Abstract
Published literatures have reported the relationship between hypoxic-inducible factor-2α (HIF-2α) expression and clinicopathological features in gastric cancer (GC), but the evaluated conclusions remain controversial.
A meta-analysis was carried to examine the clinicopathological features and prognostic values of HIF-2α in patients with GC. Systematic detailed searches were performed to Pub Med, Cochrane Library, and EBSCO until to August 2015.
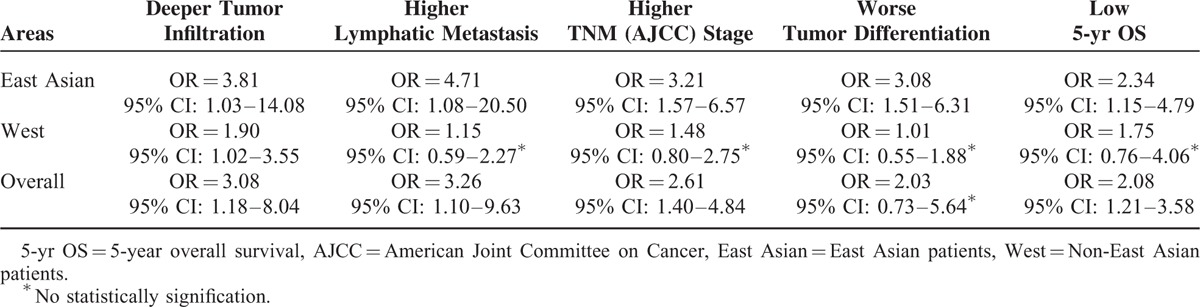
Six studies (508 specimens) were included in this meta-analysis. HIF-2α-positive expression indicates an unfavorable prognosis value and advanced clinicopathological differences for the available patient dates with GC. Further multivariate meta-analysis revealed that HIF-2α-positive expression in gastric cancer associated with deeper tumor infiltration (OR = 3.08; 95%CI: 1.18–8.04), higher rates of lymphatic metastasis (OR = 3.26; 95%CI: 1.10–9.63), higher TNM stage (III+IV) (OR = 2.61; 95%CI: 1.40–4.84), and much lower 5-year overall survival (OR = 2.08; 95%CI: 1.21–3.58). Nevertheless, there is no association between HIF-2α-positive expression and worse tumor differentiation (OR = 2.03; 95%CI: 0.73–5.64). In addition, by this subgroup analysis, HIF-2α-positive expression associated with deeper tumor infiltration (OR = 3.81; 95%CI: 1.03–14.08), higher lymphatic metastasis (OR = 4.71; 95%CI: 1.08–20.50), higher TNM stage (OR = 3.21; 95%CI: 1.57–6.57), worse tumor differentiation (OR = 3.08; 95%CI: 1.51–6.31), and lower 5-year overall survival (OR = 2.34; 95%CI: 1.15–4.79).
Our results indicate that HIF-2α overexpression can potently predict the poor prognosis and may be a potential therapeutic target for gastric carcinoma, according to the limited evidence. Meanwhile, further studies are needed to elucidate the accuracy of these results.
INTRODUCTION
Worldwide, gastric cancer (GC) is the fifth most common malignant cancers, leading to an estimated 952,000 new cases in GLOBOCAN 2012.2 Despite recent advances in medical technology have made it possible for early detection of tumors through screening, diagnosis, and treatment, the prognosis of GC still remains poor. In 2012, there were an estimated 723,000 cancer-related deaths with a poor 5-year overall survival (OS) rate.1,2 The high mortality rate of GC is due to advanced metastasis but not the primary cancer. Therefore, detecting novel and target-based biological markers are core value for improving diagnosis and treatment of GC as early as possible.
In the 1950s, Gray et al first describes the hypoxic tissue areas of tumors, and then the feature was widely found in many solid human tumors.3 Tumor hypoxia, areas of low oxygen, is associated with metabolism, differentiation, necrosis or apoptosis, and rapid growth of tumors. Also, it can adversely affect the prognosis of cancers of breast, uterine cervix, head and neck, rectum, lung, and so on.4,5,6 Some researchers have elucidated 1 mechanism by which hypoxia-inducible factors (HIFs) predominantly prompt the adaptation to hypoxia at the level of cell and tissue.13 In addition, detailed studies have also hypothesized that HIFs are essential mediators of the cellular oxygen-signaling pathway, and HIFs expression correlated with a poor patient prognosis, and regulated metastasis, differentiation from the aspects of tumorigenesis.14 Until now, hypoxia-inducible factors (HIFs) have been discovered and evaluated in GC, including hypoxia-inducible factor-1α (HIF-1α), hypoxia-inducible factor-2α (HIF-2α), and hypoxia-inducible factor-3α (HIF-3α). Among of all 3 HIFs, HIF-1α, and HIF-2α are the most extensively studied and are the well-characterized. In recent most clinical studies, HIF-1α expression, but also HIF-2α expression, was placed as an explicit critical regulator and was associated with a poor prognostic in many human solid tumors, including cancers of stomach, colorectum, ovary, and so on.14,15
Interesting, for GC, prior research focused on the association between HIF-1α expression and prognosis and clinicopathological features. Besides, 1 previous published meta-analysis has reported that HIF-1α associated with poor OS and disease-free survival (DFS) in GC.16
However, there has been no reported meta-analysis about HIF-2α in GC. So, this paper analyze the correlation of HIF-2α clinicopathological features and link with 5-year overall survival rate in gastric cancer, which were known to provide useful information for cancer prognosis and treatment. Based on the above, we summarized all the available clinical trials to finish a meta-analysis about HIF-2α expression, and further confirm prognostic value and clinicopathological differences of HIF-2α in GC. Moreover, in this paper, we investigated several high-quality studies have been published recently, and we also reviewed and explored the potential evidences that association between HIF-2α expression and clinicopathological features as well as 5-year overall survival rate for GC patients.
MATERIALS AND METHODS
Ethics Statement
This study was authorized and approved by the Clinical Research Ethics Committee of Affiliated Hospital of Qinghai University and Cancer Hospital, Chinese Academy of Medical Sciences.
Literature Retrieval
We searched the literature from PubMed, Cochrane Library, and EBSCO using the terms: “HIF-2α,” “HIF2α,” “HIF-2,” “HIF2,” “hypoxia-inducible factor 2α,” “hypoxia-inducible factor-2α,” “EPAS1,” “Gastric Cancer,” “Gastric Carcinoma,” “Stomach Cancer,” “Stomach Carcinoma,” “Stomach Neoplasm,” “Stomach Neoplasm.” Meanwhile, the search strategy also limited to next terms “prognostic value” “prognosis” or “clinicopathological.” In addition, MeSH terms as well as other free text words were used in our retrieval.
Inclusion and Exclusion Criteria
The objective of this meta-analysis was to evaluate the prognostic value of HIF-2α expression in GC patients regarding OS and give the correlation between clinicopathological features and HIF-2α expression. Therefore, only published articles in English languages were selected in our meta-analysis. In addition, to satisfy our eligibility, studies had to fulfill the following inclusion criteria: (1) gastric tissue specimens from human, not animals; (2) using immunohistochemical (IHC) analysis for HIF-2α in human gastric tissue specimens; (3) offering the IHC images of specimens; (4) the relationship between clinicopathological features and HIF-2α expression should be depicted; or (5) provides information about 5-year overall survival rate.
The exclusion criteria for our selected literature were as follows: (1) letters, case reports, and conference records without original data; (2) specimens from animals or cell lines; (3) specimens from other solid tumors; (4) studies lacking substantiated evidence of HIF-2α expression on survival; and (5) specimen information from secondary literatures or overlapping articles.
Qualitative Assessment
Quality assessment was matched with the Newcastle Ottawa quality assessment scale (NOS).17 We systematically evaluated the quality of all the studies, and 0 and 9 scores were respectively designated as lowest and highest quality. Studies with scores of 5 to 9 are considered as high quality, whereas 0 to 4 as low quality in our meta-analysis. Detailed scores on the 6 studies were listed in Table 1.
TABLE 1.
Newcastle Ottawa Quality Assessment Scale (NOS) of All Studies
Data Analysis
The paper was analyzed using the STATA statistical software (version 12.1). The pooled effect was calculated using either a fixed-effects or a random-effects model in odds ratio (OR) with 95% CI. Heterogeneity of studies was assessed by chi-square and Q statistical test. We calculated the OR with the random effects model when the studies were homogeneous (with chi-square was ≥ 50%, P < 0.1 for the Q test). Beyond that, we choose the fixed effects model. Overall, a P value < 0.1 or chi- square was ≥ 50% indicates significant heterogeneity among studies.18 The possibility of publication bias was assessed using a funnel plot when the P values < 0.1 for the Q test. In the Harbord et al study, researchers observe that an appropriate Harbord's test reality better than Egger's test with little studies.19 Therefore, publication bias was evaluated by Harbord's test not Egger's test and Begg's test. Specially, the difference was statistically significance when the P values < 0.05 in our meta-analysis.
RESULTS
Literature Research and Study Characteristics
The initial search strategy retrieved 208 publications. We screened all titles, abstracts, and full texts. Finally, 6 eligible studies,7–12 ranging from 2008 to 2015, were included in our analysis. Figure 1 described how exacting the process was, and explaining the whole work. Table 1 retrospectively analyzes and summarizes the characteristics of all the studies in the final analysis. Six studies, involving 508 GC specimens, were included to evaluate the association between HIF-2α expression and clinicopathological features. And, a total of 2 studies met our criteria and were included for analysis the correlation between HIF-2α expression and OS. To date, the morbidity and mortality of GC are comparatively higher in Eastern Asian populations, such as Chinese, Japanese, and Korean. Meanwhile, in our meta-analysis, 4 studies were conducted in China (298 specimens), 1 in England (172 specimens), and 1 in Korea (38 specimens), respectively.
FIGURE 1.
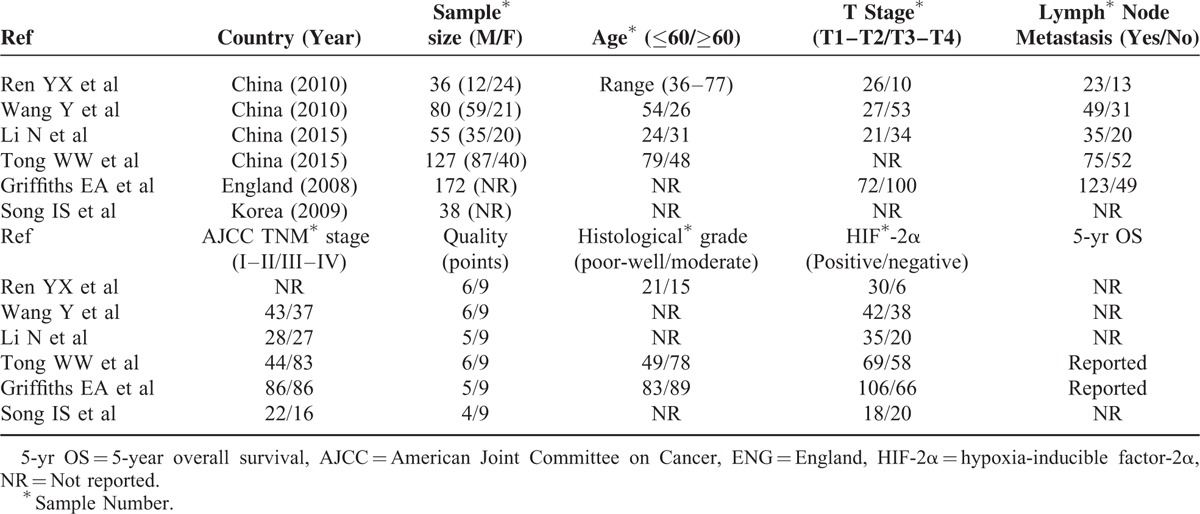
The association between HIF-2α positive and tumor infiltration: HIF-2α-positive expression in GC associated with deeper tumor infiltration (OR = 3.08; 95%CI: 1.18–8.04).CI = confidence interval, GC = gastric cancer, HIF-2α = hypoxia-inducible factor-2α, OR = odds ratio.
Correlation Between HIF-2α-Positive Expression and Clinicopathological Features of GC
Of all cancer-related clinicopathological features, all the pooled date of 6 studies showed that HIF-2α-positive expression were significantly associated with depth of invasion, lymphatic metastasis, and TNM staging of GC.
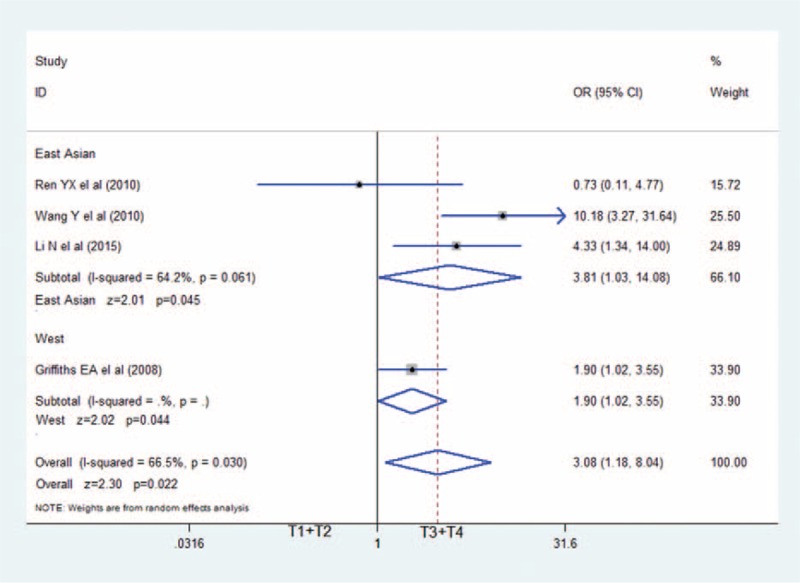
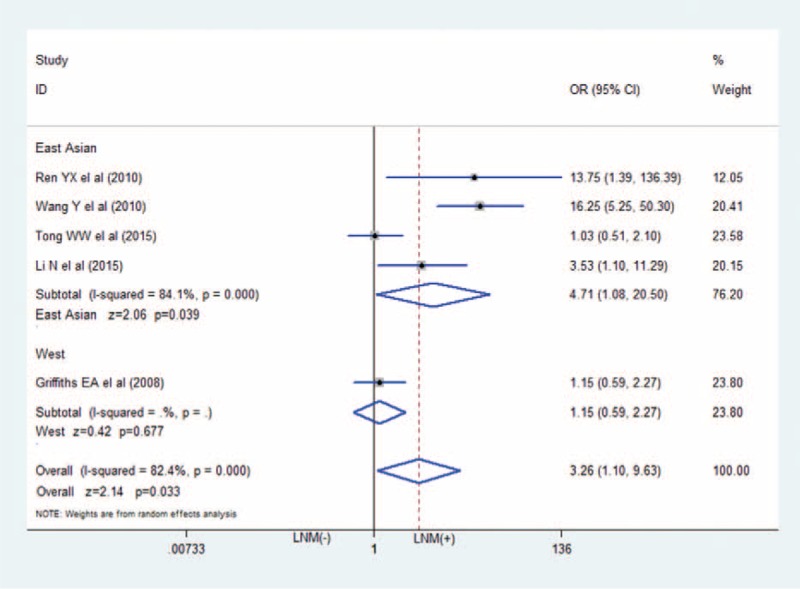
Data on the association between depth of invasion and HIF-2α expression was shown in 4 studies.7–9,11 As shown in Figure 2, patients with T3 and T4 gastric cancer had higher HIF-2α expression (343 specimens; OR = 3.08; 95%CI: 1.18–8.04; P = 0.022; random effects model) than those with T1 and T2 GC, with moderate between-study heterogeneity (I2 = 66.5%; P = 0.030). Also, we revealed the correlation between HIF-2α-positive expressions with other clinicopathological features. Figure 3 showed that positive expression of HIF-2α was closely associated with much higher lymphatic metastasis (OR = 3.26; 95%CI: 1.10–9.63; P = 0.033; random effects model),7–11 and much more advanced TNM staging in Figure 4 (OR = 2.61; 95%CI: 1.40–4.84; P = 0.002; random effects model).8–12
FIGURE 2.
The association between HIF-2α positive and lymphatic metastasis: HIF-2α-positive expression in GC associated with higher rates of lymphatic metastasis (OR = 3.26; 95%CI: 1.10–9.63). CI�=�confidence interval, GC�=�gastric cancer, HIF-2α = hypoxia-inducible factor-2α, OR = odds ratio.
FIGURE 3.
The association between HIF-2α positive and TNM stage: HIF-2α-positive expression in GC associated with higher TNM stage (III+IV) (OR = 2.61; 95%CI: 1.40–4.84). CI = confidence interval, GC = gastric cancer, HIF-2α = hypoxia-inducible factor-2α, OR = odds ratio.
FIGURE 4.
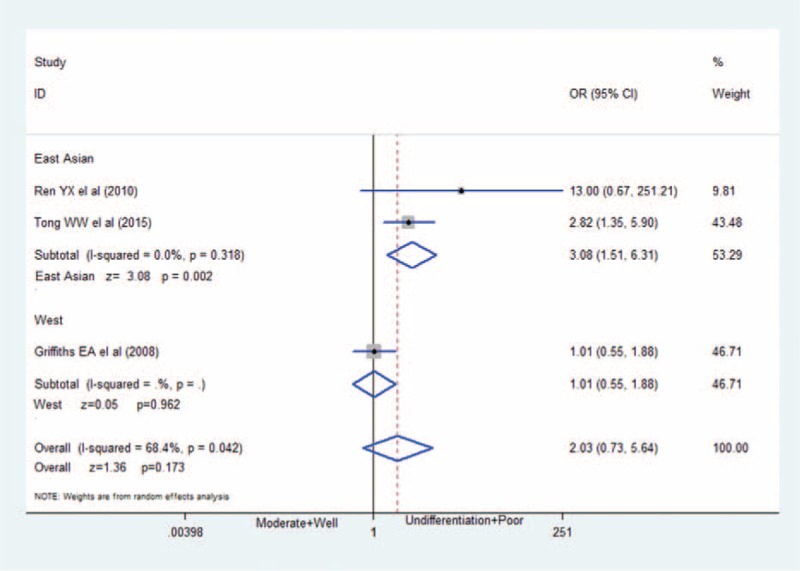
The association between HIF-2α positive and tumor differentiation: HIF-2α-positive expression has no association with worse tumor differentiation (OR = 2.03; 95%CI: 0.73–5.64) in GC. CI = confidence interval, GC = gastric cancer, HIF-2α = hypoxia-inducible factor-2α, OR = odds ratio.
In Figure 5 of our meta-analysis, 3 studies7,10,11 provides the information of grade of tumor differentiation (poor, moderate or well), but only 1 study (Tong et al) show relationship of HIF-2α expression with poor tumor differentiation. After we combine all the 3 studies, there is no statistically signification (OR = 2.03; 95%CI: 0.73–5.64; P = 0.173; random effects model), with moderate between-study heterogeneity (I2 = 68.4%; P = 0.042). We reassessed and reanalyzed aforementioned factors in subgroups defined by race, compared the differences of East Asians with Westerns, and eliminated ethnic differences. The detailed results are shown in Table 2. Results showed that that HIF-2α overexpression was closely associated with deeper tumor infiltration, higher lymphatic metastasis, higher TNM stage, worse tumor differentiation, and low 5-year overall survival among East Asians. Surprisingly, among Westerns HIF-2α overexpressions are only related with deeper tumor infiltration.
FIGURE 5.
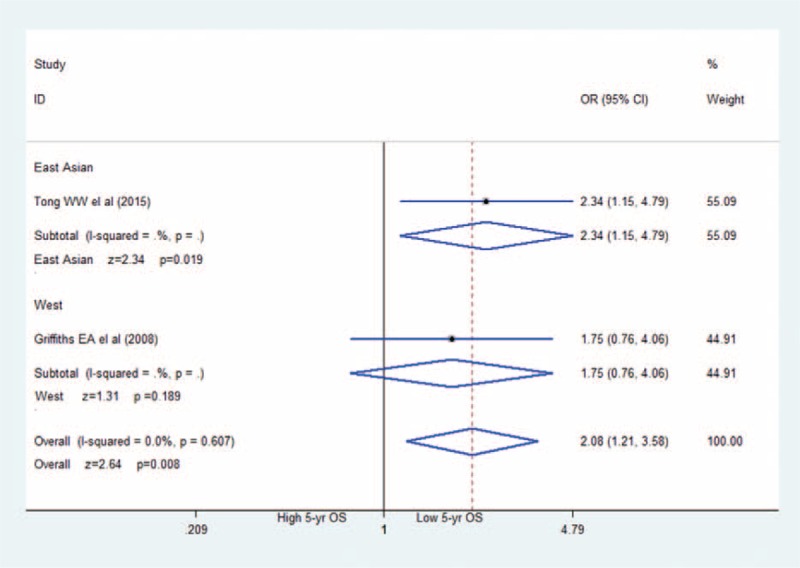
The association between HIF-2α positive and 5-year overall survival: HIF-2α-positive expression in GC associated with much lower 5-year overall survival (OR = 2.08; 95%CI: 1.21–3.58). CI = confidence interval, GC = gastric cancer, HIF-2α = hypoxia-inducible factor-2α, OR = odds ratio.
TABLE 2.
The Characteristics of Populations Based on Different Areas
Correlation Between HIF-2α-Positive Expression and 5-Year Overall Survival
In Figure 6, 6 studies7–12 mentioned corrections between poor prognosis (OS) and HIF-2α expression in GCs, but only 2 of them reported extract figure of 5-year OS. Meta-analysis revealed that patients with HIF-2α positive expression correlated significantly with poor 5-year OS. In this meta-analysis we applied the fixed effects model to obtain the result because heterogeneity was I2 = 0.0%, and P = 0.607. Of special note, the paper of Tong only provided readers a 5 years survival curve, and we obtain the exact date with the help of the responding author of this paper.
FIGURE 6.
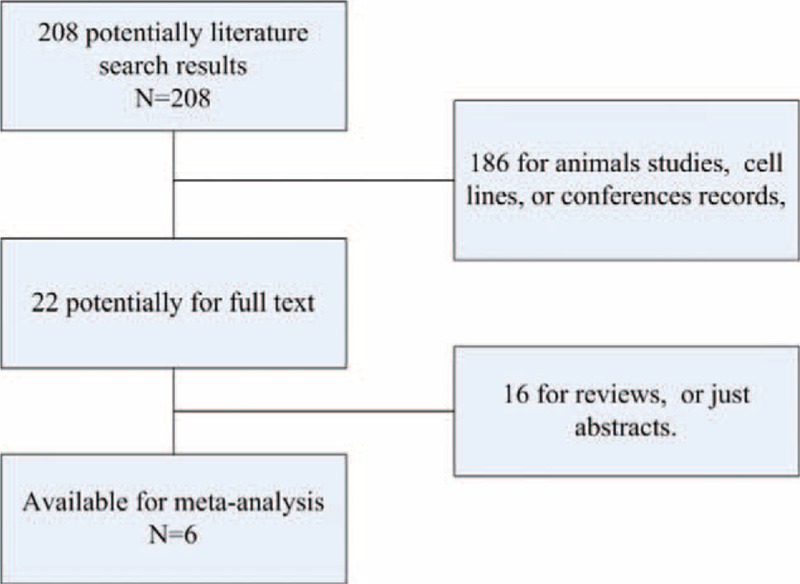
The search flow of literature: about 208 articles were retrieved, and those irrelevant articles were excluded, finally 6 articles were included.
Publication Bias
We used Harbord's test to assess publication bias for all comparisons. The results of Harbord's test showed there were no evidence of publication bias on TNM staging (P = 0.364), tumor infiltration (P = 0.991), lymphatic metastasis (P = 0.213), and tumor differentiation (P = 0.454), which means that there are no small-study effects. In our meta-analysis, all but one of the selected studies for meta-analyses was from the East Asian patients.17 So, we recalculate results after taking out that one. The detailed result is listed in Table 2.
Subgroup Analysis
Interesting, for East Asians, HIF-2α-positive expression associated with deeper tumor infiltration (OR = 3.81; 95%CI: 1.03–14.08), higher lymphatic metastasis (OR = 4.71; 95%CI: 1.08–20.50), higher TNM stage (OR = 3.21; 95%CI: 1.57–6.57), worse tumor differentiation (OR = 3.08; 95%CI: 1.51–6.31), and lower 5-year overall survival (OR = 2.34; 95%CI: 1.15–4.79) (Table 2). Also, the results of Harbord's test showed there had no publication bias. (The results were not shown.).
DISCUSSION
Hypoxia was first described in the 1990s as a prognostic value for patient, thereafter repeatedly mentioned in solid tumor. Hypoxia is a primarily pathophysiological consequence with the high uncontrolled growth of tumor, especially tumor angiogenesis. Equally important, tumor hypoxia can increase tumor aggressiveness by up- or downregulates the expression of HIFs.20,21 Briefly, HIFs plays an important role in promoting tumor. HIF-1α, its expression in various human cancers, has been reported and detected by numerous researches.
So what do we know about HIF-2α expression? Recent studies also have demonstrated that HIF-2α is overexpressed in various human malignancies. In contrast, HIF-2α expression plays a key role in the progression of renal carcinoma and neuroblastoma, whereas its expression is lost in patients of colon cancer with advanced tumor stage.22 Moreover, HIF-2α expression has been performed to assess the prognosis in many solid tumors. For renal cell carcinoma, the high expression of HIF -2α was significantly associated with poor cancer-specific survival.23 But, for GC, the association between HIF-2α expression and prognosis and clinicopathological features remains controversial.
To our best knowledge, this is the first meta-analysis that revealed the association between HIF-2α expression and clinicopathological features of GC. Meanwhile, we enacted strict and exacting quality control in its inclusion and exclusion criteria to insure that quality-reliable results.
What is more, due to the limitation of meta-analysis, data were collected from different authors and organizations. And, in this paper, tumor differentiations of GC were classified into 2 categories, including poor-well and moderate. So, the association between HIF-2α expression and tumor differentiation needed more detailed classification and made subgroup analysis in future research. Considering that HIF-2α overexpression was significant with lymph node metastasis, depth of invasion, and TNM staging, it should be specially noted in next step survival analyses. However, our study successfully confirmed elevated HIF-2α associated with poor prognosis and advanced clinicopathological features for patients with GC. In particular, we clearly observed that HIF-2α expression with deeper tumor infiltration in this paper. Additionally, we explored the correlation between the expression of HIF-2α and clinicopathologic features of GC, and found that the expression of HIF-2α was linked with deeper tumor infiltration, lymph node metastasis, and higher TNM staging (III+IV), but no with poor tumor differentiation.
Again in this meta-analysis, we also found that the 5-year OS in the HIF-2α positive group was significantly lower than that in the HIF-2α negative group. We used the Harbord's test to assess publication bias for all 5 comparisons in our meta-analysis; fortunately, the result showed that there has no small-study effect. And the results, analyzed by Harbord's test, could further explain our retrieved articles with high quality and reliability.
From our subgroup analysis, there is significantly association between HIF-2α-positive expression and clinicopathologic features, and low 5-year overall survival among East Asians. Specifically, for East Asians, HIF-2α-positive expression associated with deeper tumor infiltration, higher lymphatic metastasis, higher TNM stage, worse tumor differentiation, and lower 5-year overall survival. However, for West, HIF-2α-positive expression had no association with lymphatic metastasis, TNM stage, tumor differentiation, and 5-year overall survival (Table 2). Of special note, the conclusions of East Asians come from 5 studies, but only 1 study in West study.7–12 Based on above, the study of East Asians come from different center and may eliminate possible single center bias. Therefore, the conclusions indicate that meta-analysis is appropriate to analyze the populations from Eastern Asia. As far as Westerns is concerned, more prospective studies are needed to eliminate potent confounding factor. Again, this study may provide some reference for further studies about HIF-2α in gastric cancer.
Though we followed strict quality standard, several limitations of this meta-analysis should also be acknowledged. First, the sample size of our included studies was relatively small, which might influence the validity of our analysis; hence, a larger sample size is needed. Second, the assessments of HIF-2α overexpression were different; it may affect the interpretation of results of meta-analysis due to artificial errors of immunohistochemistry. Third, although we recalculate results after taking out that one from England, it may be necessary to more accurate results to reflect the ethnicity.
Taken together, the meta-analysis suggested that HIF-2α-positive expression could be a useful prognostic marker may be a potential therapeutic target for GC patients. In addition, our meta-analysis will provide useful information for clinical decision clinical prognosis in GC. But today, further studies are needed to elucidate the accuracy of these results.
Acknowledgements
The authors would like to thank all of the patients and clinical investigators who were involved in the studies selected for this meta-analysis.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, GC = gastric cancer, HIF-1α = hypoxia-inducible factor-1α, HIF-2α = hypoxia-inducible factor-2α, HIF-3α = hypoxia-inducible factor-3α, HIFs = hypoxia-inducible factors, OS = overall survival.
Funding: this work was supported by grants from the National Natural Science Foundation of China (No. 81360318).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No.11. Lyon: International Agency for Research on Cancer; 2013. [2013-12-12, 2014-01-18]. URL: http://globocan.iarc.fr. [Google Scholar]
- 2.Colquhoun A, Arnold M, Ferlay J, et al. Global patterns of cardia and non-cardia GC incidence in 2012. Gut 2015; 64:1881–1888. [DOI] [PubMed] [Google Scholar]
- 3.Gray LH, Conger AD, Ebert M, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radio 1953; 26:638–648. [DOI] [PubMed] [Google Scholar]
- 4.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 2007; 26:225–239. [DOI] [PubMed] [Google Scholar]
- 5.Brizel DM, Sibley GC, Prosnitz LR, et al. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1997; 38:285–289. [DOI] [PubMed] [Google Scholar]
- 6.Sivridis E, Giatromanolaki A, Gatter KC, et al. Tumor and Angiogenesis Research Group. Association of hypoxia-inducible factors 1alpha and 2alpha with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer 2002; 95:1055–1063. [DOI] [PubMed] [Google Scholar]
- 7.Ren YX, Liu HT, Li SL, et al. Clinical significance of hypoxia-inducible factor-2α expression in gastric carcinoma. World Chin J Digestol 2010; 18:1923–1927. [Google Scholar]
- 8.Wang Y, Li Z, Zhang H, et al. HIF-1α and HIF-2α correlate with migration and invasion in GC. Cancer Biol Ther 2010; 10:376–382. [DOI] [PubMed] [Google Scholar]
- 9.Li N, Wang HX, Qin C, et al. Relationship between clinicopathological features and HIF-2α in gastric adenocarcinoma. Genet Mol Res 2015; 14:1404–1413.doi: 10.4238/2015. [DOI] [PubMed] [Google Scholar]
- 10.Tong WW, Tong GH, Chen XX, et al. HIF2α is associated with poor prognosis and affects the expression levels of survivin and cyclin D1 in gastric carcinoma. Int JOncol 2015; 46:233–242.doi:10.3892/ijo.2014.2719. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths EA, Pritchard SA, McGrath SM, et al. Hypoxia-associated markers in gastric carcinogenesis and HIF-2a in gastric and gastro-oesophageal cancer prognosis. Br J Cancer 2008; 98:965–973.doi: 10.1038/sj.bjc.6604210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song IS, Wang AG, Yoon SY, et al. Regulation of glucose metabolism-related genes and VEGF by HIF-1α and HIF-1β, but not HIF-2α, in gastric cancer. Exp Mol Med 2009; 41:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Du F, Luo Y, et al. The emerging role of hypoxia-inducible factor-2 involved in chemo/radioresistance in solid tumors. Cancer Treat Rev 2015; 41:623–633. [DOI] [PubMed] [Google Scholar]
- 14.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 2008; 15:678–685.doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Du F, Shen G, et al. The role of hypoxia-inducible factor-2 in digestive system cancers. Cell Death Dis 2015; 6:e1600.doi: 10.1038/cddis.2014.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S, Ma R, Zheng XY, et al. Meta-analysis of immunohistochemical expression of hypoxia inducible factor-1α as a prognostic role in gastric cancer. World J Gastroenterol 2014; 20:1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2010; Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta- analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 18.Dersimonian R, Nan L. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 19.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006; 25:3443–3457. [DOI] [PubMed] [Google Scholar]
- 20.Greijer AE, van der Groep P, Kemming D, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J Pathol 2005; 206:291–304. [DOI] [PubMed] [Google Scholar]
- 21.Joshi S, Singh AR, Zulcic M, et al. A macrophage-dominant PI3K isoform controls hypoxia-induced HIF1α and HIF2α stability and tumor growth, angiogenesis, and metastasis. Mol Cancer Res 2014; 12:1520–1531. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene 2013; 32:4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan Y, Li H, Ma X, et al. Prognostic significance of hypoxia-inducible factor expression in renal cell carcinoma: a PRISMA-compliant systematic review and meta-analysis. Medicine 2015; 94:e1646. [DOI] [PMC free article] [PubMed] [Google Scholar]


