Abstract
The aim of this study was to compare the effects of botulinum toxin injection between subacute and chronic stroke patients.
Eighteen stroke patients (9 subacute and 9 chronic) with spasticity of 1+ or higher in the hemiplegic elbow or wrist joint, based on the modified Ashworth scale were recruited. Modified Ashworth scale, modified Tardieu scale, manual muscle testing, passive range of motion, Brunnstrom stage, modified Barthel index, and Fugl-Meyer scale evaluations of the hemiplegic upper extremity were performed just before the injection and 4 weeks later. A total dose of 200 U of botulinum toxin type A was injected into each patient. One or more of the elbow flexor muscles and one or more of the wrist flexor or finger flexor muscles were included.
Modified Ashworth scale, manual muscle testing, passive range of motion, and modified Barthel index results were improved in subacute patients only. However, modified Tardieu scale for the elbow and Fugl-Meyer scale results were improved in both groups, and the improvement was comparable.
In conclusion, botulinum toxin injection in subacute patients was more helpful for spasticity, contracture, and function than in chronic patients. However, beneficial effects of botulinum toxin injection on spasticity and function in chronic patients were found in the assessments of the modified Tardieu scale and Fugl-Meyer scale.
INTRODUCTION
Spasticity is common after stroke, limiting range of motion and causing functional derangement. It is found in 19% of patients 3 months after stroke onset1 and in 20% after 18 months.2 Botulinum toxin treatment for spasticity has been reported to be effective in terms of the modified Ashworth scale and patients’ global assessment scores; however, significant improvement was not demonstrated in global functional assessment scores, such as the Barthel index and the functional independence measure.3 Studies on botulinum toxin injection for post-stroke spasticity have mostly dealt with chronic stroke patients.4 Early use of botulinum toxin is recommended to prevent soft-tissue shortening5 because spasticity contributes to contracture development in acute-to-subacute stroke.6 There have been several reports about successful results of early botulinum toxin treatment in subacute stroke patients7,8; however, the effects of botulinum toxin were not compared between subacute and chronic stroke patients.
The goals of this study were to demonstrate differences in botulinum toxin treatment outcomes between subacute and chronic stroke patients, and to suggest proper timing of botulinum toxin injections.
METHODS
Participants
Patients with hemiplegic stroke owing to cerebral infarction or hemorrhage were recruited. Patients with spasticity of 1+ or higher on the modified Ashworth scale, in the hemiplegic elbow or wrist joint, were eligible. Exclusion criteria were quadriplegic stroke, fracture in the upper extremity, recent (<3 months) botulinum toxin injection, history of nerve block, intrathecal medication, upper arm operation to treat spasticity, unstable medical conditions, hypersensitivity to botulinum toxin, neuromuscular junction disease, pregnancy, and lactation. The study protocol was approved by the local institutional review board and conducted in accordance with the regulatory standards of Good Clinical Practice and the Declaration of Helsinki. All study participants or their legal representatives provided written informed consent.
Only patients undergoing inpatient or outpatient rehabilitation treatment ≥2 days per week were included. From these, 2 groups were selected. Patients with elapsed time from stroke of between 4 weeks and 6 months were identified as the subacute group, whereas patients with elapsed time of >5 years were the chronic group.
Intervention
A total dose of 200 U of botulinum toxin type A (BOTOX; Allergan Inc., Irvine, CA) was used for each patient. The muscles to be injected and the appropriate doses for the individual muscle were selected according to the spasticity pattern of each patient. One or more of the elbow flexor muscles (biceps brachii, brachioradialis, and brachialis) were included for each patient, as well as one or more of the wrist flexor muscles (flexor carpi radialis and flexor carpi ulnaris) or one or more of the finger flexor muscles (flexor digitorum profundus, flexor digitorum superficialis, and flexor pollicis longus), which also flex the wrist. For the clear measurement of flexion and extension without rotation, the pronator teres muscle was excluded. One vial containing 100 U of botulinum toxin type A was diluted with 2 mL of normal saline. Botulinum toxin was injected by one experienced physiatrist with electrical stimulation and/or ultrasound guidance.
Measurements
Spasticity was evaluated with the modified Ashworth scale9 and the modified Tardieu scale10 in the elbow and wrist joints of the hemiplegic upper extremity. Modified Ashworth scale scores of 1+, 2, 3, and 4 were changed respectively to 2, 3, 4, and 5 for statistical analysis.11 For modified Tardieu scale measurement, the angle where the catch was beginning to be felt was defined as R1, the angle of maximum passive range of motion was R2, and the difference between R1 and R2 was designated D (dynamic range).10,11 Manual muscle testing, passive range of motion, and Brunnstrom stage were also checked in the same joints. Modified Tardieu scale and passive range of motion were evaluated with a manual goniometer. Functional evaluation was performed with the modified Barthel index12 and the Fugl-Meyer scale13 of the hemiplegic upper extremity. Patients were assessed just before the injection (pre) and 4 weeks later (post). Assessment was performed by one of the investigators who was blind to the group allocation.
Statistical Analysis
The sample size was calculated based on standardized effect sizes obtained from previously published data from patients with brain lesions.14 Power analysis was performed using the G∗Power software version 3.1 (University of Dusseldorf, Dusseldorf, Germany). We expected only small numbers of chronic stroke patients to be undergoing rehabilitation, and a priori analysis with the Mann-Whitney U test indicated that a sample size of at least 8 patients in each group would provide a statistical power of 80% (β = 0.20 and α = 0.05). Taking into account the 10% dropout rate, a total of 18 patients were needed.
Statistical analysis was performed using SPSS version 22.0 (SPSS Inc., Chicago, IL). Baseline characteristics between the 2 groups were compared with the Mann–Whitney U test for continuous variables and with Fisher exact test for categorical variables. Changes within each group were analyzed with the Wilcoxon signed-rank test. Comparisons between the 2 groups at each time-point were performed by the Mann–Whitney U test. A P value <0.05 was considered statistically significant.
RESULTS
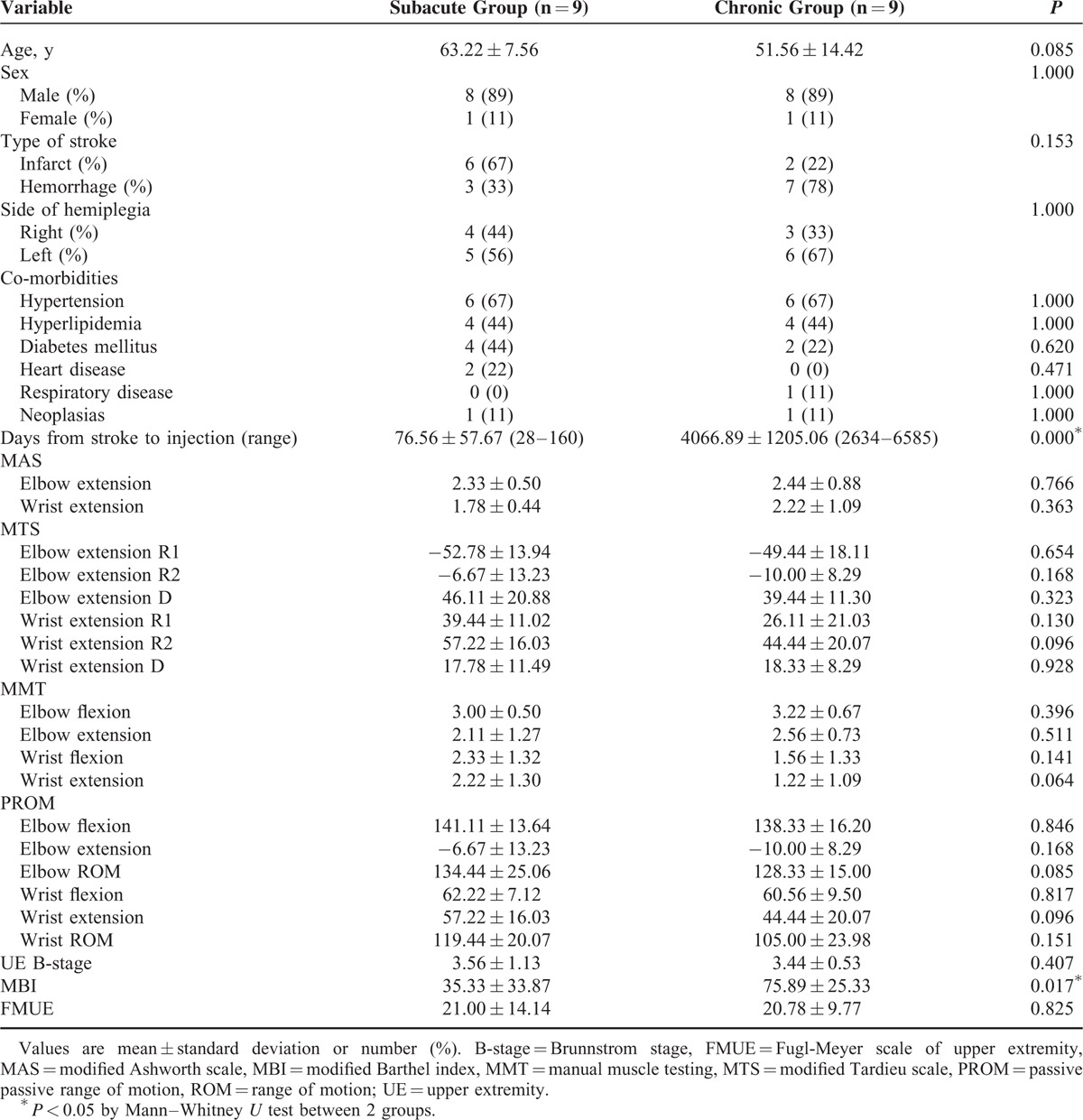
Fifteen subacute stroke patients and 13 chronic stroke patients were recruited, and 9 subacute patients and 9 chronic patients completed the study (Figure 1). No adverse events related to botulinum toxin injection occurred during this study. No significant differences were found between the 2 groups in terms of age, sex, type of stroke, side of hemiplegia, or comorbidities. The days from stroke to botulinum toxin injection differed between the 2 groups. The range of duration was 28 to 160 days in subacute group, and 2634 to 6585 days (7–18 years) in chronic group. The results of the initial evaluation showed no significant differences except on the modified Barthel index (Table 1).
FIGURE 1.
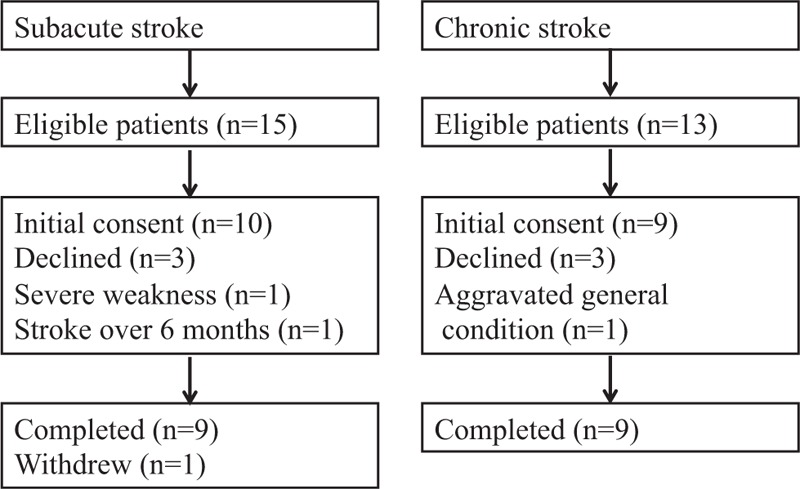
Flow diagram of the study.
TABLE 1.
Baseline Characteristics of Patients
In the aspect of spasticity, the modified Ashworth scale score was improved significantly in the elbow and wrist extensors of subacute patients only, and the modified Ashworth scale score after 4 weeks was different between the 2 groups (P = 0.004 for the elbow and P = 0.007 for the wrist). Results of the modified Tardieu scale showed improved R1 in the elbow and wrist extensors of both groups, and R1 of the wrist extensor after 4 weeks was different between the 2 groups (P = 0.019). Elbow extensor D was improved in both groups. Wrist extensor R2, which was the same value as wrist extension range of motion, was improved in the subacute patients, and was different after 4 weeks between the 2 groups (P = 0.008) (Table 2).
TABLE 2.
Assessment Just Before the Injection (Pre) and 4 Weeks After (Post)
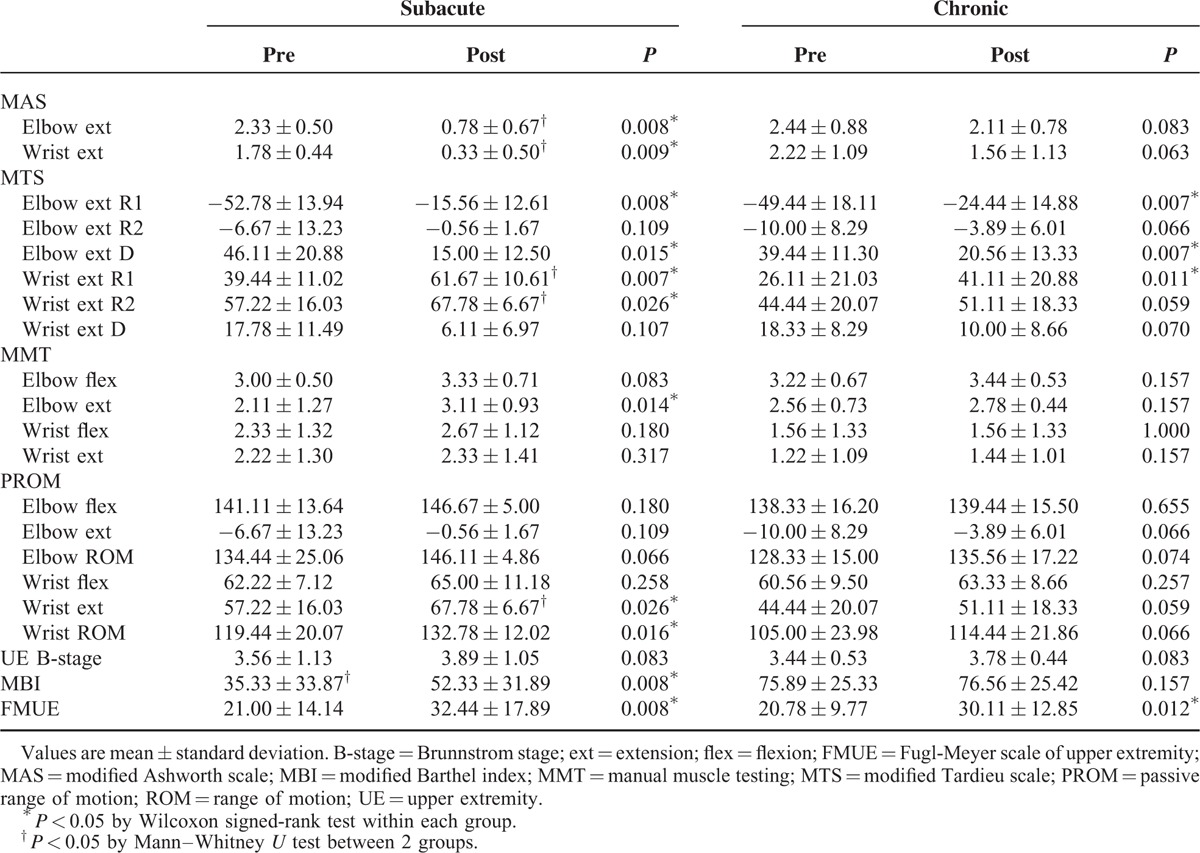
There was no change in manual muscle testing of the elbow and wrist in the chronic patients. Manual muscle testing of the elbow extensor was improved in the subacute patients. Range of motion was improved only in the wrist of the subacute patients. Brunnstrom stage of the upper extremity was not changed in both groups. The chronic patients showed no change in the modified Barthel index, whereas the subacute patients showed improvement in the modified Barthel index. The Fugl-Meyer scale score of the hemiplegic upper extremity was improved in both groups (Table 2).
DISCUSSION
In this study, botulinum toxin injection improved general aspects of spasticity in the subacute patients; however, the effects on modified Tardieu scale scores of the elbow and Fugl-Meyer scale scores of the hemiplegic upper extremity were comparable between the subacute and chronic groups.
To the best of our knowledge, this is the first study to compare the effects of botulinum toxin between subacute and chronic stroke patients in the same setting. Previous studies investigated the effects of botulinum toxin in stroke patients with relatively similar duration (acute, subacute, or chronic) from stroke onset or at a determined time from onset (3 months or 6 months were frequently used). Systematic reviews and meta-analyses pooled multiple studies without considering stroke duration.
The main effect of botulinum toxin injection in the upper extremity was the improvement of spasticity. Meta-analyses3,15 have reported improvement on the modified Ashworth scale and the modified Tardieu scale; most of the studies used the modified Ashworth scale and some measured the modified Tardieu scale. However, in this study, the modified Ashworth scale score was improved in subacute patients only. In chronic patients, the modified Ashworth scale score of the wrist extensor was decreased without statistical significance, and the modified Ashworth scale score of the elbow extensor was almost unchanged. The modified Ashworth scale score of the elbow and wrist extensor after 4 weeks was different between the 2 groups. There have been studies16,17 on botulinum toxin injection in which the results showed no improvement on the Ashworth scale or the modified Ashworth scale. The effect of botulinum toxin injection on the modified Ashworth scale score in chronic patients could be lower than expected, and further studies with stricter grouping of stroke duration are needed.
On the contrary, modified Tardieu scale scores were improved in both groups. R1 in the elbow and wrist extensors and the elbow extensor D showed statistically significant changes in the 2 groups, and R1 and D of the elbow extensor after 4 weeks did not differ between the 2 groups. Improvement of Tardieu scale scores with botulinum toxin injection was also reported previously.18 The Tardieu scale and the modified Tardieu scale are supposed to reflect spasticity more clearly than the modified Ashworth scale, with the possible ability to differentiate contracture from spasticity.11,19 This study showed similar results, favoring the modified Tardieu scale for spasticity evaluation. A study on the effect of botulinum toxin injection in children with cerebral palsy suggested D of the modified Tardieu scale as a more valuable tool,11 whereas in the present study, the changes in R1 and D were statistically significant in both groups. Because the change in R2 would be small in chronic patients with contracture, R1 might be more suitable for spasticity measurement.
Weakness of injected muscles is an expected but undesirable effect of botulinum toxin. However, manual muscle testing of the elbow flexors and the wrist flexors was unchanged in both groups. Due to the reduced flexor muscle tone, manual muscle testing of the elbow extensor was improved in the subacute patients. Wrist extension range of motion (wrist extensor R2) and passive range of motion of the wrist were improved in subacute patients only. Improvement of passive range of motion after botulinum toxin injection in patients with brain lesions that had developed >1 year previously was reported,20 but in the chronic patients, the effect could be lower than expected. Although botulinum toxin injection with rehabilitation, dynamic splinting, or serial casting improved contracture in several reports,21,22 botulinum toxin injection during the subacute period is recommended to prevent contracture.
The modified Barthel index was improved in the subacute patients, whereas it was nearly fixed in the chronic patients. Improvement of the Barthel index after botulinum toxin injection was not supported in a systematic review.4 The Barthel index was not correlated to the degree of spasticity in botulinum toxin-treated patients because mobility and continence items are included in it.23 Therefore, improvement of the modified Barthel index in subacute patients might be the result of spontaneous recovery. Only small room for improvement of the modified Barthel index remained in the chronic patients because the modified Barthel index of these patients had already reached the maximum score before botulinum injection.
In contrast, the initial Fugl-Meyer scale score of the hemiplegic upper extremity was far off the full score of 66 in the chronic patients. After botulinum toxin injection, the Fugl-Meyer scale score was improved in both groups, and there was no difference after 4 weeks. There have been several studies using the Fugl-Meyer scale as a measure after botulinum toxin injection, and some reported improvement,24,25 whereas others did not.7 The Fugl-Meyer scale for the upper extremity reflects motor function with more detail because of the 1 to 66 point system. Although botulinum toxin injection did not improve Brunnstrom stage or the modified Barthel index in the chronic patients, it did bring improvement of the Fugl-Meyer scale score for the hemiplegic upper extremity, which means functional improvement.
In this study, improvement of spasticity after botulinum toxin injection seemed to be better visualized in the subacute patients; however, improvement of the modified Tardieu scale for the elbow and the Fugl-Meyer scale score for the hemiplegic upper extremity was not different between the 2 groups. Consequently, improvement of spasticity and function was shown even in the chronic patients, and the change could be easily visualized by assessments such as the modified Tardieu scale and the Fugl-Meyer scale for the hemiplegic upper extremity.
The present study has several limitations. First, the comparability of the 2 groups was not affirmed in some variables. Although the differences were not statistically significant, the subacute group was mainly composed of older patients with cerebral infarction, whereas the majority of the chronic group consisted of relatively younger patients with cerebral hemorrhage. For economic reasons, it is not easy for chronic stroke patients to be in rehabilitation treatment continuously, and 5 of the 9 chronic patients in this study were undergoing rehabilitation with the help of Workers’ Compensation insurance. Cerebral infarction is more difficult than cerebral hemorrhage to accept as an industrial disaster, and this situation might affect the different rates of stroke causes and ages in this study. However, it was reported that spasticity and changes after botulinum toxin injection were not different between cerebral infarction and hemorrhage,26 and the causes would not influence the results of this study. Another difference between the 2 groups was the initial value of the modified Barthel index, which was fixed in the chronic patients while the subacute patients were in the process of improving it. In other words, the chronic patients acquired maximum independence despite their spasticity, but the subacute patients did not. The second limitation was that this study was comprised of a small number of patients. This was because of the small number of chronic patients undergoing continuous rehabilitation treatment. Further studies with large numbers of patients are needed. Third, the assessor was aware of the evaluation time, which was pre- or post-botulinum toxin injection; the blinding was only to the group allocation. The reason for single-assessor assignment was to eliminate inter-rater reliability issues in the measurements. Further large-scale studies with a double-blind design are needed.
In conclusion, botulinum toxin injection in the subacute period is recommended because it can improve spasticity, contracture, and function. However, botulinum toxin injection can improve spasticity as evaluated with the modified Tardieu scale for the elbow and function as evaluated with the Fugl-Meyer scale for the hemiplegic upper extremity in chronic patients, and the effects are comparable to those in subacute patients. In addition, studies on the effects of botulinum toxin injection might consider stroke duration more strictly.
Footnotes
Abbreviations: D = dynamic range of modified Tardieu scale, R1 = angle of muscle reaction of modified Tardieu scale, R2 = angle of full range of motion of modified Tardieu scale.
This study was supported by Hallym University Research Fund, 2013 (HURF-2013-25). The funding source played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sommerfeld DK, Eek EU, Svensson AK, et al. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke 2004; 35:134–139. [DOI] [PubMed] [Google Scholar]
- 2.Welmer AK, von Arbin M, Widen Holmqvist L, et al. Spasticity and its association with functioning and health-related quality of life 18 months after stroke. Cerebrovasc Dis 2006; 21:247–253. [DOI] [PubMed] [Google Scholar]
- 3.Ozcakir S, Sivrioglu K. Botulinum toxin in poststroke spasticity. Clin Med Res 2007; 5:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley N, Pereira S, Salter K, et al. Treatment with botulinum toxin improves upper-extremity function post stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil 2013; 94:977–989. [DOI] [PubMed] [Google Scholar]
- 5.Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins 2012; 4:913–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ada L, O’Dwyer N, O’Neill E. Relation between spasticity, weakness and contracture of the elbow flexors and upper limb activity after stroke: an observational study. Disabil Rehabil 2006; 28:891–897. [DOI] [PubMed] [Google Scholar]
- 7.Hesse S, Mach H, Frohlich S, et al. An early botulinum toxin A treatment in subacute stroke patients may prevent a disabling finger flexor stiffness six months later: a randomized controlled trial. Clin Rehabil 2012; 26:237–245. [DOI] [PubMed] [Google Scholar]
- 8.Rosales RL, Kong KH, Goh KJ, et al. Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: a randomized controlled trial. Neurorehabil Neural Repair 2012; 26:812–821. [DOI] [PubMed] [Google Scholar]
- 9.Johnson GR. Outcome measures of spasticity. Eur J Neurol 2002; 9 suppl 1:10–16. [DOI] [PubMed] [Google Scholar]
- 10.Yam WK, Leung MS. Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. J Child Neurol 2006; 21:1031–1035. [DOI] [PubMed] [Google Scholar]
- 11.Jang DH, Sung IY, Kang YJ. Usefulness of the tendon reflex for assessing spasticity after botulinum toxin-a injection in children with cerebral palsy. J Child Neurol 2013; 28:21–26. [DOI] [PubMed] [Google Scholar]
- 12.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 1989; 42:703–709. [DOI] [PubMed] [Google Scholar]
- 13.Sanford J, Moreland J, Swanson LR, et al. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther 1993; 73:447–454. [DOI] [PubMed] [Google Scholar]
- 14.Simpson DM, Gracies JM, Yablon SA, et al. Bo NTTZDST. Botulinum neurotoxin versus tizanidine in upper limb spasticity: a placebo-controlled study. J Neurol Neurosurg Psychiatry 2009; 80:380–385. [DOI] [PubMed] [Google Scholar]
- 15.Baker JA, Pereira G. The efficacy of Botulinum Toxin A for spasticity and pain in adults: a systematic review and meta-analysis using the Grades of Recommendation, Assessment, Development and Evaluation approach. Clin Rehabil 2013; 27:1084–1096. [DOI] [PubMed] [Google Scholar]
- 16.Brashear A, McAfee AL, Kuhn ER, et al. Botulinum toxin type B in upper-limb poststroke spasticity: a double-blind, placebo-controlled trial. Arch Phys Med Rehabil 2004; 85:705–709. [DOI] [PubMed] [Google Scholar]
- 17.Marco E, Duarte E, Vila J, et al. Is botulinum toxin type A effective in the treatment of spastic shoulder pain in patients after stroke? A double-blind randomized clinical trial. J Rehabil Med 2007; 39:440–447. [DOI] [PubMed] [Google Scholar]
- 18.Lam K, Lau KK, So KK, et al. Can botulinum toxin decrease carer burden in long term care residents with upper limb spasticity? A randomized controlled study. J Am Med Dir Assoc 2012; 13:477–484. [DOI] [PubMed] [Google Scholar]
- 19.Patrick E, Ada L. The Tardieu Scale differentiates contracture from spasticity whereas the Ashworth Scale is confounded by it. Clin Rehabil 2006; 20:173–182. [DOI] [PubMed] [Google Scholar]
- 20.Wang HC, Hsieh LF, Chi WC, et al. Effect of intramuscular botulinum toxin injection on upper limb spasticity in stroke patients. Am J Phys Med Rehabil 2002; 81:272–278. [DOI] [PubMed] [Google Scholar]
- 21.Lai JM, Francisco GE, Willis FB. Dynamic splinting after treatment with botulinum toxin type-A: a randomized controlled pilot study. Adv Ther 2009; 26:241–248. [DOI] [PubMed] [Google Scholar]
- 22.Singer BJ, Dunne JW, Singer KP, et al. Non-surgical management of ankle contracture following acquired brain injury. Disabil Rehabil 2004; 26:335–345. [DOI] [PubMed] [Google Scholar]
- 23.Dionyssiotis Y, Kiourtidis D, Karvouni A, et al. Consequences of neurologic lesions assessed by Barthel Index after Botox((R)) injection may be underestimated. Ther Clin Risk Manag 2012; 8:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardoso E, Pedreira G, Prazeres A, et al. Does botulinum toxin improve the function of the patient with spasticity after stroke? Arq Neuropsiquiatr 2007; 65 (3A):592–595. [DOI] [PubMed] [Google Scholar]
- 25.Takekawa T, Kakuda W, Taguchi K, et al. Botulinum toxin type A injection, followed by home-based functional training for upper limb hemiparesis after stroke. Int J Rehabil Res 2012; 35:146–152. [DOI] [PubMed] [Google Scholar]
- 26.Schramm A, Ndayisaba JP, Auf dem Brinke M, et al. Spasticity treatment with onabotulinumtoxin A: data from a prospective German real-life patient registry. J Neural Transm 2014; 121:521–530. [DOI] [PubMed] [Google Scholar]


