Abstract
The clinical utility of leukocytosis in risk assessment for ST-elevation myocardial infarction (STEMI) is still unclear. We aim to demonstrate the prognostic value of leukocyte counts independent from traditional risk factors and the TIMI risk score (TRS) for STEMI and to propose a practical model comprising leukocyte count for early triage in STEMI undergoing primary angioplasty.
A prospective database (n = 796) of consecutive STEMI cases receiving primary angioplasty at a tertiary medical center was retrospectively analyzed in the period from February 1, 2007 through December 31, 2012. Primary endpoints were 30-day and 1-year mortality. Propensity score-adjusted Cox regression models and subdivision analysis were performed.
Leukocytosis group (n = 306) had higher 30-day mortality (5.9% vs 3.1%, P = 0.048) and 1-year mortality (9.2% vs 5.1%, P = 0.022). After adjustment by propensity score and TRS, leukocyte count (per 103/μL) was an independent predictor of 1-year mortality (HR: 1.086, 95% CI: 1.034–1.140, P = 0.001). Subdivision analysis demonstrated the correlation between leukocytosis and higher 1-year mortality within both high and low TRS strata (divided by 4, the median of TRS). Additionally, 24% (191 out of 796) of patients were characterized by nonleukocytosis and TRS < 4, having 0% of mortality rate at 1-year follow-up.
In conclusion, leukocyte count is an independent prognostic factor adding incremental value to TRS for STEMI. Nonleukocytosis in conjunction with TRS < 4 identifies a large patient group at extremely low risk and thus provides rapid early triage for STEMI patients undergoing primary PCI. This finding is worth validation in the future.
INTRODUCTION
Numerous studies have documented the important role of inflammation in atherosclerotic diseases.1–3 Inflammatory processes promote the initiation and evolution of atheroma, contribute to acute thrombosis, and take part in the pathophysiology of acute coronary syndrome (ACS).3,4 Elevation of several inflammatory markers, such as C-reactive protein, serum amyloid A, interleukin-6, and interleukin-1 receptor antagonist has been associated with unfavorable outcomes in patients with ACS.3,5 Leukocyte count is a widely implicated and convenient indicator of inflammation in clinical practice, and the key role that leukocytes play in ACS has already been demonstrated.6 The correlation of leukocyte count with cardiovascular risk factors and acute myocardial infarction has been reported in several studies.7–10 In patients with ST-elevation myocardial infarction (STEMI), the most fulminant manifestation of coronary atherosclerotic disease, leukocyte count also plays a role in predicting prognosis. Many studies have shown an association between elevated admission leukocyte count and poor outcomes, including larger infarct, worse left ventricular function, and increased long-term mortality.11–17 However, the clinical utility of the above findings is still unclear because the usefulness of a new biomarker depends on the ability to demonstrate that it adds incremental value to prognostic models traditionally used in clinical practice.18 One widely utilized and validated prognostic model is the Thrombolysis in Myocardial Infarction (TIMI) risk score for STEMI. Comprising age, coronary risk factors, body weight, vital signs, Killip classification, the culprit vessel, and symptom-to-balloon time, the TIMI risk score (TRS) for STEMI is easily accessible and able to predict early both 30-day and 1-year mortality.19 Whether leukocyte count brings independent prognostic value beyond such a powerful traditional risk prediction tool is unknown. We intended to use a widely acknowledged cutoff point of leukocyte count to define leukocytosis. According to the definition of systemic inflammatory response syndrome (SIRS) used in daily practice, a leukocyte count ≥12,000/μL is generally accepted to indicate an inflammatory process.20 Our study's purpose was to clarify whether elevated leukocyte count brings independent prognostic value to the traditional TRS for STEMI and to propose a practical and simple model to integrate leukocyte count into clinical risk stratification in STEMI.
METHODS
Ethics Statement
This study was a retrospective analysis of a prospective database approved by the Institutional Review Board of Far Eastern Memorial Hospital. All data were stored in the hospital database and used only for academic research.
Study Population
The database enrolled consecutive patients of STEMI diagnosed at the emergency department (ED) who had the onset of chest pain within 12 hours before presentation and underwent primary percutaneous coronary intervention (PCI) at Far Eastern Memorial Hospital, the only tertiary medical center in New Taipei City, which is an administrative region with more than 3 million residents in northern Taiwan. Patients receiving coronary artery bypass grafting during the admission were not registered to the database.
We analyzed the data collected in the period from February 1, 2007 through December 31, 2012, and excluded those without available TRS for STEMI. The registry used the electrocardiographic criteria for STEMI: either ≥0.2 mV (anterior myocardial infarction) or 0.1 mV (nonanterior myocardial infarction) of ST elevation in 2 contiguous electrocardiographic leads or left bundle branch block that was new or presumed to be new.
Treatment Protocol
Loading doses of oral dual-antiplatelet therapy and intravenous heparin were administered at the ED. Our hospital is a PCI center performing elective PCI for more than 1000 cases per year and primary PCI for more than 100 cases per year. Cardiac catheterization was performed routinely via right femoral artery. The decision to use glycoprotein IIb/IIIa inhibitors was left to the discretion of the interventionists. Balloon angioplasty and stenting if feasible were performed in the culprit vessel. Postangioplasty care was given in standard fashion according to contemporary guidelines.21 Treatment with oral beta blockers, statins, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers was initiated during admission.
Data Collection
Plasma leukocyte counts were measured in venous blood drawn on admission before angiography, and we divided the patients into a leukocytosis group and a nonleukocytosis group using the cutoff point of 12,000/μL. Mortality data were collected from the registration data of Department of Health and phone interview. All patients were followed for 1 year, and there was no loss of follow-up at 1 year. TRS was calculated by summation of the following: 1 point for anterior myocardial infarction, 1 point for history of diabetes mellitus, hypertension or angina pectoris, 1 point for body weight < 67 kg, 1 point for symptom to treatment time > 4 hours, 2 points for Killip II–IV, 2 points for heart rate > 100 beats per minute, 2 points for age of 65 to 74 years, 3 points for systolic blood pressure < 100 mmHg and 3 points for age ≥ 75 years.19
Endpoints and Statistical Analysis
The primary study endpoints were 30-day and 1-year mortality. Categorical variables are expressed as numbers and percentage and compared using a Chi-square test. Continuous variables are presented as medians and interquartile ranges and were compared using the Mann–Whitney U test. Thirty-day and 1-year mortality were compared using Kaplan–Meier estimate with generalized Wilcoxon test. All P values were 2-tailed, and P < 0.05 was considered statistically significant. All statistical analyses were reviewed and revised by a professional statistical consultant of our hospital and were performed using SPSS statistical software version 19.0 (IBM Corp., Armonk, NY).
Propensity-Score Adjustment
We constructed 2 propensity-score models. In model A, a propensity score was created for each patient by a logistic regression model comprising all potential predictors or confounders of 1-year mortality as covariates, including age, gender, body mass index, history of cardiovascular risk factors (diabetes mellitus, hypertension, hyperlipidemia, former or current smokers, previous myocardial infarction, and previous ischemic stroke), laboratory data on admission (creatinine and hemoglobin levels) and presentation factors (Killip classification, anterior myocardial infarction, multivessel diseases, and door-to-balloon time). Leukocyte count was then entered into the propensity score-adjusted Cox-proportional hazard regression analysis (by including the propensity score as a covariate) for prediction of 1-year mortality. In model B, a new propensity score was created for each patient the same as in model A except the exclusion of covariates involved in calculation of TRS (age, body mass index, diabetes mellitus, hypertension, Killip classification, anterior myocardial infarction, and door-to-balloon time). The propensity score-adjusted Cox regression analysis (by including the new propensity score as a covariate) containing both leukocyte count and TRS was performed.
Subdivision Analysis
We subdivided patients into nonleukocytosis and leukocytosis respectively within strata of high and low TRS (using the median of TRS as a cutoff point) to investigate the interplay between leukocyte count and TRS and predictive performance after combining both. In both strata, Kaplan–Meier method with generalized Wilcoxon test was used to compare 1-year mortality of nonleukocytosis and leukocytosis groups.
RESULTS
Characteristics of Study Subjects
From February 2007 to December 2012, our database included 847 patients, but 51 of them did not have an available TRS. Among 796 analyzable patients, 306 had a leukocyte count on admission ≥12,000/μL (the leukocytosis group), and the other 490 patients had a leukocyte count <12,000/μL (the nonleukocytosis group).
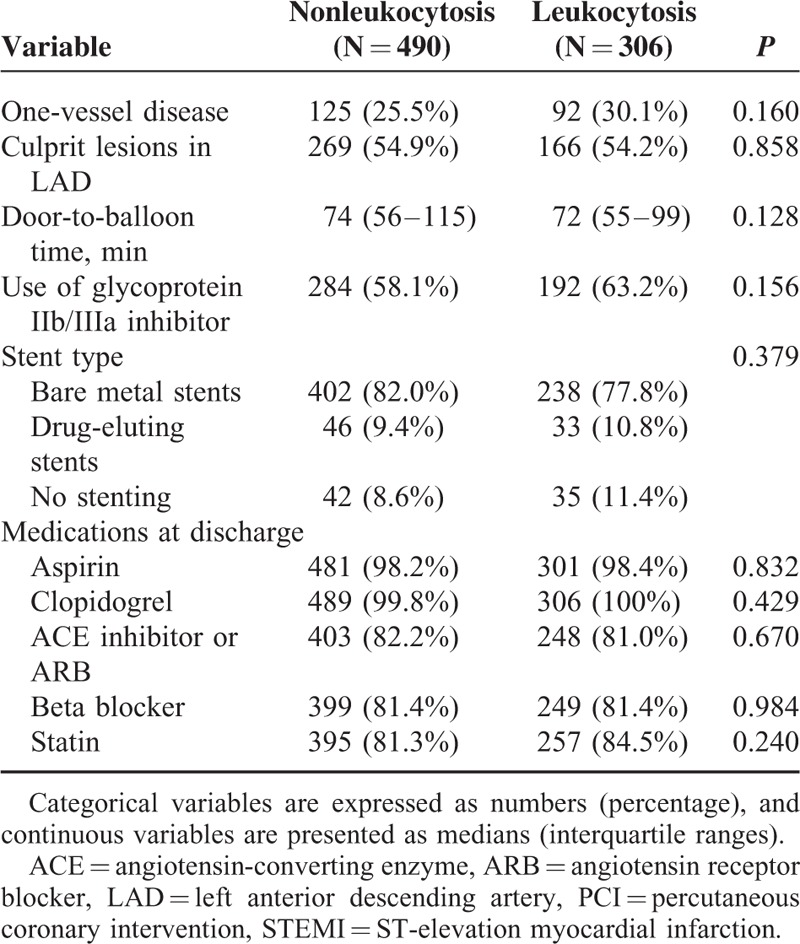
Leukocytosis on admission was associated with younger age, male gender, less extensive history of hypertension or dyslipidemia, current or former smoker status, elevated hemoglobin levels, higher triglyceride levels, and lower TRS (Table 1). Angiographic, procedural, and pharmacological data are demonstrated in Table 2.
TABLE 1.
Baseline Clinical Characteristics of the Study Population
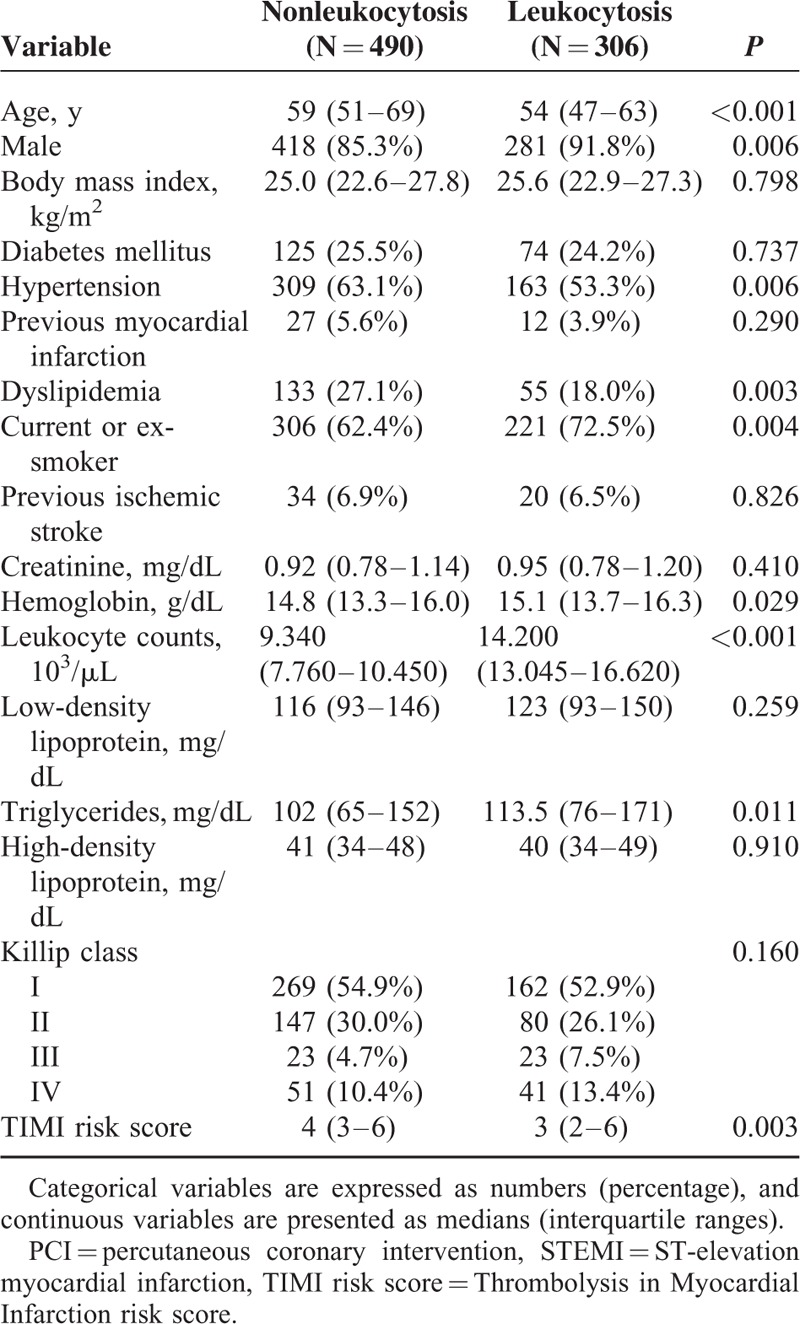
TABLE 2.
Angiographic, Procedural, and Pharmacological Baseline Data of the Study Population
Primary Endpoints and Propensity-Score Adjustment
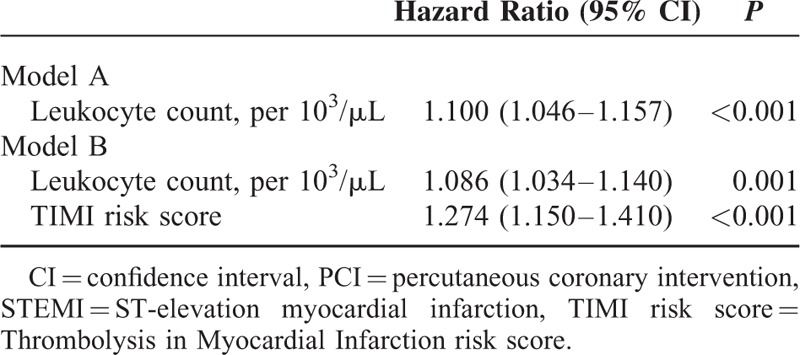
Compared with nonleukocytosis patients, those with leukocytosis had higher 30-day mortality (5.9% vs 3.1%, P = 0.048) and 1-year mortality (9.2% vs 5.1%, P = 0.022) (Table 3). In addition, enzymatic infarct sizes were also significantly larger in the leukocytosis group. After performing propensity-score analysis by model A, we found leukocyte count (per 103/μL) an independent predictor of 1-year mortality (HR: 1.100, 95% CI: 1.046–1.157, P < 0.001) (Table 4). In model B, leukocyte count (per 103/μL) remained an independent predictor of 1-year mortality (HR: 1.086, 95% CI: 1.034–1.140, P = 0.001) after adjustment by the propensity score and TRS (Table 4). In addition, the combination with leukocyte count resulted in an increase in C-statistics of TRS from 0.774 to 0.805.
TABLE 3.
Enzymatic Infarct Sizes and Crude Mortality of Nonleukocytosis and Leukocytosis Groups
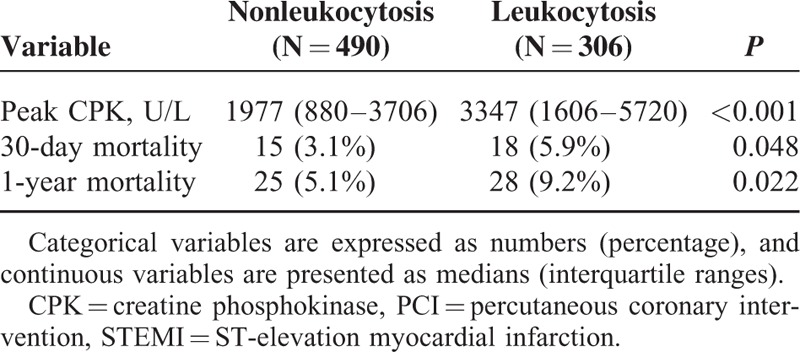
TABLE 4.
Propensity Score-Adjusted Cox Regression Models Including Leukocyte Count and TIMI Risk Score for Predicting 1-Year Mortality
Subdivision Analysis
The median of TRS was 4, by which stratification of high and low TRS was made. Further subdivision by leukocytosis or not showed significant difference in 1-year mortality between nonleukocytosis and leukocytosis groups within both strata (0% vs 2.6%, P = 0.026 within low TRS and 8.3% vs 15.9%, P = 0.012 within high TRS, respectively) (Figure 1).
FIGURE 1.
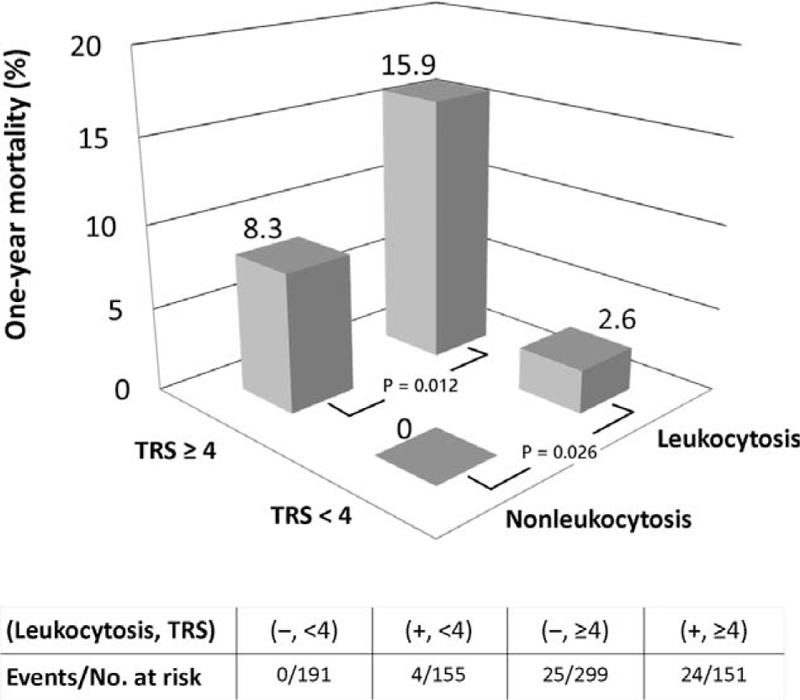
Subdivision analysis. Leukocytosis was correlated with significantly higher 1-year mortality within high and low TRS stata. Nonleukocytosis along with TRS < 4 identified a low-risk group having literally 0% risk of death at 1-year follow-up. Oppositely, leukocytosis with TRS ≥ 4 predicted grave prognosis. TRS = Thrombolysis in Myocardial Infarction (TIMI) risk score.
DISCUSSION
Our study's novel finding is that leukocyte count of STEMI patients on admission not only predict 1-year mortality independently of all known traditional risk factors but also has prognostic value beyond the well-validated, widely accepted risk-prediction tool—in this case, the TRS for STEMI. To our knowledge, this is the first study to demonstrate the independent prognostic value of elevated leukocyte count beyond the TRS and introduce a practical tool, as discussed later, integrating leukocyte count and traditional risk assessment for STEMI patients undergoing primary PCI.
Compared with the nonleukocytosis group, patients with leukocytosis tended to be younger, more often male and current or former smokers. They also have higher hemoglobin levels, higher triglyceride levels and lower TRS. Leukocytosis was inversely correlated with the number of coronary risk factors. These baseline characteristics were similar to those described in previous studies, including one of the largest-scale studies by Palmerini et al.16 Although all the above features had been reportedly associated with better outcomes in STEMI,21–25 patients with leukocytosis still carried an approximately 2-fold relative risk for both 30-day and 1-year mortality. These results reflect a patient group that has fewer traditional cardiovascular risk factors and even lower TRS but whose higher inflammatory and thrombotic status, which is hardly reflected by traditional parameters, result in their more fulminant presentation and grave prognosis. The linkage between elevated leukocyte counts and larger infarct sizes and worse left ventricular function may explain the extension of heightened mortality to 1 year.13,17
Smit et al26 demonstrated that the correlation between leukocyte counts and 1-year mortality was not as statistically significant as that of C-reactive protein and 1-year mortality. However, the patients in this analysis came from the ON-TIME trial, which excluded those in Killip class III or IV, and only 16% of whom were in Killip class > I. Another example was the Stent PAMI Trial, in which less than 10% of study subjects were in Killip > I. By retrospective analysis of the registry, Pellizzon et al stated that the admission leukocyte count was a strong independent predictor of reinfarction but not of mortality.27 Lack of association between leukocyte count and mortality in above-mentioned registries might be driven by low disease severity. In contrast, our cohort included nonselective patients with any disease severity, including cardiogenic shock or severe heart failure, only approximately half of whom were in Killip class I. We believe that this sample is compatible with real-world scenarios and truly reflects the effects of provoked inflammation in the acute phase of myocardial infarction.
Patients in some of the previous studies were stratified by tertiles or quartiles according to leukocyte count, a method that clinicians never use in real-world practice. Our study demonstrated that leukocyte count can be a prognostic predictor of STEMI in daily clinical practice by using the most common and widely accepted cutoff point used in the SIRS definition.
Given the independent prognostic value of leukocyte count beyond the TRS, combination of both may yield more powerful risk assessment. None of the 191 patients with nonleukocytosis and TRS < 4 died in 1-year follow-up, that is, this patient group had literally 0% risk of mortality. In contrast, patients with leukocytosis and TRS ≥4 had particularly poor outcomes. Figure 1 illustrates that a leukocyte count along with a TRS can pick out a large number of patients in particularly low or high risk (24% without death at 1-year follow-up and 18.9% with extremely high mortality).
The ability of a risk-assessing model to identify low-risk population is important. Less frequent monitoring, earlier discharge, a shorter hospital stay, and longer follow-up intervals can be planned for patients at very low risk. Better allocation of medical resources is expected by cost saving in those at very low risk and focusing on those at high risk. Being the most widely accepted and well validated risk-prediction model for STEMI, the TRS is able to identify those with 1-year mortality < 1% in about 10% of patients.19 Using the TRS < 4 in conjunction with nonleukocytosis, however, we were able to identify patients at very low risk with 0% of 1-year mortality who constituted 24% (191 out of 796) of our study cohort. This makes a simple method of rapid and reliable triage for STEMI in the very early clinical course to predict extremely low risk in a large proportion of patients.
Whether elevated leukocyte count directly leads to poor prognosis is still unclear because the mechanisms underlying this correlation have not been fully uncovered. What is known is that leukocytes take part in the production of tissue factors, thrombin generation, platelet aggregation, and the release of proinflammatory factors.28–30 In addition, reduced microcirculatory perfusion may result from the adhesion or aggregation of increased leukocytes.31 In turn, these effects may contribute to reperfusion injury, acute no-reflow phenomenon or further infarct extension.31–34 The association between elevated leukocyte count and enlarged infarct sizes is consistent with these mechanisms.
This study had several limitations to be acknowledged. First, the possibility of coexisting infectious diseases was not excluded. However, the median of length of hospital stay (5 days) did not differ significantly between the leukocytosis and nonleukocytosis groups. Thus, it is reasonable to conclude that no serious cases of infectious disease were found in this STEMI cohort. Second, information on leukocyte differential counts was unavailable. Third, it is still unknown whether the association between elevated leukocyte count and mortality was causal or merely a result of provoked inflammation or larger infarct sizes.
CONCLUSIONS
In conclusion, for STEMI patients undergoing primary PCI, elevated leukocyte counts predict 1-year mortality independently from known traditional cardiovascular risk factors or the TRS. Leukocytosis-or-not on admission in conjunction with the TRS (≥4 or <4) provides rapid and early triage in clinical practice to identify a large proportion of patients at extremely low long-term risk. It is worth validation by studies in the future.
Acknowledgments
The authors thank the patients who participated in the study. We thank the statistical consultant, Dr. Chien-Hao Chen, for his assistance in statistical analysis. We also thank Yung-Cheng Chen and Yung-Chu Tsai as research coordinators for their valuable efforts.
Footnotes
Abbreviations: ACS = acute coronary syndrome, ED = emergency department, PCI = percutaneous coronary intervention, SIRS = systemic inflammatory response syndrome, STEMI = ST-elevation myocardial infarction, TIMI = Thrombolysis in Myocardial Infarction, TRS = TIMI risk score.
This work was supported in part by grants FEMH-2013-HHC-002 and FEMH-2014-HHC-002 from the Far Eastern Memorial Hospital, New Taipei City, Taiwan, and grant MOST 103-2325-B-418-001 from the Ministry of Science and Technology of Taiwan.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Boyle J. Association of coronary plaque rupture and atherosclerotic inflammation. J Pathol 1997; 181:93–99. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999; 340:115–126. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105:1135–1143. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Tabas I, Fredman G, et al. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res 2014; 114:1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayr A, Klug G, Schocke M, et al. Late microvascular obstruction after acute myocardial infarction: relation with cardiac and inflammatory markers. Int J Cardiol 2012; 157:391–396. [DOI] [PubMed] [Google Scholar]
- 6.van der Laan AM, Hirsch A, Robbers LF, et al. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: monocytes and myocardial infarction. Am Heart J 2012; 163:57.e2–65.e2. [DOI] [PubMed] [Google Scholar]
- 7.Ernst E, Hammerschmidt D, Bagge U, et al. Leukocytes and the risk of ischemic diseases. JAMA 1987; 257:2318–2324. [PubMed] [Google Scholar]
- 8.Friedman G, Klatsky A, Siegelaub A. The leukocyte count as a predictor of myocardial infarction. N Engl J Med 1974; 290:1275–1278. [DOI] [PubMed] [Google Scholar]
- 9.Friedman G, Tekawa I, Grimm R, et al. The leucocyte count: correlates and relationship to coronary risk factors: the CARDIA study. Int J Epidemiol 1990; 19:889–893. [DOI] [PubMed] [Google Scholar]
- 10.Ingram D, Gillum R. Leukocyte count and cardiovascular risk factors. J Natl Med Assoc 1992; 84:1041–1043. [PMC free article] [PubMed] [Google Scholar]
- 11.Barron HV, Cannon CP, Murphy SA, et al. Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: a thrombolysis in myocardial infarction 10 substudy. Circulation 2000; 102:2329–2334. [DOI] [PubMed] [Google Scholar]
- 12.Chia S, Nagurney J, Brown D, et al. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol 2009; 103:333–337. [DOI] [PubMed] [Google Scholar]
- 13.Chung S, Song YB, Hahn JY, et al. Impact of white blood cell count on myocardial salvage, infarct size, and clinical outcomes in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a magnetic resonance imaging study. Int J Cardiovasc Imaging 2014; 30:129–136. [DOI] [PubMed] [Google Scholar]
- 14.Grzybowski M, Welch R, Parsons L, et al. The association between white blood cell count and acute myocardial infarction in-hospital mortality: findings from the National Registry of Myocardial Infarction. Acad Emerg Med 2004; 11:1049–1060. [DOI] [PubMed] [Google Scholar]
- 15.Palmerini T, Brener SJ, Genereux P, et al. Relation between white blood cell count and final infarct size in patients with ST-segment elevation acute myocardial infarction undergoing primary percutaneous coronary intervention (from the INFUSE AMI trial). Am J Cardiol 2013; 112:1860–1866. [DOI] [PubMed] [Google Scholar]
- 16.Palmerini T, Mehran R, Dangas G, et al. Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions: analysis from the Harmonizing Outcome with Revascularization and Stent in Acute Myocardial Infarction trial. Circulation 2011; 123:2829–2837.7 p following 2837. [DOI] [PubMed] [Google Scholar]
- 17.Prasad A, Stone G, Stuckey T, et al. Relation between leucocyte count, myonecrosis, myocardial perfusion, and outcomes following primary angioplasty. Am J Cardiol 2007; 99:1067–1071. [DOI] [PubMed] [Google Scholar]
- 18.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. [Erratum appears in Circulation. 2009 Jun 30;119(25):e606 Note: Hong, Yuling [added]]. Circulation 2009; 119:2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000; 102:2031–2037. [DOI] [PubMed] [Google Scholar]
- 20.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 21.Benamer H, Tafflet M, Bataille S, et al. Female gender is an independent predictor of in-hospital mortality after STEMI in the era of primary PCI: insights from the greater Paris area PCI Registry. EuroIntervention 2011; 6:1073–1079. [DOI] [PubMed] [Google Scholar]
- 22.Newell MC, Henry JT, Henry TD, et al. Impact of age on treatment and outcomes in ST-elevation myocardial infarction. Am Heart J 2011; 161:664–672. [DOI] [PubMed] [Google Scholar]
- 23.Vrsalovic M, Pintaric H, Babic Z, et al. Impact of admission anemia, C-reactive protein and mean platelet volume on short term mortality in patients with acute ST-elevation myocardial infarction treated with primary angioplasty. Clin Biochem 2012; 45:1506–1509. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y-T, Liu T-J, Lai H-C, et al. Lower serum triglyceride level is a risk factor for in-hospital and late major adverse events in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention—a cohort study. BMC Cardiovasc Disord 2014; 14:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aune E, Roislien J, Mathisen M, et al. The “smoker's paradox” in patients with acute coronary syndrome: a systematic review. BMC Med 2011; 9:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smit J, Ottervanger J, Slingerland R, et al. Comparison of usefulness of C-reactive protein versus white blood cell count to predict outcome after primary percutaneous coronary intervention for ST elevation myocardial infarction. Am J Cardiol 2008; 101:446–451. [DOI] [PubMed] [Google Scholar]
- 27.Pellizzon GG, Dixon SR, Stone GW, et al. Relation of admission white blood cell count to long-term outcomes after primary coronary angioplasty for acute myocardial infarction (The Stent PAMI Trial). Am J Cardiol 2003; 91:729–731. [DOI] [PubMed] [Google Scholar]
- 28.Goel MS, Diamond SL. Neutrophil enhancement of fibrin deposition under flow through platelet-dependent and -independent mechanisms. Arterioscler Thromb Vasc Biol 2001; 21:2093–2098. [DOI] [PubMed] [Google Scholar]
- 29.Palmerini T, Coller BS, Cervi V, et al. Monocyte-derived tissue factor contributes to stent thrombosis in an in vitro system. J Am Coll Cardiol 2004; 44:1570–1577. [DOI] [PubMed] [Google Scholar]
- 30.Vita JA, Brennan ML, Gokce N, et al. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 2004; 110:1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiagarajan RR, Winn RK, Harlan JM. The role of leukocyte and endothelial adhesion molecules in ischemia-reperfusion injury. Thromb Haemost 1997; 78:310–314. [PubMed] [Google Scholar]
- 32.Kaminski KA, Bonda TA, Korecki J, et al. Oxidative stress and neutrophil activation—the two keystones of ischemia/reperfusion injury. Int J Cardiol 2002; 86:41–59. [DOI] [PubMed] [Google Scholar]
- 33.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007; 357:1121–1135. [DOI] [PubMed] [Google Scholar]
- 34.Botto N, Sbrana S, Trianni G, et al. An increased platelet-leukocytes interaction at the culprit site of coronary artery occlusion in acute myocardial infarction: a pathogenic role for “no-reflow” phenomenon? Int J Cardiol 2007; 117:123–130. [DOI] [PubMed] [Google Scholar]


