Abstract
Thyroid storm is a life-threatening and emergent manifestation of thyrotoxicosis. However, predictive features associated with fatal outcomes in this crisis have not been clearly defined because of its rarity.
The objective of this study was to investigate the associations of patient characteristics, treatments, and comorbidities with in-hospital mortality.
We conducted a retrospective observational study of patients diagnosed with thyroid storm using a national inpatient database in Japan from April 1, 2011 to March 31, 2014.
Of approximately 21 million inpatients in the database, we identified 1324 patients diagnosed with thyroid storm. The mean (standard deviation) age was 47 (18) years, and 943 (71.3%) patients were female. The overall in-hospital mortality was 10.1%. The number of patients was highest in the summer season. The most common comorbidity at admission was cardiovascular diseases (46.6%). Multivariable logistic regression analyses showed that higher mortality was significantly associated with older age (≥60 years), central nervous system dysfunction at admission, nonuse of antithyroid drugs and β-blockade, and requirement for mechanical ventilation and therapeutic plasma exchange combined with hemodialysis.
The present study identified clinical features associated with mortality of thyroid storm using large-scale data. Physicians should pay special attention to older patients with thyrotoxicosis and coexisting central nervous system dysfunction. Future prospective studies are needed to clarify treatment options that could improve the survival outcomes of thyroid storm.
INTRODUCTION
Thyroid storm, an emergent manifestation of thyrotoxicosis, is a life-threatening metabolic crisis.1 The occurrence of this disorder is rare; the estimated incidence of thyroid storm in Japan was reported to be 2.0 per million per year in a nationwide questionnaire survey from 2004 to 2008, conducted by the Japan Thyroid Association.2
Owing to its rarity, predictive features associated with enhanced survival or mortality of this disorder remain to be further elucidated. Currently, administration of antithyroid drugs (ATDs), including methimazole (MMI) and propylthiouracil (PTU), is regarded as a standard approach for treatment of thyroid storm induced by severe thyrotoxicosis.3–5 Plasmapheresis can be used as a rescue treatment to remove thyroid hormones, catecholamines, and cytokines if conventional medical treatments such as ATDs, steroids, iodine, and β-blockade are ineffective or contraindicated.6,7 However, any recommendations for the treatment of thyroid storm have merely been based on clinical experience and case series studies.3–7
The reported mortality rates of thyroid storm vary widely, being 10.7% (38 of 356 patients), 25.0% (7 of 28 patients), and 8.0% (2 of 25 patients).2,8,9 The above-mentioned hospital-based questionnaire survey conducted by the Japan Thyroid Association suggested that shock, disseminated intravascular coagulation, and multiple organ failure were associated with mortality in patients with thyroid storm, but did not include microdata of individual patients.2 Therefore, which factors are associated with mortality of thyroid storm remains incompletely defined.
The objectives of the present study were: to describe the patient characteristics and current clinical practices for treating thyroid storm, including ATD therapy and supportive measures; and to examine the factors associated with in-hospital mortality of thyroid storm, using a national inpatient database in Japan.
METHODS
Data Source
The Diagnosis Procedure Combination database is a discharge abstract and national administrative claims database for acute-care inpatients in Japan, the details of which have been described elsewhere.10–13 Briefly, 1061 hospitals participated in the database and provided data for approximately 7 million inpatient admissions in 2013. The latter figure represented approximately 50% of all inpatient admissions to acute-care hospitals in Japan. The diagnostic records are linked to a payment system, and the attending physicians are required to record the diagnoses of diseases accurately. The database includes the following data: dates of admission and discharge; patient sex and age; primary and secondary diagnoses, comorbidities at admission, and complications after admission; procedures; medications and devices used; consciousness level at admission measured with the Japan Coma Scale (JCS); and in-hospital mortality. Diagnoses are recorded using International Classification of Diseases Tenth Revision (ICD-10) codes and text data in Japanese. The JCS scores are defined as follows14: 0, alert consciousness; 1–3, wakefulness without any stimuli; 10–30, arousal by some stimuli; and 100–300, coma. JCS assessments and the Glasgow Coma Scale are well-correlated.15 The database does not include any laboratory data for serum thyroid hormone levels before or during treatment.
The Institutional Review Board at The University of Tokyo approved the present study. Because of the anonymous nature of the data, written informed consent was not required
Patient Selection and Data
From the database, we retrospectively extracted the records for all patients diagnosed as thyroid storm (ICD code: E055) from April 1, 2011 to March 31, 2014. We excluded patients with a suspected diagnosis of thyroid storm.
We examined patient sex, age, JCS score at admission, season at admission, medications, and requirements for supportive measures. Age was categorized into <20, 20–39, 40–59, 60–79, and ≥80 years. We also examined the practice patterns for treating thyroid storm in terms of variation in ATD therapy (none, MMI alone, PTU alone, and both MMI and PTU), and use of steroids, iodine, acetaminophen, cholestyramine, β-blockade, and αβ-blockade. Supportive measures included mechanical ventilation, hemodialysis (HD; intermittent or continuous renal replacement therapy), therapeutic plasma exchange (TPE), countershock, intraaortic balloon pumping, extracorporeal membrane oxygenation, and therapeutic hypothermia. Requirement for hemodiafiltration was categorized into none, HD or TPE, and both HD and TPE.
Season at admission was divided into spring (March to May), summer (June to August), autumn (September to November), and winter (December to February). We identified the following comorbidities at admission: cardiovascular diseases, respiratory diseases, gastrointestinal and hepatic diseases, renal diseases, cerebrovascular diseases, neuromuscular and psychiatric diseases, infections, pneumonia, adrenal insufficiency, diabetic ketoacidosis, trauma, disseminated intravascular coagulation, malignancy, and pregnancy. We also counted the numbers of comorbidities at admission and categorized them into 0, 1, 2, and ≥3.
Statistical Analyses
In-hospital mortality was compared between the groups using Chi-square tests. Multivariable logistic regression analyses were performed to evaluate factors associated with in-hospital mortality. In the multivariable regression model, the independent variables included age, sex, variation in ATD therapy, and number of comorbidities at admission, along with variables having values of P < 0.05 in the initial Chi-square tests. All tests were 2-tailed, and P < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS statistical package, version 22.0 (IBM, Armonk, NY).
RESULTS
Among approximately 21 million inpatient admissions in the database during the study period, we identified 1324 patients who were diagnosed with thyroid storm.
Patient Characteristics at Admission
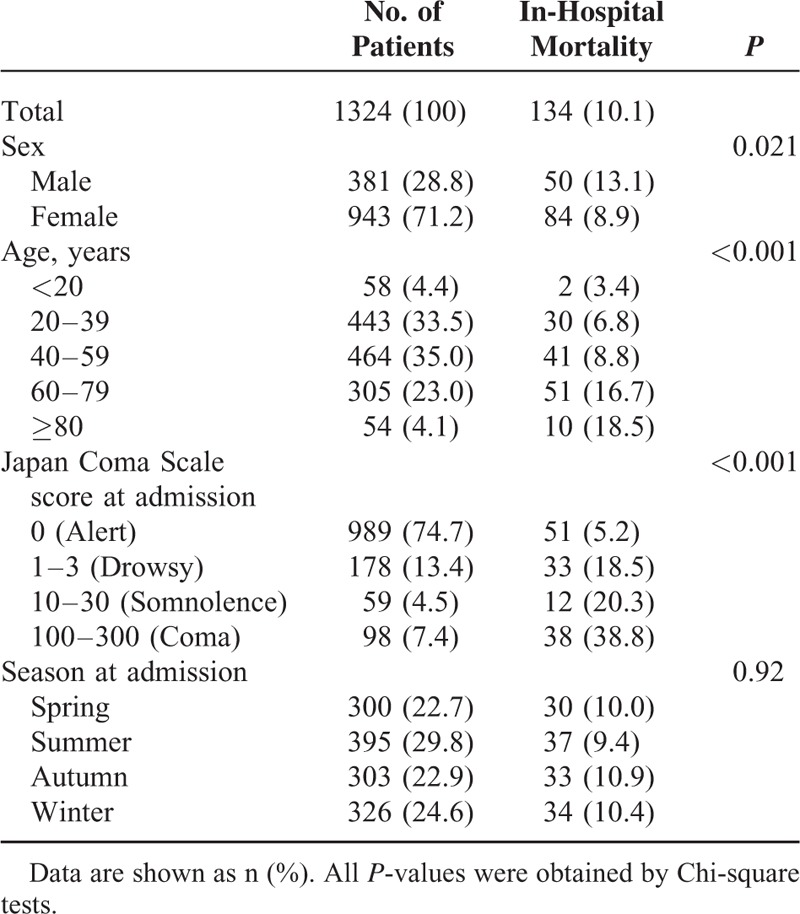
The characteristics of the patients and the in-hospital mortality rates in the subgroups are shown in Table 1. Overall, 71.2% of the patients were female. The mean (standard deviation) age was 47 (18) years. The ratio of females to males was 2.5. The summer season was associated with the highest number of patients with thyroid storm. The overall in-hospital mortality rate was 10.1%. The Chi-square tests showed significantly higher in-hospital mortality rates for male patients, higher age, and higher JCS score at admission. No significant associations with in-hospital mortality were observed for seasonality.
TABLE 1.
Patient Characteristics at Admission
Comorbidities at Admission
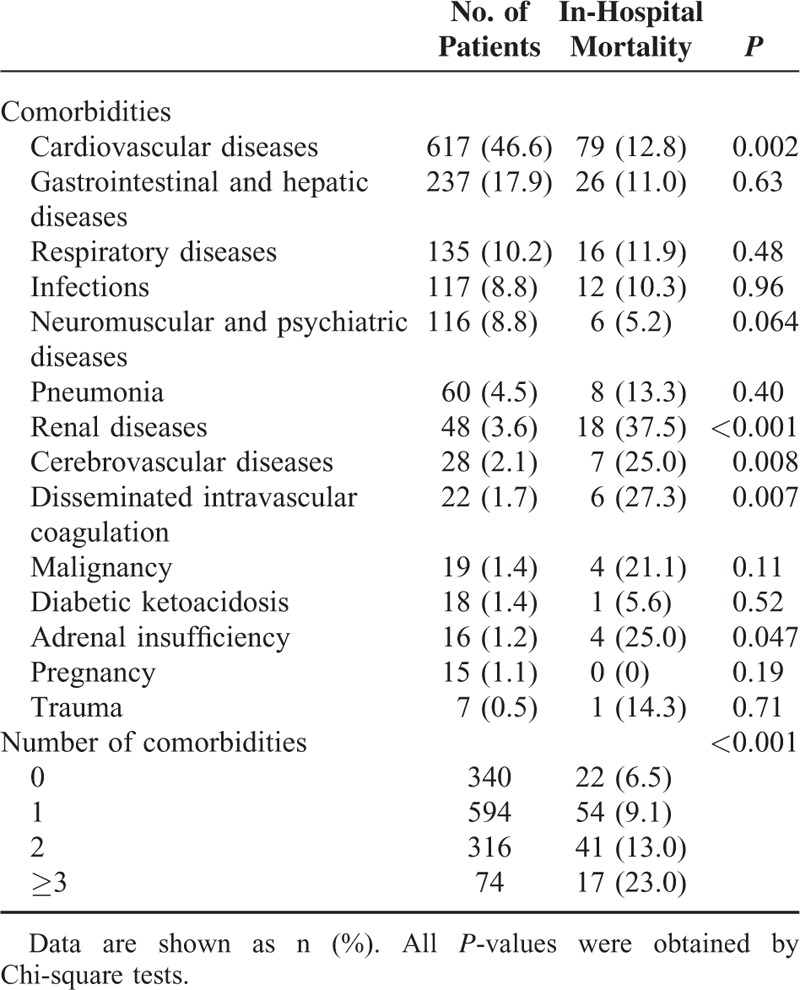
Cardiovascular diseases were the most common comorbidity at admission, followed by gastrointestinal and hepatic diseases, respiratory diseases, infections, and neuromuscular and psychiatric diseases (Table 2). The comorbidities significantly associated with in-hospital mortality were cardiovascular diseases, renal diseases, cerebrovascular diseases, disseminated intravascular coagulation, and adrenal insufficiency. The number of comorbidities at admission was significantly associated with in-hospital mortality.
TABLE 2.
Comorbidities at Admission in Patients With Thyroid Storm
Clinical Practice Patterns for Thyroid Storm
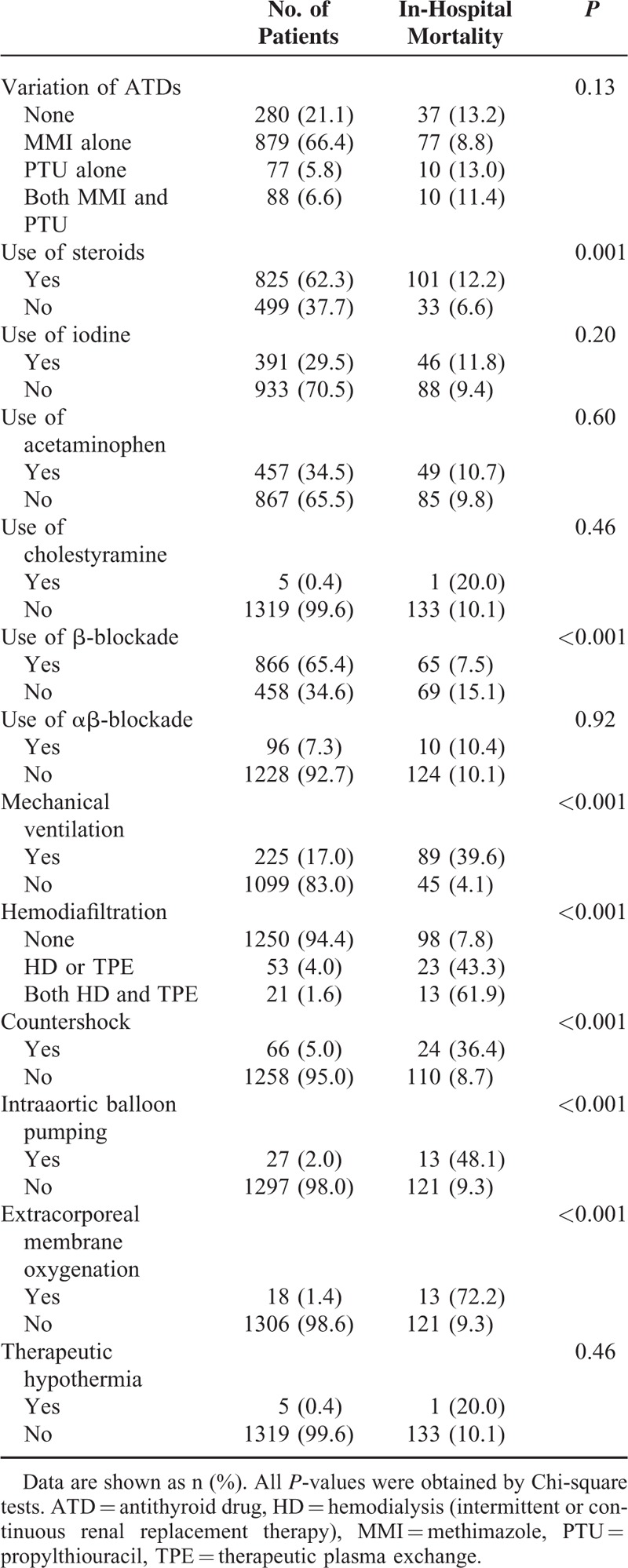
The clinical practice patterns for thyroid storm are shown in Table 3. Patients with thyroid storm were more likely to be treated with MMI than with PTU. The patients treated with MMI alone showed a relatively low mortality rate compared with those treated with PTU alone, but the variation in ATD therapy was not significantly associated with in-hospital mortality (P = 0.13). Among the total of 967 patients treated with MMI alone (n = 879) or both MMI and PTU (n = 88), we identified 131 patients treated with intravenous MMI, of whom 27 (20.6%) died. The numbers of patients treated with MMI and PTU were 46 with initial MMI, 30 with initial PTU, and 12 with initial MMI and PTU. Significant associations with in-hospital mortality were observed for use of steroids, nonuse of β-blockade, and requirement for mechanical ventilation, hemodiafiltration, countershock, intraaortic balloon pumping, and extracorporeal membrane oxygenation.
TABLE 3.
Clinical Practice Patterns for Thyroid Storm
Risk Factors for In-Hospital Mortality
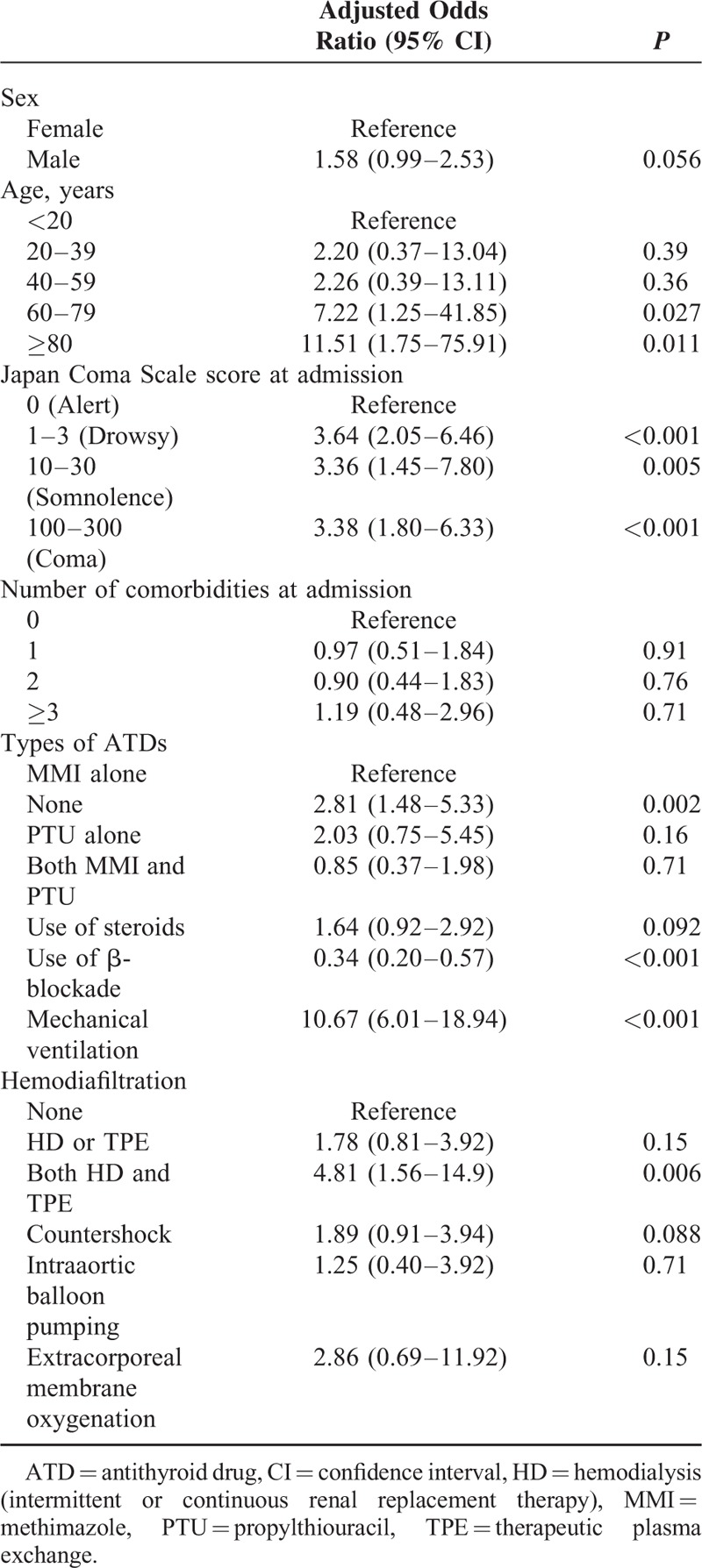
The factors significantly associated with higher mortality detected by multivariable logistic regression analyses for in-hospital mortality in patients with thyroid storm were: age above 60 years, higher JCS score at admission, nonuse of ATDs and β-blockade, and requirement for mechanical ventilation and TPE combined with HD (Table 4).
TABLE 4.
Multivariable Logistic Regression Model for In-Hospital Mortality in Patients With Thyroid Storm
DISCUSSION
We examined 1324 patients with thyroid storm using a nationwide inpatient database in Japan. The overall in-hospital mortality rate was 10.1%. In-hospital mortality was significantly associated with older age (≥60 years), central nervous system dysfunction at admission, nonuse of ATDs and β-blockade, and requirement for mechanical ventilation and TPE combined with HD. The strength of the present study is the large number of patients with thyroid storm. The distributions of sex, age, and in-hospital mortality were similar to those in the previous nationwide questionnaire survey in Japan.2
The number of patients with thyroid storm in our study (n = 1324 over 3 years) was higher than the estimated number of the patients reported in a previous questionnaire survey conducted in Japan (n = 1283 in a 5-year period).2 We speculate that this discrepancy is due to the questionnaire survey's relatively low response rate (52.5%), which likely means the questionnaire survey was not completely representative of the population and consequently underestimated the number of patients with thyroid storm. In this study, patients with a suspected diagnosis of thyroid storm were excluded. We consider that the number of patients with thyroid storm in our study was more accurate than the previous questionnaire survey.
The number of patients with thyroid storm was greatest in the summer season. It is well-known that the most common underlying cause of thyroid storm is Graves’ disease.3 A previous study showed that Graves’ thyrotoxicosis was aggravated by seasonal allergic rhinitis induced by cedar pollen.16 In Japan, the cedar pollen season is from late February to late April every year, and seasonal allergic rhinitis is a common disease.17 A previous report indicated that serum thyroid-stimulating hormone receptor antibody (TRAb) production was likely to increase in the summer season after cedar pollen-induced allergic rhinitis attacks in patients with Graves’ disease coexisting with seasonal allergic rhinitis.18 We speculate that delayed allergic reactions to cedar pollen stimulated the immune system and activated TRAb production in the thyroid grand. Another possible explanation is that the seasonal climate change with increased temperatures aggravated the thyrotoxicosis, because heat-intolerance is a common symptom of the disease.1
Approximately 75% of patients with thyroid storm had JCS score 0 (alert consciousness) at admission. We speculate that the patients with JCS score 0 at admission developed central nervous system manifestations after admission, because 5.2% of these patients had a fatal outcome. Another possible explanation is that prudent physicians may have promptly started treatment for patients with potential thyroid storm in the early phases to prevent increasing severity and irreversible outcomes. The most important clinical point is to treat patients with potential thyroid storm promptly and aggressively based on clinical impressions, rather than to wait until the patients fulfill the diagnostic criteria for thyroid storm.3,7
The diagnosis of thyroid storm is based on the combination of clinical signs and laboratory findings. In clinical settings in Japan, physicians diagnose patients with thyroid storm using the Japan Thyroid Association criteria and/or Burch-Wartofsky Point Scale.2,19 In our study, the diagnosis of thyroid storm was based on an ICD-10 code in the database, and independent criteria or diagnostic scoring systems were not applied. However, we believe that the validity of the recorded diagnoses was robust, because the mortality rate in our study was compatible with the rate in the previous nationwide survey in Japan (10.1% vs 10.7%).
Cardiovascular diseases were the most frequent comorbidity at admission in patients with thyroid storm. This may have arisen because severe hyperthyroidism can lead to sinus tachycardia, heart failure, and atrial fibrillation associated with thromboembolism including cerebrovascular events.20–22 In older patients with underlying coronary artery ischemia, angina pectoris or myocardial infarction can be caused by an increase in myocardial oxygen demand following the onset of thyroid storm.21 Generally, Graves’ disease improves during pregnancy through a decrease in stimulating TRAb levels.23,24 However, it has been reported that thyroid storm can occur during pregnancy, triggered by infection, preeclampsia, labor, placental presentation, or Cesarean section.24 Indeed, in our data, 15 patients were diagnosed with thyroid storm during pregnancy. The previous Japanese survey suggested that thyroid storm could be triggered by infection, diabetic ketoacidosis, trauma, pregnancy, cerebrovascular disease, ischemic heart disease, and adrenal insufficiency.2 Although the comorbidities at admission in our data included these disorders, we could not confirm whether these comorbidities were triggers or complications.
There are no globally validated treatment guidelines for thyroid storm, because it is practically infeasible to conduct a randomized controlled trial in patients with this rare and emergent disorder. The issue of whether thyroid storm should be initially treated with MMI or PTU is controversial.1,3–5 Traditionally, initial treatment with PTU for thyroid storm has been preferred, because PTU has an advantage over MMI in blocking peripheral conversion of thyroxine to triiodothyronine.25 Our data showed that two-thirds of patients with thyroid storm were treated with MMI. We speculate that physicians were more likely to use MMI than PTU within our data because an intravenous preparation of MMI is commercially available in Japan. However, because of the limited sample sizes and unknown severity of thyroid storm, our results could not provide recommendations regarding the optimal ATD for thyroid storm.
It has been recommended that patients with thyroid storm should be treated with steroids at stress doses until the possibility of coexisting relative adrenal insufficiency has been excluded.1,3,4,19 Indeed, a previous study indicated that cortisol secretion in response to low-dose adrenocorticotropin stimulation after dexamethasone suppression was lower in patients with severe thyrotoxicosis than in those with euthyroid state.26 Steroids have another unique effect in blocking peripheral conversion of thyroxine to triiodothyronine.1,3,4,19 In our study, patients treated with steroids showed higher mortality than those without such treatment, possibly because severe patients were more likely to receive steroids.
In the present study, patients treated with mechanical ventilation and TPE combined with HD showed significantly higher mortality rates than those without these treatments. One possible interpretation of our findings is that more severe patients with multiple organ failure were more likely to receive treatments with mechanical ventilation and hemodiafiltration.
The present study confirmed an association between central nervous system dysfunction and higher mortality, which was reported in a previous study.8 Our results indicated that the Japan Thyroid Association criteria and Burch-Wartofsky Point Scale are valid because these criteria consider central nervous system dysfunction to be important.2,19 All physicians should pay special attention to the presence of central nervous system dysfunction in patients with thyrotoxicosis.
Our study had several limitations. First, the recorded diagnoses in the database, by its retrospective nature, are less well-validated than those in planned prospective studies. Owing to the lack of detailed clinical information including symptoms or laboratory data in the database, we could not confirm the severity of each individual thyroid storm event or whether the patients fulfilled the Japan Thyroid Association criteria or Burch-Wartofsky Point Scale in the diagnosis of thyroid storm.2,19 We were also unable to confirm whether serum thyroid hormone levels at the time of diagnosis of thyroid storm were associated with patient mortality. Second, patient selection was not based on a random sampling method because participation in the database was voluntary for each hospital. Finally, in the database, postdischarge mortality information is unknown.
In conclusion, this large retrospective observational study using a nationwide database in Japan showed that the overall in-hospital mortality rate of thyroid storm was 10.1%. Factors associated with higher mortality were older age (≥60 years), central nervous system dysfunction, nonuse of ATDs and β-blockade, and a requirement for mechanical ventilation and TPE combined with HD. For future research to improve the survival outcome of thyroid storm, a well-designed prospective case-registration database including detailed clinical information should be established.
Footnotes
Abbreviations: ATD = antithyroid drug, HD = hemodialysis, JCS = Japan Coma Scale, MMI = methimazole, PTU = propylthiouracil, TPE = therapeutic plasma exchange, TRAb = thyroid-stimulating hormone receptor antibody.
YO and HY are the guarantors of this work and had full access to all of the data in the study, and take responsibility for the integrity of the data and accuracy of the data analysis.
All authors approved the final version of the manuscript.
Study concept and design: YO, HY, and YT; Acquisition, analysis, or interpretation of data: YO, SO, HY, HM, and YT; Drafting of the manuscript: YO, SO, and HY; Critical revision of the manuscript for important intellectual content: HY and YT; Statistical analysis: YO, SO, and HY; Administrative, technical, or material support: HY, HM, KF, and YT; Study supervision: HY, KF, and YT; This study was funded by grants from the Ministry of Health, Labour and Welfare of Japan (grant numbers: H27-Policy-Designated-009 and H27-Policy-Strategy-011).
The funding institution had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Devereaux D, Tewelde SZ. Hyperthyroidism and thyrotoxicosis. Emerg Med Clin N Am 2014; 32:277–292. [DOI] [PubMed] [Google Scholar]
- 2.Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid 2012; 22:661–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin N Am 2006; 35:663–686. [DOI] [PubMed] [Google Scholar]
- 4.Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid 2011; 21:593–646. [DOI] [PubMed] [Google Scholar]
- 5.Gwiezdzinska JK, Wartofsky L. Thyroid emergencies. Med Clin N Am 2012; 96:385–403. [DOI] [PubMed] [Google Scholar]
- 6.Muller C, Perrin P, Faller B, et al. Role of plasma exchange in the thyroid storm. Ther Apher Dial 2011; 15:522–531. [DOI] [PubMed] [Google Scholar]
- 7.Szczepiorkwski ZM, Winters JL, Bandarenko N, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher 2010; 25:83–177. [DOI] [PubMed] [Google Scholar]
- 8.Swee DS, Chng CL, Lim A. Clinical characteristics and outcome of thyroid storm: a case series and review of neuropsychiatric derangements in thyrotoxicosis. Endocr Pract 2015; 21:182–189. [DOI] [PubMed] [Google Scholar]
- 9.Angell TE, Lechner MG, Nguyen CT, et al. Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J Clin Endocrinol Metab 2015; 100:451–459. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Yasunaga H, Matsui H, et al. Impact of systemic steroids on posttonsillectomy bleeding: analysis of 61430 patients using a national inpatient database in Japan. JAMA Otolaryngol Head Neck Surg 2014; 140:906–910. [DOI] [PubMed] [Google Scholar]
- 11.Tagami T, Matsui H, Fushimi K, et al. Intravenous immunoglobulin and mortality in pneumonia patients with septic shock: an observational nationwide study. Clin Infect Dis 2015; 61:385–392. [DOI] [PubMed] [Google Scholar]
- 12.Tagami T, Matsui H, Horiguchi H, et al. Low-dose corticosteroid use and mortality in severe community-acquired pneumonia patients. Eur Respir J 2015; 45:463–472. [DOI] [PubMed] [Google Scholar]
- 13.Sako A, Yasunaga H, Matsui H, et al. Hospitalization for hypoglycemia in Japanese diabetic patients: a retrospective study using a national inpatient database, 2008–2012. Medicine 2015; 94:e1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Todo T, Usui M, Takakura K. Treatment of severe intraventricular hemorrhage by intraventricular infusion of urokinase. J Neurosurg 1991; 74:81–86. [DOI] [PubMed] [Google Scholar]
- 15.Ono K, Wada K, Takahata T, et al. Indications for computed tomography in patients with mild head injury. Neurol Med Chir (Tokyo) 2007; 47:291–298. [DOI] [PubMed] [Google Scholar]
- 16.Hidaka Y, Amino N, Iwatani Y, et al. Recurrence of thyrotoxicosis after attack of allergic rhinitis in patients with Graves’ disease. J Clin Endocrinol Metab 1993; 77:1667–1670. [DOI] [PubMed] [Google Scholar]
- 17.Amino N, Hidaka Y, Takano T, et al. Association of seasonal allergic rhinitis is high in Graves’ disease and low in painless thyroiditis. Thyroid 2003; 13:811–814. [DOI] [PubMed] [Google Scholar]
- 18.Takeoka K, Hidaka Y, Hanada H, et al. Increase in serum levels of autoantibodies after attack of seasonal allergic rhinitis in patients with Graves’ disease. Int Arch Allergy Immunol 2003; 132:268–276. [DOI] [PubMed] [Google Scholar]
- 19.Burch HB, Wartofsky L. Life-threatening thyrotoxicosis: thyroid storm. Endocrinol Metab Clin N Am 1993; 22:263–277. [PubMed] [Google Scholar]
- 20.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001; 344:501–509. [DOI] [PubMed] [Google Scholar]
- 21.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev 2005; 26:704–728. [DOI] [PubMed] [Google Scholar]
- 22.Grais IM, Sowers JR. Thyroid and the heart. Am J Med 2014; 127:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brent GA. Graves’ disease. N Engl J Med 2008; 358:2594–2605. [DOI] [PubMed] [Google Scholar]
- 24.Chan GW, Mandel SJ. Therapy insight: management of Graves’ disease during pregnancy. Nat Clin Pract Endocrinol Metab 2007; 3:470–478. [DOI] [PubMed] [Google Scholar]
- 25.Cooper DS. Antithyroid drugs. N Engl J Med 2005; 352:905–917. [DOI] [PubMed] [Google Scholar]
- 26.Tsatsoulis A, Johnson EO, Kalogera CH, et al. The effect of thyrotoxicosis on adrenocortical reserve. Eur J Endocrinol 2000; 142:231–235. [DOI] [PubMed] [Google Scholar]


