Abstract
Matrix metalloproteinases (MMPs) exhibit an important function in extracellular matrix degradation. MMPs modulate the activation of growth factors, cytokines and metastasis. At present, the effect of exercise on serum levels of MMP-2 and −9 remains unclear. The aim of the present study was to investigate the effect of various physical activities on the circulating levels of MMP-2 and −9 in breast cancer (BC) survivors and healthy subjects. A total of 66 female subjects were enrolled in the present study. The cohort included 46 BC survivors and 20 healthy subjects divided into 5 groups: Group A (17 BC survivors, participating in recreational dragon boat paddling), group B (14 BC survivors, participating in recreational physical activity), group C (15 sedentary BC survivors), group D (10 healthy subjects, participating in recreational physical activity) and group E (10 sedentary healthy subjects). ELISA assays revealed a significant increase in the level of circulating MMP-2 in group B compared with all other groups. Recreational physical activity increased the levels of MMP-9 in healthy subjects (group D vs. E), however, the differences were not statistically significant, while in the BC survivor groups the results were opposite, with exercise reducing MMP-9 levels (group B vs. C). Furthermore, a significant increase in MMP-2 was observed in group B lymph node metastasis-positive (N+) subjects compared with group A and C N+ subjects. Thus, the results of the present study indicate that various physical activities modulate the levels of circulating MMP-2 and −9 in BC survivors, and the same exercise program induces a different effect when undertaken by healthy subjects and BC survivors. These results may have important implications with regard to the selection of appropriate physical activities for BC survivors, leading to improvements to their survival and prevention of recurrence, as well as amelioration of physical function, quality of life and fatigue.
Keywords: physical exercise, matrix metalloproteinase-2, matrix metalloproteinase-9, breast cancer, metastasis
Introduction
Breast cancer (BC) is the most common malignancy in women worldwide. Novel breast cancer cases and cancer-associated mortalities accounted for 29 and 15%, respectively, of all female cancers in 2015 (1). Lifestyle is considered an important risk factor for BC. All types of physical activity appear to reduce the risk of BC, although the evidence of this association is stronger for BC occurring in post-menopausal women than pre-menopausal women (2,3).
Studies have shown that the risk of BC is ~25% lower in the most active trained women compared with sedentary women (4,5). The BC risk is 17% higher in sedentary women compared with women that perform physical activity (6). In addition, the risk of BC decreases by 5% for every 2 h per week in women performing moderate or vigorous recreational activity (7). Physical activity has also been demonstrated to reduce the risk of BC recurrence in BC survivors (8,9). Therefore, The American Cancer Society recommends that cancer survivors partake in 150 min of physical activity per week (10,11).
The mechanism underlying the health benefits of regular exercise may involve the cumulative anti-inflammatory effects of repeated physical exercise (12,13). Regular exercise promotes the synthesis of myokines that modulate the inflammatory response (14). In addition, exercise may affect the levels of matrix metalloproteinases (MMPs) (15,16).
MMPs are a family of structurally and functionally related proteinases that are characterized by their ability to degrade the extracellular matrix (ECM), and their involvement in the normal functioning of various tissues during growth, development and aging (16,17). MMP expression is induced by a number of factors, including inflammatory cytokines, hormones, growth factors and oncogenes (18,19). In addition, MMPs are implicated in tumor invasion and metastasis (20,21). MMP-2 and −9 have been reported to exhibit an important ECM homeostatic function during muscle growth, development and repair processes (16). Previous studies have demonstrated that physical exercise affects the levels of MMPs following acute and chronic therapies in humans (22–24). A marginal increase in MMP-9, but not MMP-2, serum concentration has been demonstrated to occur following acute physical exercise (25). In particular, MMP-2 and −9 serum levels increase following exercise training that incorporates resistance-type exercise, as the mechanical stressors result in ECM remodeling (26). MMP-2 and −9 hydrolyze components of the basement membrane, and regulate tumor growth and metastasis (27). Activated MMP-2 is located in the protruding section of the cell-penetrating matrix. Its activation facilitates the passage of tumor cells through the ECM and the basal membrane of the blood vessel wall, thus promoting invasion of tumor cells (28). In addition, MMP-2 and −9 are gelatinases; thus, their role in the degradation of gelatin leads to the release of signaling molecules from the ECM that aid cell migration and angiogenesis (29). Previous studies have investigated the effects of acute short duration exercise on MMP concentrations in skeletal muscle and plasma (30,31). However, it remains unclear whether different exercise training programs promote different MMP responses (32). To the best of our knowledge, no previous studies have investigated the effect of exercise on the modulation of MMP-2 or −9 serum concentration in BC survivors.
In our previous study, it was demonstrated that muscle-derived cytokines are released following a dragon boat training program and the resulting decreased oxidative stress conditions are responsible for the health benefits observed in patients with BC (14). In the present study, the effect of two different exercise training programs on MMP-2 and −9 serum concentration was investigated in BC survivors and healthy subjects. In addition, the association between MMP serum concentration and lymph node metastasis was investigated in BC survivors. The patients enrolled in the current study were active individuals involved in dragon boat paddling or other recreational physical activities.
Dragon boat paddling is a competitive sport that originated in China >2,000 years ago (33). Dragon boat paddling is a repetitive, vigorous physical exercise that increases flexibility, aerobic capacity and strength, and provides a novel approach for upper body rehabilitation following breast surgery and radiation treatment. This exercise has been reported to reduce the risk of developing lymphedema in women at the highest risk by 70% (34).
The present study may have important implications for BC survivors, with regard to their lifestyle choices and participation in physical activity.
Patients and methods
Patients
A total of 66 women were enrolled in the present study, between May and December 2014, in collaboration with Associazione Pagaie Rosa Dragon Boat Onlus (www.pagaierosa.org). The cohort included 46 women who were BC survivors and 20 healthy subjects. Blood from patients was drawn at the Day Hospital of Don Gnocchi Foundation (Rome, Italy). BC survivors were divided into three different groups: Group A, 17 women who participated in recreational dragon boat paddling (mean ± standard deviation age, 53.4±8.8 years); group B, 14 women who participated in other recreational physical activities (age, 53.9±11.2 years); and group C, 15 sedentary women (age, 59.6±9.9 years). The 20 healthy women were divided in two groups: Group D, 10 women who participated in other recreational physical activities (age, 49.4±7.8 years); and group E, 10 sedentary women (age, 51.0±8.1 years) (Table I). All the BC survivors were enrolled 10±5 years post-surgery. The exclusion criteria included cardiovascular disease, acute somatic symptoms, including fever or infection, uncontrolled hypertension, uncontrolled pain or any other condition that contraindicated exercise training in BC survivors or healthy subjects (35). It was not possible to enroll a control group of healthy subjects who participate in dragon boat racing as there is only a small number of individuals who play this sport in Rome. In addition, the effects of dragon boat mediated-release of MMPs in healthy subjects was not part of the scope of the present study.
Table I.
Anthropometric parameters of enrolled subjects.
| Group | Subject status | Type of physical activity | Enrolled subjects, n | Age, yearsa | Height, cma | Weight, kga | BMIa |
|---|---|---|---|---|---|---|---|
| A | BC | Dragon boat paddling | 17 | 53.4±8.8 | 161.9±7.5 | 60.2±9.4 | 22.9±2.4 |
| B | BC | Recreational physical activity | 14 | 53.9±11.2 | 164.2±7.9 | 64.3±10.2 | 23.9±3.5 |
| C | BC | Sedentary | 15 | 59.6±9.9 | 164.1±5.8 | 67.4±9.1 | 25.0±3.3 |
| D | H | Recreational physical activity | 10 | 49.4±7.8 | 164.3±2.5 | 61.6±5.4 | 22.9±2.4 |
| E | H | Sedentary | 10 | 51.1±8.1 | 163.3±4.1 | 56.3±6.0 | 21.2±3.0 |
Mean ± standard deviation. BMI, body mass index; BC, breast cancer; H, healthy.
Anthropometric parameters, including height, weight and body mass index (BMI), were determined for all participants at the beginning of the study (Table I). The TNM status was also determined for all the patients (Table II) (36). No clinical or radiographic evidence of distant metastases were present.
Table II.
Tumor stage and lymph node metastasis of breast cancer survivors (n=46).
| Parameter | Patients, n |
|---|---|
| Tumor stage | |
| I | 24 |
| II | 14 |
| III | 8 |
| Lymph node metastasis | |
| Present | 25 |
| Absent | 21 |
| Histological type | |
| Ductal | 42 |
| Lobular | 4 |
The present study was performed in accordance with the Declaration of Helsinki and written informed consent was provided by all subjects prior to enrolling in the study. The study was approved by the ethics committee of the Fondazione Policlinico Tor Vergata (Study Design DRAGON BOAT-REGISTRO SPERIMENTAZIONI protocol number 150/13).
Training program
The present study did not involve analysis of MMPs prior to training. Thus, the training program did not have a specific duration, and patients could be enrolled at any time during their training. All subjects from groups A, B and D were already performing identical physical activities to those described below prior to the start of the study. Sedentary subjects were only in groups C and E.
Group A
All subjects in group A participated in recreational dragon boat paddling once a week for 120 min, and were supervised by an exercise trainer.
The 120-min dragon boat paddling session included a 30 min dry land warm up as activation exercise for the whole body, followed by 90 min of dragon boat paddling training. The dragon boat paddling training consisted of: A 20-min rowing warm up on the right and left sides of the boat (changing side every 10 min for symmetric training); 50 min rowing with stroke-rate regulated by sound feedback (tambourine) and rowing technical skill exercises (resting in relation to the intensity of the exercise); and 10 min training on competition distances (200 or 500 meters repeated at last twice). Finally, the subjects performed a 10 min cool-down and 10 min of stretching exercises, focusing on the upper limbs.
Groups B and D
All subjects in groups B and D participated in other recreational physical activities (the same for the two groups) 3 times a week for 60 min, and were supervised by an exercise trainer.
The 60-min recreational physical activities included a 10-min warm-up, 30 min endurance training (treadmill 6.5–8.0 km/h, cyclette 55–65 rpm or aerobic dance) and 20 min strength training (2–4 kg). The warm up involved large muscle groups with low-intensity activities (slow run and execution of joint mobility exercises), gradually preparing the body to pass from the rest condition to the state of activation, with progressive increase of intensity. Aerobic dance, performed to music, was physical exercise that combined rhythmic aerobic exercise with strength training routines to improve flexibility, muscular strength and cardiovascular fitness. Finally, the subjects performed 10 min of stretching exercises. Each subject enrolled in groups B and D performed treadmill, cyclette or aerobic dance alternately during the week.
Measurement of MMP-2 and −9 levels
Serum samples were obtained during the training program. Blood was drawn without anticoagulant a total of 48 h after training (and between two training sessions) from all subjects between 9:00 and 10:00 a.m., following an overnight fast, and was allowed to coagulate for 20–30 min. Blood samples were not obtained before the training program began as the aim of the present study was to compare the effects of various physical activities on the circulating levels of MMP-2 and MMP-9 in well-trained subjects. Serum was separated by centrifugation (800 × g for 10 min at 4°C), and all specimens were aliquoted, frozen and stored at −80°C within 2 h of collection. Commercially available human ELISA kits (high-sensitivity Quantikine; catalog nos., MMP200 and DMP900; R&D Systems, Inc., Minneapolis, MN, USA) were used to determine the MMP-2 and −9 levels, according to the manufacturer's instructions. Optical density was measured using a microplate reader at a wavelength of 450 nm (Model 550; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data were initially entered into a Microsoft Excel for Office 2007 database (Microsoft Corporation, Redmond, WA, USA) and statistical analysis was performed using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA). Data are presented as the mean ± standard deviation for parameters with (normal) Gaussian distributions (following confirmation with histograms and the Kolgomorov-Smirnov test) or as frequencies (%) for occurrences.
All data obtained were compared by using one-way analysis of variance. Significant results were examined by Bonferroni's test for one or more factors, respectively. P<0.05 was considered to indicate a statistically significant difference.
To determine the number of samples necessary in each group, power analysis was performed based on an α of 5% and variation of 40% of the variable outcome MMP-2. The t-test showed that an amplitude of n=8 for both groups reached a power (1-β) of 94%. This was only calculated for MMP-2 as it was the only significantly modified parameter. The variation of MMP-9 concentration was not significant.
Results
No significant differences in age or height were observed between the groups. Conversely, the weight (group C vs. group E, P=0.024) and BMI (group C vs. group E, P=0.035) were significantly higher in sedentary BC survivors in comparison with healthy sedentary women (Table I). Table II shows tumor stage and lymph node metastasis of breast cancer survivors enrolled in the study.
To determine the effect of physical exercise on MMP-2 and −9 serum concentration in BC survivors and healthy subjects, two different exercises training programs were compared. The results revealed a difference in MMP serum concentration between BC survivors and healthy subjects participating in different exercise programs.
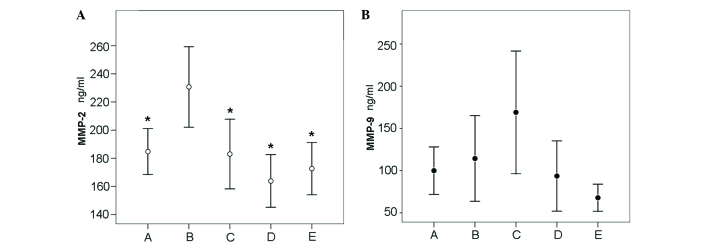
Specifically, the MMP-2 serum concentration was significantly increased in the sera of BC survivors participating in recreational physical activity (group B) compared with those participating in dragon boat paddling (group A; B vs. A, P=0.028), or with sedentary BC survivors (group C; B vs. C, P=0.008; Fig. 1A). Statistically significant differences were observed between group B (230.7±49.5) and group A (184.7±31.7; P=0.028) and between group B and group C (182.9±44.8; P=0.008).
Figure 1.
MMP-2 and MMP-9 serum concentration in breast cancer survivors and healthy subjects. (A) Significant differences in MMP-2 levels were identified in group B when compared with groups A (P=0.028), C (P=0.008), D (P=0.001) and E (P=0.02). *P<0.05 vs. B. (B) No significant differences in MMP-9 levels were identified between groups A-E. MMP, matrix metalloproteinase.
However, no significant differences in MMP-2 serum concentration were identified between BC patients participating in dragon boat paddling (group A) and sedentary BC survivors (group C; P=0.999). Notably, the mean MMP-2 serum concentration was significantly higher in BC survivors participating in recreational physical activity (group B, 230.7±49.5) compared with healthy subjects participating in recreational physical activity (group D; 163.8±26.1; P=0.001) and between group B and sedentary healthy subjects in group E (172.6±25.9; P=0.021). By contrast, no significant differences in mean MMP-2 levels were identified between the sedentary BC survivors (group C; 182.9±44.8) and sedentary healthy subjects (group E; 172.6±25.9; P=0.999).
The effect of exercise on MMP-9 serum concentration revealed a different trend between BC survivors and healthy subjects. Notably, physical exercise decreased the MMP-9 levels in BC survivors in group A and group B compared to group C, while it increased the MMP-9 serum levels in healthy subjects in group D compared with group E (Fig. 1B).
Although variations in mean MMP-9 levels were identified between dragon boat paddling BC survivors and sedentary BC survivors (group A, 99.9±54.5; group C, 169.0±131.2; P=0.679), recreational physical activity BC survivors and sedentary BC survivors (group B, 114.3±87.8; group C, 169.0±131.2; P=0.999), and healthy recreational physical activity and healthy sedentary individuals (group D, 93.6.3±58.3; group E, 67.8±22.5; P=0.999), these differences were not statistically significant (Table III).
Table III.
Serum matrix metalloproteinase-9 concentration in BC dragon boat group (A), BC physical activity group (B), BC sedentary group (C), H physical activity group (D) and H sedentary group (E).
| Dragon boat/physical activity group (mean ± sd) | Sedentary group (mean ± sd) | P-value |
|---|---|---|
| A (99.9±54.5) | C (160.9±131.2) | 0.679 |
| B (114.3 ± 87.8) | C (160.9±131.2) | 0.999 |
| D (93.6 ± 58.3) | E (67.8 ± 22.5) | 0.999 |
BC, breast cancer; H, healthy; sd, standard deviation.
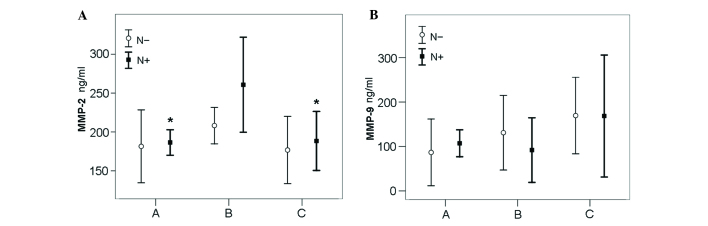
The association between MMP-2 and −9 serum levels, and the presence of lymph node metastasis were also investigated in groups A-C. No statistical differences in mean MMP-2 serum levels were identified between lymph node metastasis positive (N+) BC survivors of the dragon boat paddling group (group A; 186.5±24.4) and N+ sedentary BC survivors (group C; 188.4±45.3) or between the lymph node metastasis negative (N-) BC survivors of the dragon boat paddling group (group A; 181.5±44.7) and the N- sedentary BC survivors (group C; 176.7±46.8) (Fig. 2).
Figure 2.
MMP-2 and MMP-9 serum levels in N+ and N- breast cancer survivors. (A) Significant differences in MMP-2 levels were identified in the BN+ group when compared with the CN+ (P=0.013) and AN+ (P=0.007) groups. *P<0.05 vs. N- group. (B) No significant differences in MMP-9 levels were identified between groups A-C. Number of survivors: Group A, N- 6; N+ 11; group B, N- 8; N+ 6; group C, N- 7; N+ 8. MMP, matrix metalloproteinase; N+, lymph node metastasis positive; N-, lymph node metastasis negative.
However, significant differences in mean MMP-2 serum levels were identified between N+ BC survivors that participated in recreational physical activity (group B; 260.7±58.2) and N+ sedentary BC survivors (group C; 188.4±45.3; P=0.013). No significant differences were identified between N- BC survivors participating in recreational physical activity (group B; 208.2±28.1) and N- sedentary BC survivors (group C; 176.7±46.8; P=0.913). MMP-2 serum levels were significantly higher in N+ BC survivors of the recreational physical activity group (group B; 260.7±58.2) compared with N+ BC survivors of the dragon boat paddling group (group A; 186.5±24.4; P=0.007) (Fig. 2). No significant differences were observed in MMP-9 serum levels between the N- and N+ subgroups among groups A-C.
Discussion
Regular physical exercise improves physical function, quality of life and reduces fatigue in BC survivors (37,38). It also decreases the risk of BC recurrence and reduces BC-specific mortality in BC survivors (39,40).
Physical exercise is considered an essential factor for improving cancer survival. Previous studies have indicated that exercise interventions are efficacious in men with prostate cancer (41,42) and in multiple myeloma (43,44). In endometrial, ovarian and colorectal cancer survivors, lifestyle interventions that include regular participation in physical activity are associated with positive health outcomes (45,46). Furthermore, it has been demonstrated that individuals who are physically active after being diagnosed with breast or colon cancer exhibit a higher rate of survival compared with those that are physically inactive (47).
Circulating MMPs are involved in the breakdown of the ECM in carcinogenesis and cardiovascular diseases (48). MMPs modulate the activation of growth factors, cytokines and angiogenesis, facilitating physiological adaptations to exercise (18,49).
However, high levels of mechanical stress, such as that induced by high physical impact exercise, may activate the local production of MMPs in skeletal muscle. Rullman et al (50) reported that a single session of exercise induces MMP-9 activation, causing a marked increase in serum MMP-9 concentration. Kadoglou et al (24) demonstrated that exercise reduces MMP-9 serum levels, but not MMP-2 levels, in patients with type 2 diabetes. Additional studies have investigated the function of circulating MMP-2 and MMP-9 in BC patients (51,52); the results revealed increased MMP-9 expression and activity in malignant tumors, and enhanced MMP-2 activity in malignant tumors exhibiting high estrogen receptor expression. These studies indicate that serum levels of MMP-2 or −9 may present useful markers for the staging and prognosis of BC and other types of cancer (53), such as prostate adenocarcinoma, in association with prostate-specific antigen levels, gastric cancer and lung carcinoma (54–56).
At present, the effect of different exercise training programs on MMP-9 and −2 serum levels in human subjects remains unclear (57). It was previously demonstrated that endurance training decreases MMP-9 concentration (58) and aerobic training reduces MMP-2 plasma levels in individuals with pathological conditions (25). Urso et al (26) observed that the variation in MMP-2 and −9 serum levels is dependent on different exercise training regimens. Thus, understanding which type of exercise and intensity may reduce the risk of recurrence in BC patients is important.
The aim of the present study was to determine the effect of two different exercise training programs on the serum MMP-2 and −9 levels of healthy subjects and BC survivors. The results did not identify any significant differences in MMP-2 serum levels in healthy subjects that participated in recreational physical activity; there were no significant differences in MMP-2 serum levels in group D compared with group E, and although MMP-9 levels increased in group D compared with group E, these differences were not statistically significant. Among the BC survivors, no significant differences in circulating MMP-2 levels were identified between the dragon boat paddling (group A) and sedentary (group C) groups. However, a significant increase in serum MMP-2 was identified in the recreational physical activity BC group (group B) compared with the sedentary BC group (group C). This difference in circulating MMP-2 levels following exercise appears to be dependent on the different exercise training programs undertaken by the BC survivors; however, the same exercise program did not induce significant increases in the MMP-2 levels of healthy subjects.
MMP-9 serum levels were higher in sedentary BC survivors (group C) than in healthy subjects (group E). Furthermore, MMP-9 serum levels were decreased in both groups of recreationally active BC survivors (groups A and B) compared with sedentary BC survivors (group C). This result may be associated with tumor necrosis factor-α (TNF-α) expression, as TNF-α is a stimulator of MMP-9 production and is decreased following exercise (59,60).
However, exercise increased MMP-9 serum levels in the healthy subjects (group D vs. E). This difference reflects the impact of exercise training and may be associated with an inflammatory response (61). Rullman et al (62) reported that endurance exercise enhances MMP-9 but not MMP-2 levels in human skeletal muscle in healthy subjects.
Our previous study regarding exercise in BC patients contributed to the understanding of the modulatory effects of exercise on MMP-2 and MMP-9 regulation. It was demonstrated that muscle-derived cytokines are released following a training program, while decreasing the oxidative stress (14). Changes in MMP serum levels may reflect the impact of exercise on the production of inflammatory molecules. It is hypothesized that exercise causes a reduction in free radicals and an increase in antioxidant defenses, which may influence the inflammatory conditions and subsequently alter MMP serum concentrations. The different effects of exercise observed on MMP-2 and −9 serum levels may be associated with different dose-dependent responses to physical activity (57).
The results of the present study indicate that the type of physical exercise (intensity, volume, frequency and length) may effect MMP-2 and MMP-9 plasma levels. The effect of the same exercise program was different in healthy subjects and BC survivors. To the best of our knowledge, it remains to be elucidated why this differential response occurs. To understand these findings, it is helpful to analyze the weekly frequency of exercise, as well as the period of rest and the muscle strength applied. In the present study, exercise was performed once a week for 120 min with a rest time of 6 days in the dragon boat paddling group (group A). The muscle strength applied during paddling is proportionate to an anti-gravity exercise in accordance with the variables of hydrodynamics. The other recreational physical activity groups (group B and D) trained 3 times a week for 60 min, and thus the rest time was shorter than that of the dragon boat paddling group. For groups B and D, the muscle strength applied was proportionate to the extra load used during the exercise session without the benefit of hypogravity. All these variables have been shown to affect MMP-2 serum levels in BC survivors.
Coagulation enzymes are involved in MMP-2 activation for example, urokinase plasminogen converts plasminogen to plasmin, which promotes tumor growth and angiogenesis, degrading the ECM and basement membrane, and activating pro-MMPs (63). MMPs and urokinase plasminogen activators are involved in tumor invasion and metastasis. The results of the present study demonstrated that BC survivors exhibit increased MMP-2 levels when compared with healthy subjects. These results may be due to an increase in urokinase plasminogen activator expression, as it has been demonstrated that exercise increases plasma fibrinolytic activity. Furthermore, exhaustive exercise is associated with increased expression of plasminogen activator in plasma (64), and exercise-stimulated thrombin and plasmin generation were previously identified in healthy subjects that exercised for a significantly longer duration (65). Prolonged physical exercise is associated with numerous changes in blood hemostasis and higher levels of aerobic fitness are associated with increased fibrinolytic activity (66,67), while MMP-2 activation is inhibited by plasmin inhibitors (68).
Previous studies have reported that serum levels of MMP-2 and MMP-9 are significantly higher in BC patients than in control subjects (69,70). Furthermore, patients with N+ cancer exhibit significantly higher MMP-2 and MMP-9 activity than those with N- cancer (53). The results obtained in the present study revealed a significant increase in MMP-2 but not MMP-9 concentration in N+ BC survivors compared with N- BC survivors. Furthermore, different exercise regimes exhibited different effects on circulating MMP-2 and MMP-9 levels.
In conclusion, the contradictory results obtained in the present study may be due to the fact that exercise of various intensities were performed in previous studies. Therefore, further studies are required to determine the biological significance of MMP levels in the adaptation of skeletal muscle to physical activity in cancer patients and to explore how different types of physical exercise affect MMP production. Overall, the results of the present study indicate that various physical activities modulate the levels of circulating MMP-2 and −9 in BC survivors, and an identical exercise program induces a contrasting effect when undertaken by healthy subjects and BC survivors.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund/American Institute for Cancer Research: Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. AICR; Washington DC: 2007. [Google Scholar]
- 3.Hildebrand JS, Gapstur SM, Campbell PT, Gaudet MM, Patel AV. Recreational physical activity and leisure-time sitting in relation to postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2013;22:1906–1912. doi: 10.1158/1055-9965.EPI-13-0407. [DOI] [PubMed] [Google Scholar]
- 4.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170:1758–1764. doi: 10.1001/archinternmed.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen D, Mao W, Liu T, Lin Q, Lu X, Wang Q, Lin F, Ekelund U, Wijndaele K. Sedentary behavior and incident cancer: A meta-analysis of prospective studies. PLoS One. 2014;9:e105709. doi: 10.1371/journal.pone.0105709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137:869–882. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 8.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 9.Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, Baron JA, Egan KM, Willett WC. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 10.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 11.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 12.Pinto A, Di Raimondo D, Tuttolomondo A, Buttà C, Milio G, Licata G. Effects of physical exercise on inflammatory markers of atherosclerosis. Curr Pharm Des. 2012;18:4326–4349. doi: 10.2174/138161212802481192. [DOI] [PubMed] [Google Scholar]
- 13.Crisafulli A, Tocco F, Melis F, Milia R, Concu A. Natural killer cells responsiveness to physical exercise: A brief review. Open J Immunol. 2013;3:190–200. doi: 10.4236/oji.2013.34024. [DOI] [Google Scholar]
- 14.Tresoldi I, Foti C, Masuelli L, Frajese GV, Rossi P, Modesti A, Bei R, Giganti MG. Effects of dragon boat training on cytokine production and oxidative stress in breast cancer patients: A pilot study. Open J Immunol. 2014;4:22–29. doi: 10.4236/oji.2014.41004. [DOI] [Google Scholar]
- 15.Ramin A, Abbas M, Ali Abbas G, Asghar R, Amir L, Farshid S, Hussein B. Effects of exhaustive aerobic exercise on matrix metaloproteases activity in athletes and non-athletes. World J Sport Sci. 2011;4:185–191. [Google Scholar]
- 16.Madden MC, Byrnes WC, Lebin JA, Batliner ME, Allen DL. Plasma matrix metalloproteinase-9 response to eccentric exercise of the elbow flexors. Eur J Appl Physiol. 2011;111:1795–1805. doi: 10.1007/s00421-010-1806-y. [DOI] [PubMed] [Google Scholar]
- 17.Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: A brief review. Muscle Nerve. 2004;29:191–197. doi: 10.1002/mus.10529. [DOI] [PubMed] [Google Scholar]
- 18.Benz CC. Transcriptional factors and breast cancer. Endoc Rel Cancer. 1998;5:271–282. doi: 10.1677/erc.0.0050271. [DOI] [Google Scholar]
- 19.Giunciuglio D, Culty M, Fassina G, Masiello L, Melchiori A, Paglialunga G, Arand G, Ciardiello F, Basolo F, Thomson EW, et al. Invasive phenotype of MCF10A cells overexpressing c-Ha-ras and c-erbB-2 oncogenes. Int J Cancer. 1995;63:815–822. doi: 10.1002/ijc.2910630612. [DOI] [PubMed] [Google Scholar]
- 20.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 21.Cocket MI, Murohy G, Birch ML, O'Connell JP, Crabbe T, Millican AT, Hart IR, Dochery AJ. Matrix metalloproteinase and metastatic cancer. Biochem Soc Symp. 1998;63:295–313. [PubMed] [Google Scholar]
- 22.Lucotti P, Monti LD, Setola E, Galluccio E, Gatti R, Bosi E, Piatti P. Aerobic and resistance training effects compared to aerobic training alone in obese type2 diabetic patients on diet treatment. Diabetes Res Clin Pract. 2011;94:395–403. doi: 10.1016/j.diabres.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Bellafiore M, Battaglia G, Bianco A, Farina F, Palma A, Paoli A. The involvement of MMP-2 and MMP-9 in heart exercise-related angiogenesis. J Transl Med. 2013;11:283. doi: 10.1186/1479-5876-11-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadoglou NP, Vrabas IS, Sailer N, Kapelouzou A, Fotiadis G, Noussios G, Karayannacos PE, Angelopoulou A. Exercise ameliorates serum MMP-9 and TIMP-2 levels in patients with type 2 diabetes. Diabetes Metab. 2010;36:144–151. doi: 10.1016/j.diabet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Koskinen SO, Höyhtyä M, Turpeenniemi-Hujanen T, Martikkala V, Mäkinen TT, Oksa J, Rintamäki H, Löfberg M, Somer H, Takala TE. Serum concentrations of collagen degrading enzymes and their inhibitors after downhill running. Scand J Med Sci Sports. 2001;11:9–15. doi: 10.1034/j.1600-0838.2001.011001009.x. [DOI] [PubMed] [Google Scholar]
- 26.Urso ML, Pierce JR, Alemany JA, Harman EA, Nindl B. Effects of exercise training on the matrix metalloprotease response to acute exercise. Eur J Appl Physiol. 2009;106:655–663. doi: 10.1007/s00421-009-1063-0. [DOI] [PubMed] [Google Scholar]
- 27.Jacob A, Jing J, Lee J, Schedin P, Gilbert SM, Peden AA, Junutula JR, Prekeris R. Rab40b regulates trafficking of MMP2 and MMP9 during invadopodia formation and invasion of breast cancer cells. J Cell Sci. 2013;126:4647–4658. doi: 10.1242/jcs.126573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu RR, Li MD, Li T, Tan Y, Zhang M, Chen JC. Matrix metalloproteinase 2 (MMP2) protein expression and laryngeal cancer prognosis: A meta analysis. Int J Clin Exp Med. 2015;8:2261–2266. [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob A, Prekeris R. The regulation of MMP targeting to invadopodia during cancer metastasis. Front Cell Dev Biol. 2015;3:4. doi: 10.3389/fcell.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackey AL, Donnelly AE, Swanton A, Murray F, Turpeenniemi-Hujanen T. The effects of impact and non-impact exercise on circulating markers of collagen remodeling in humans. J Sports Sci. 2006;24:843–848. doi: 10.1080/02640410500231470. [DOI] [PubMed] [Google Scholar]
- 31.Mackey AL, Donnelly AE, Turpeeniemi-Hujanen T, Proper HP. Skeletal muscle collagen content in humans after high-force eccentric contractions. J Appl Physiol (1985) 2004;97:197–203. doi: 10.1152/japplphysiol.01174.2003. [DOI] [PubMed] [Google Scholar]
- 32.Niessner A, Richter B, Penka M, Steiner S, Strasser B, Ziegler S, Heeb-Elze E, Zorn G, Leitner-Heinschink A, Niessner C, et al. Endurance training reduces circulating inflammatory markers in persons at risk of coronary events: Impact plaque stabilization? Atheroscleorosis. 2006;186:160–165. doi: 10.1016/j.atherosclerosis.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 33.Harris SR. “We're all in the same boat”: A review of the benefits of dragon boat racing for women living with breast cancer. Evid Based Complement Alternat Med. 2012;2012:167651. doi: 10.1155/2012/167651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane K, Jespersen D, McKenzie DC. The effect of a whole body exercise programme and dragon boat training on arm volume and arm circumference in women treated for breast cancer. Eur J Cancer Care (Engl) 2005;14:353–358. doi: 10.1111/j.1365-2354.2005.00595.x. [DOI] [PubMed] [Google Scholar]
- 35.Lucia A, Earnest C, Pérez M. Changed-related fatigue: Can exercise physiology assist oncologys? Lancet Oncol. 2003;4:616–625. doi: 10.1016/S1470-2045(03)01221-X. [DOI] [PubMed] [Google Scholar]
- 36.Sobin LH, Gospodarowicz MK, Wittekind Ch, editors. TNM Classification of Malignant Tumors. 7th. Wiley-Blackwell; Oxford: 2009. pp. 4–15. [Google Scholar]
- 37.Irwin ML. Physical activity interventions for cancer survivors. Br J Sports Med. 2009;43:32–38. doi: 10.1136/bjsm.2008.053843. [DOI] [PubMed] [Google Scholar]
- 38.Winters-Stone KM, Schwartz A, Nail LM. A review of exercise interventions to improve bone health in adult cancer survivors. J Cancer Surviv. 2010;4:187–201. doi: 10.1007/s11764-010-0122-1. [DOI] [PubMed] [Google Scholar]
- 39.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 40.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma: The health, eating, activity and lifestyle (HEAL) study. Cancer. 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsen TS, Thorsen L, Kirkegaard C, Ugelstad I, Fosså SD, Raastad T. The effect of strength training on muscle cellular stress in prostate cancer patients on ADT. Endocr Connect. 2016;5:74–82. doi: 10.1530/EC-15-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skinner TL, Peeters GG, Croci I, Bell KR, Burton NW, Chambers SK, Bolam KA. Impact of a brief exercise program on the physical and psychosocial health of prostate cancer survivors: A pilot study. Asia Pac J Clin Oncol. 2016 Feb 28; doi: 10.1111/ajco.12474. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 43.Shallwani S, Dalzell MA, Sateren W, O'Brien S. Exercise compliance among patients with multiple myeloma undergoing chemotherapy: A retrospective study. Support Care Cancer. 2015;23:3081–3088. doi: 10.1007/s00520-015-2680-2. [DOI] [PubMed] [Google Scholar]
- 44.Jones LW, Courneya KS, Vallance JK, Ladha AB, Mant MJ, Belch AR, Stewart DA, Reiman T. Association between exercise and quality of life in multiple myeloma cancer survivors. Support Care Cancer. 2004;12:780–788. doi: 10.1007/s00520-004-0668-4. [DOI] [PubMed] [Google Scholar]
- 45.Smits A, Lopes A, Das N, Bekkers R, Massuger L, Galaal K. The effect of lifestyle interventions on the quality of life of gynaecological cancer survivors: A systematic review and meta-analysis. Gynecol Oncol. 2015;139:546–552. doi: 10.1016/j.ygyno.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Meyerhardt J, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 47.Bourke L, Smith D, Steed L, Hooper R, Carter A, Catto J, Albertsen PC, Tombal B, Payne HA, Rosario DJ. Exercise for men with prostate cancer: A systematic review and meta-analysis. Eur Urol. 2016;26:693–703. doi: 10.1016/j.eururo.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 48.Parks WC, Mecham RP. Matrix metaloproteinasis. Academic Press; San Diego: 1998. pp. 299–356. [Google Scholar]
- 49.Carmeli E, Haimovitch TG, Nemcovsky CE. Expression of matrix metalloproteinase 2 and heat shock protein-72 in immobilized muscle in rats. J Musculoskelet Neuronal Interact. 2006;6:96–102. [PubMed] [Google Scholar]
- 50.Rullman E, Olsson K, Wagsater D, Gustafsson T. Circulating MMP-9 during exercise in humans. Eur J Appl Physiol. 2013;113:1249–1255. doi: 10.1007/s00421-012-2545-z. [DOI] [PubMed] [Google Scholar]
- 51.Somiari SB, Somiari RI, Heckman CM, Olsen CH, Jordan RM, Russell SJ, Shriver CD. Circulating MMP2 and MMP9 in breast cancer: Potential role in classification of patients into low risk, high risk, benign disease and breast cancer categories. Int J Cancer. 2006;119:1403–1411. doi: 10.1002/ijc.21989. [DOI] [PubMed] [Google Scholar]
- 52.Jinga DC, Blidaru A, Condrea I, Ardeleanu C, Dragomir C, Szegli G, Stefanescu M, Matache C. MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast cancer: Correlations with prognostic factors. J Cell Mol Med Vol. 2006;10:499–510. doi: 10.1111/j.1582-4934.2006.tb00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li HC, Cao DC, Liu Y, Hou YF, Wu J, Lu JS, Di GH, Liu G, Li FM, Ou ZL, et al. Prognostic value of matrix metalloproteinase (MMP-2 and MMP-9) in patients with lymph node-negative breast carcinoma. Breast Cancer Res Treat. 2004;88:75–85. doi: 10.1007/s10549-004-1200-8. [DOI] [PubMed] [Google Scholar]
- 54.Morgia G, Falsaperla M, Malaponte G, Madonia M, Indelicato M, Travali S, Mazzarino MC. Matrix metalloproteinases as diagnostic (MMP-13) and prognostic (MMP-2, MMP-9) markers of prostate cancer. Urol Res. 2005;33:44–50. doi: 10.1007/s00240-004-0440-8. [DOI] [PubMed] [Google Scholar]
- 55.Mönig SP, Baldus SE, Hennecken JK, Spiecker DB, Grass G, Schneider PM, Thiele J, Dienes HP, Hölscher AH. Expression of MMP-2 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology. 2001;39:597–602. doi: 10.1046/j.1365-2559.2001.01306.x. [DOI] [PubMed] [Google Scholar]
- 56.Hoikkala S, Pääkkö P, Soini Y, Mäkitaro R, Kinnula V, Turpeenniemi-Hujanen T. Tissue MMP-2 and MMP-9 [corrected] are better prognostic factors than serum MMP-2/TIMP-2-complex or TIMP-1 [corrected] in stage [corrected] I–III lung carcinoma. Cancer Lett. 2006;236:125–132. doi: 10.1016/j.canlet.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Nascimento Dda C, Durigan Rde C, Tibana RA, Durigan JL, Navalta JW, Prestes J. The response of matrix metalloproteinase-9 and −2 to exercise. Sports Med. 2015;45:269–278. doi: 10.1007/s40279-014-0265-8. [DOI] [PubMed] [Google Scholar]
- 58.Niessner A, Richter B, Penka M, Steiner S, Strasser B, Ziegler S, Heeb-Elze E, Zorn G, Leitner-Heinschink A, Niessner C, et al. Endurance training reduces circulating inflammatory markers in persons at risk of coronary events: Impact on plaque stabilization? Atherosclerosis. 2006;186:160–165. doi: 10.1016/j.atherosclerosis.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 59.Steenport M, Khan KM, Du B, Barnhard SE, Dannenberg AJ, Falcone DJ. Matrix metalloproteinase (MMP)-1 and MMP-3 induce macrophage MMP-9: Evidence for the role of TNF-alpha and cyclooxygenase-2. J Immunol. 2009;183:8119–8127. doi: 10.4049/jimmunol.0901925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 61.Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582:1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rullman E, Norrbom J, Strömberg A, Wågsäter D, Rundqvist H, Haas T, Gustafsson T. Endurance exercise activates matrix metalloproteinases in human skeletal muscle. J Appl Physiol (1985) 2009;106:804–812. doi: 10.1152/japplphysiol.90872.2008. [DOI] [PubMed] [Google Scholar]
- 63.Roomi MW, Kalinovsky T, Rath M, Niedzwiecki A. Down-regulation of urokinase plasminogen activator and matrix metalloproteinases and up-regulation of their inhibitors by a novel nutrient mixture in human prostate cancer cell lines PC-3 and DU-145. Oncol Rep. 2011;26:1407–1413. doi: 10.3892/or.2011.1434. [DOI] [PubMed] [Google Scholar]
- 64.Booth NA, Walker E, Maughan R, Bennett B. Plasminogen activator in normal subjects after exercise and venous occlusion: t-PA circulates as complexes with C1-inhibitor and PAI-1. Blood. 1987;69:1600–1604. [PubMed] [Google Scholar]
- 65.Small M, Simpson I, McGhie I, Douglas JT, Lowe GD, Forbes CD. The effect of exercise on thrombin and plasmin generation in middle-aged men. Haemostasis. 1987;17:371–376. doi: 10.1159/000215772. [DOI] [PubMed] [Google Scholar]
- 66.Francis RM, Romeyn CL, Coughlin AM, Nagelkirk PR, Womack CJ, Lemmer JT. Age and aerobic training status effects on plasma and skeletal muscle tPA and PAI-1. Eur J Appl Physiol. 2014;114:1229–1238. doi: 10.1007/s00421-014-2857-2. [DOI] [PubMed] [Google Scholar]
- 67.Sumann G, Fries D, Griesmacher A, Falkensammer G, Klingler A, Koller A, Streif W, Greie S, Schobersberger B, Schobersberger W. Blood coagulation activation and fibrinolysis during a downhill marathon run. Blood Coagul Fibrinolysis. 2007;18:435–440. doi: 10.1097/MBC.0b013e328136c19b. [DOI] [PubMed] [Google Scholar]
- 68.Baramova EN, Bajou K, Remacle A, L'Hoir C, Krell HW, Weidle UH, Noel A, Foidart JM. Involvement of PA/plasmin system in the processing of pro-MMP-9 and in the second step of pro-MMP-2 activation. FEBS Lett. 1997;405:157–162. doi: 10.1016/S0014-5793(97)00175-0. [DOI] [PubMed] [Google Scholar]
- 69.Ranuncolo SM, Armanasco E, Cresta C, Bal De Kier Joffe E, Puricelli L. Plasma MMP-9 (92 kDa-MMP) activity is useful in the follow-up and in the assessment of prognosis in breast cancer patients. Int J Cancer. 2003;106:745–751. doi: 10.1002/ijc.11288. [DOI] [PubMed] [Google Scholar]
- 70.La Rocca G, Pucci-Minafra I, Marrazzo A, Taormina P, Minafra S. Zymographic detection and clinical correlations of MMP-2 and MMP-9 in breast cancer sera. Br J Cancer. 2004;90:1414–1421. doi: 10.1038/sj.bjc.6601725. [DOI] [PMC free article] [PubMed] [Google Scholar]