double-blinded in these trials, and we thought that the lack of blinding may not have effect on the primary outcome. The evaluation results were limited in our opinion.
FIGURE 2.
Risk of bias summary.
Pooled Analysis
Different from previous studies that provided the whole rate in all patients or total sample, we also conducted a meta-analysis to estimate different types of rates. Table 1 lists out the occurrence rates of different outcomes, and Table 2 shows the risk difference of different outcomes between the ICP monitoring group and the non-ICP group.
TABLE 1.
Summary of Prevalence for Different Outcomes in Patients With TBI
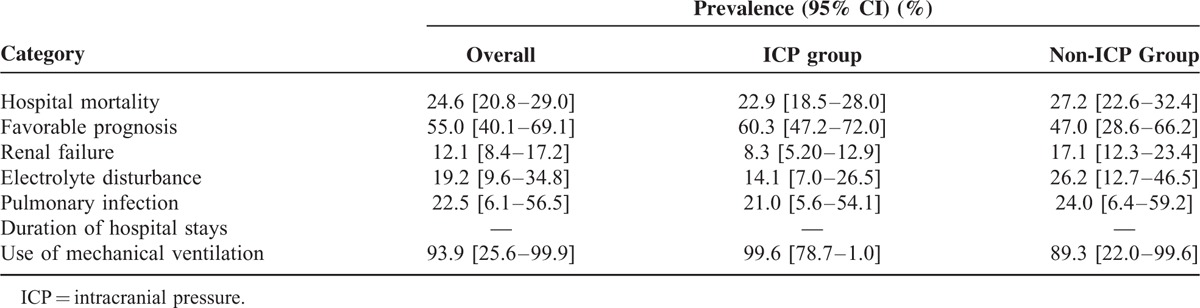
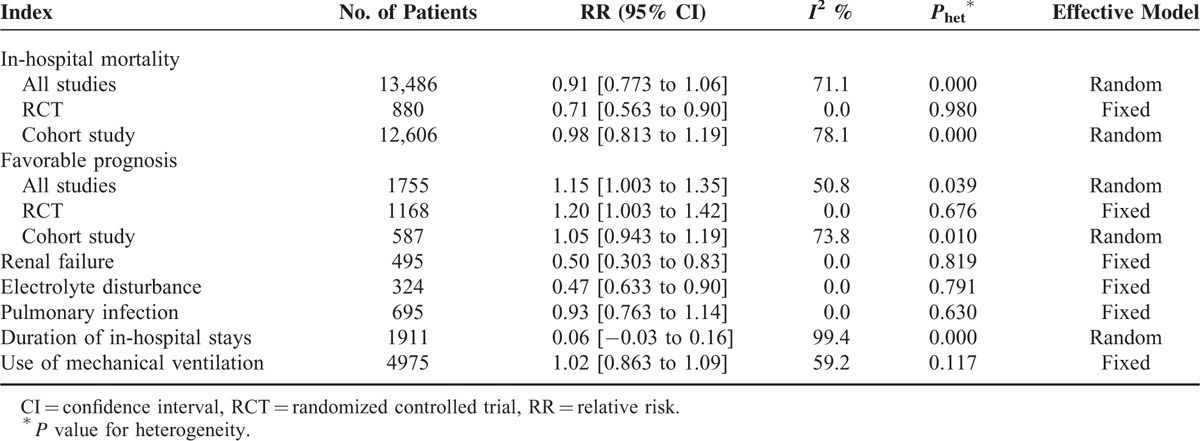
TABLE 2.
Summary of Different Category Results
In-Hospital Mortality
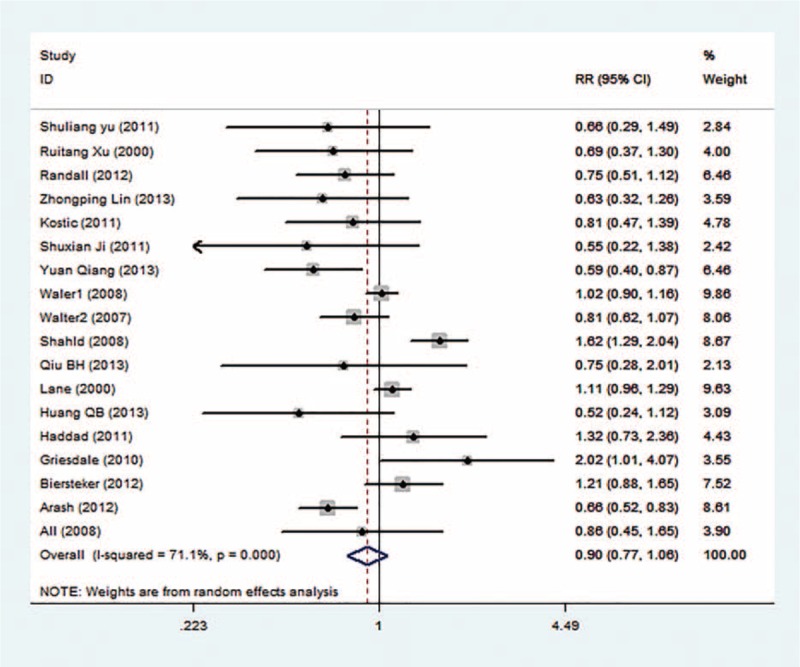
Mortality was observed in all studies with 13,486 patients. The meta-analysis shows that the in-hospital mortality rate is 24.6% in all TBI patients, but it is not significantly different between the ICP group and the non-ICP group (22.9% vs 27.2%), with pooled RR = 0.91 (95% CI: 0.77–1.06, Figure 3) in the random-effect model (I2 = 71.1%, P = 0.000). The mortality rates are significantly different between groups in the RCTs (RR = 0.71, 95% CI: 0.86–0.90), but not significant in the cohort studies (RR = 0.98, 95% CI: 0.81–1.19). The heterogeneity within studies and selection of effect model is shown in Table 2.
FIGURE 3.
Forest plot of ICP monitoring in the prevention of mortality.
Favorable Prognosis
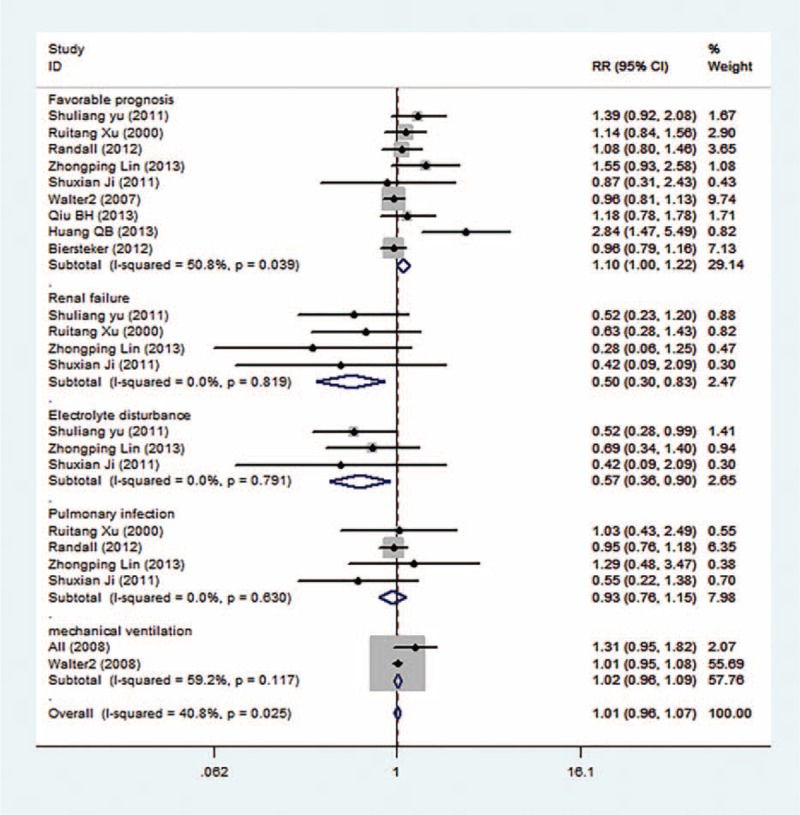
There was heterogeneity within studies, so a random-effective model was used (I2 = 50.8%, P = 0.039, Figure 4). The rate of favorable prognosis for all TBI patients is 55.0%, but it is significantly higher in the ICP group versus the non-ICP group (60.3% vs 47.0%). There was no heterogeneity within studies, so a fixed-effective model was used. The ICP group got a 1.15-fold higher prognosis versus the non-ICP group (RR = 1.15, 95% CI: 1.00–1.35).
FIGURE 4.
Forest plots of ICP monitoring in the prevention of different complications.
Renal Failure and Electrolyte Disturbance
The occurrence rate of renal failure for all TBI patients is 12.1%, but it is lower in the ICP group versus the non-ICP group (8.3% vs 17.1%). The difference analysis shows that the risk of renal failure for the ICP group is lower versus the non-ICP group (RR = 0.50, 95% CI: 0.30–0.83, Figure 4) in the fixed-effective model (I2 = 0.0%, P = 0.819). The rate of electrolyte disturbance is 19.2% among all TBI patients, but it is significantly lower in the ICP group versus the non-ICP group (14.1% vs 26.2%). The risk ratio was 0.47, with 95% CI from 0.63 to 0.90 under the fixed-effective model (I2 = 0.0%, P = 0.791). The non-ICP patients tend to suffer from electrolyte disturbance.
Pulmonary Infection
The rate of pulmonary infection among TBI patients is 22.5%, but it is almost equal between the ICP group and the non-ICP group (21.0% vs 24.0%). The difference analysis shows that ICP monitoring cannot relieve pulmonary infection (RR = 0.93, 95% CI: 0.76–1.14, Figure 4) in the fixed-effective model (I2 = 0.0%, P = 0.630).
Duration of Hospital Stays and Mechanical Ventilation
The ICP monitoring seemingly did not shorten the in-hospital duration for severe TBI patients (WMD = 0.06, 95% CI: −0.03, 0.16, Figure 4). There was no significant difference between 2 groups in the random-effective model (I2 = 99.4%, P = 0.000). The use rate of mechanical ventilation is 93.9% for all TBI patients, and the rates are not significantly different between the 2 groups (99.6% vs 89.3%) (RR = 1.02, 95% CI: 0.86–1.09) under the fixed-effective model (I2 = 59.2%, P = 0.117).
Sensitivity Analysis
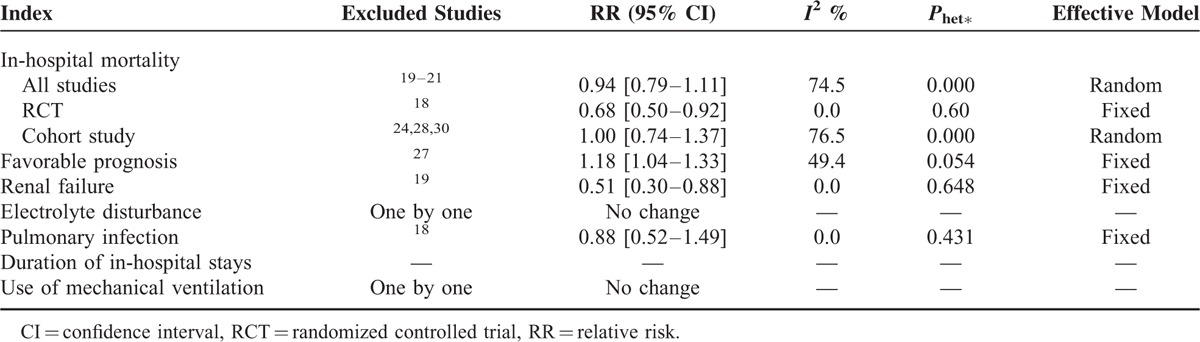
We used 2 different ways to perform sensitivity analysis. First, we sequentially excluded individual studies one by one, and the summary RRs were not significantly altered, which indicated that the pooled results were stable (Figure S2). Second, we purposely exclude some studies such study with few sample size, higher weight in the meta-analysis. We compare the original results with the ones with excluding studies, the results also did not change significantly (Table 3).
TABLE 3.
Sensitivity Analysis of Different Category for Various Outcomes
Publication Bias
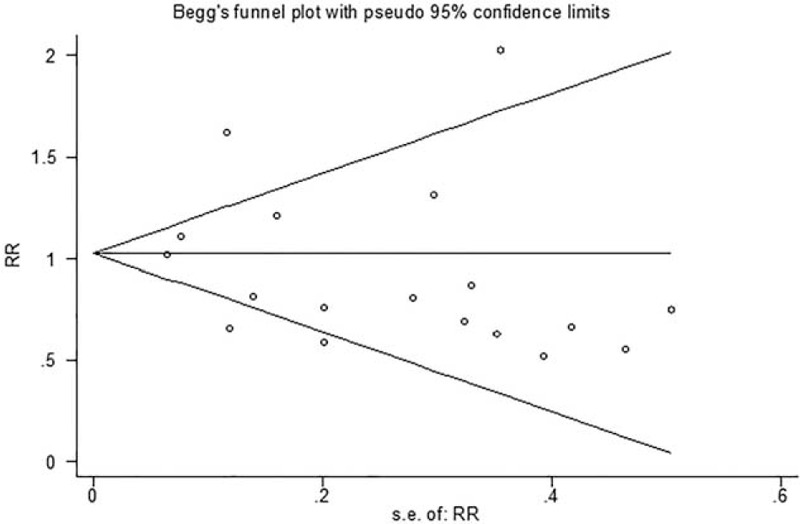
Begg funnel plot and Egger tests were performed to evaluate the publication bias of the included articles. We treated the pooled mortality analysis with the most studies as the assessment standard. The Begg test and modified Egger linear regression test indicated that no significant publication bias (Z = 0.08, P = 0.940; t = −0.95, P = 0.357, Figure 5).
FIGURE 5.
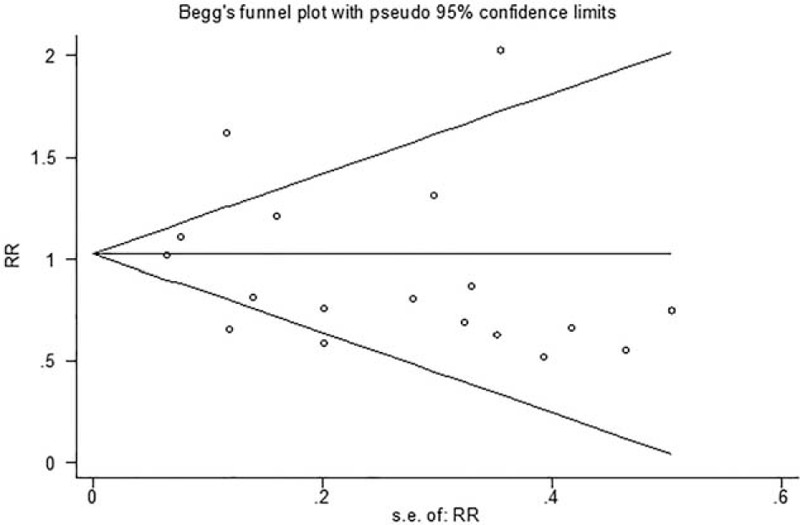
Funnel plots of publication bias for mortality.
DISCUSSION
The present meta-analysis consisted of 6 RCTs and 12 cohort studies with a sample size of 13,486 severe TBI patients, which ensures the reliability of the results. The comprehensive analysis found that ICP monitoring for severe TBI patients cannot reduce the mortality of hospital, pulmonary infection rate, use of mechanical ventilation and duration of hospital stays, but lower the occurrence rate of electrolyte disturbance and renal failure and improving prognosis for these patients.
Our results are different from the previous meta-analysis involved fewer studies and smaller sample size.13 The previous meta-analysis failed to find any benefit in TBI patients from ICP monitoring. Compared with this finding, our study has several strengths. First, we include more comprehensive studies and a larger sample size while the previous study missed 4 RCTs19,21–23 and 5 cohort studies.8,30–33 Second, the previous meta-analysis had some shortcomings in statistic methods as it used odds ratio (OR). However, OR is inappropriate for RCTs or cohort studies, which should be evaluated by relative risk (RR).34 Moreover, the previous meta-analysis directly calculated the mortality of in-hospital and occurrence rates of other outcomes by using the sums of the inputted data. Actually, there are special methods of meta-analysis. The estimator assumes a model of the form: x[i] = mu + b[i] + e[i] in which b[i] is drawn from N (0, tau2) and e[i] is drawn from N (0, sigma[i]2). The estimator forms a direct calculation of tau, and uses this to form revised estimates of standard error sqrt (s[i]2 + tau2) in x, calculates weights as the inverse of these and in turn calculates a weighted mean, allowing for any calculated excess variance tau2.35 Third, the previous meta-analysis found that ICP monitoring could not lower the mortality while we give an opposite result. Although the total synthesis shows no significance, the results only included RCTs found that TBI patients could benefit from ICP monitoring. Considering that RCT is the optimum design to evaluate the effect of intervention, we supposed that the present results are quite reliable. Finally, our study shown some outcome indices that were not mentioned in the previous meta-analysis, and the findings further broaden the potential benefits and support of ICP monitoring. We also noticed that a recent study also reported the Impact of ICP on prognosis of patients with brain injury.36 Our study is different from the recently published studies. The population of published study was limited in all TBI patients, and ours included severe TBI patients. The difference in study population could affect the results. Actually, it indeed has some effects. The published study found no evidences that ICP monitoring overall is significantly superior to no ICP monitoring in terms of the mortality of TBI patients. However, our RCT results found ICP could reduce the mortality of severs TBI patients. Besides, the published only consisted of one outcome (mortality) and the present study reported more comprehensive results.
The previous study has reported that ICP monitoring have good accuracy. The mean error has few influences on ICP measuring results although there are some errors. This may be one of the reasons that ICP monitoring is widely used in clinical practice, especially for TBI patients.37 Severe TBI usually causes the increase of ICP and reduction of cerebral perfusion pressure38 The traditional nerve function examination could not predict ICP because of its dynamic change over time. In the past 30 years, we found that the ICP elevation is directly associated with unfavorable outcomes, which highlights the importance of consecutive ICP monitoring.39 Most scholars agree on the following criteria: IPV = 5 to 15, 15 to 20, 20 to 40, and >40 mmHg indicate normal, slight elevation, moderate elevation, and severe elevation, respectively.40 In clinical practice, ICP > 20 mmHg is usually treated as the cutoff value of pressure reduction. The Guidelines for Severe TBI Management VI list the following indications for ICP monitoring: 3 < GSW < 8 after injury, abnormal findings on skull CT; 3 < GSW < 8 after injury, normal skull CT, but meeting 2 or more of the following criteria: age > 40, unilateral or bilateral decorticated syndromes, systolic pressure < 11.97 kPa.41 The criteria in China are a little different from the above criteria. The Chinese scholars treat ICP ≤ 2.66 kPa as the target for treatment. In spite of the controversy since its appearance, ICP monitoring has been widely used in clinical practice. The rate of ICP monitoring is 77.4% in America, 44.5% in Australia, and 57% in UK.24,42,43 However, the rate is less than 5% according to the Chinese TBI data from December 2008 to August 2009.44 Therefore, China has a long way to standardize diagnosis and treatment.
The morbidity and mortality of TBI patients are significant causes of death and disability among the emergency room visits and hospital stays. The present study finds no evidences that ICP could reduce the hospital mortality. The reasons could be the following. First, it is known that patients with ICP monitoring could receive increased treatment interventions according to the acquired information such as oxygen supply. It is possible for these patients to improve outcomes. However, some clinical therapy such as sedation could be related to adverse outcomes. Lack of knowledge could be a confounding factor on the negative results. Second, it is likely that ICP monitoring could benefit a special type of TBI patients. It is a pointcut that the future study should focus on the more specific patterns of TBI patients. Third, it should arouse our attention that there is still a gap from information from ICP monitoring to clinical practice. The interventions usually change with ICP or cerebral perfusion pressure.45 However, the cutoff values of cerebral perfusion pressure have not been built. The TBI patients could benefit from these regardless of the current findings. Finally, there are some differences between results from observational and RCT. The latter obtained obvious significant findings, but the results still need to be confirmed in the future research because the number of RCT only have 6 studies.
LIMITATIONS
There are still some limitations in this study. First, our literature search was limited in the online electronic databases and did not include some unpublished data. These unpublished may exert some effect on the results. Second, the study number of electrolyte disturbance and pulmonary infections indeed are in limited quantities and we admit that there may weaken the reliability of results to some extent. But it is still stronger than a single study. We should be more cautious when explain this point. Third, the sample size of several RCTs was small, and study with larger sample size is needed in the future. Finally, in spite that Begg test and modified Egger linear regression test and indicated that no significant publication bias, the funnel plot shows slight asymmetry. It shown that there existed some publication bias.
CONCLUSION
In conclusion, ICP monitoring may play a role in decreasing the rate of electrolyte disturbances, rate of renal failure, and increasing favorable functional outcome. However, there was no significant effect for reducing the risk of hospital mortality, lowering occurrence rate of pulmonary infection, use of mechanical ventilation, and duration of hospital stays. RCTs with larger sample size are necessary to further support the current results.
Supplementary Material
Supplementary Material
Acknowledgment
We thank all our colleagues working in the First Affiliated Hospital of Chinese PLA General Hospital.
Footnotes
Abbreviations: AIS = Abbreviated Injury Scale, CI = confidence interval, CT = computed tomography, GCS = Glasgow Coma Scale, ICP = intracranial pressure monitoring, NOS = Newcastle-Ottawa scale, OR = odd ratios, RCT = randomized controlled trial, RR = relative risk, SMD = standardized mean difference, TBI = traumatic brain injury, WMD = weighted mean difference.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Pearson WS, Sugerman DE, McGuire LC, et al. Emergency department visits for traumatic brain injury in older adults in the United States: 2006–08. West J Emerg Med 2012; 13:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein SC, Georgoff P, Meghan S, et al. 150 years of treating severe traumatic brain injury: a systematic review of progress in mortality. J Neurotrauma 2010; 27:1343–1353. [DOI] [PubMed] [Google Scholar]
- 3.Narayan RK, Kishore PR, Becker DP, et al. Intracranial pressure: to monitor or not to monitor? A review of our experience with severe head injury. J Neurosurg 1982; 56:650–659. [DOI] [PubMed] [Google Scholar]
- 4.Kelts EA. Traumatic brain injury and visual dysfunction: a limited overview. NeuroRehabilitation 2010; 27:223–229. [DOI] [PubMed] [Google Scholar]
- 5.Alhashemi HH. Dysphagia in severe traumatic brain injury. Neurosciences (Riyadh) 2010; 15:231–236. [PubMed] [Google Scholar]
- 6.Lavinio A, Menon DK. Intracranial pressure: why we monitor it, how to monitor it, what to do with the number and what's the future? Curr Opin Anaesthesiol 2011; 24:117–123. [DOI] [PubMed] [Google Scholar]
- 7.Sahuquillo J, Biestro A. Is intracranial pressure monitoring still required in the management of severe traumatic brain injury? Ethical and methodological considerations on conducting clinical research in poor and low-income countries. Surg Neurol Int 2014; 5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farahvar A, Gerber LM, Chiu YL, et al. Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg 2012; 117:729–734. [DOI] [PubMed] [Google Scholar]
- 9.Alali AS, Fowler RA, Mainprize TG, et al. Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma 2013; 30:1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talving P, Karamanos E, Teixeira PG, et al. Intracranial pressure monitoring in severe head injury: compliance with Brain Trauma Foundation guidelines and effect on outcomes: a prospective study. J Neurosurg 2013; 119:1248–1254. [DOI] [PubMed] [Google Scholar]
- 11.Shafi S, Diaz-Arrastia R, Madden C, et al. Intracranial pressure monitoring in brain-injured patients is associated with worsening of survival. J Trauma-Injury Infect Crit Care 2008; 64:335–340. [DOI] [PubMed] [Google Scholar]
- 12.Cremer OL, van Dijk GW, van Wensen E, et al. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit Care Med 2005; 33:2207–2213. [DOI] [PubMed] [Google Scholar]
- 13.Su SH, Wang F, Hai J, et al. The effects of intracranial pressure monitoring in patients with traumatic brain injury. PLoS ONE 2014; 9:e87432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558.doi: 10.1002/sim.1186.PMID:12111919. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med 2012; 367:2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xian J, Liao J, Su H, et al. Intracranial pressure and cerebral perfusion pressure monitoring in the preventive nursing care of severe traumatic brain injury. Sci Res Essays 2011; 6:1053–1056. [Google Scholar]
- 20.Kostic A, Stefanovic I, Novak V, et al. Prognostic significance of intracranial pressure monitoring and intracranial hypertension in severe brain trauma patients. Med Pregl 2011; 64:461–465. [PubMed] [Google Scholar]
- 21.Lin ZP, Wang XT. Application of intracranial pressure monitoring in patients with severe brain trauma. Lingnan J Emergency Med 2013; 18:61–62. [Google Scholar]
- 22.Yu SL, Chen H, Ye ZH, et al. Application of continuous intracranial pressure monitoring in patients with severe traumatic craniocerebral injury. Clin Med Chin 2011; 27:612–614. [Google Scholar]
- 23.Xu RT, Zhai CM. Clinical significance of continuous intracranial pressure monitoring in patients with severe traumatic craniocerebra injury. J Postgraduates Med 2000; 23:36–37. [Google Scholar]
- 24.Mauritz W, Steltzer H, Bauer P, et al. Monitoring of intracranial pressure in patients with severe traumatic brain injury: an Austrian prospective multicenter study. Intensive Care Med 2008; 34:1208–1215. [DOI] [PubMed] [Google Scholar]
- 25.Salim A, Hannon M, Brown C, et al. Intracranial pressure monitoring in severe isolated pediatric blunt head trauma. Am Surg 2008; 74:1088–1093. [PubMed] [Google Scholar]
- 26.Haddad S, Aldawood AS, Alferayan A, et al. Relationship between intracranial pressure monitoring and outcomes in severe traumatic brain injury patients. Anaesth Intensive Care 2022; 39:1043–1050. [DOI] [PubMed] [Google Scholar]
- 27.Mauritz W, Janciak I, Wilbacher I, et al. Severe traumatic brain injury in Austria IV: intensive care management. Wien Klin Wochenschr 2007; 119:46–55. [DOI] [PubMed] [Google Scholar]
- 28.Lane PL, Skoretz TG, Doig G, et al. Intracranial pressure monitoring and outcomes after traumatic brain injury. Can J Surg 2000; 43:442–448. [PMC free article] [PubMed] [Google Scholar]
- 29.Griesdale DE, McEwen J, Kurth T, et al. External ventricular drains and mortality in patients with severe traumatic brain injury. Can J Neurol Sci 2010; 37:43–48. [DOI] [PubMed] [Google Scholar]
- 30.Biersteker HA, Andriessen TM, Horn J, et al. Factors influencing intracranial pressure monitoring guideline compliance and outcome after severe traumatic brain injury. Crit Care Med 2012; 40:1914–1922. [DOI] [PubMed] [Google Scholar]
- 31.Qiu BH, Qi ST, Zeng H, et al. Study on treatment of continuous intracranial pressure monitoring in patients with severe traumatic craniocerebra injury. Chin J Neurosurg 2013; 29:933–936. [Google Scholar]
- 32.Huang QB, Zhang Y, Su YH, et al. Prognostic correlation of intracranial pressure monitoring in patients with severe craniocerebral injury. Natl Med J China 2013; 93:1788–1790. [PubMed] [Google Scholar]
- 33.Yuang Q, Liu H, Yao HJ, et al. Impact of intracranial pressure monitoring on severe traumatic brain injury prognosis and disease afflictions. Chin J Neurosurg 2013; 29:120–124. [Google Scholar]
- 34.Stuart A, Ord JK. Kendall's Advanced Theory of Statistics. 6th ed. London: Edward Arnold; 1994. [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 36.Yuan Q, Wu X, Sun Y, et al. Impact of intracranial pressure monitoring on mortality in patients with traumatic brain injury: a systematic review and meta-analysis. J Neurosurg (United States) 2015; 122:574–587. [DOI] [PubMed] [Google Scholar]
- 37.Zacchetti L, Magnoni S, Di Corte F, et al. Accuracy of intracranial pressure monitoring: systematic review and meta-analysis. Crit Care 2015; 19:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horton JC. Continuous intracranial pressure monitoring: a last resort in pseudotumor cerebri. J Neuroophthalmol 2011; 31:199–201. [DOI] [PubMed] [Google Scholar]
- 39.Marmarou A, Saad A, Aygok G, et al. Contribution of raised ICP and hypotension to CPP reduction in severe brain injury: correlation to outcome. Acta Neurochir Suppl 2005; 95:277–280. [DOI] [PubMed] [Google Scholar]
- 40.Dubost C, Pasquier P, Merat S. Intracranial-pressure monitoring in traumatic brain injury. N Engl J Med 2013; 368:1750–1751. [DOI] [PubMed] [Google Scholar]
- 41.Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma 2007; 24:S37–S44. [DOI] [PubMed] [Google Scholar]
- 42.Matta B, Menon D. Severe head injury in the United Kingdom and Ireland: a survey of practice and implications for management. Crit Care Med 1996; 24:1743–1748. [DOI] [PubMed] [Google Scholar]
- 43.Hesdorffer DC, Ghajar J. Marked improvement in adherence to traumatic brain injury guidelines in United States trauma centers. J Trauma 2007; 63:841–847. [DOI] [PubMed] [Google Scholar]
- 44.Jiang JY, Gao GY. Chinese craniocerebral trauma in the past ten years. Chin J Neurosurg 2013; 29:109–111. [Google Scholar]
- 45.Huang SJ, Hong WC, Han YY, et al. Clinical outcome of severe head injury in different protocol-driven therapies. J Clin Neurosci 2007; 14:449–454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


