Abstract
Video-assisted thoracoscopic surgery (VATS) has been known to be a stressful event for patients, and dexmedetomidine is known to attenuate surgery-induced sympathetic responses and potentiate analgesia in perioperative periods. The present was designed to evaluate the effects of intraoperative dexmedetomidine administration on the quality of recovery (QoR) and pulmonary function after VATS.
Patients with lung cancer undergoing VATS were randomized to Dex group (loading of 1.0 μg/kg for 20 minutes before the termination of surgery, n = 50) or Control group (comparable volume of normal saline, n = 50). The QoR-40 questionnaire assesses postoperative recovery and validates the overall surgical and general anesthesia outcomes. The QoR-40 scores, forced expiratory volume for 1 second (FEV1) on postoperative day (POD) 1 and 2, and emergence agitation were evaluated.The global QoR-40 score (162.3 ± 17.8 vs 153.3 ± 18.7, P = 0.016 on POD 1; 174.3 ± 16.0 vs 166.8 ± 16.7, P = 0.028 on POD 2) and FEV1 (2.1 ± 0.4 vs 1.9 ± 0.5 L, P = 0.034 on POD 1; 2.2 ± 0.5 vs 2.0 ± 0.4 L, P = 0.030 on POD 2) were significantly higher in the Dex group compared with the Control group on POD1 and POD 2. The score of emergence agitation was lower in the Dex group compared with the Control group (3 [2–5] vs 5 [3–7], P < 0.001). The number of patients indicating severe emergence agitation was shorter in the Dex group than Control group (0 [0%] vs 7 [14%], P = 0.048). The length of hospital stay was significantly shorter (6.7 [3–9] vs 8.4 [4–9] days, P = 0.045) in the Dex group compared with the Control group.
Intraoperative dexmedetomidine administration improved QoR, postoperative pulmonary function, and emergence agitation in patients undergoing VATS. Consequently, intraoperative dexmedetomidine administration could improve postoperative outcomes and reduced the length of hospital stay in patients undergoing VATS.
INTRODUCTION
Video-assisted thoracoscopic surgery (VATS) has a wide range of applicability, including the diagnosis and treatment of lung cancer, and has gradually replaced conventional thoracotomy. VATS is minimally invasive compared with open thoracotomy, with known benefits including lesser postoperative pain and the possibility of earlier mobilization.1,2 However, patients undergoing VATS also complain of a moderate degree of acute postoperative pain.2,3 Furthermore, the incidence of chronic postoperative pain has been reported to be similar to that with open thoracotomy.4–6 Present study indicate that approximately 40% patients undergoing surgery for lung cancer experience psychological distress and mood changes, which somewhat higher than the incidence associated with cancers of other organs.7 Therefore, patients who undergo VATS should not be underestimated and neglected in regards to appropriate treatment for acute postoperative pain and psychological stress to avoid adverse effects on the postoperative quality of life and morbidity.
Dexmedetomidine is a selective α2-agonist and a sedative with anti-inflammatory,8,9 analgesic,10,11 and antiemetic effects,12 and its use for procedural sedation during endoscopy13 or ablation for atrial fibrillation has been increasing.14 It is also used for sedation in the intensive care unit. Recently, it was reported that dexmedetomidine is often used as an adjuvant for general anesthesia.15 Sympatholysis of dexmedetomidine can attenuate the increased sympathetic tone after surgery and result in antistress effects. In addition, opioid-sparing and analgesic effects are promoted by the perioperative administration of dexmedetomidine.10,16,17
The Quality of Recovery questionnaire (QoR-40) is a tool used to assess the quality of recovery after surgery through questions pertaining to 40 items related to 5 domains. It has been validated as a global and reliable measurement tool18,19 and is considered important not only for surgeons but also anesthesiologists from the perspective of providing high-quality anesthetic services and ensuring improved postoperative outcomes and QoR. Although it has been reported that the perioperative administration of dexmedetomidine improves QoR after major abdominal and spinal surgeries,20,21 the outcomes for patients who undergo thoracic surgery including VATS remain poorly investigated.
The aims of this study were to evaluate the effects of intraoperative dexmedetomidine administration on QoR by using the QoR-40 and postoperative pulmonary function in patients who undergo VATS. The hypothesis was that intraoperative dexmedetomidine administration results in an improved QoR after VATS.
METHODS
Study Population
This study received approval from the Institutional Review Board of Severance Hospital (Ref. 4–2015–0284) on August 5, 2015 and was registered at clinicalTrials.gov (NCT02537249) on August 24, 2015. All participants provided written informed consent before participation. The inclusion criteria were as follows: an American Society of Anesthesiologists physical status of II or III, age >20 years, and the presence of non-small cell lung cancer requiring VATS. Patients with a history of arrhythmia, severe bradycardia (heart rate <45 beats/min), severe functional liver or kidney disease, and psychiatric/central nervous system disturbances that could preclude completion of the QoR-40 were excluded.
Study Protocol
Enrolled patients were randomly allocated to Control group or Dex group using a randomized sequence generated by a computer, and randomization process was centralized. Allocations were concealed in sealed envelopes, which were sent to the anesthesia nurses who prepared dexmedetomidine or saline solutions with comparable volume. The participating anesthesiologist who infused the drug was blinded to randomization. Therefore, the surgeons, anesthesia and recovery nurses, and patients were all blinded to randomization.
Patients were premedicated with glycopyrrolate 0.2 mg. On arrival to the operating room, the patient's blood pressure, oxygen saturation, electrocardiograms, and bispectral index (BIS; A-200 monitor, Aspect Medical System Inc, Newton, MA) were monitored. Anesthesia was induced with propofol 1.5 to 2.0 mg/kg and remifentanil 1.0 μg/kg. Following the loss of consciousness, rocuronium 0.8 mg/kg was administered to facilitate tracheal intubation. Anesthetic maintenance was achieved with desflurane at 1 minimum alveolar concentration and remifentanil infused at 0.05 to 0.15 μg /kg/min before skin closure. After a loading dose of fentanyl 1.0 μg/kg was intravenously injected, fentanyl at a dose 0.4 μg/kg/h and ramosetron 0.6 mg were infused through a patient-controlled analgesia (PCA) system (Accufuser Plus; Woo Young Medical, Seoul, Korea; flow rate 1 mL/h, bolus volume 1 mL, lock out time 15 minutes) was started from 30 minutes before the end of surgery. After residual block was reversed, desflurane was discontinued and the inspired oxygen flow was increased to 5 L/min. Patients were extubated after BIS > 70 and a train-of-four ratio of >0.9 were verified.
Intervention
In the Dex group, dexmedetomidine (Precedex; Hospira, Lake Forest, IL) 200 μg mixed with normal saline to achieve a total volume of 50 mL was administered. Dexmedetomidine 1.0 μg/kg was administrated for 20 minutes in Dex group. A comparable volume of saline was administered in the Control group. The dexmedetomidine or saline was administered for 20 minutes before the termination of surgery.
Outcome Measures
The multidimensional QoR-40 questionnaire was used as a measure for postoperative QoR, with the global score ranging from 40 to 200. In total, the questionnaire contains 40 questions pertaining to 5 domains: emotional state, physical comfort, psychological support, physical independence, and pain.19 The patients completed this questionnaire before, at postoperative day (POD) 1 and 2, respectively; the scoring system was explained before the surgery, and an accurate understanding of all questions was confirmed. After surgery, the questionnaires were performed by an investigator blinded to randomization. Postoperative pulmonary function was assessed using an automated flow-sensing spirometer (SPM-7; Bionet, Seoul, Korea) for forced expiratory volume in 1 second (FEV1) measurement on POD 1 and 2. The patients were assessed for signs of emergence agitation for 2 minutes after extubation using the Riker sedation-agitation scale22: 1 = unarousable—unresponsive to noxious stimuli, cannot follow commands; 2 = very sedated—easily arouses to physical stimuli and/or may have spontaneous movements, cannot follow commands; 3 = sedated—difficult to arouse but arouses by verbal or physical stimuli, follows commands; 4 = calm and cooperative—calm and easily arouses, follows commands; 5 = agitated—shows mild physical and/or emotional agitation or anxiety, calms with verbal commands; 6 = very agitated—show strong agitation/anxiety, unable to calm with verbal commands and may require restraints; 7 = dangerous agitation—shows severe agitation/anxiety, trying to remove endotracheal tube and/or catheters, may attack staff. Severe agitation was defined as Riker sedation-agitation scale 7. The times from cessation of desflurane to eye opening as well as extubation were verified during emergence. Also, we assessed the incidence of coughing during emergence in both groups. Numeric rating scale (NRS: 0–10, 0 = no pain; 5 = moderate pain; 10 = worst possible pain) at rest and coughing were recorded at 10 and 30 minutes after arrival to the postanesthetic care unit (PACU). In PACU, arterial gas analysis was performed at room air, and the partial pressure of arterial oxygen (PaO2) and carbon dioxide (PaCO2) were assessed. Patients were discharged from PACU when the modified Aldrete score 23 was ≥9 and the time of discharge was recorded. The frequencies of postoperative opioid and rescue antiemetic consumption on POD 1 and 2 were recorded. Perioperative data were collected by an investigator not involved in treatment. Postoperative pulmonary complications including atelectasis and pneumonia, time to chest tube removal, incidence of prolonged air leakage, and length of hospital stay were verified for all patients.
Statistical Analysis
The primary outcome was the global QoR-40 score on POD 1. The sample size was estimated on the basis of the premise that a difference in the global QoR-40 score of 10 points (standard deviation [SD], 14) between the Dex and Control groups was clinically significant because 10 points difference represents 15% improvement in the quality of recovery.24 We found that 42 patients were required to achieve 90% power with a significance level of (alpha) 0.05. Assuming a dropout rate of 20%, we enrolled 50 patients per group. The data were tested for normality using the Kolmogorov–Smirnov test. Inter-group comparisons were performed using an unpaired t test, Mann–Whitney U test for continuous variables and Fisher exact test for categorical variables. Values are expressed as means ± SD for continuous variables and numbers (percentages) for categorical variables. Parameters showing a non-normal distribution are expressed as medians and interquartile ranges (IQR). All statistical analyses were performed using SPSS 20.0 (SPSS Inc, Chicago, IL) and P < 0.05 was considered statistically significant.
RESULTS
A total of 113 patients scheduled to undergo VATS were assessed for eligibility between August 2015 and October 2015. Two patients were excluded, and 111 patients were randomized after obtaining informed consent (Figure 1). Eleven patients were withdrawn from the study and 50 patients were randomly assigned to each group. As shown in Table 1, demographic and clinical characteristics showed no significant differences between the groups.
FIGURE 1.
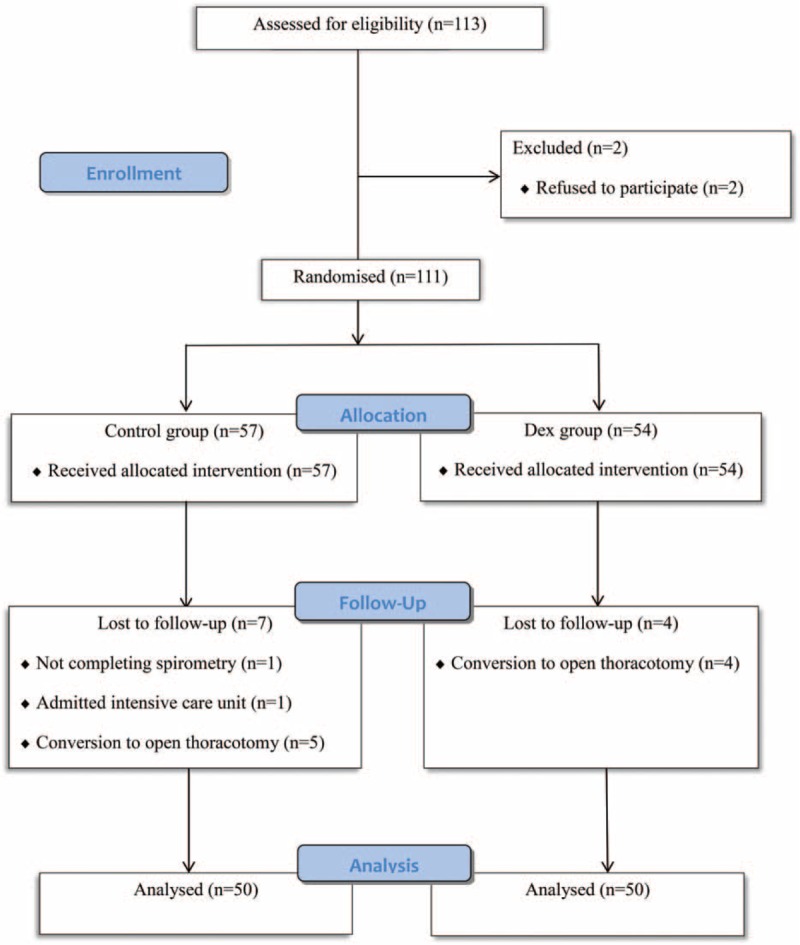
CONSORT flow diagram.
TABLE 1.
Baseline Demographic and Clinical Characteristics

As shown in Table 2, baseline QoR-40 global and dimensional scores were not different between the groups. The global scores on POD 1 and 2 were significantly higher in the Dex group compared with the Control group. On POD 1 and 2, scores for the emotional state, physical comfort, and pain were higher in the Dex group compared with the Control group (Figure 2).
TABLE 2.
Global and Dimensions Score of Quality of Recovery

FIGURE 2.
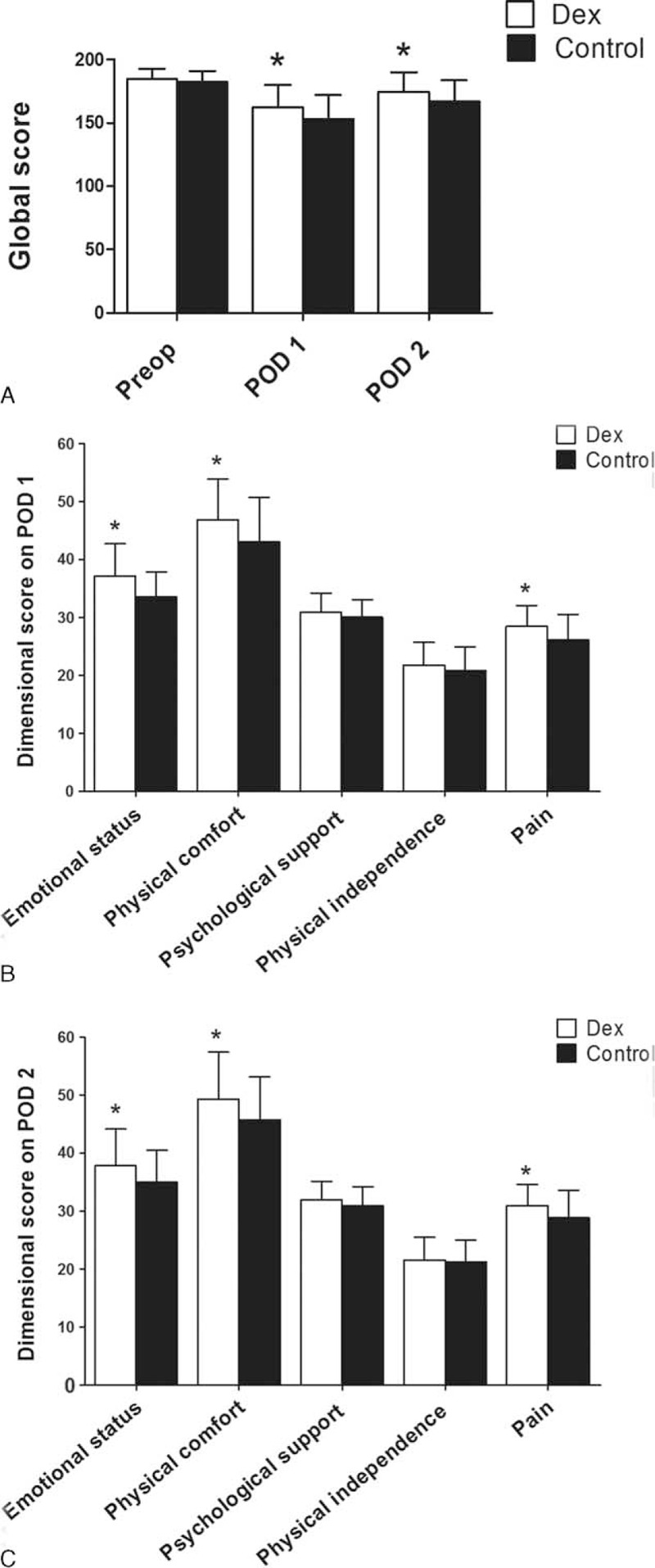
Changes in QoR-40 global and dimensional scores between Dex and Control groups. Variables presented as mean ± standard deviation. POD = postoperative day, Preop = preoperative, QoR-40 = quality of recovery 40 questionnaire. ∗P < 0.05 compared with Control group.
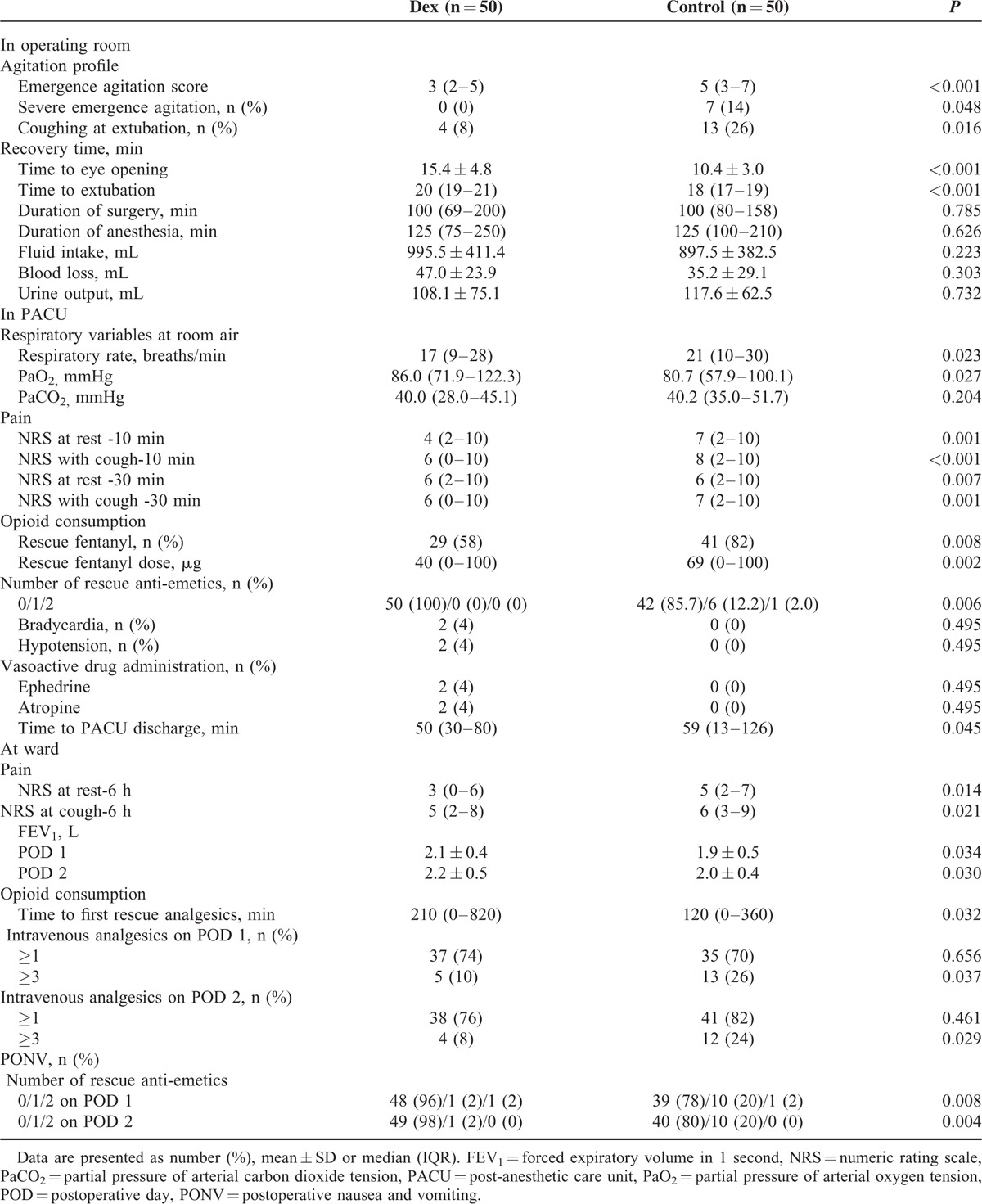
Perioperative data are presented in Table 3. The score of emergence agitation was lower in the Dex group compared with the Control group. The number of patients showing severe emergence agitation was greater in the Control group than Dex group. The incidence of coughing at extubation was significantly lower in Dex group compared with the Control group. The times from cessation of desflurane to eye opening as well as extubation were delayed in the Dex group compared with the Control group. PaO2 measured in PACU was higher in the Dex group compared with the Control group. Although the respiratory rate was lower in the Dex group compared with the Control group, PaCO2 was not different between the groups. Both NRS at rest and coughing, and opioid consumption in PACU were significantly lower in the Dex group compared with the Control group. The postoperative nausea and vomiting were more frequent in the Control group; thus, incidence of administrated anti-emetics was significantly higher in the Control group compared with the Dex group. There were no differences in the incidence of hypotension or bradycardia between the 2 groups. The discharge time from PACU was shorter in the Dex group compared with the Control group. NRS at rest and cough at ward were significantly lowered in the Dex group compared with the Control group. FEV1 was significantly greater in the Dex group compared with the Control group on POD 1 and 2. Intravenous analgesia after surgery was required significantly earlier in the Control group compared with the Dex group. Moreover, the incidence of >3 requests for intravenous opioids on POD 1 and 2 was significantly lower in the Dex group compared with the Control group. Rescue anti-emetics were administrated more frequently in the Control group compared with the Dex group on POD 1 and 2.
TABLE 3.
Perioperative Data
As shown in Table 4, the incidence of postoperative complications such as atelectasis, pneumonia, chylothorax, and atrial fibrillation were not significantly different between the groups. The length of hospital stay was significantly shorter in the Dex group compared with the Control group.
TABLE 4.
Postoperative Complication and Length of Hospital Stay

DISCUSSION
This study demonstrated that the quality of recovery was significantly enhanced with intraoperative dexmedetomidine administration in patients undergoing VATS. Postoperative pulmonary function and oxygenation were also improved in the Dex group, without increase in pulmonary complications. Finally, pain scores, emergence agitation, postoperative nausea and vomiting, and opioid consumption were decreased with dexmedetomidine administration. Although dexmedetomidine administration prolonged the time to return of consciousness from general anesthesia, discharge time from PACU and length of hospital stay were significantly reduced.
The validity and reliability of the QoR-40 is clinically significant for patient-related psychometric assessment in verifying patient satisfaction.18,19, In our study, emotional state, physical comfort, and pain among 5 dimensions of QoR-40 were improved by dexmedetomidine administration. The physical comfort dimension consists of postoperative nausea/vomiting, shivering, restlessness, and fatigue. Nausea and vomiting are triggered by opioid use and high catecholamine levels,25 and dexmedetomidine is known to have beneficial effects on early postoperative nausea and vomiting, particularly in high-risk patients.12 Although there were no differences in surgery-related complications between the groups, the length of hospital stay was significantly reduced in the Dex group. These results provide further evidence for dexmedetomidine attenuating the general condition, as it can improve the quality of recovery in respect of the patients’ physical comfort and emotional status. Therefore, dexmedetomidine acts as an important contributor in raising patient satisfaction and relieving postoperative pain.
In this study, moderate pain after VATS was sustained despite of the application of PCA. Several studies have confirmed the analgesic and opioid-sparing effects of dexmedetomidine after major surgery.10,17,20 Intraoperative dexmedetomidine administration decreased immediate postoperative pain and improved QoR-40 for the pain dimension from after surgery until POD 2. Dexmedetomidine has a relatively short elimination half-life of 2 to 2.5 hours.26 However, during that time, dexmedetomidine acts as a sedative by its sympatholytic properties. Dexmedetomidine enhances the analgesic effects of coadministered analgesics16; distinctively, a synergic antinociceptic action occurs when coadministering α2-adrenergic agonists and μ-receptor agonists.27 Yet, it is not fully clarified whether the analgesic properties of dexmedetomidine have any long-lasting effects. According to Ge et al,20 in addition to the downstream mechanism of α2-adrenergic receptor, dexmedetomidine extends the analgesics time of other analgesics, thereby indirectly having a long-lasting proanalgesic effect. In accordance, our result reflected that dexmedetomidine could have a synergistic effect with fentanyl, a μ-receptor agonist. Therefore, the improved postoperative pain score can be attributable to both direct effects and indirect effects of dexmedetomidine with coadministration of fentanyl-based intravenous patient-controlled analgesia. In this study, dexmedetomidine induced smooth emergence after VATS. To our knowledge, there were no previous studies focusing on emergence agitation after thoracic surgery. Emergence agitation is multifactorial and has been linked to severe pain from the surgical site and factors that may cause physical irritation such as urinary catheterization.28 In our study, the average NRS after VATS was 7 for the Control group, and a causal relationship between severe pain and emergence agitation can be considered. Consequently, dexmedetomidine administration may be an effective way to ameliorate emergence agitation for patient undergoing VATS.
In our result, intraoperative dexmedetomidine administration improved arterial oxygenation in the PACU and pulmonary function on POD 1 and 2. Postoperative pulmonary function was known to relate with pain after thoracic surgery.29,30 Incision pain prevents patients from sighing and taking deep breaths and suppresses coughing to expectorate sputum; this can cause ineffective ventilation. Therefore, the pain-modulating effect of dexmedetomidine could have improved postoperative oxygenation. Also, opioid-sparing effect of dexmedetomidine could contribute to reduce the opioid-induced respiratory depression. Although dexmedetomidine administration delayed the emergence time from general anesthesia by extending the time to eye opening and extubation; it paradoxically shortened the discharge time from PACU by attennuating pain, PONV, and respiratory function.
The length of hospital stay after VATS can be influenced by several factors including age, performance status, and postoperative pulmonary function.31 In addition to these socioenvironmental and clinical factors, it is important to consider psychological factors, which can also manipulate postoperative length of hospital stay.32 In the present study, there were no significant differences of social demographic factors or clinical factors including comorbidity and acquired postoperative complications between the 2 groups. Nevertheless, the Dex group showed superior QoR scores and emotional scores, and overall psychological statuses of the patients were improved. Ameliorating the psychological distress of patients reduced the length of hospital stay, which in return can also yield larger hospital cost savings. Although we were not able to confirm the long-term effects of dexmedetomidine, Myles et al33 reported poor quality of recovery can predict a poor quality of life after surgery. Thus, dexmedetomidine administration during VATS can increase QoR, promote quality of life, and lead to a higher satisfaction rate for patients after surgery.
This study has several limitations. First, we enrolled patients with lung cancer whose cardiopulmonary function is relatively normal before surgery. Therefore, our results are not applicable to patients with high marginal cardiopulmonary function. Although dexmedetomidine administration has been related to severe bradycardia, hypotension, and cardiac arrest, such side-effects were not appeared in our study. Therefore, additional studies are needed to investigate the effects of dexmedetomidine on patients with compromised cardiopulmonary function undergoing VATS. Second, previous studies20,21 investigated the use of dexmedetomidine on its effects of adequate analgesia or postoperative recovery with both loading doses and maintenance infusion of dexmedetomidine. In this study, dexmedetomidine was administrated as much as loading dose without maintenance dose. Our results suggest that to improve QoR and postoperative pulmonary function, a large dose of dexmedetomdine is not necessary to achieve such beneficial postoperative outcomes. Further studies are needed to specify the range of dexmedetomidine dosage required to achieve such beneficial postoperative outcomes.
In conclusion, our results suggested that intraoperative dexmedetomidine administration could improve the quality of recovery and postoperative pulmonary function in patients undergoing VATS. These findings were related with the decrease in pain, postoperative nausea and vomiting, emergence agitation, and opioid consumption by dexmedetomidine administration. Consequently, intraoperative dexmedetomidine administration could improve postoperative outcomes and reduced the hospital stay in patients undergoing VATS.
Footnotes
Abbreviations: BIS = bispectral index, Dex = dexmedetomidine, FEV1 = forced expiratory volume in 1 second, NRS = numeric rating scale, PaCO2 = partial pressure of arterial carbon dioxide, PACU = post-anesthetic care unit, PaO2 = partial pressure of arterial oxygen, PCA = patient-controlled analgesia, POD = postoperative day, QoR = quality of recovery, VATS = video-assisted thoracoscopic surgery.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2059283).
The authors have no fundings and conflicts of interest to disclose.
REFERENCES
- 1.Li WW, Lee RL, Lee TW, et al. The impact of thoracic surgical access on early shoulder function: video-assisted thoracic surgery versus posterolateral thoracotomy. Eur J Cardiothorac Surg 2003; 23:390–396. [DOI] [PubMed] [Google Scholar]
- 2.Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001; 72:362–365. [DOI] [PubMed] [Google Scholar]
- 3.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006; 81:421–426. [DOI] [PubMed] [Google Scholar]
- 4.Passlick B, Born C, Sienel W, et al. Incidence of chronic pain after minimal-invasive surgery for spontaneous pneumothorax. Eur J Cardiothorac Surg 2001; 19:355–359. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand PC, Regnard JF, Spaggiari L, et al. Immediate and long-term results after surgical treatment of primary spontaneous pneumothorax by VATS. Ann Thorac Surg 1996; 61:1641–1645. [DOI] [PubMed] [Google Scholar]
- 6.Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J Thorac Cardiovasc Surg 1994; 107:1079–1085. [DOI] [PubMed] [Google Scholar]
- 7.Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology 2001; 10:19–28. [DOI] [PubMed] [Google Scholar]
- 8.Ueki M, Kawasaki T, Habe K, et al. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia 2014; 69:693–700. [DOI] [PubMed] [Google Scholar]
- 9.Xiang H, Hu B, Li Z, et al. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation 2014; 37:1763–1770. [DOI] [PubMed] [Google Scholar]
- 10.Ren C, Chi M, Zhang Y, et al. Dexmedetomidine in postoperative analgesia in patients undergoing hysterectomy: a CONSORT-prospective, randomized, controlled trial. Medicine (Baltimore) 2015; 94:e1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajan S, Hutcherson MT, Sessler DI, et al. The effects of dexmedetomidine and remifentanil on hemodynamic stability and analgesic requirement after craniotomy: a randomized controlled trial. J Neurosurg Anesthesiol 2015; In press. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Shim J, Song J, et al. Dexmedetomidine added to an opioid-based analgesic regimen for the prevention of postoperative nausea and vomiting in highly susceptible patients: A randomised controlled trial. Eur J Anaesthesiol 2016; 33:75–83. [DOI] [PubMed] [Google Scholar]
- 13.Hoy SM, Keating GM. Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs 2011; 71:1481–1501. [DOI] [PubMed] [Google Scholar]
- 14.Sairaku A, Yoshida Y, Hirayama H, et al. Procedural sedation with dexmedetomidine during ablation of atrial fibrillation: a randomized controlled trial. Europace 2014; 16:994–999. [DOI] [PubMed] [Google Scholar]
- 15.Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation 2013; 127:1576–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin TF, Yeh YC, Lin FS, et al. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br J Anaesth 2009; 102:117–122. [DOI] [PubMed] [Google Scholar]
- 17.Blaudszun G, Lysakowski C, Elia N, et al. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 2012; 116:1312–1322. [DOI] [PubMed] [Google Scholar]
- 18.Gornall BF, Myles PS, Smith CL, et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth 2013; 111:161–169. [DOI] [PubMed] [Google Scholar]
- 19.Myles PS, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth 2000; 84:11–15. [DOI] [PubMed] [Google Scholar]
- 20.Ge DJ, Qi B, Tang G, et al. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal colectomy: a CONSORT-prospective, randomized, controlled clinical trial. Medicine (Baltimore) 2015; 94:e1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekker A, Haile M, Kline R, et al. The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol 2013; 25:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med 1999; 27:1325–1329. [DOI] [PubMed] [Google Scholar]
- 23.Chung F. Are discharge criteria changing? J Clin Anesth 1993; 5:64–68. [DOI] [PubMed] [Google Scholar]
- 24.Myles PS, Hunt JO, Nightingale CE, et al. Development and psychometric testing of a quality of recovery score after general anesthesia and surgery in adults. Anesth Analg 1999; 88:83–90. [DOI] [PubMed] [Google Scholar]
- 25.Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 1992; 77:162–184. [DOI] [PubMed] [Google Scholar]
- 26.Scheinin H, Aantaa R, Anttila M, et al. Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha2-adrenoceptor antagonist atipamezole: a pharmacodynamic and kinetic study in healthy volunteers. Anesthesiology 1998; 89:574–584. [DOI] [PubMed] [Google Scholar]
- 27.Fairbanks CA, Kitto KF, Nguyen HO, et al. Clonidine and dexmedetomidine produce antinociceptive synergy in mouse spinal cord. Anesthesiology 2009; 110:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HC, Lee YH, Jeon YT, et al. The effect of intraoperative dexmedetomidine on postoperative catheter-related bladder discomfort in patients undergoing transurethral bladder tumour resection: A double-blind randomised study. Eur J Anaesthesiol 2015; 32:596–601. [DOI] [PubMed] [Google Scholar]
- 29.Ried M, Schilling C, Potzger T, et al. Prospective, comparative study of the On-Q(R) PainBuster(R) postoperative pain relief system and thoracic epidural analgesia after thoracic surgery. J Cardiothorac Vasc Anesth 2014; 28:973–978. [DOI] [PubMed] [Google Scholar]
- 30.Bauer C, Hentz JG, Ducrocq X, et al. Lung function after lobectomy: a randomized, double-blinded trial comparing thoracic epidural ropivacaine/sufentanil and intravenous morphine for patient-controlled analgesia. Anesth Analg 2007; 105:238–244. [DOI] [PubMed] [Google Scholar]
- 31.Ueda K, Kaneda Y, Sakano H, et al. Obstacles for shortening hospitalization after video-assisted pulmonary resection for lung cancer. Ann Thorac Surg 2003; 76:1816–1820. [DOI] [PubMed] [Google Scholar]
- 32.Oxlad M, Stubberfield J, Stuklis R, et al. Psychological risk factors for increased post-operative length of hospital stay following coronary artery bypass graft surgery. J Behav Med 2006; 29:179–190. [DOI] [PubMed] [Google Scholar]
- 33.Myles PS, Hunt JO, Fletcher H, et al. Relation between quality of recovery in hospital and quality of life at 3 months after cardiac surgery. Anesthesiology 2001; 95:862–867. [DOI] [PubMed] [Google Scholar]


