Abstract
Xingnaojing (XNJ) is commonly extracted from Angongniuhuang, a classic Chinese emergency prescription, and widely used in the treatment of nervous system disorders including consciousness disturbance in China.
To evaluate the beneficial and adverse effects of XNJ injection, on consciousness disturbance.
Seven major electronic databases were searched to retrieve randomized controlled trials designed to evaluate the clinical efficacy of XNJ alone or combined with Western medicine in treating consciousness disturbance caused by conditions such as high fever, poisoning, and stroke. The methodological quality of the included studies was assessed using criteria from the Cochrane Handbook for Systematic Review of Interventions, and analyzed using the RevMan 5.3.0 software.
Seventeen randomized controlled trials on XNJ were included in this study and the trials generally showed low methodological quality. The results revealed that XNJ alone or in combination with other medicines and adjuvant methods had a positive effect on patients with fever-, poisoning-, and stroke-induced coma.
XNJ effectively treated consciousness disturbances that were caused by high fever, poisoning, or stroke.
INTRODUCTION
Disturbance of consciousness, specifically coma, is a state in which patients become unresponsive to external stimuli, lose motor and sensory functions, and only retain autonomic nervous system functions.1 There are numerous causes of consciousness disturbance such as high fever, poisoning, and stroke. High fever-induced coma, which causes the body temperature to rise and, thereby, increases the release of excitatory amino acid neurotransmitters and oxygen-free radicals, aggravates brain damage. Moreover, increase in the body temperature can subsequently increase the levels of lactic acid in the entire or parts of the brain, resulting in accelerated neuronal death.2 Toxic comas, which primarily result from exogenous poisoning or intoxication, can induce different levels of consciousness in patients depending on the poisoning severity. Stroke-related comas, whether induced by cerebral infarction or hemorrhage, have been associated with cerebral edema and the brain edema severity directly affects the patients’ states of consciousness.3
Xingnaojing (XNJ) is extracted from Angongniuhuang, a classic Chinese emergency prescription, and is widely used to treat nervous system disorders including consciousness disturbance in China.4 Its main components are musk, borneol, gardenia, and Yu gold. A recent research study found that musk ketone excites the central nervous system (CNS), inhibiting vascular permeability while borneol synergistically enhances the effects of musk ketone.5 Furthermore, a combination of musk ketone and borneol increases the excitability of the respiratory center and improves blood composition.5 Briefly, XNJ excites respiration and the vasomotor center to improve cerebral edema and hypoxia, increase the metabolic rate and activity of brain cells, and thereby enhance brain function and promote the recovery of consciousness; therefore, it has a positive effect on consciousness disorders.6 Furthermore, experimental studies have shown that XNJ influences free radical damage through its antioxidant effect, which can reduce the associated brain damage to a certain extent.7–9 Clinical studies have also found that for patients with heat-induced unconsciousness, XNJ effectively reduced body temperature, improved their state of consciousness, and reduced brain damage.2 Furthermore, XNJ reduced the content of plasma beta-endorphin in the brain, and showed good therapeutic effects on acute alcoholism in combination with naloxone.10 Another study demonstrated the good awakening function of XNJ injection in cerebral hemorrhage, especially after a 7-day treatment.11
This present study was designed to perform a comprehensive systematic review and evaluation of the efficacy of XNJ injection for the treatment of consciousness disturbance compared with existing drug therapy.
METHODS
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Ethical approval was not necessary for this review study.
Database and Search Strategies
A literature search of the Chinese National Knowledge Infrastructure (CNKI), the Chinese Biomedical Literature (CBMdisc), the Chinese Scientific Journal Database (VIP), the Wanfang Database, EMbase, PubMed, and the Cochrane Library was conducted, which was concluded in May 2015. Furthermore, other relevant research papers were searched manually. The following search terms were used individually or in combination: “Xingnaojing,” “Xingnaojing injection,” “XNJ,” “coma,” “disturbance of consciousness,” and “randomized controlled trial.” The references of the selected studies were also searched for additional relevant studies. In addition, we used a flow chart to make the search process more rigorous and exhaustive (Figure 1).
FIGURE 1.
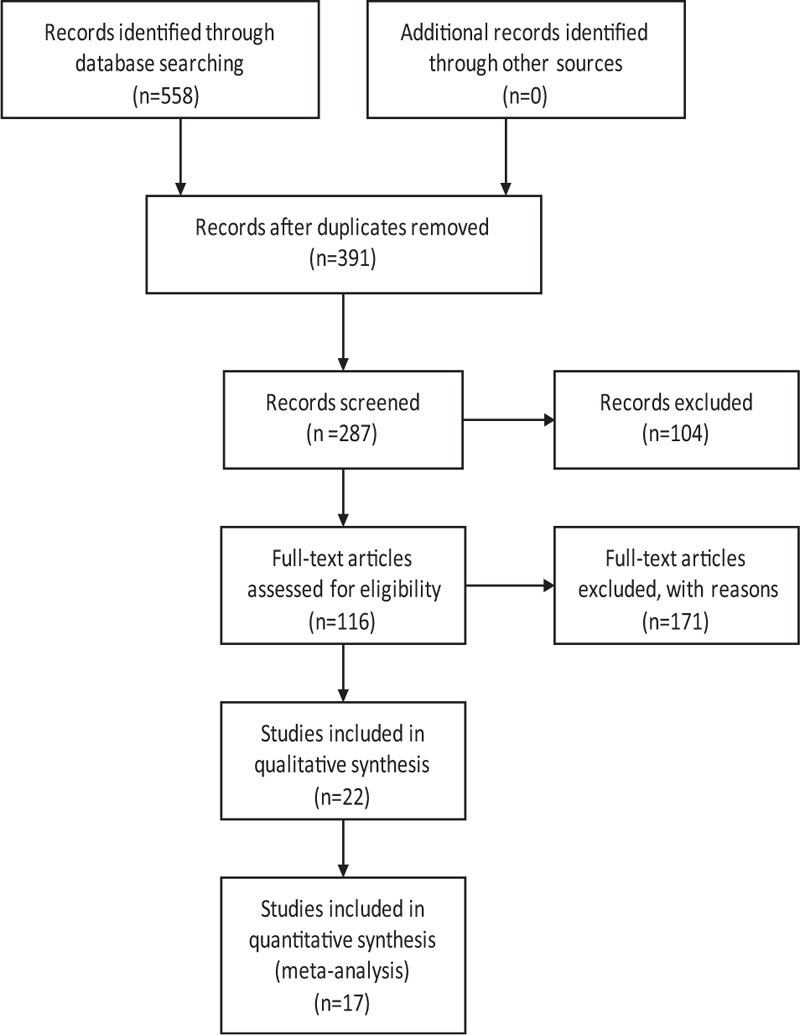
Flow diagram of the systematic review.
Inclusion and Exclusion Criteria
Inclusion Criteria
There were no restrictions placed on the studies included based on language, population characteristics, and publication type. All RCTs of patients with disturbance of consciousness that studied prescriptions based on XNJ alone or in combination with Western medicine compared with no medicine or Western medicine alone were included. Studies that used the Glasgow Coma Score (GCS) in combination with neurological deficits or disease diagnostic criteria were all included. The primary, secondary, and tertiary outcome measurements were the GCS, significant efficiency, and wake-up time, respectively.
Exclusion Criteria
Duplicated publications that reported on the same groups of participants were excluded.
Data Extraction and Quality Assessment
Two authors independently conducted the literature search and screening, as well as the data extraction. One author each was in charge of the Chinese and English literature retrievals, and then 1 author scrutinized the first selection while the other performed a secondary check. If an author questioned the relevance of any study or its content, a third individual was invited to arbitrate and make a decision, which contributed to improving the quality of the final selection and facilitated its adherence to our requirements. The extracted data included the title and authors of the study, year of publication, article source, study size, the total number of cases, grouping diagnosis criteria, details of the methodological approaches, and treatment process. In addition, the details of the control interventions, outcomes, and adverse effects were collected for each study. To ensure that the selected literature was of high quality, we used the RevMan version 5.3.0 to assess the studies systematically and comprehensively, according to the 7-parameter set. These were sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. Then, we constructed the “Risk of bias graph” and “Risk of bias summary” (Figures 2 and 3) to display the results of the bias risk assessment.12 Finally, we prepared the funnel plot to evaluate the publication bias and further verify the reliability of the results.
FIGURE 2.
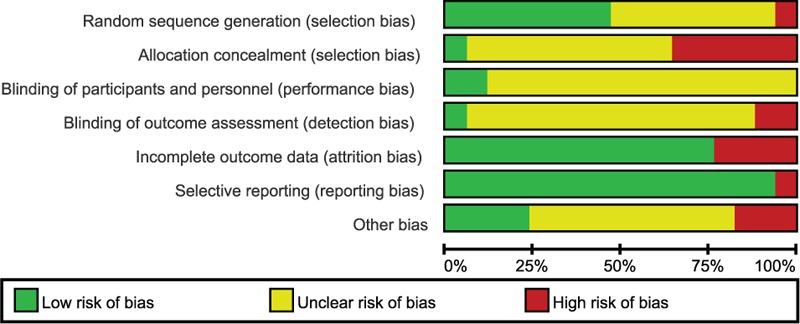
Risk of bias graph. Judgments of reviewing authors about each risk of bias item are presented as percentages of all included studies. Quality of selected studies was assessed according to the Cochrane criteria.12
FIGURE 3.
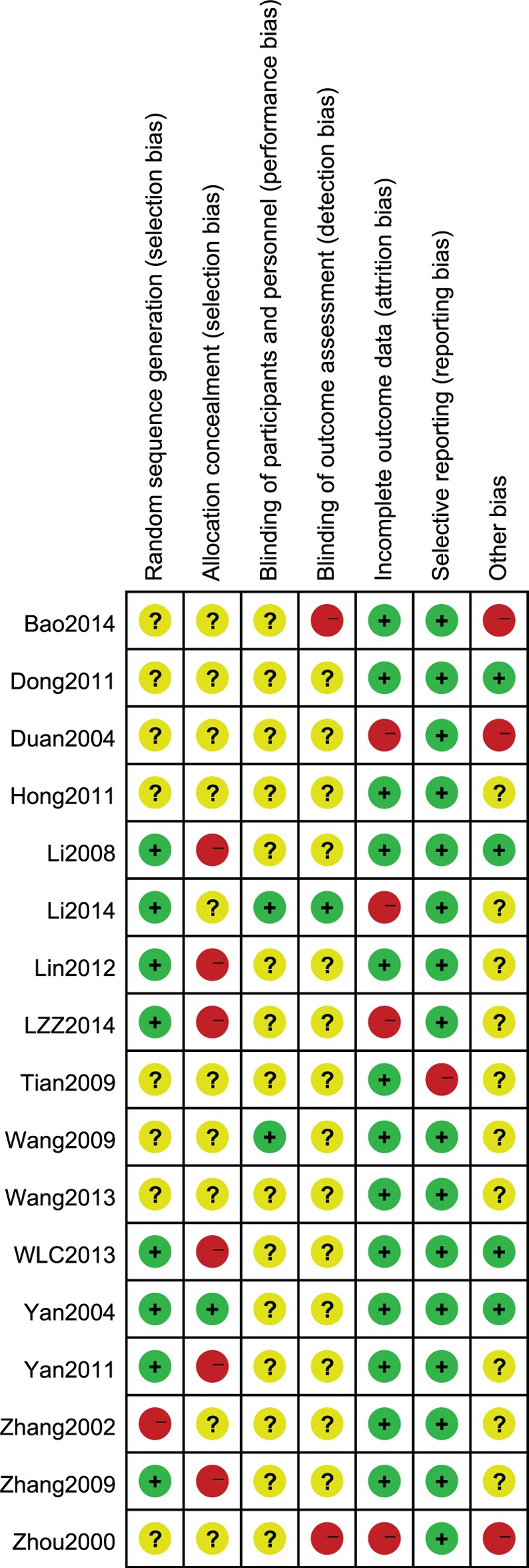
Risk of bias summary. Judgments of reviewing authors about each risk of bias item for each study included are summarized.
Data Synthesis
The RevMan 5.3 software provided by the Cochrane Collaboration was used for data analysis. Dichotomous data are expressed as relative risk (RR), continuous outcomes are presented as weighted mean difference (WMD), and the 95% confidence intervals (CIs) were calculated for both. The meta-analysis was performed if the intervention and control groups, as well as the outcomes, were the same or similar. The statistical heterogeneity was considered significant if the I2 index exceeded 50% or P < 0.1. In the absence of significant heterogeneity, we pooled the data using fixed (I2 < 50%) or random (I2 > 50%) effects models.
RESULTS
A total of 17 studies that were relevant to the literature were selected to more intuitively incorporate the basic information (Table 1).13–29
TABLE 1.
Characteristics of Included Studies
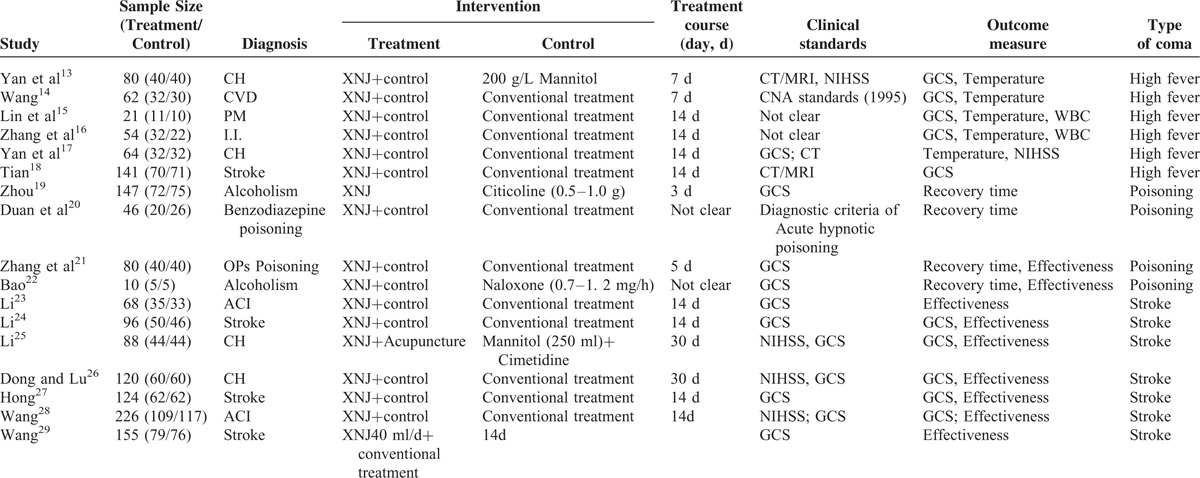
Description of Included Trials
After the initial search, we retrieve 558 relevant studies from 7 commonly used databases. The studies included 248, 130, 98, 12, and 65, 0, and 5 articles from the CNKI, Wanfang Database, CBMdisc, PubMed, VIP, EMbase, and the Cochrane Library, respectively. However, XNJ is currently only clinically used domestically in China and, therefore, we retrieved very few specific articles from the literature search of the foreign language databases containing clinical research literature and from the subsequent screening (Figure 1). In the initial search, articles that were not relevant contained duplicated content, or were incomplete were excluded. Despite the different databases that were searched including the VIP, most articles were retrieved from the CNKI database and, therefore, numerous duplicated articles (167) were obtained and subsequently removed. Consequently, 116 eligible articles remained and after we analyzed the specific content including whether a coma GCS was included and the RCT analysis was comprehensive, 17 studies were finally selected. All of the studies focused on the clinical treatment of disturbance of consciousness with XNJ, and the following 3 causes of consciousness disturbance: high fever-, poisoning-, and stroke-induced comas. Each cause was analyzed separately using comparative analysis of fever clearance time and wake time, wake time, and the GCSs for high fever-, poisoning-, and stroke-induced comas, respectively.
Methodological Quality of Included Trials
The majority of the included RCTs were assessed to be of low methodological quality, and only 17 articles used the random sequence generation method. One study26 used the ballot and numbers method, whereas some others15,16,23,25,29 used the table of random numbers method but did not provide any detailed information. The information provided was insufficient and, therefore, we were untenable to assess the quality of the allocation methods. Moreover, allocation concealment was only mentioned in one of the studies.13 Two trials14,24 used the blinding method for the participants and personnel but information on the outcome assessment blinding was not provided in any trial. Only 1 trial17 reported participant dropouts or withdrawal rates while none mentioned follow-up activities.
Effects of Interventions
High Fever-Induced Coma
The results of the meta-analysis of 3 RCTs showed that the use of XNJ injection had a statistically significant benefit on the efficacy rate compared with that observed with the oral administration of conventional drugs in patients with high fever-induced coma (n = 280; OR, 2.24; 95% CI, 1.38–3.64; I2 = 7%; P = 0.001; Figure 4).
FIGURE 4.
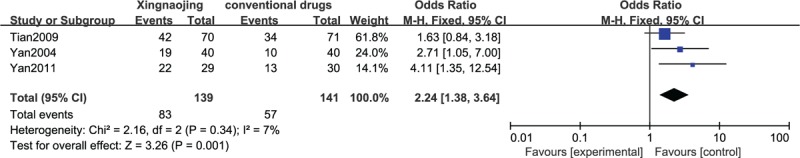
Efficacy in high fever-induced coma cases.
The results of the meta-analysis of 2 RCTs revealed that the use of XNJ had a statistically significant benefit on recovery compared with the oral administration of conventional drugs in patients with high fever-induced coma (n = 142; OR, 2.53; 95% CI, 1.28–4.99; I2 = 0%; P = 0.007, Figure 5). The meta-analysis of 2 other RCTs showed similar results (n = 75; MD, 1.01; 95% CI, 0.83–1.19; I2 = 0%; P < 0.00001; Figure 6). The differences between Figures 5 and 6 are not very obvious, which may be attributable to the small sample sizes or the inclusion of an insufficient number of relevant studies.
FIGURE 5.
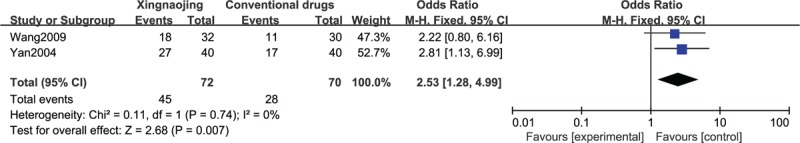
Glasgow Coma Score (GCS, dichotomous) of high fever-induced coma cases.
FIGURE 6.
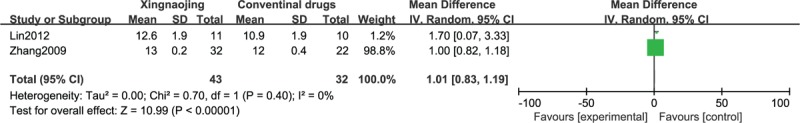
Glasgow Coma Score (GCS, continuous) of high fever-induced coma cases.
Toxic Coma
The results of the meta-analysis of 3 RCTs showed that the use of XNJ had a statistically significant benefit on recovery compared with the oral administration of conventional drugs in patients experiencing toxic coma (n = 75; MD, −4.62; 95% CI, −7.14 to −2.10; I2 = 66%; P = 0.0003; Figure 7). As seen in this figure, XNJ apparently promoted the recovery of patient awareness to a certain extent.
FIGURE 7.
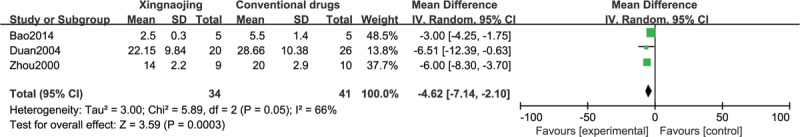
Recovery time of toxic coma cases.
Stroke-Induced Coma
The results of the meta-analysis of 3 RCTs showed that the use of XNJ had a statistically significant benefit in terms of the effectiveness rate compared with the oral administration of conventional drugs in patients with stroke-induced coma (n = 563; OR, 2.17; 95% CI, 1.54–3.06; I2 = 0%; P < 0.00001; Figure 8).
FIGURE 8.
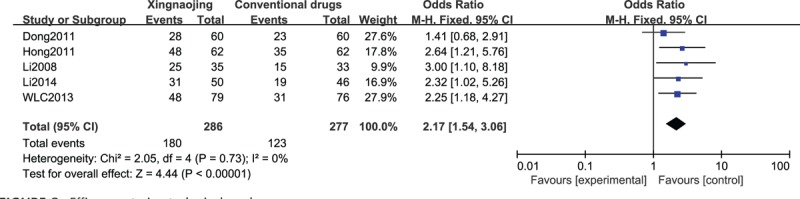
Efficacy rate in stroke-induced coma cases.
The GCS scores of patients with stroke-induced coma from 4 RCTs were compared using a forest plot and the results revealed that XNJ administration to these patients effectively improved their symptoms (n = 558; MD, 2.84; 95% CI, 1.23–4.46; I2 = 87%; P = 0.0006; Figure 9).
FIGURE 9.
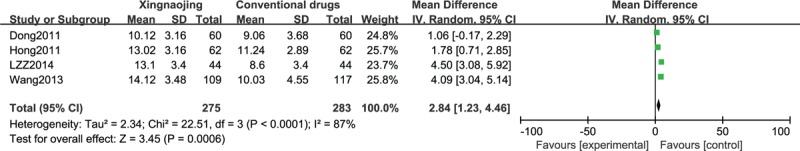
Glasgow Coma Score (GCS) in stroke-induced coma cases.
Meta-Analysis of All Included Studies Based on GCS and Efficacy
We comprehensively analyzed the included literature based on efficacy analysis and GCS values and discovered that XNJ had advantages over conventional medicine in the clinical treatment of patients who were comatose. In particular, it effectively improved the state of consciousness of patients (n = 923; OR, 2.22; 95% CI; 1.70–2.91; I2 = 0%; P < 0.00001; Figure 10; and n = 633; MD, 2.32; 95% CI; 1.06–3.57; I2 = 91%; P = 0.0003, Figure 11).
FIGURE 10.
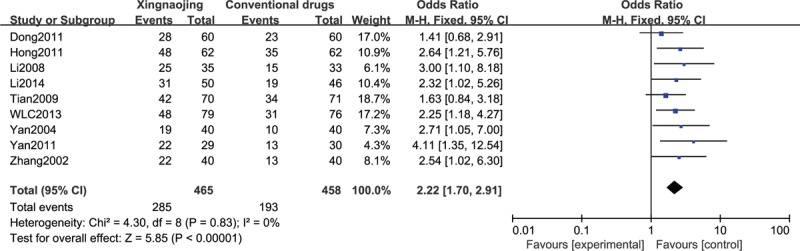
Efficacy rate in all included studies.
FIGURE 11.
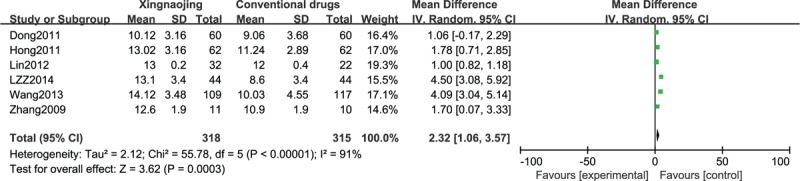
Comparison of Glasgow Coma Score (GCS) of all included studies.
Adverse Effects
Among all the included RCTs, only 1 trial mentioned adverse effects and contained few details. Therefore, the authenticity and scientific value of the studies, as well as the comprehensive nature of the provided information, were slightly in doubt. We subsequently opined that we may need to conduct studies specifically focused on drug safety to fully elucidate the safety of XNJ use.
Publication Bias
We used the Revman software to construct the funnel plot and evaluate the publication bias of the RCTs. Because some of the 17 included studies were continuous variables while others were 2 classification variables there different indicators and, therefore, we prepared 2 funnel plots (Figures 12 and 13).
FIGURE 12.
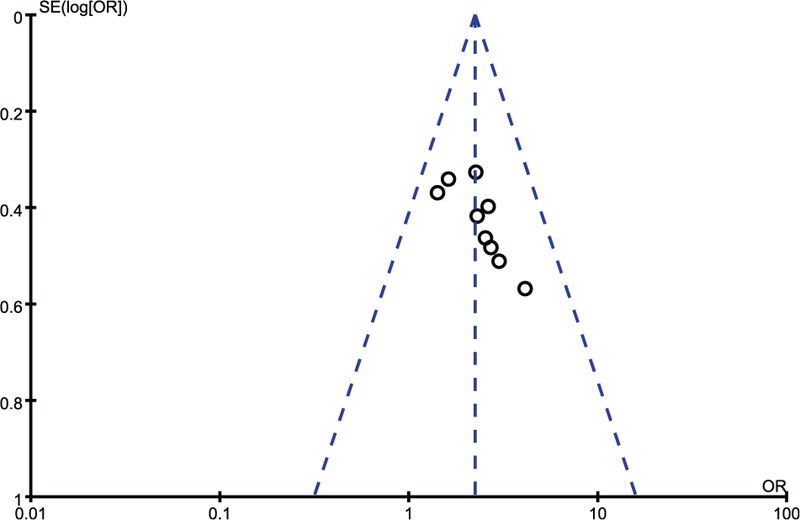
Funnel plot of included studies with efficacy rates.
FIGURE 13.

Funnel plot of included studies with Glasgow Coma Score (GCS).
DISCUSSION
Disturbance of consciousness, specifically coma, is a state in which patients become unresponsive to external stimuli, lose motor and sensory functions, and only retain autonomic nervous system functions. There are various causes of consciousness disturbance and in recent years, XNJ has been widely used to treat patients experiencing comas. In addition, literature reviews have demonstrated that most studies that investigated the protective mechanisms of XNJ only assessed its effects on certain types of poisoning-induced comas. Therefore, to gain a better understanding of the effects of XNJ, we assessed its activity in the treatment of the comas induced by the 3 causes using a systematic analysis and evaluation of the literature. For this purpose, we selected 17 trials with 1582 patients who were eligible for analysis (Table 1).13–29 In summary, we found that XNJ was beneficial in the recovery of patients with high-fever coma, poisoning coma, and stroke-induced comas, using 1 or several common indicators to evaluate the RTCs.
Exposure to heat may cause tissue hypoxia and microcirculation, which increases capillary permeability, edema, and microcirculation further. These processes can subsequently lead to the following conditions: dysfunction of important organs such as the heart, lungs, brain, kidney, and liver; water and electrolyte metabolism and acid–base disorders; and even multiple organ failure with coma, shock, and other life-threatening symptoms. Figures 4–6 compared the data on the GCS and significant efficiency from selected RTCs and the results showed that XNJ effectively induced recovery of consciousness, and was an effective antipyretic in patients with high-fever-induced comas.
Numerous toxic materials can produce deleterious effects on the CNS. For example, the stability of β-endorphin may be perturbed, which can inhibit the CNS. Therefore, in clinical practice patients who have been exposed to poisons exhibit different levels of consciousness. We analyzed the waking time and discovered that XNJ was beneficial in promoting the recovery of the consciousness and awareness of patients with poisoning-induced comas. Cerebral hemorrhage or infarction-associated strokes can lead to cerebral edema. If the condition is severe or not adequately treated, the patients may lose consciousness. The data presented in Figures 8 and 9 suggest that XNJ exhibited obvious therapeutic effects in patients with stroke-induced coma.
Regardless of these observations, our objective determination was that the quality of the studies that were included in the meta-analyses was not very good. Figures 1 and 2 (risk of bias and risk of bias summary) revealed that numerous included studies had various inconsistencies. One example is the allocation concealment bias; specifically, although 1 study13 adopted the drawing lots method, the rest of the included studies did not use allocation concealment or their methods were unclear. Therefore, there is a risk that patients may have reported subjective experiences in relation to the drug effect. However, the data integrity and selective reporting of the studies were relatively objective and unbiased.
The systematic review of the literature and the meta-analyses had some disadvantages or limitations, which are worth mentioning. The evaluated RTCs were mainly published in national journals and periodicals that were not of high quality. Many selected publications were outdated (such as13,19–21 published 10 years ago), and the content and research methods may be outdated. The study contents were not exhaustive and thorough enough (e.g., 1 study17 contained efficacy data that only included a few cases and no other details related to the data and other indicators). Few studies used the same index, which reduced the strength of the comparisons (e.g., high-fever-induced comas were only compared in 2 studies and, therefore, there was insufficient evidence).
However, the systematic evaluation also had some advantages that are worth mentioning. Before the systematic evaluation was conducted, we set up a discussion group and brainstormed extensively on the modalities to be used in summarizing the steps and research ideas, as well as in developing a clear, logical purpose for the study. The main criterion of the GCS was the final discussion. This may be attributed to the abundance of relevant literature, which can increase the value of the comparison and improve the credibility of the conclusion. Because the scope of our search was broad, we were able to avoid overlooking excellent studies. First, we started by attempting to fully understand the basis of each potential study; second, we integrated the main contents of each study; and third, we selected the most suitable studies. We adopted a multiangle and multilevel analytical approach to evaluating the studies. Specifically, we first analyzed the mechanisms of the 3 static types of coma-inducing stimuli and the clinical effects of XNJ from the start of treatment until recovery. This was followed by an analysis of the ratio of each type based on different indicators. The meta-analysis involved a heterogeneity test, which used the degree of freedom to fix the numerical size regardless of the changes in the number of studies. Compared with other methods, heterogeneity tests are more robust and reliable. The I2 statistic reflects the heterogeneity of the proportion of the total variation in the effect quantity. An I2 = 0% (if I2 was negative, we set it to 0) indicated that heterogeneity was not observed while the larger the I2 statistic was, the greater the heterogeneity. Therefore, an I2 > 50% was indicative of obvious heterogeneity30 and at a value <32% the study was considered homogeneous.31 The forest map results indicated that the I2 values of Figures 5, 6, 8, and 10 were 0%; Figure 4 was 7%; and Figures 7, 9, and 11 were 66, 87, and 91% respectively. Therefore, we can conclude that the results shown in Figures 4–6, 8, and 10 can be considered to lack heterogeneity, which suggests they are relatively reliable. Therefore, this provides some guarantee that XNL treatment of coma would exhibit similar effects in new patients. In addition, the I2 values of Figures 7, 9, and 11 were higher than 50%, which indicated that the analysis revealed heterogeneity. This may be attributable to the following factors. The sample size is too small and, therefore, the I2 test created a degree of uncertainty. There may be heterogeneity between studies such as differences in treatment regimens, experimental design, treatment populations, and data analysis.32Figures 7, 9, and 11 are all continuous variables, and high heterogeneity is more likely to occur with continuous than with dichotomous variables.33 For the publication bias, we performed a qualitative analysis of the studies using a funnel plot, and adopted a fixed effect model for the 2 classification variables, which showed slight heterogeneity. In addition, we used a more balanced random effects model for the continuous variable with large heterogeneity. Finally, the results shown in Figures 12 and 13 revealed that the 2 groups of studies were approximately symmetrically distributed and, therefore, we can conclude that there was no obvious bias in the studies and the statistical analyses are relatively reliable.
In summary, we found that XNJ played an important role not only in relieving cerebral edema and improving stroke sequelae but also in facilitating awakening from comas. In addition, as a traditional Chinese medicine, XNJ is relatively safe and, therefore, can play a potentially greater role in clinical practice in the future. Finally, our future research studies will be committed to further assessing the therapeutic range of XNJ for the treatment of other diseases, and elucidating a clear underlying mechanism for its action, including understanding the specific targets that play a critical role in its medicinal effects.
Footnotes
Abbreviations: ACI = acute cerebral infarction, CBMdisc = Chinese Biomedical Literature Database, CH = cerebral hemorrhage, CI = confidence intervals, CNKI = Chinese National Knowledge Infrastructure, CVD = cerebrovascular disease, GCS = Glasgow coma scores, I.I. = Intracranial Pyogenic Infectious, MD = mean difference, NIHSS = National Institutes of Health Stroke Scale, Ops = Organophosphorus, OR = odds ratio, PM = Purulent Meningitis, RCTs = randomized controlled trials, RR = relative risk, VIP = the Chinese Scientific Journal Database, WMD = weighted mean difference, XNJ = Xingnaojing Injection.
LW, HZ, YX contributed equally to this work as first authors.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wang WZ. Neurology. PMPH 2006; 211–213. [Google Scholar]
- 2.Yan JC, Jiang XJ, Zhang XL, et al. Effect of Xingnaojing injection on tumor necrosis factor α in patients with consciousness disturbance combined with fever after cerebral hemorrhage. Chin J Clin Rehabil 2004; 19:3828–3830. [Google Scholar]
- 3.Li FK, Yu CD, Liu Q. 30 cases of Xingnaojing injection on the treatment of stroke, consciousness disorders. China Pharmaceu 2000; 6:39–40. [Google Scholar]
- 4.Guo Y, Yan SH, Xu LP, et al. Use of Angong Niuhuang in treating central nervous system diseases and related research. Evid Based Complement Alternat Med 2014; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen QM. Studies on pharmacological foundation of Xingnaojing and clinical application. Pract Clin First Aid Combination TCM Western Med 1999; 4:191. [Google Scholar]
- 6.Liu GH, Wang SS, Zhang JW, et al. One case of Xingnaojing injection on TOB. Clin J Anhui Traditional Chin Med 1999; 2:107. [Google Scholar]
- 7.He XJ, Qing LM, Liu YL. The protective effect of borneol on experimental cerebral ischemia. J Guangdong Coll Pharmacy 2006; 2:171. [Google Scholar]
- 8.He XJ, Qin XH, Liu YL. Borneol on anti-cerebral ischemia. Shanxi Med J 2007; 9:794. [Google Scholar]
- 9.Ma B, Liu L, Zhang Y, et al. Xingnaojing injection attenuates neurologic deficits against focal ischemic injury in stroke-prone renovascular hypertensive rats. Chin J Integr Med Cardio-/Cerebrovasc Dis 2014; 2:212–214. [Google Scholar]
- 10.Shi J. Analysis of clinical efficacy of Xingnaojing combined with naloxone in treatment of acute alcohol poisoning. Pract Clin J Integr Traditional Chin Western Med 2014; 4:40–41. [Google Scholar]
- 11.Gu HJ, Li G, Wan D. Xingnaojing injection for the treatment of acute cerebral hemorrhage and its effect on serum hs-CRP and NSE. Chin J Exp Traditional Med Formulae 2014; 14:178–181. [Google Scholar]
- 12.Higgins J, Altman D. Assessing risk of bias in included studies. Cochrane Handbook Systematic Rev Interventions 2008; 187–242. [Google Scholar]
- 13.Yan JC, Jiang XJ, Zhang XL, et al. Effect of Xingnaojing injection on tumor necrosis factor α in patients with consciousness disturbance combined with fever after cerebral hemorrhage. Chin J Clin Rehab 2004; 19:3828–3830. [Google Scholar]
- 14.Wang XJ. Clinical observation of Xingnaojing on treatment of acute cerebrovascular disease consciousness disorders. Chin Traditional Patent Med 2009; 6:827–828. [Google Scholar]
- 15.Lin XJ, Duan DQ, Zhuang WD. Efficacy of adult purulent meningitis XNJI treatment. China Pract Med 2012; 3:10–11. [Google Scholar]
- 16.Zhang ZS, Han F, Huang T, et al. Efficacy of XNJI on the treatment of suppurative intracranial infection. J Guangzhou Univ Traditional Chin Med 2009; 4:332–334. [Google Scholar]
- 17.Yan XL, Cui ZG, Wang ZP. XNJI on treatment of cerebral hemorrhage fever clinical efficacy. Mod J Integr Traditional Chin Western Med 2011; 35:4516–4517. [Google Scholar]
- 18.Tian YC. Clinical observation of XNJI on treatment of 70 patients of coma patients with acute stroke fever. Heibei J TCM 2009; 8:1215–1216. [Google Scholar]
- 19.Zhou YT. Xingnaojing treatment of alcoholism consciousness 72 cases—with citicoline treatment of 75 cases as control. Zhejiang J Traditional Chin Med 2001; 1:45–46. [Google Scholar]
- 20.Duan F, Ji PZ, Liu ZX, et al. XNJI severe benzodiazepine poisoning efficacy of tables. Tianjin Med J 2004; 3:186. [Google Scholar]
- 21.Zhang WQ, Bi LZ, Qi R. Efficacy of XNJI treatment of 40 cases of organophosphorus pesticide poisoning consciousness. Med Forum 2002; 3:18–19. [Google Scholar]
- 22.Bao WJ. Efficacy of Xingnaojing on acute severe alcoholism combined with naloxone. Harbin Med J 2014; 6:377–379. [Google Scholar]
- 23.Li XM. Influence of XNJI on acute cerebral infarction level of consciousness. Fujian J TCM 2008; 3:8–9. [Google Scholar]
- 24.Li J. Clinical efficacy of XNJI on stroke patients with impaired consciousness. Clin Med 2014; 8:71–72. [Google Scholar]
- 25.Li ZZ. Clinical observation on treating 88 cases of acute cerebral hemorrhage with Xingnaojing injection plus acupuncture. Clin J Chin Med 2014; 13:40–41. [Google Scholar]
- 26.Dong F, Lu GJ. Efficacy of XNJI on the treatment of acute cerebral hemorrhage associated with consciousness. JETCM 2011; 2:191–227. [Google Scholar]
- 27.Hong JX, Lin F. Efficacy of XNJI on the treatment of consciousness in acute stroke. Xinjiang J Traditional Chin Med 2011; 4:39–40. [Google Scholar]
- 28.Wang KJ. Efficacy of XNJI on the treatment of cerebral infarction with consciousness. World J Integr Traditional Western Med 2013; 33–35. [Google Scholar]
- 29.Wang LC. Efficacy of XNJI on the treatment of stroke patients consciousness. Internal Med China 2013; 3:247–248. [Google Scholar]
- 30.Wang D, Qu JX, Mou ZY. Heterogeneity and treatment method in meta-analysis. Chin J Evid Based Med 2009; 10:1115–1118. [Google Scholar]
- 31.He HQ, Chen K. Method of heterogeneity in meta-analysis. China J Health Statist 2006; 6:486–490. [Google Scholar]
- 32.von Hippel PT. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med Res Methodol 2015; 15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alba AC, Alexander PE, Chang J, et al. High statistical heterogeneity is more frequent in meta-analysis of continuous than binary outcomes. J Clin Epidemiol 2015; 19:1707–1728. [DOI] [PubMed] [Google Scholar]


