Abstract
Adhesion G protein–coupled receptors (aGPCRs) have emerging roles in development and tissue maintenance and is the most prevalent GPCR subclass mutated in human cancers, but to date, no drugs have been developed to target them in any disease. aGPCR extracellular domains contain a conserved subdomain that mediates self-cleavage proximal to the start of the 7-transmembrane domain (7TM). The two receptor protomers, extracellular domain and amino terminal fragment (NTF), and the 7TM or C-terminal fragment remain noncovalently bound at the plasma membrane in a low-activity state. We recently demonstrated that NTF dissociation liberates the 7TM N-terminal stalk, which acts as a tethered-peptide agonist permitting receptor-dependent heterotrimeric G protein activation. In many cases, natural aGPCR ligands are extracellular matrix proteins that dissociate the NTF to reveal the tethered agonist. Given the perceived difficulty in modifying extracellular matrix proteins to create aGPCR probes, we developed a serum response element (SRE)-luciferase–based screening approach to identify GPR56/ADGRG1 small-molecule inhibitors. A 2000-compound library comprising known drugs and natural products was screened for GPR56-dependent SRE activation inhibitors that did not inhibit constitutively active Gα13-dependent SRE activation. Dihydromunduletone (DHM), a rotenoid derivative, was validated using cell-free aGPCR/heterotrimeric G protein guanosine 5′-3-O-(thio)triphosphate binding reconstitution assays. DHM inhibited GPR56 and GPR114/ADGRG5, which have similar tethered agonists, but not the aGPCR GPR110/ADGRF1, M3 muscarinic acetylcholine, or β2 adrenergic GPCRs. DHM inhibited tethered peptide agonist-stimulated and synthetic peptide agonist-stimulated GPR56 but did not inhibit basal activity, demonstrating that it antagonizes the peptide agonist. DHM is a novel aGPCR antagonist and potentially useful chemical probe that may be developed as a future aGPCR therapeutic.
Introduction
Adhesion G protein–coupled receptors (aGPCRs) are a 33-member subclass of family B G protein–coupled receptors (GPCRs) that have critical roles in tissue specification and replenishment and human cancers (Langenhan et al., 2013; Hamann et al., 2015). It was recently discovered that the extracellular peptide stalks emanating from the first transmembrane-spanning helix of multiple adhesion GPCRs are tethered-peptide agonists that activate aGPCR-mediated heterotrimeric G protein signaling (Liebscher et al., 2014; Demberg et al., 2015; Stoveken et al., 2015; Wilde et al., 2016). Tethered-agonist regulation is intimately linked to the ability of aGPCRs to execute a precise autocatalytic, self-cleavage event in which the P1′ residue becomes the N terminus of the ∼17–26 amino acid tethered-agonist stalks (Lin et al., 2004; Arac et al., 2012; Liebscher et al., 2014; Stoveken et al., 2015). Self-cleavage is thought to be constitutive, and the resultant receptor protomers or fragments remain noncovalently bound while in residence at the plasma membrane. In this state, the N terminus of the postcleaved tethered-agonist stalk binds firmly within a hydrophobic β-strand network that would preclude it from engaging the carboxy-terminal fragment (CTF)/seven-transmembrane domain (7TM) orthosteric site.
A current hypothesis which we favor that describes the dynamism of receptor activation consists of two components: 1) anchored protein ligands bind aGPCR N-terminal fragment (NTF) binding determinants, and 2) the action of shear force created by cell movement serves to dissociate the NTF from the CTF to release or decrypt the tethered agonist (Karpus et al., 2013; Langenhan et al., 2013; Scholz et al., 2015; Stoveken et al., 2015). We reconstituted aGPCRs, GPR56 (ADGRG1) and GPR110 (ADGRF1), with purified G protein heterotrimers and provided a biochemical demonstration that experimentally induced NTF dissociation dramatically enhanced aGPCR-mediated G protein activation (Stoveken et al., 2015). The known protein ligands of aGPCRs are fibrillar extracellular matrix proteins or, in some instances, proteins presented by neighboring cells (Hamann et al., 1996; Sugita et al., 1999; Stacey et al., 2003; Xu et al., 2006; Bolliger et al., 2011; Chiang et al., 2011; Luo et al., 2011; Silva et al., 2011; Boucard et al., 2012; O'Sullivan et al., 2012; Paavola and Hall, 2012; Paavola et al., 2014; Petersen et al., 2015; Scholz et al., 2015). Based on the proposed two-component mode of ligand-mediated aGPCR activation, we anticipate that natural NTF ligands or ligand-based NTF-binding mimetics will be of limited, stand-alone pharmacological use to manipulate receptor activities. Indeed, application of dilute, soluble extracellular matrix protein ligands to aGPCR cell culture–based signaling assays most likely does not recapitulate authentic aGPCR pharmacology. Hence, there is a critical need to develop small-molecule modulators that can bypass the two-component ligand-mediated regulation process and directly modulate receptor activities. A number of adhesion GPCR small-molecule screening efforts are ongoing, but to our knowledge, only a few candidate small-molecule activators were proposed to regulate GPR97 (Gupte et al., 2012; Southern et al., 2013).
We conducted a chemical-screening effort to identify small-molecule inhibitors of GPR56, an attractive therapeutic target due to its proposed roles in neurogenesis, neuromaintenance, and cancer progression (Piao et al., 2004; Shashidhar et al., 2005; Xu et al., 2006, 2010; Yang et al., 2011; Saito et al., 2013; Hamann et al., 2015). GPR56 signals through G13 and multiple laboratories showed that GPR56 robustly activates serum response element (SRE) or serum response factor luciferase gene reporters (Iguchi et al., 2008; Kim et al., 2010; Wu et al., 2013; Stoveken et al., 2015; Kishore et al., 2016). We adapted our construct that produces the full tethered-agonist-activated GPR56 CTF for use as an SRE luciferase human embryonic kidney 293 (HEK293) cell high-throughput inhibitor screening platform (Stoveken et al., 2015). A counterscreen was developed in which each library compound was tested for inhibition of constitutively active Gα13-Q226L–activated SRE luciferase. Since G13 activation is the first step downstream of GPR56, all compound hits that inhibited the GPR56 CTF, but not Gα13-Q226L, are likely to act at the receptor level and not at points downstream in the pathway. Sixty-six compounds from the ∼2000-compound Spectrum Collection chemical library inhibited GPR56-CTF–dependent luciferase activity, 63 of which also inhibited Gα13-Q226L and were therefore eliminated. The identified compounds were dihydromunduletone (DHM), a derivative of the natural-product mundulone, and two structurally related natural products that we have temporarily termed “H.M.S.1” and “H.M.S.2,” pending chemical syntheses (Burrows et al., 1959; Ollis and Sutherland, 1961).
Here, we present evidence that DHM is a bona fide selective antagonist for a subset of aGPCRs. This is the first identification of a small-molecule aGPCR inhibitor that has potential to uncover novel aGPCR biology and represents a first step in the development of a therapeutic strategy targeting aGPCRs.
Materials and Methods
Reagents and Antibodies.
The GPR56 C-terminal antibody was a gift from Dr. Randy Hall (Emory University, Atlanta, GA) (Paavola et al., 2011). Dihydromunduletone, isorotenone, mundulone, and deguelin were from the Spectrum Collection chemical library and were purchased individually as powders from MicroSource Discovery Systems, Inc. (Gaylordsville, CT). Rotenone and polyclonal hemagglutinin (HA) antibody were purchased from Sigma-Aldrich (St. Louis, MO). Latrunculin B was from Calbiochem (San Diego, CA). Quant-iT PicoGreen double stranded DNA reagent was from Invitrogen (Carlsbad, CA). Streptavidin Sepharose High Performance was from GE Healthcare (Chicago, IL). Sulfo-NHS-Biotin was from Thermo Scientific (Waltham, MA). 5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) and the phRLuc-N1 plasmid were from PerkinElmer (Waltham, MA). The pGL4.33 [luc2/SRE/Hygro] plasmid was purchased from Promega (Madison, WI).
High-Throughput Screen.
HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum (FBS). Cells were transiently transfected with GPR56 7TM (Met-Thr383 to Ile693) pcDNA3.1 or GNA13Q226L pcDNA3.1 (cDNA.org) and the SRE-luciferase reporter using a polyethylenimine transfection method (Wang et al., 2010; Oner et al., 2013; Stoveken et al., 2015). Five hours after transfection, cells were trypsinized, counted, and seeded at 15,000 cells per well in 384-well plates in 20 µl of phenol red–free DMEM buffered with 25 mM Hepes, pH 7.4, and incubated overnight with ∼3–5 µM compounds in dimethylsulfoxide (DMSO); Spectrum Collection, at the URMC High Throughput Screening Core (Microsource Discovery Systems, Inc. Gaylordsville, CT). The next morning, 20 µl of SteadyLite luciferase reagent (PerkinElmer) was robotically dispensed into each well and incubated for 15 minutes at room temperature. Luminescence was read on a PerkinElmer Envision Plate Reader.
Directed Dual SRE-Luciferase Assay.
HEK293T cells maintained in DMEM +10% (v/v) FBS were transiently transfected in 24-well format using the polyethylenimine reagent with 200 ng of GPR56 7TM (encodes Met-Thr383 to Ile693), 25 ng of GPR56 A386M 7TM (encodes Ala386Met to Ile687), or 200 ng of GNA13Q226L pcDNA3.1 plasmids; 100 ng of the SRE-luciferase reporter; and 1 ng of phRLuc (Stoveken et al., 2015). Total DNA levels were balanced with empty pcDNA3.1. Cells were serum starved for 10 hours the following day; DHM or synthetic-peptide agonist was added to the cell medium at 2 and 4 hours, respectively, during the serum-starving phase. Cells were harvested in culture medium by trituration, washed in Tyrode’s solution (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 0.2 mM Na2HPO4, 12 mM NaHCO3, and 5.5 mM d-glucose), and lysed in firefly luciferase reagent (NanoLight Technologies, Inc., Pinetop, AZ). Firefly luminescence was quenched using Renilla luciferase buffer containing 3 µM coelenterazine H (Dyer et al., 2000). Luminescence was read using a TriStar2 plate reader (Berthold, Wildbad, Germany). All firefly luciferase data were normalized to the Renilla luciferase signal and expressed as fold increase over the signal obtained from cells transfected with SRE-luciferase only.
Insect Cell Culture, Baculovirus Generation, and Adhesion GPCR Membrane Preparation.
Spodoptera frugiperda 9 and High-Five insect cells were maintained as described (Stoveken et al., 2015). Baculoviruses were generated per the manufacturer’s protocol (Bac-to-Bac manual; Invitrogen). For adhesion GPCR membrane preparations, High-Five insect cells were infected with a 1/50 dilution (1° amplification) or a 1/100 dilution (2° amplification) of recombinant adhesion GPCR baculovirus stocks for 48 hours prior to cell harvest and preparation of native and urea-treated adhesion GPCR membranes as described (Stoveken et al., 2015).
Adhesion GPCR:G Protein Reconstitution Assays.
Recombinant heterotrimeric G protein α subunits (Gαi1, Gα13, Gαs, Gαq) were purified from High-Five insect cells using the GST-Ric-8A or B association method (Chan et al., 2011). Recombinant Gβ1γ2 was purified from High-Five membranes using the Gαi-His6 association method (Kozasa and Gilman, 1995). Adhesion GPCR membranes (1–5 µg) were reconstituted with purified G proteins (100 nM Gα of interest and 500 nM Gβ1γ2) in preincubation buffer [50 mM Hepes (pH 7.4), 1 mM dithiothreitol, 1 mM EDTA, and 3 µg/ml bovine serum albumin] with 0 or 20 µM GDP. Compounds (DHM, deguelin, mundulone, isorotenone, rotenone), synthetic-peptide agonist, or DMSO vehicle control (<5% v/v) was added to the membrane/G protein mixture and incubated for 30 minutes at 25°C. An equal volume of 5′-3-O-(thio)triphosphate (GTPγS) binding buffer (preincubation buffer containing 10 mM MgCl2 and 50 mM NaCl) with 2 µM GTPγS, [35S]GTPγS (20,000–50,000 cpm/pmol), and the indicated concentrations of compounds or synthetic-peptide agonist was added to initiate the kinetic assay (compounds or peptides were included in both incubation mixtures to maintain the final concentrations). Triplicate samples were removed from the reaction mixtures and quenched at the indicated time points with 20 mM Tris (pH 7.7), 100 mM NaCl, 10 mM MgCl2, 1 mM GTP, and 0.08% (m/v) deionized polyoxyethylene 10 lauryl ether C12E10. Quenched samples were filtered through BA85 nitrocellulose filters (GE Healthcare Chicago, IL), dried, and counted by liquid scintillation counting as described (Stoveken et al., 2015).
Mitochondrial Complex I (NADH:Ubiquinone Oxidoreductase) Activity Assay.
Complex I activity was measured from frozen C57BL/6J mouse heart mitochondria isolated by differential centrifugation (Wojtovich et al., 2011). Mitochondria (25 µg/ml) were suspended in assay buffer (25 mM K2HPO4/KH2PO4, 10 mM MgCl2, 2.5 mg/ml bovine serum albumin, 1 mM KCN, 75 µM NADH, pH 7.2, 37°C) in the presence of the indicated isoflavone compounds. After the addition of coenzyme Q1 (0.1 mM), complex I activity was measured as the rotenone-sensitive rate (nmol/min/mg) of NADH oxidation (ε = 6180 M−1 at 340 nm) and expressed as percentage of inhibition (Nadtochiy et al., 2007).
Rho GTPase Activation Assay.
HEK293T cells were plated at 4 × 106 cells per 10-cm plate. Twenty-four hours later, cells were transfected with 6.5 µg GPR56 A386M 7TM pcDNA3.1 and 1 µg 3XHA-RhoA pcDNA3.1 (cDNA.org) using the polyethylenimine transfection method (Oner et al., 2013). The next day, cells were trypsinized, pooled, and plated at 4 × 106 cells per 10-cm plate. The seeded transfected cells were allowed to attach for 8 hours and then serum starved overnight. Serum-starved cells were treated with 10 µM DHM or DMSO vehicle for 15 minutes. P7 synthetic peptide agonist or DMSO vehicle was added to the cells and incubated for an additional 5 minutes. Cells were washed once with phosphate-buffered saline + protease inhibitor mixture (23 µg/ml phenylmethylsulfonyl fluoride, 21 µg/ml Nα-p-tosyl-l-lysine-chloromethyl ketone, 21 µg/ml L-1-p-tosylamino-2-phenylethyl-chloroketone, 3.3 µg/ml leupeptin, and 3.3 µg/ml lima bean trypsin inhibitor) and lysed in 1 ml of lysis buffer [25 mM Hepes (pH 7.2), 150 mM NaCl, 5 mM MgCl2, 1% Nonidet P-40, 5% glycerol, protease inhibitor mixture]. Lysates were cleared by centrifugation at 21,000g, and the total protein concentration of each lysate sample was determined by amido black protein assay (Schaffner and Weissmann, 1973). Equal amounts of protein from each lysate were added to tubes containing 60 µg of Rhotekin-RBD agarose (Cytoskeleton, Inc., Denver, CO) and tumbled for 45 minutes. Rhotekin-RBD agarose was washed three times with lysis buffer, and beads were suspended in 50 µl of 2× sample buffer [200 mM Tris (pH 6.8), 20% glycerol, 4% SDS, 200 mM dithiothreitol, 0.02% bromophenol blue] (Laemmli, 1970). Relative levels of active Rho recovered from Rhotekin agarose were compared with levels of total Rho obtained from input samples by immunoblotting with the HA antibody.
PicoGreen Cell Detachment Assay.
HEK293T cells were plated at 2.5 × 104 cells/well in 96-well plates in DMEM + 10% FBS (v/v). Twenty hours later, compounds (DHM, deguelin, isorotenone, mundulone, and rotenone) or DMSO vehicle control was serially diluted in DMEM + 10% FBS from 50 µM to 100 nM, and the cell medium was exchanged. Culture medium was replaced 24 hours later with 100 µl TE buffer [10 mM Tris-HCl (pH 7.7) and 1 mM EDTA) for 1 hour to induce hypotonic cell lysis. Quant-iT PicoGreen double stranded DNA reagent was diluted 1:200 in TE, and 100 µl was added to each well for 15 minutes to allow dye/DNA complex formation. The fluorescent dye was excited at 485 nm using 0.1% lamp energy, and emission was measured at 535 nm using the Berthold TriStar2 Plate Reader. Relative fluorescent units were plotted as functions of compound concentration, and curve fitting was performed using GraphPad Prism (GraphPad Software, La Jolla, CA).
Results
Identification of GPR56 Small-Molecule Inhibitors through High-Throughput Screening.
Isolated adhesion GPCR CTFs/7TM domains are highly active due to the engaged state of the tethered-peptide agonist (Paavola et al., 2011, 2014; Liebscher et al., 2014; Stoveken et al., 2015). We exploited this property of the GPR56 7TM to develop a high-throughput inhibitor screening assay using the SRE-luciferase gene reporter. GPR56 activates G13 robustly and thus, Gα13-sensitive SRE- or serum response factor–luciferase reporters (Shashidhar et al., 2005; Iguchi et al., 2008; Stoveken et al., 2015; Kishore et al., 2016). For the high-throughput screen and counterscreen, HEK293 cells were cotransfected with an SRE-luciferase reporter plasmid and GPR56 7TM or GNA13Q226L pcDNA3.1 plasmids, respectively. Vehicle-treated cells were the positive controls (top limit of the assays), and cells treated with latrunculin B, which disrupts the signaling axis downstream of both GPR56 and Gα13, were the negative controls (bottom limit of the assays) (Evelyn et al., 2007). The Z′ score was 0.67 for the screening assay and 0.8 for the counterscreening assay (Supplemental Fig. 1) (Zhang et al., 1999).
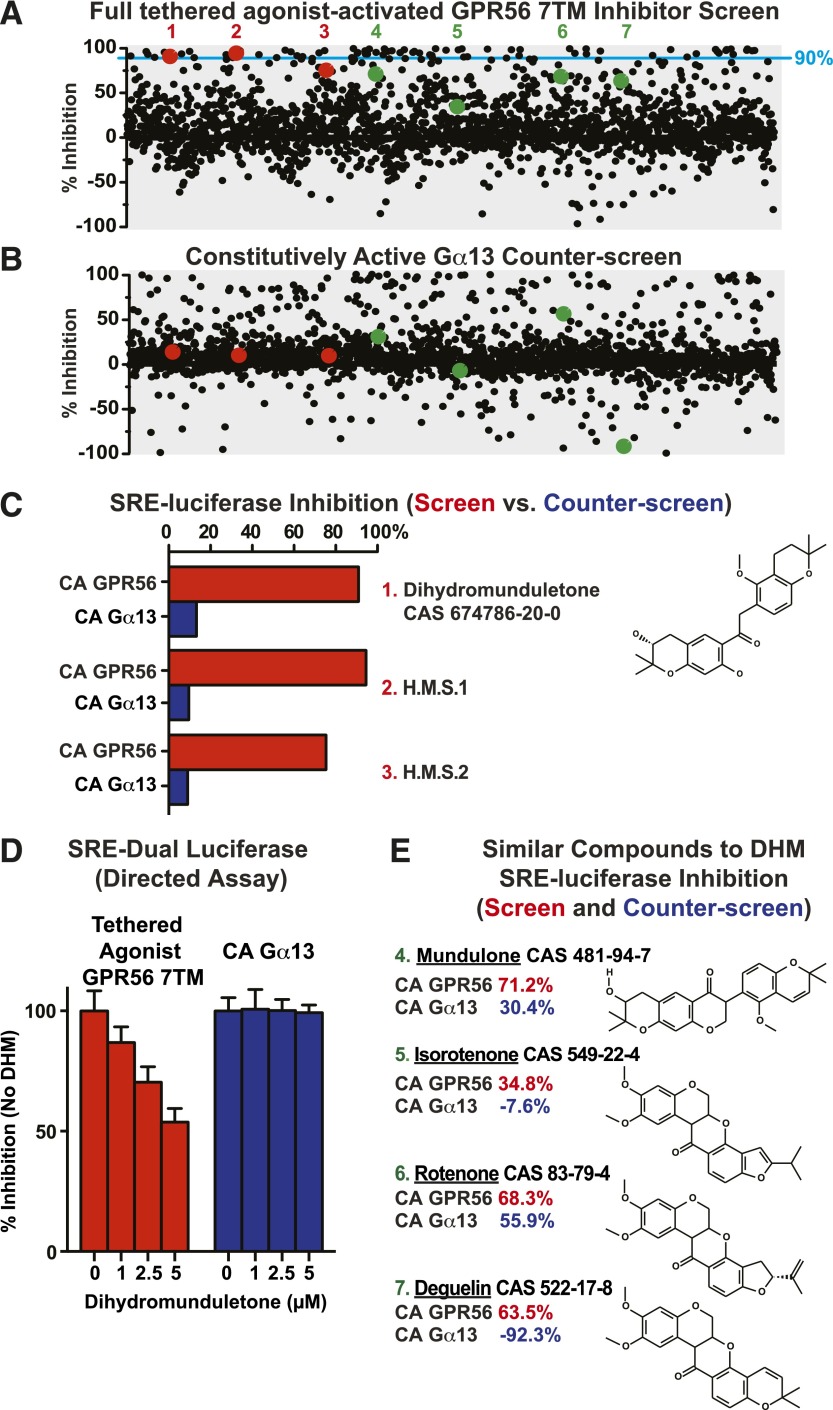
The 2000-compound Spectrum Collection chemical library (estimated ∼3–5 µM each) was then screened and counterscreened. In the screen, 66 compounds were identified that inhibited activity >90% (≥3 standard deviations above control), and 63 of these compounds also inhibited activity in the counterscreen, and were thus eliminated (Fig. 1, A and B). The three hits were DHM and two compounds we have termed H.M.S.1 and H.M.S.2. Since DHM was highly efficacious and commercially available, we decided to characterize this molecule in detail.
Fig. 1.
A cell-based high-throughput screen and counterscreen to identify candidate small-molecule GPR56 inhibitors. HEK293T cells were cotransfected with the SRE-luciferase reporter plasmid and full tethered-agonist-activated GPR56 7TM pcDNA3.1 (A) or constitutively active GNA13Q226L pcDNA3.1 (B). Transfected cells were incubated overnight in 384-well format with ∼2000 individual compounds (5 µM each) from the Spectrum Collection. SRE reporter activity was measured in response to each compound and reported as percentage of inhibition over DMSO control for the screen and counterscreen. Top hits for individual compounds are represented as red, single dots, and compounds structurally similar to the top hits are represented as green dots. The 90% inhibition threshold is shown with a light-blue line. (C) Compounds 1 (DHM), 2 (H.M.S.1), and 3 (H.M.S.2) were identified as top hits from the GPR56 7TM inhibitor screen that did not appreciably inhibit the constitutively active (CA) Gα13-Q226L activity in the counterscreen. (D) Purchased dihydromunduletone was tested for concentration-dependent GPR56 7TM or Gα13-Q226L inhibition using a distinct, directed HEK293T cell SRE-luciferase assay. Error bars are the average ± the s.d. of three experimental replicates. (E) DHM-related flavonoid compounds present in the Spectrum Collection [4 (mundulone), 5 (isorotenone), 6 (rotenone), and 7 (deguelin)] all inhibited GPR56 7TM–activated SRE luciferase to a greater extent than Gα13-Q226L–activated SRE luciferase.
The concentration dependence of DHM inhibition of GPR56 7TM or GNA13Q226L was evaluated using an independent dual SRE-luciferase assay (Fig. 1D). At concentrations of DHM up to 5 µM, GPR56 7TM activity was inhibited and Gα13-QL activity was not, confirming the results obtained in the screening assay. The library was then scanned for compounds structurally related to DHM to evaluate performance in the screen and counterscreen, even if subpar. Four isoflavonoid compounds were found that are structurally related to DHM, the isoflavone mundulone and the rotenoids isorotenone and deguelin, all of which inhibited GPR56 7TM activity more than the Gα13-QL activity (Fig. 1E), although each result constituted a single trial due to the nature of high throughput screening. The fourth compound, the rotenoid, rotenone inhibited activity in the screen only marginally better than it did in the counterscreen (n = 1) (Fig. 1E).
A potential indirect mode of DHM action would be to negatively regulate GPR56 biosynthesis or trafficking or both. To rule out this possibility, GPR56 7TM High-Five insect cells were cultured in the presence or absence of 5 µM DHM for 24 hours. The cells were collected and the relative amounts of cell-surface receptor levels were found to be identical (Supplemental Fig. 2; Supplemental Method 1). However, when rotenone, isorotenone, deguelin, or mundulone (5 µM) was included in an overnight High-Five insect cell culture, the cells appeared to die or arrest growth, and thus relative GPR56 cell surface levels were not measured. This is not a surprising outcome given that rotenoids, predominantly rotenone, are used commercially as piscicides and insecticides. Upon application to a natural water source, rotenoids efficiently enter the bloodstream through fish gills, potently inhibit complex I of the electron transport chain (ETC), and cause death (Chance et al., 1963; Li et al., 2003; Ling, 2003; Tomlin, 2009).
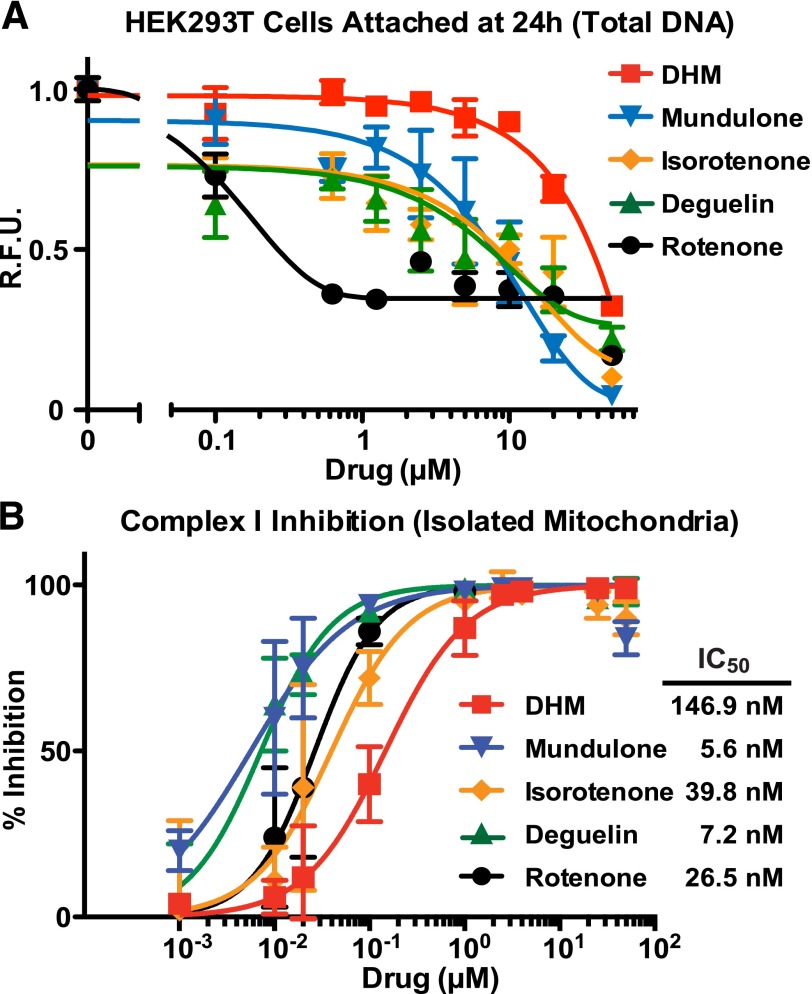
We were concerned that this known property of rotenoids to induce cell/organism death through ETC inhibition might prevent the usefulness of DHM as an adhesion GPCR inhibitor. A comparative analysis was conducted on DHM, mundulone, and rotenoid inhibition of ETC complex I versus the ability to induce death of cultured cells. HEK293 cells were treated with increasing concentrations of compounds, and 24 hours later, the cell medium and detached cells were removed. The adherent cells that remained were measured by a proxy assay in which the relative amounts of total DNA were quantified by PicoGreen assay. All of the compounds induced some degree of HEK293 cell detachment, but DHM was the least potent and could safely be used in cultured HEK293 cell assays at concentrations up to 10 µM (Fig. 2A). Similarly, we tested each compound to determine potency of ETC complex I inhibition in an isolated mitochondria assay. Again, DHM was the least-potent complex I inhibitor, which correlated with its property as the least-potent mediator of HEK293 cell detachment (Fig. 2B). It is important to note that the apparently very potent IC50 values of compound inhibition of complex I (range 5.6–146.9 nM) were generated using an assay with isolated and permeabilized mitochondria. The nature of this assay does not address questions of compound bioavailability to intact cells or organisms. In sum, DHM is the most efficacious compound at inhibiting GPR56 in the cell-based SRE-luciferase assays and the least-potent inhibitor of complex I and inducer of cultured cell death.
Fig. 2.
The rank potency of flavonoid derivative–induced HEK293 cell detachment correlates with electron transport chain complex I inhibition. (A) Adherent HEK293 cells were grown for 24 hours in the presence of the indicated concentrations of the five flavonoids (DHM, mundulone, rotenone, deguelin, isorotenone). Detached cells were removed and the relative levels of adhered cells remaining were measured by proxy assay of PicoGreen quantification of total DNA. (B) Isolated mitochondria were supplied with NADH and an electron acceptor (Q1), and the loss of NADH over 5 minutes at 340 nM was measured in the presence of the indicated amounts of each flavonoid. IC50 values were calculated from monoexponential association curves fitted using GraphPad Prism. R.F.U., relative fluorescent units. Error bars are the average ± the s.d. of three experimental replicates.
Secondary Assay Validation of DHM Inhibition of GPR56 Activity.
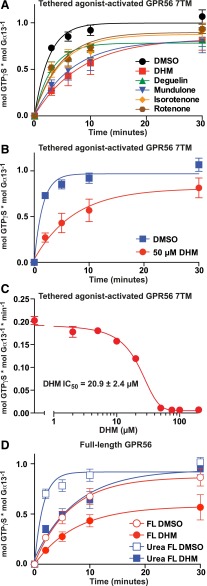
Our measurements of adhesion GPCR activation of reconstituted G proteins provide an entirely independent, cell-free assay system to evaluate small-molecule modulators obtained from cell-based screens (Stoveken et al., 2015). Insect cell membranes expressing the recombinant adhesion GPCR of interest are prepared and reconstituted with purified, recombinant Gα and Gβ1γ2. Assays are initiated by the addition of [35S]GTPγS, and the rates of aGPCR-stimulated G protein activation ([35S]GTPγS binding to Gα) are measured with or without the influence of added compounds. DHM and related compounds (deguelin, mundulone, isorotenone, and rotenone; 50 µM each) inhibited the kinetics of GPR56 7TM–stimulated G13 GTPγS binding to varying degrees (Fig. 3A). DHM was the best inhibitory compound and reduced the rate at which GPR56 7TM activated G13 >75% (from 0.18 to 0.04 minute−1) (Fig. 3B).
Fig. 3.
Secondary validation assays demonstrating that DHM and other rotenoids inhibit GPR56. (A–C) Prepared membranes containing the full tethered-agonist-activated GPR56 7TM receptor were reconstituted with purified G13 heterotrimer. The kinetics of G13 GTPγS binding were measured in the presence of 50 µM isoflavonoids (DHM, deguelin, mundulone, isorotenone, or rotenone) or DMSO vehicle control (A), or 50 µM DHM or DMSO control with 20 µM GDP present (B). Error bars are the average ± the s.d. of three experimental replicates. (C) The initial rates of full tethered-agonist-activated GPR56 7TM stimulation of G13 GTPγS binding were determined from four point (t = 0,1,2,3 min.) linear functions for each concentration of DHM. The IC50 of DHM inhibition was derived from the semilog plot of a one-phase monoexponential association function using GraphPad Prism. Error bars are the average of each linear line slope (rate) ± the s.d. of three technical replicates. (D) Membranes containing full-length (Karpus et al., 2013) GPR56 receptor were untreated or treated with ice-cold 7 M urea to mimic the proposed process of ligand-mediated receptor activation via N-terminal extracellular fragment dissociation. The kinetics of receptor-activated G13 GTPγS binding were measured in the presence of 50 µM DHM or DMSO control. Error bars were frequently smaller than the plotted symbols and are the average ± the s.d. of three experimental replicates. Full-length FL, full-length.
The potency of DHM inhibition was determined by measuring the initial linear rates (0– to 3-minute time courses) of GPR56 7TM–stimulated G13 activation in response to increasing DHM concentrations. The IC50 of DHM inhibition was ∼21 µM, which is quite potent considering that DHM may interfere with the first-order action of a tethered-peptide agonist (Fig. 3C). DHM (50 µM) also inhibited intact full-length GPR56 activation of G13 4-fold (from 0.16 to 0.04 minute−1) and full-length GPR56, in which we chemically dissociated the NTF with 7 M urea to induce the tethered-agonist-activated state (from 0.70 to 0.18 minute−1) (Fig. 3D). The high constitutive activity (0.16 minute−1) of the “intact” receptor may represent two populations: one that is truly intact and has the NTF bound to the CTF, and a second population in which the NTF has been shed or dissociated from the CTF either spontaneously during live cell culture or procedurally during preparation of native receptor membranes, which involves multiple washing and Dounce homogenization steps. The latter population would be active with a decrypted tethered-peptide agonist. These points are important when considering the potential mechanisms of DHM inhibition as a competitive antagonist to the tethered agonist or as a negative allosteric modulator.
DHM Inhibits GPR56-Related GPR114, but Not GPR110 or Class A GPCRs.
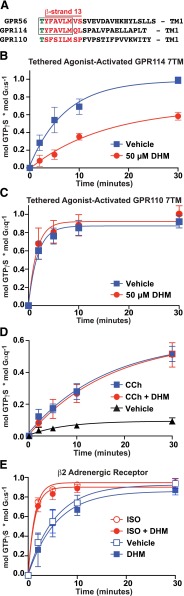
GPR114/ADGRG5 is an adhesion GPCR that promotes cyclic AMP production in cells and shares an identical amino acid sequence with GPR56 at the N-terminal tip of its tethered agonist (Gupte et al., 2012; Wilde et al., 2016) (Fig. 4A). We reconstituted the GPR114 7TM domain with purified Gαsshort and Gβ1γ2 and showed that it robustly activates Gs GTPγS binding. At a concentration of DHM that maximally inhibited GPR56 (50 µM), the rate of GPR114 7TM–stimulated Gs activity was also inhibited dramatically (Fig. 4B). GPR110/ADGRF1, a Gq-coupled group VI aGPCR, contains an amino acid sequence at the N-terminal tip of its tethered agonist that is distinct from GPR56/114 (Fig. 4A). When DHM (50 µM) was applied to the GPR110 7TM, it failed to inhibit GPR110 stimulation of Gq GTPγS binding (Fig. 4C). To determine if DHM receptor inhibition was specific to select adhesion GPCRs, we tested it with two class A GPCRs. The influence of DHM (50 µM) on receptor-stimulated G protein activation was measured for the Gq-coupled M3 muscarinic acetylcholine receptor and the Gs-coupled β2 adrenergic receptor in the absence and presence of agonists (carbachol and isoproterenol, respectively). DHM was unable to inhibit G protein activation by either of these receptors under basal or agonist-stimulated conditions (Fig. 4, D and E).
Fig. 4.
DHM exhibits specificity for group VIII adhesion GPCRs, but does not inhibit GPR110 or agonist-stimulated β2 adrenergic or M3 muscarinic acetylcholine class A GPCRs. (A) Alignment of the tethered agonist regions of group VI and group VIII adhesion GPCRs. Membranes containing the tethered-agonist-activated GPR114 7TM (B) or GPR110 7TM (C) receptors were reconstituted with purified Gs or Gq heterotrimers, respectively. The kinetics of heterotrimeric G protein GTPγS binding were measured in the presence of DHM (50 µM) or an equivalent volume of DMSO vehicle control. (D) M3 muscarinic acetylcholine receptor membranes were incubated with Gq and the indicated combinations of 50 µM carbachol (CCh), 50 µM DHM, or vehicle prior to measurement of Gq GTPγS binding kinetics. (E) β2 Adrenergic receptor membranes were incubated with Gs and the indicated combinations of 10 µM isoproterenol (ISO), 50 µM DHM, or vehicle prior to measurement of Gs GTPγS binding kinetics. Error bars are the average ± the s.d. of three experimental replicates.
DHM Inhibits Peptide Agonist–Stimulated but Not Basal Receptor Activity.
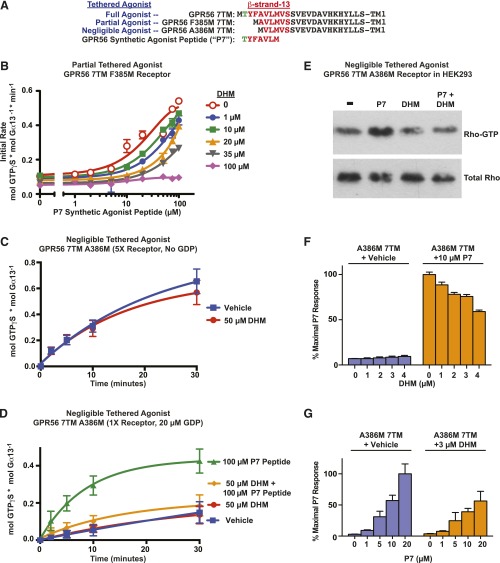
To delve into the mechanism by which DHM inhibits select aGPCRs, we tested DHM inhibition of a GPR56 7TM receptor with a partially defective tethered agonist. GPR56 F385M 7TM lacks the first two amino acids of the tethered agonist, and the third is mutated to an initiator methionine (TYFAVLM… to MAVLM…) (Fig. 5A). The activity of this receptor is diminished by ∼80%, but can be rescued by stimulation with the synthetic peptide agonist P7 (TYFAVLM) supplied in trans (Stoveken et al., 2015). To test if DHM is an aGPCR orthosteric antagonist, we attempted a Schild analysis using the F385M receptor to measure DHM/P7 competitive action/binding (Schild, 1949; Arunlakshana and Schild, 1959; Bindslev, 2008). As the peptide-agonist concentration response was performed in response to increasing concentrations of DHM, the sigmoidal curves exhibited clear rightward shifts. However, sufficient synthetic peptide agonist could not be supplied to achieve maximal efficacies, as concentrations above 100–150 µM typically decreased activity by an unknown means (Fig. 5B). These results clearly demonstrate the effectiveness of DHM as an aGPCR inhibitor and leave open the possibility that DHM is an orthosteric antagonist.
Fig. 5.
DHM inhibits tethered- and synthetic-peptide agonist–stimulated GPR56, but does not inhibit basal receptor activity, a mode of action consistent with that of a neutral antagonist. (A) Alignment of stalk regions of intact tethered agonist and compromised tethered agonist GPR56 7TM domain receptors. The seven amino acid synthetic GPR56 agonist peptide is aligned underneath. (B) Partially compromised tethered agonist GPR56 F385M 7TM receptor membranes were reconstituted with G13, and P7 peptide concentration–dependent activation of G13 GTPγS binding was measured in response to increasing concentrations of DHM. Initial rates are plotted and were determined from four point (t = 0,1,2,3 min.) linear functions. Error bars are the average of each linear line slope (rate) ± the s.d. of three technical replicates. (C) The ability to measure near-basal activity of the GPR56 A386M 7TM receptor with a negligibly active tethered agonist was enhanced by use of five times more receptor membranes and excluding GDP from the reconstitution assay. GPR56 A386M 7TM basal activation of G13 GTPγS binding was measured in the presence of 50 µM DHM or the DMSO vehicle control. Error bars are the average ± the s.d. of three experimental replicates. (D) DHM inhibits P7 synthetic-peptide agonist stimulation of the compromised tethered agonist GPR56 A386M 7TM receptor. GPR56 A386M 7TM membranes were reconstituted with G13, and receptor-stimulated G13 GTPγS binding kinetics were measured in response to 100 µM P7 agonist peptide and/or 50 µM DHM in the presence of 20 µM GDP. Error bars are the average ± the s.d. of three experimental replicates. (E) P7 agonist-peptide (10 µM) stimulation of the GPR56 A386M 7TM receptor in intact HEK293 cells activated RhoA GTPase, an effect blocked by DHM (10 µM) (n = 3); shown is a representative immunoblot. (F) P7 agonist-peptide (10 µM) stimulation of the GPR56 A386M 7TM receptor in intact HEK293 cells activated SRE-luciferase. Increasing concentrations of DHM (1–4 µM) inhibited the P7 stimulus. Error bars are the average ± the s.d. of three technical replicates. (G) Increasing P7 agonist peptide (up to 20 µM) activated the SRE-luciferase reporter in a concentration-dependent manner, and 3 µM DHM inhibited P7 activation. Error bars are the average ± the s.d. of three experimental replicates.
To test antagonism in another manner, we used the GPR56 A386M 7TM receptor, which has a more severely compromised tethered agonist that essentially renders the receptor a model of basal activity (Fig. 5A) (Stoveken et al., 2015). The sensitivity of our G protein reconstitution assay was increased by the use of five times more GPR56 A386M 7TM receptor and by not including 20 µM GDP in the assay to promote the most efficient G13:receptor precoupling. Under these conditions, GPR56 A386M 7TM provided modest G13 activation. However, DHM (50 µM) failed to inhibit this basal, tethered-agonist-independent activity (Fig. 5B). DHM did not inhibit basal signaling of GPR56 A386M 7TM when standard assay conditions were used (Fig. 5D). The synthetic P7 peptide agonist TYFAVLM (100 µM) was then used as a surrogate to the tethered agonist to markedly stimulate GPR56 A386M 7TM activation of G13. When DHM (50 µM) and P7 (100 µM) were coapplied to GPR56 A386M 7TM, DHM provided nearly complete inhibition of the synthetic peptide-agonist-induced receptor activation (Fig. 5D).
To verify GPR56 inhibition by DHM in cells, Rho GTPase activation assays were performed to test acute receptor inhibition. GPR56 activates G13 to stimulate Rho guanine nucleotide exchange factor-mediated Rho-GTP production (Iguchi et al., 2008; Luo et al., 2011; Paavola et al., 2011). HEK293T cells transfected with HA-tagged RhoA and GPR56 A386M 7TM were treated with DHM (10 µM) or vehicle. RhoA-GTP production was stimulated by application of P7 peptide agonist (10 µM) or vehicle to the cell culture medium for 5 minutes. RhoA-GTP was isolated from each treated cell group by Rhotekin pull-down assay and visualized by anti-HA immunoblotting (Fig. 5E). Treatment with DHM alone did not affect basal RhoA-GTP levels. P7 treatment alone provided an increase in the amount of RhoA-GTP produced, but P7 peptide activation was clearly blocked by DHM.
DHM inhibition of the P7 peptide response was then recapitulated using GPR56 SRE-luciferase assays. Cells transfected with GPR56 A386M 7TM were incubated with increasing concentrations of DHM. P7 peptide agonist was added, and SRE-luciferase activity was measured. DHM inhibited the P7 peptide–induced luciferase activity in a concentration-dependent manner (Fig. 5F). Cells were also treated with a fixed concentration of 3 µM DHM or vehicle and then stimulated with an increasing concentration of P7 peptide agonist. DHM treatment blunted P7 peptide activation at each concentration (Fig. 5G). In conclusion, DHM antagonizes synthetic-peptide agonist and tethered-peptide agonist–mediated aGPCR activation in isolated membranes and HEK293T cell–based assays, but it does not inhibit basal receptor signaling. This strongly indicates that the mode of DHM inhibition is to act as an antagonist that interferes with peptide agonist binding, but does not preclude the possibility that DHM may act as a negative allosteric modulator.
Discussion
We support a hypothesis where adhesion GPCR activation requires at least two separable events to occur in a prescribed order: 1) adhesion GPCR NTFs bind to anchored protein ligands, and 2) shear force generated by cell movement in relation to the anchored-ligand:NTF complex overcomes large energetic and entropic barriers that are required to dissociate the NTF from the CTF (Yona et al., 2008; Karpus et al., 2013; Langenhan et al., 2013; Scholz et al., 2015; Stoveken et al., 2015). NTF dissociation results in decryption of the adhesion GPCR tethered-peptide agonist, which rapidly and perhaps irreversibly binds its orthosteric site on the 7TM domain (CTF). This model nearly mandates that adhesion GPCR signaling deactivation follows an internalization/desensitization mechanism. Based on this model, the development of natural adhesion GPCR ligand mimetics seems to be a very unlikely means to create receptor modulators. It may be far better to develop synthetic-peptide modulators based on the tethered-peptide agonists that activate multiple adhesion GPCRs (Liebscher et al., 2014; Demberg et al., 2015; Stoveken et al., 2015; Wilde et al., 2016). Such peptides would act akin to protease activated receptors (PAR) agonist peptides that modulate protease-activated receptors (PAR GPCRs) (Vu et al., 1991; Scarborough et al., 1992). Alternatively, and perhaps even more effectively, small-molecule modulators could be identified that would bypass the two event-mediated activation processes and regulate adhesion GPCR activities directly.
Using a cell-based high-throughput screening assay to uncover small-molecule inhibitors of tethered-agonist-activated GPR56, we identified DHM as a novel adhesion GPCR antagonist. In direct receptor-stimulated G protein–activation assays, DHM inhibited two of three adhesion GPCRs tested, but not two class A GPCRs, indicating that DHM has selective action for a subset of adhesion GPCRs. DHM inhibition of GPR56 was verified using a cell-based RhoA GTPase effector enzyme assay and by an independent, dual luciferase-gene-reporter assay. Mechanistically, DHM did not inhibit basal GPR56 activity, but did inhibit GPR56 activity stimulated by its tethered-peptide agonist or by a synthetic-peptide agonist. These data establish that the mode of DHM inhibition may be as a neutral antagonist that competes with the tethered-peptide agonist for binding to its orthosteric site. If DHM indeed acts as an orthosteric antagonist, this provides a solidifying pharmacological argument supporting the emerging hypothesis that adhesion GPCRs are activated by tethered-peptide agonists (Liebscher et al., 2014; Stoveken et al., 2015).
Four additional compounds in the Spectrum Collection that are structurally related to DHM were assayed by SRE-luciferase and GPR56 reconstitution assays. Mundulone, rotenone, isorotenone, and deguelin all inhibited GPR56-mediated G13 activation, but with lower efficacies than DHM. Rotenone has a well established cellular target; it directly inhibits complex I of the mitochondrial ETC (Chance et al., 1963). Complex I inhibition explains the toxic effects of rotenone as an insecticide and potent piscicide (Chance et al., 1963; http://www.doc.govt.nz/documents/science-and-technical/SFC211.pdf). Rotenone is less toxic to humans but can cause death if a large quantity is ingested (Wood et al., 2005). We directly compared the ability of all five compounds to inhibit ETC complex I versus toxicity toward cultured HEK293 cells using a proxy assay that measured compound ability to induce cell detachment over 24 hours (Fig. 2). DHM was clearly the least-potent inhibitor of complex I, which correlated with it being the poorest mediator of cell detachment. Importantly, DHM was the most efficacious inhibitor of GPR56 and GPR114. For practical considerations, we recommend use of DHM as a probe compound in cell-based adhesion GPCR studies at concentrations <10 µM to avoid cytotoxicity (Fig. 2A).
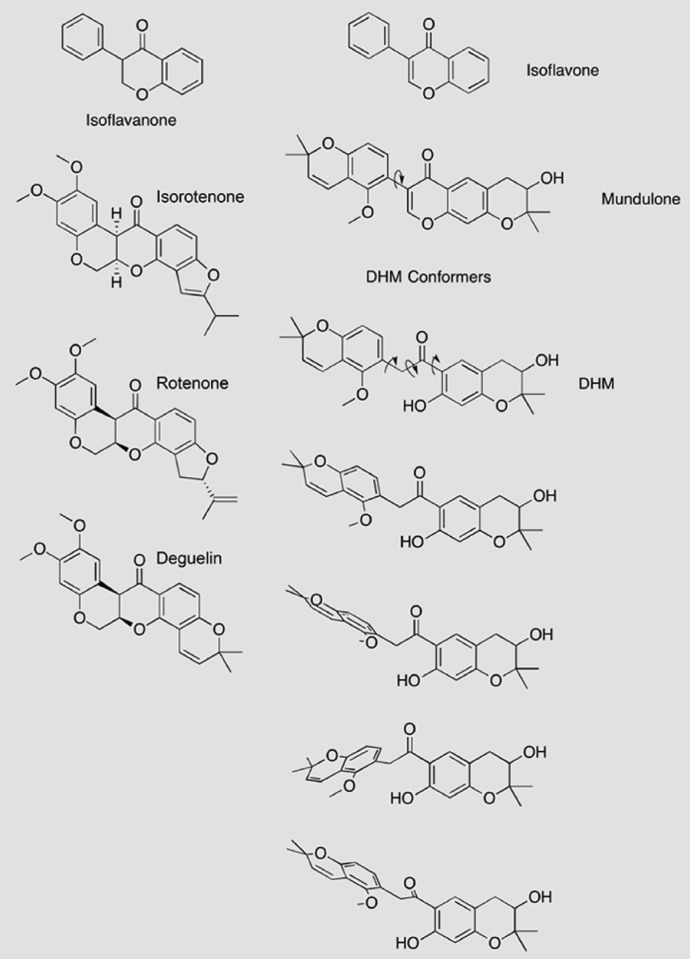
Our future and ongoing optimization of DHM will use a dual structure-activity relationship approach to seek derivatives that widen the gap between higher-potency adhesion GPCR inhibition and lower-potency complex I inhibition (and associated cytotoxicity). A comparison of the chemical structures of the five isoflavonoid compounds provides insight into the key structural features to focus on and may explain the different potencies of adhesion GPCR inhibition (Fig. 6). Rotenone, isorotenone, and deguelin consist of five joined hydrocarbon rings that share an isoflavanone-like core. The contiguous nature of these rings imparts a rigid, planar structural orientation. DHM and mundulone each possess a moiety that disrupts the contiguousness of the five hydrocarbon rings to confer increased flexibility. DHM and mundulone differ in that the former has a 2-phenylacetophenone core, and the latter has an isoflavone core. The 2-phenylacetophenone core in DHM would confer additional flexibility over mundulone, and thus, DHM has the most flexibility of the five studied isoflavonoids. Examples of potential DHM conformers are shown in Fig. 6. Additionally, the phenolic hydroxyl of DHM, which is blocked in mundulone, could be important for adhesion GPCR target interaction.
Fig. 6.
Structural features of DHM and related compounds. The rotenoids (isorotenone, rotenone, and deguelin) are relatively rigid, planar compounds with isoflavanone-like cores. In contrast, mundulone, a true isoflavone, and DHM both lack the isoflavanone core, which allows for increased flexibility and the predicted ability to adopt multiple conformations. Mundulone may rotate around one central carbon-carbon bound, indicated by the arrow. The core of DHM is a 2-phenylacetophenone that would permit rotation about the three indicated carbon-carbon bonds, strongly suggesting that DHM is the most flexible compound of the five compounds studied. Representative conformers of DHM are shown to illustrate the predicted flexibility of the compound [conformational analysis was done with the Merck Molecular Force Field (MMFF) force field, iSpartan (www.wavefun.com); structures were drawn with ChemDraw Pro (Perkin Elmer, Waltham, MA)].
The mode of DHM inhibitory action remains a partially open question, although our data are consistent with those of a neutral antagonist that competes with a tethered-peptide agonist for binding to its orthosteric site. Still, the data do not eliminate the possibility that DHM may serve as a negative allosteric modulator. A Schild analysis was attempted to directly measure DHM competitive action to the GPR56 synthetic peptide agonist P7 (Fig. 4B) (Schild, 1949; Arunlakshana and Schild, 1959; Bindslev, 2008). When the P7 peptide agonist concentration response was performed with increasing DHM concentrations, the response curves exhibited clear rightward shifts but could not be fit to full sigmoidal functions because maximum efficacy of P7-mediated activation (rate of G protein GTPγS binding) was unachievable. Activation of GPR56 with P7 peptide agonist concentrations >150 µM results in diminishing efficacy. The reason for this is unknown, although we speculate that use of very high adhesion GPCR synthetic peptide agonist concentrations results in nonproductive aggregation of an essential assay component. Since sufficient peptide agonist cannot be used to overcome DHM antagonism, the observed curve flattening might be construed to preclude DHM action as an orthosteric antagonist. However, it could also indicate that DHM has greater affinity for the orthosteric site than the peptide agonist (Arunlakshana and Schild, 1959). Our future work in this area will include development of labeled synthetic peptide agonist or labeled DHM probes that will be used to perform direct adhesion GPCR competition binding analyses.
An important prospective use of a labeled DHM probe will be to determine the active fraction of adhesion GPCR present at the cell surface in response to perturbations intended to alter receptor activation (e.g., peptide agonists, small-molecule modulators, or natural ligands). Our previous work showed that the P7 synthetic-peptide agonist failed to activate an intact (full-length) GPR56 receptor with its NTF bound to the CTF (Stoveken et al., 2015). We proposed that the synthetic-peptide agonist was sterically blocked by the NTF from accessing its CTF orthosteric binding site. If the same holds true for a DHM probe, then quantitative measurement of its binding to cell surfaces would provide a measurement of the population of active adhesion GPCR CTF that has had its NTF dissociated. DHM inhibits the GPR56 7TM and urea-activated full-length GPR56 (NTF dissociated), which are both tethered-agonist-activated receptors. However, DHM also provided very good inhibition of intact, full-length GPR56 that did not have its NTF dissociated via the urea-treatment activation mimetic (Fig. 3D). Intact or holoreceptor GPR56 has substantial basal activity, and we speculate that, in our assays, “intact” GPR56 is actually a mixed population of NTF bound to CTF (a low-activity-state holoreceptor) and free CTF (tethered-agonist-activated), which was generated by spontaneous NTF shedding during cell culture and/or by the mechanical treatments used during receptor membrane preparation. If a labeled DHM probe can only bind with high affinity to the free GPR56 CTF, it could be used as a means to determine whether intact GPR56 is a mixed population of free CTF and holoreceptor or mostly holoreceptor. If the receptor proves to be mainly intact, then this would corroborate recent findings that intact GPR56 has high basal signaling activity (Kishore et al., 2016).
Here, we have identified and validated DHM as a new inhibitor of select adhesion GPCRs (GPR56/GPR114). Work with additional receptors is underway to determine a more complete profile of adhesion GPCR substrate specificity. To our knowledge, DHM is the first reported small-molecule adhesion GPCR antagonist, and its development as a probe compound will meet a critical need for a larger pharmacological toolbox to study adhesion GPCRs. DHM also represents a lead compound that may be developed for increased selectivity and potency toward specific adhesion GPCRs to treat adhesion GPCR–directed disease.
Supplementary Material
Abbreviations
- aGPCR
adhesion G protein–coupled receptor
- CTF
carboxy-terminal fragment
- DHM
dihydromunduletone
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethylsulfoxide
- ETC
electron transport chain
- FBS
fetal bovine serum
- GPCR
G protein–coupled receptor
- GTPγS
5′-3-O-(thio)triphosphate
- HA
hemagglutinin
- HEK293
human embryonic kidney 293
- NTF
amino terminal fragment
- PAR
protease activated receptors
- [35S]GTPγS
5′-O-(3-[35S]thio)triphosphate
- SRE
serum response element
- 7TM
seven-transmembrane domain
Authorship Contributions
Participated in research design: Stoveken, Wojtovich, Smrcka, Tall.
Conducted experiments: Stoveken, Bahr, Wojtovich, Tall.
Performed data analysis: Stoveken, Wojtovich, Anders, Tall.
Wrote or contributed to the writing of the manuscript: Stoveken, Anders, Tall.
Footnotes
This work was supported by a PhRMA Foundation predoctoral fellowship in pharmacology to H.M.S. The production of recombinant G proteins used for this work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM088242 to G.G.T.].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Araç D, Boucard AA, Bolliger MF, Nguyen J, Soltis SM, Südhof TC, Brunger AT. (2012) A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J 31:1364–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. (1959) Some quantitative uses of drug antagonists. Br Pharmacol Chemother 14:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindslev N(2008) The Schild against other theories, in Drug-Acceptor Interactions pp 283–298, Coaction Publishing, Stockholm. [Google Scholar]
- Bolliger MF, Martinelli DC, Südhof TC. (2011) The cell-adhesion G protein-coupled receptor BAI3 is a high-affinity receptor for C1q-like proteins. Proc Natl Acad Sci USA 108:2534–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Ko J, Südhof TC. (2012) High affinity neurexin binding to cell adhesion G-protein-coupled receptor CIRL1/latrophilin-1 produces an intercellular adhesion complex. J Biol Chem 287:9399–9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows BF, Finch N, Ollis WD, Sutherland IO.(1959) Mundulone. Proceedings of the Chemical Society: 150-152. [Google Scholar]
- Chan P, Gabay M, Wright FA, Kan W, Oner SS, Lanier SM, Smrcka AV, Blumer JB, Tall GG. (2011) Purification of heterotrimeric G protein alpha subunits by GST-Ric-8 association: primary characterization of purified G alpha(olf). J Biol Chem 286:2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Williams GR, Hollunger G. (1963) Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J Biol Chem 238:418–431. [PubMed] [Google Scholar]
- Chiang NY, Hsiao CC, Huang YS, Chen HY, Hsieh IJ, Chang GW, Lin HH. (2011) Disease-associated GPR56 mutations cause bilateral frontoparietal polymicrogyria via multiple mechanisms. J Biol Chem 286:14215–14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demberg LM, Rothemund S, Schöneberg T, Liebscher I. (2015) Identification of the tethered peptide agonist of the adhesion G protein-coupled receptor GPR64/ADGRG2. Biochem Biophys Res Commun 464:743–747. [DOI] [PubMed] [Google Scholar]
- Dyer BW, Ferrer FA, Klinedinst DK, Rodriguez R. (2000) A noncommercial dual luciferase enzyme assay system for reporter gene analysis. Anal Biochem 282:158–161. [DOI] [PubMed] [Google Scholar]
- Evelyn CR, Wade SM, Wang Q, Wu M, Iñiguez-Lluhí JA, Merajver SD, Neubig RR. (2007) CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther 6:2249–2260. [DOI] [PubMed] [Google Scholar]
- Gupte J, Swaminath G, Danao J, Tian H, Li Y, Wu X. (2012) Signaling property study of adhesion G-protein-coupled receptors. FEBS Lett 586:1214–1219. [DOI] [PubMed] [Google Scholar]
- Hamann J, Aust G, Araç D, Engel FB, Formstone C, Fredriksson R, Hall RA, Harty BL, Kirchhoff C, Knapp B, et al. (2015) International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol Rev 67:338–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann J, Vogel B, van Schijndel GM, van Lier RA. (1996) The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J Exp Med 184:1185–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi T, Sakata K, Yoshizaki K, Tago K, Mizuno N, Itoh H. (2008) Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J Biol Chem 283:14469–14478. [DOI] [PubMed] [Google Scholar]
- Karpus ON, Veninga H, Hoek RM, Flierman D, van Buul JD, Vandenakker CC, vanBavel E, Medof ME, van Lier RA, Reedquist KA, et al. (2013) Shear stress-dependent downregulation of the adhesion-G protein-coupled receptor CD97 on circulating leukocytes upon contact with its ligand CD55. J Immunol 190:3740–3748. [DOI] [PubMed] [Google Scholar]
- Kim JE, Han JM, Park CR, Shin KJ, Ahn C, Seong JY, Hwang JI. (2010) Splicing variants of the orphan G-protein-coupled receptor GPR56 regulate the activity of transcription factors associated with tumorigenesis. J Cancer Res Clin Oncol 136:47–53. [DOI] [PubMed] [Google Scholar]
- Kishore A, Purcell RH, Nassiri-Toosi Z, Hall RA. (2016) Stalk-dependent and Stalk-independent Signaling by the Adhesion G Protein-coupled Receptors GPR56 (ADGRG1) and BAI1 (ADGRB1). J Biol Chem 291:3385–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa T, Gilman AG. (1995) Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. Characterization of alpha 12 and inhibition of adenylyl cyclase by alpha z. J Biol Chem 270:1734–1741. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. [DOI] [PubMed] [Google Scholar]
- Langenhan T, Aust G, Hamann J. (2013) Sticky signaling--adhesion class G protein-coupled receptors take the stage. Sci Signal 6:re3. [DOI] [PubMed] [Google Scholar]
- Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278:8516–8525. [DOI] [PubMed] [Google Scholar]
- Liebscher I, Schön J, Petersen SC, Fischer L, Auerbach N, Demberg LM, Mogha A, Cöster M, Simon KU, Rothemund S, et al. (2014) A tethered agonist within the ectodomain activates the adhesion G protein-coupled receptors GPR126 and GPR133. Cell Reports 9:2018–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Chang GW, Davies JQ, Stacey M, Harris J, Gordon S. (2004) Autocatalytic cleavage of the EMR2 receptor occurs at a conserved G protein-coupled receptor proteolytic site motif. J Biol Chem 279:31823–31832. [DOI] [PubMed] [Google Scholar]
- Ling N. (2003) Rotenone—a review of its toxicity and use for fisheries management. Science for Conservation 211:40. [Google Scholar]
- Luo R, Jeong SJ, Jin Z, Strokes N, Li S, Piao X. (2011) G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci USA 108:12925–12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Burwell LS, Brookes PS. (2007) Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol 42:812–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollis WD and Sutherland IO(1961) Isoprenoid units in natural phenolic compounds. Symposium Publication Division, Pergamon Press, Oxford, New York [Google Scholar]
- Oner SS, Blumer JB, Lanier SM. (2013) Group II activators of G-protein signaling: monitoring the interaction of Gα with the G-protein regulatory motif in the intact cell. Methods Enzymol 522:153–167. [DOI] [PubMed] [Google Scholar]
- O’Sullivan ML, de Wit J, Savas JN, Comoletti D, Otto-Hitt S, Yates JR, 3rd, Ghosh A. (2012) FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron 73:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola KJ, Hall RA. (2012) Adhesion G protein-coupled receptors: signaling, pharmacology, and mechanisms of activation. Mol Pharmacol 82:777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola KJ, Sidik H, Zuchero JB, Eckart M, Talbot WS. (2014) Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci Signal 7:ra76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola KJ, Stephenson JR, Ritter SL, Alter SP, Hall RA. (2011) The N terminus of the adhesion G protein-coupled receptor GPR56 controls receptor signaling activity. J Biol Chem 286:28914–28921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SC, Luo R, Liebscher I, Giera S, Jeong SJ, Mogha A, Ghidinelli M, Feltri ML, Schöneberg T, Piao X, et al. (2015) The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron 85:755–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, Dobyns WB, Qasrawi B, Winter RM, Innes AM, et al. (2004) G protein-coupled receptor-dependent development of human frontal cortex. Science 303:2033–2036. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kaneda K, Suekane A, Ichihara E, Nakahata S, Yamakawa N, Nagai K, Mizuno N, Kogawa K, Miura I, et al. (2013) Maintenance of the hematopoietic stem cell pool in bone marrow niches by EVI1-regulated GPR56. Leukemia 27:1637–1649. [DOI] [PubMed] [Google Scholar]
- Scarborough RM, Naughton MA, Teng W, Hung DT, Rose J, Vu TK, Wheaton VI, Turck CW, Coughlin SR. (1992) Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J Biol Chem 267:13146–13149. [PubMed] [Google Scholar]
- Schaffner W, Weissmann C. (1973) A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem 56:502–514. [DOI] [PubMed] [Google Scholar]
- Schild HO. (1949) pAx and competitive drug antagonism. Br Pharmacol Chemother 4:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz N, Gehring J, Guan C, Ljaschenko D, Fischer R, Lakshmanan V, Kittel RJ, Langenhan T. (2015) The adhesion GPCR latrophilin/CIRL shapes mechanosensation. Cell Reports 11:866–874. [DOI] [PubMed] [Google Scholar]
- Shashidhar S, Lorente G, Nagavarapu U, Nelson A, Kuo J, Cummins J, Nikolich K, Urfer R, Foehr ED. (2005) GPR56 is a GPCR that is overexpressed in gliomas and functions in tumor cell adhesion. Oncogene 24:1673–1682. [DOI] [PubMed] [Google Scholar]
- Silva JP, Lelianova VG, Ermolyuk YS, Vysokov N, Hitchen PG, Berninghausen O, Rahman MA, Zangrandi A, Fidalgo S, Tonevitsky AG, et al. (2011) Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc Natl Acad Sci USA 108:12113–12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern C, Cook JM, Neetoo-Isseljee Z, Taylor DL, Kettleborough CA, Merritt A, Bassoni DL, Raab WJ, Quinn E, Wehrman TS, et al. (2013) Screening β-arrestin recruitment for the identification of natural ligands for orphan G-protein-coupled receptors. J Biomol Screen 18:599–609. [DOI] [PubMed] [Google Scholar]
- Stacey M, Chang GW, Davies JQ, Kwakkenbos MJ, Sanderson RD, Hamann J, Gordon S, Lin HH. (2003) The epidermal growth factor-like domains of the human EMR2 receptor mediate cell attachment through chondroitin sulfate glycosaminoglycans. Blood 102:2916–2924. [DOI] [PubMed] [Google Scholar]
- Stoveken HM, Hajduczok AG, Xu L, Tall GG. (2015) Adhesion G protein-coupled receptors are activated by exposure of a cryptic tethered agonist. Proc Natl Acad Sci USA 112:6194–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Khvochtev M, Südhof TC. (1999) Neurexins are functional alpha-latrotoxin receptors. Neuron 22:489–496. [DOI] [PubMed] [Google Scholar]
- Tomlin C (2009) The Pesticide Manual: A World Compendium, 15th ed, (Tomlin CDS ed) British Crop Production Council, Alton, UK. [Google Scholar]
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. (1991) Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64:1057–1068. [DOI] [PubMed] [Google Scholar]
- Wang C, Tao W, Wang Y, Bikow J, Lu B, Keating A, Verma S, Parker TG, Han R, Wen XY. (2010) Rosuvastatin, identified from a zebrafish chemical genetic screen for antiangiogenic compounds, suppresses the growth of prostate cancer. Eur Urol 58:418–426. [DOI] [PubMed] [Google Scholar]
- Wilde C, Fischer L, Lede V, Kirchberger J, Rothemund S, Schöneberg T, Liebscher I. (2016) The constitutive activity of the adhesion GPCR GPR114/ADGRG5 is mediated by its tethered agonist. FASEB J 30:666–673. [DOI] [PubMed] [Google Scholar]
- Wojtovich AP, Sherman TA, Nadtochiy SM, Urciuoli WR, Brookes PS, Nehrke K. (2011) SLO-2 is cytoprotective and contributes to mitochondrial potassium transport. PLoS One 6:e28287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Alsahaf H, Streete P, Dargan PI, Jones AL. (2005) Fatality after deliberate ingestion of the pesticide rotenone: a case report. Crit Care 9:R280–R284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MP, Doyle JR, Barry B, Beauvais A, Rozkalne A, Piao X, Lawlor MW, Kopin AS, Walsh CA, Gussoni E. (2013) G-protein coupled receptor 56 promotes myoblast fusion through serum response factor- and nuclear factor of activated T-cell-mediated signalling but is not essential for muscle development in vivo. FEBS J 280:6097–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Begum S, Barry M, Crowley D, Yang L, Bronson RT, Hynes RO. (2010) GPR56 plays varying roles in endogenous cancer progression. Clin Exp Metastasis 27:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Begum S, Hearn JD, Hynes RO. (2006) GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc Natl Acad Sci USA 103:9023–9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Chen G, Mohanty S, Scott G, Fazal F, Rahman A, Begum S, Hynes RO, Xu L. (2011) GPR56 Regulates VEGF production and angiogenesis during melanoma progression. Cancer Res 71:5558–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Lin HH, Dri P, Davies JQ, Hayhoe RP, Lewis SM, Heinsbroek SE, Brown KA, Perretti M, Hamann J, et al. (2008) Ligation of the adhesion-GPCR EMR2 regulates human neutrophil function. FASEB J 22:741–751. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. (1999) A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen 4:67–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.