Abstract
Glycine receptors (GlyR) are inhibitory Cys-loop ion channels that contribute to the control of excitability along the central nervous system (CNS). GlyR are found in the spinal cord and brain stem, and more recently they were reported in higher regions of the CNS such as the hippocampus and nucleus accumbens. GlyR are involved in motor coordination, respiratory rhythms, pain transmission, and sensory processing, and they are targets for relevant physiologic and pharmacologic modulators. Several studies with protein crystallography and cryoelectron microscopy have shed light on the residues and mechanisms associated with the activation, blockade, and regulation of pentameric Cys-loop ion channels at the atomic level. Initial studies conducted on the extracellular domain of acetylcholine receptors, ion channels from prokaryote homologs—Erwinia chrysanthemi ligand-gated ion channel (ELIC), Gloeobacter violaceus ligand-gated ion channel (GLIC)—and crystallized eukaryotic receptors made it possible to define the overall structure and topology of the Cys-loop receptors. For example, the determination of pentameric GlyR structures bound to glycine and strychnine have contributed to visualizing the structural changes implicated in the transition between the open and closed states of the Cys-loop receptors. In this review, we summarize how the new information obtained in functional, mutagenesis, and structural studies have contributed to a better understanding of the function and regulation of GlyR.
Introduction
Inhibitory glycine receptors (GlyR) are anion-selective ligand-gated ion channels (LGICs), which together with GABAA receptors (GABAAR), the nicotinic acetylcholine receptors (nAChR) and serotonin type 3 receptors (5HT-3) form the eukaryotic Cys-loop family. In mature neurons, the activation of GlyR leads to a fast increase in the passive diffusion of anions, mainly chloride ions, into the neurons, resulting in membrane hyperpolarization and reduction in neuronal excitability (Lester et al., 2004; Miller and Smart, 2010; Zeilhofer et al., 2012). It is well accepted that inhibitory GlyR function is critical for the control of several physiologic processes, namely muscle tone, motor coordination, sensory processing, respiratory rhythms, and pain (Lynch, 2009; Callister and Graham, 2010; Zeilhofer et al., 2012; Alvarez et al., 2013). In addition, the critical role of GlyR inhibition in normal physiology is further highlighted by genetic studies in humans that have linked mutations in GlyR genes with hyperekplexia (Harvey et al., 2008).
Functional chloride-permeable GlyR ion channels are formed by 5α subunits (homomeric) or by the mixture of α and β subunits (heteropentameric), which are molecular complexes with either a 2α–3β or 3α–2β stoichiometry (Grudzinska et al., 2005; Durisic et al., 2012). At present, four α (α1–4) subunits and one β subunit have been described that have a regionally and temporally controlled expression during development and maturation of the central nervous system (CNS).
The α1 subunit is widely expressed in the adult CNS, but low levels have been detected in the spinal cord during early development and in newborn animals with an increased expression toward postnatal day 15 (Aguayo et al., 2004; Lynch, 2009). In contrast, α2 subunits show a high level of expression during embryonic development in the spinal cord, brainstem, and some supraspinal regions such as the cortex, hippocampus, and thalamus (Kuhse et al., 1991; Malosio et al., 1991). The predominant α2 expression declines extensively during advanced stages of brain development. However, it has been suggested that α2 expression is maintained in higher brain regions such as the hippocampus and cortex (Kuhse et al., 1991; Chau et al., 2010; Avila et al., 2013; Blednov et al., 2015). The α3 subunits are expressed after the third postnatal week in the hippocampus, retina, and lamina II of the spinal dorsal horn (Malosio et al., 1991; Aguayo et al., 2004; Harvey et al., 2004). Finally, the α4 subunit is found as a pseudogene in humans, but it is expressed in the spinal cord, sympathetic nervous system, kidney, liver, spermatozoids, and retina of other species (Harvey et al., 2000; Simon et al., 2004).
The α subunits, but not the β subunit, can form functional homopentameric ion channels. When present in heteropentameric α/β GlyR, the β subunit displays structural and regulatory functions, including GlyR clustering in synaptic locations through interaction of the intracellular loop domain (IL) of β with the scaffolding protein gephyrin, and control of pharmacologic responses to agonist and several other modulators (van Zundert et al., 2005; Calamai et al., 2009; Dutertre et al., 2012). In the present review, we define the term modulator to refer to a ligand (small molecule or protein) that binds to a site that is distinct to the agonist site, but allosterically affects the function of the protein in question.
The current state of GlyR pharmacology has been reviewed previously elsewhere (Lynch, 2009; Yevenes and Zeilhofer, 2011; Zeilhofer et al., 2012; Burgos et al., 2015b) and is composed of a limited collection of agonists, antagonists, and a growing number of modulators. Until recently, most of our knowledge about individual residues and mechanisms involved in GlyR function and pharmacologic modulations has been derived from studies using mutated GlyR in combination with electrophysiologic studies and molecular modeling with the nAChR or prokaryotic Cys-loop receptors as templates (Absalom et al., 2003; Bertaccini et al., 2007; Speranskiy et al., 2007; Cheng et al., 2008; Harris et al., 2008; Vijayan et al., 2012; Olsen et al., 2014; Yu et al., 2014). In recent years, however, several reports have shed important new light on the residues and mechanisms at the atomic level associated with the activation, blockade, and modulation of pentameric Cys-loop ion channels. Here, we intend to summarize the most recent advances in GlyR structure with a special focus on the molecular sites underlying its functional regulation by several modulators of physiologic and clinical relevance.
General Structure of Cys-Loop Receptors
Complete atomic structures of Cys-loop receptors, including GlyR, have been very challenging to achieve so far. Technically, there is still a need for optimized expression systems that can produce large concentrations of highly purified proteins with mature posttranslational modifications and high solubility (Carpenter et al., 2008). The principal structures available to date are shown in Table 1. Data from acetylcholine-binding protein (AChBP) from the snail Lymnaea stagnalis at 2.7 Å resolution provided the initial experimentally determined data for the agonist binding site on a Cys-loop receptor (Brejc et al., 2001). Later, the nAChR structure obtained by cryoelectron microscopy at 4 Å resolution provided novel information on the atomic structure of one of the most studied LGIC, describing both a detailed structure of the agonist binding-site and proposing some sequential events that might lead to channel opening (Unwin, 2005). More recently, the high-resolution structures of the prokaryotic ligand-gated ion channel (LGIC) activated by GABA from Erwinia chrysanthemi (ELIC) (Hilf and Dutzler, 2008; Zimmermann and Dutzler, 2011) and the proton-gated ion channel from Gloeobacter violaceus (GLIC) were published (Prevost et al., 2012; Sauguet et al., 2013b). In comparison with GlyR, the analysis showed some differences likely related to the functionality of the proteins. First, they presented a lower sequence identity of 25% (α1/ELIC: 24%; α1/GLIC: 23%). Second, unlike GlyR, they had cationic selectivity and a poor extent of pharmacologic modulations.
TABLE 1.
Experimentally resolved structures of Cys-loop ion channels
Summary of the principal structures of prokaryote and eukaryote receptors obtained by X-ray diffraction (XRD), electron cryomicroscopy (ECM), or solution nuclear magnetic resonance (NMR). Additionally, the states of the ion channel in each structure are indicated—open (O), closed (C), locally closed (LC), resting (R) states, or not applicable (NA)—with corresponding codes of the Protein DataBank (http://www.rcsb.org/pdb/home/home.do).
| Receptor | Structure | State | Method | Reference | Release | |
|---|---|---|---|---|---|---|
| ELIC | 2VL0: 3.3 Å | C | XRD | Hilf and Dutzler, 2008 | 2008 | |
| 3RQU: 3.09 Å | C | Pan et al., 2012b | 2012 | |||
| ELIC F246A mutant | 2YKS: 3.30 Å | C | XRD | Zimmermann and Dutzler, 2011 | 2011 | |
| ELIC F16ʹS mutant | 4TWH: 3.60 Å | C | XRD | Ulens et al., 2014 | 2014 | |
| ELIC L9ʹ F16ʹ double mutant | 3UQ5: 4.2 Å | O | XRD | Gonzalez-Gutierrez et al., 2012 | 2012 | |
| 3UQ7: 3.8 Å | ||||||
| GLIC | 3EAM: 2.90 Å | O | XRD | Bocquet et al., 2009 | 2008 | |
| 3EHZ: 3.10 Å | O | Hilf and Dutzler, 2009 | 2008 | |||
| 4HFI: 2.4 Å | O | Sauguet et al., 2013b | 2013 | |||
| 4NPQ: 4.35 Å | R | Sauguet et al., 2014 | 2013 | |||
| GLIC H11ʹF mutant | 3TLT: 3.3 Å | LC | XRD | Prevost et al., 2012 | 2012 | |
| GLIC E19ʹP mutant | 3TLS: 3.2 Å | LC | XRD | Prevost et al., 2012 | 2012 | |
| GLIC F14ʹA mutant | 4HFB: 2.75 Å | O | XRD | Sauguet et al., 2013a | 2013 | |
| GLIC I9ʹA T25ʹA double mutant | 4LML: 3.8 Å (lig-bounded) | C | XRD | Gonzalez-Gutierrez et al., 2013 | 2013 | |
| GluCl | 3RHW: 3.26 Å | O | XRD | Hibbs and Gouaux, 2011 | 2011 | |
| 4TNV: 3.60 Å | C | Althoff et al., 2014 | 2014 | |||
| nAChR 2αβδγ | 2BG9: 4 Å | C | ECM | Unwin, 2005 | 2005 | |
| 4AQ9: 6.2 Å | O | Unwin and Fujiyoshi, 2012 | 2012 | |||
| 4AQ5: 6.2 Å | C | Unwin and Fujiyoshi, 2012 | 2012 | |||
| nAChR α7 (TMs) | 2MAW | NA | NMR | Mowrey et al., 2013b | 2013 | |
| 5-HT3A | 4PIR: 3.5 Å | O | XRD | Hassaine et al., 2014 | 2014 | |
| GABAA β3 | 4COF: 2.97 Å | C | XRD | Miller and Aricescu, 2014 | 2014 | |
| GlyR α1 | 3JAE: 3.9 Å | O | ECM | Du et al., 2015 | 2015 | |
| 3JAD: 3.9 Å | C | |||||
| GlyR α3 | 5CFB: 3.04 Å | C | XRD | Huang et al., 2015 | 2015 | |
| GlyR α1 (TMs) | 2M6B | NA | NMR | Mowrey et al., 2013a | 2013 | |
| 2M6I | O | |||||
| GlyR α1 (TM2–TM3) | 1VRY | NA | NMR | Ma et al., 2005 | 2005 | |
| Chimera GlyR-GLIC | 4X5T: 3.5 Å | C | XRD | Moraga-Cid et al., 2015 | 2015 | |
| Chimera ELIC-GLIC | 4YEU: 4.60 Å | R | XRD | Unpublished | 2015 |
A more significant contribution for the understanding of GlyR structural characteristics was recently achieved by studying the crystallographic structure of the glutamate-gated chloride channels (GluCl) from Caenorhabditis elegans, a eukaryotic LGIC, which allowed the homology modeling of GlyR because it presented a higher percentage of identity (α1/GluCl: 44%) (Hibbs and Gouaux, 2011). A key advancement in the resolution of GlyR structure was obtained from studies of homopentamers formed by α1 or α3 subunits and the chimeric receptor GLIC/α1 GlyR called Lily (Du et al., 2015; Huang et al., 2015; Moraga-Cid et al., 2015). All these recent studies have contributed to understand the nature of conformational changes that might occur during open-closed states, desensitization, and pharmacologic modulation.
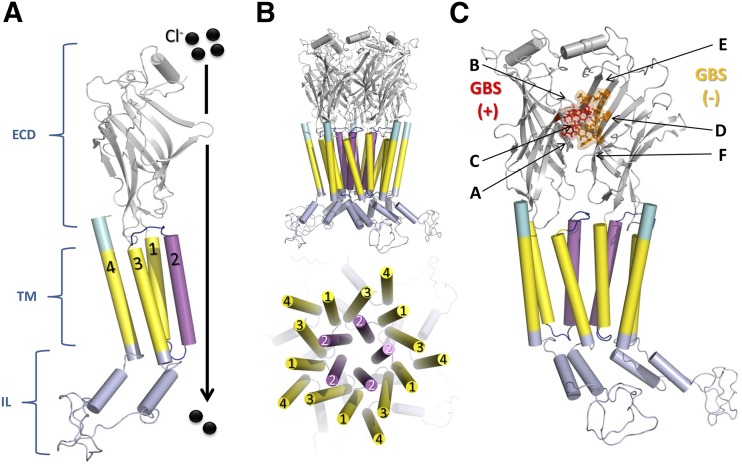
The resolution of GlyR structures confirmed the classic topology of the Cys-loop family, which includes a large extracellular N-terminal domain (ECD), four transmembrane domains (TM1–4), a large IL between TM3 and TM4, and a small extracellular C-terminal region (Moss and Smart, 2001; Thompson et al., 2010) (Fig. 1A). These protein structures also confirmed the conformation of functional pentameric ion channel complexes, with the TM2 domains shaping the ion channel pore at the central axis and the TM4 domains facing the plasma membrane (Fig. 1B).
Fig. 1.
Topology and schematic structure of GlyR. (A) Representation of a monomer of α1 GlyR where the different regions of the receptor are presented: extracellular domain (ECD, gray), transmembrane domains (TM, yellow) highlighting the TM2 which is part of the channel pore (magenta), the intracellular loop domain (IL, light blue), and C-terminal region (cyan). (B) Pentameric arrangement of subunits to form functional GlyR (C) Representation of dimer α1–α1 GlyR where the glycine binding site (GBS) is located. The loops (A–C) and β-strands (D–F) that comprise the GBS are also labeled. The amino acids from the principal (F44, F63, R65, L117, L127, S129) and complementary subunit (F158, Y202, T204, F207) are colored red and orange, respectively. The IL is based on the α1 GlyR full model described previously by Burgos et al. (2015a). All images were created using PyMOL.
In addition, some elements of the secondary structure and most of the residues interacting with extracellular ligands have been identified through the analyses of these structures. The ECD, for example, showed a classic barrel-like structure formed by 10 β-strands (β1–β10) accompanied by two α-helices at the N-terminal between β-strands 3 and 4 (Fig. 1A). The neurotransmitter binding site is located between two neighboring subunits and is formed by three loops (A, B, C) of the principal subunit (+) along with three β-strands (D, E, F) from the complementary subunit (−) (Unwin, 2005; Du et al., 2015; Huang et al., 2015). A more detailed analysis revealed that amino acids R65, T204, Y202, S129, F159, and P207 interact with glycine in the agonist-binding site (the numbers relate to the mature sequence of human α1 GlyR) (Du et al., 2015; Huang et al., 2015) (Fig. 1C). These residues are in agreement with the data obtained from functional and mutagenesis studies regarding agonist binding (Rajendra et al., 1995; Laube et al., 2002; Pless et al., 2011; Yu et al., 2014).
There are five identical agonist-binding sites on homopentameric α1 GlyR. In heteropentameric GlyR, the presence of α/β and β/β binding interfaces play a role in modifying the agonist affinity and pharmacologic properties (Dutertre et al., 2012; Shan et al., 2012). The TM, on the other hand, consist of four amphipathic α-helices (TM1–4), where TM2 helices form the channel pore while TM4s are located on the external face corresponding to the region that interacts with lipid components of the neuronal membrane (Ma et al., 2005; Mowrey et al., 2013a) (Fig. 1, A and B). Unfortunately, to facilitate crystallization of these proteins, the large intracellular domain that connects TM3 and TM4 was truncated or replaced by short peptide linkers, so it is currently not experimentally resolved (Thompson et al., 2010; Langlhofer et al., 2015).
Despite the lack of detailed structural information, data from 5-HT3 receptors and nAChR strongly suggest the presence of α helices in intracellular regions (Unwin and Fujiyoshi, 2012; Hassaine et al., 2014). In addition, a recent study using in silico and circular dichroism methodologies has proposed the existence of a helical conformation at both the N- and C-terminal regions of the IL in α1 GlyR near the TMs (Burgos et al., 2015a).
Transitions between Open and Closed States
The available structures of eukaryotic Cys-loop receptors in expected open/close conformations have allowed depicting some events that could well represent key steps leading to ion channel opening. For instance, structures for nAChR (Unwin and Fujiyoshi, 2012), GluCl (Hibbs and Gouaux, 2011; Althoff et al., 2014), and α1 GlyR (Du et al., 2015) have been described in the agonist-bound open state and in the antagonist-bound closed state. Structural alignments of each receptor between its open and closed conformations showed that the major conformational changes in the Cys-loop receptors are well conserved.
Briefly, upon activation by agonist binding to the ECD, the upper section of ECD twists around the pore axis while the lower ECD section tilts toward the center of the pore, accompanied by a significant displacement of loop C (Althoff et al., 2014; Du et al., 2015). To transfer the conformational changes in the ECD to the TM domains and open the ion channel, the upper section of TM2 seems to rotate outward because of the displacement of the Cys-loop through β10, producing the coupling of the TM2–TM3 extracellular loop to the β1–β2 loop.
It was also shown that the lower section of TM2, connected to TM1 and coupled to the β8–β9 loop, moves toward the pore axis as a consequence of displacement of the β8–β9 loop and subsequent rotation of TM1 (Du et al., 2015). Additionally, TM3 and TM4 rotate clockwise around the center of the helix bundle, producing the apparent complete rotation of the TM domains (Althoff et al., 2014).
It is currently assumed that all these conformational changes gated by the binding of the agonist to ECD might actually lead to the opening of the ion channel. Nevertheless, a finer picture, where intermediate and desensitized states of the receptor are also incorporated, is pending further studies.
Molecular Sites for the Functional Regulation of GlyR
The availability of Cys-loop family receptor structures provides information about the location and possible mechanisms of action of several physiologically and other clinically relevant modulators (see Table 2). For instance, these studies have provided structural information for GLIC and ELIC bound to key pharmacologic modulators such as general anesthetics (desflurane, isoflurane, propofol, ketamine) (Nury et al., 2011; Pan et al., 2012a; Kinde et al., 2015), benzodiazepines (Spurny et al., 2012), ions (Hilf et al., 2010; Hibbs and Gouaux, 2011; Zimmermann and Dutzler, 2011), and ethanol (Sauguet et al., 2013a). Interestingly, some of these modulators have opposite pharmacologic effects to those reported in GlyR (Table 2). Unfortunately, only a single study has provided the structure of GlyR bound to one modulator, ivermectin, showing the molecular interactions of this antiparasitic drug with the TM domains of GlyR α1 subunits (Du et al., 2015). Despite these limitations, the current evidence makes it possible to describe the molecular sites for the regulation of GlyR by important modulators.
TABLE 2.
Experimentally obtained structures of prokaryotic LGIC with pharmacologic and physiologically relevant compounds
All modulators described here were cocrystallized with their respective receptors allowing the analysis and description of each binding site.
| Receptor | Modulator | PDB Code | Molecular or Pharmacologic Effects | GlyRa | Reference |
|---|---|---|---|---|---|
| GLIC | Desflurane | 3P4W | Inhibition (IC50 = 27 ± 13 μM) | + | Nury et al., 2011 |
| Propofol | 3P50 | Inhibition (IC50 = 24 ± 6.3 μM) | + | Nury et -al., 2011 | |
| Ketamine | 4F8H | Inhibition (IC50 = 58 μM) | + | Pan et al., 2012a | |
| Bromoform | 4HFH | Inhibition in GLIC wild type | + | Sauguet et al., 2013a | |
| 4HFD | Potentiation by 1 mM (F14ʹA ethanol-sensitive mutant) | Sauguet et al., 2013a | |||
| 5HCM | Locally closed conformation | Laurent et al., 2016 | |||
| (2–21ʹ cross-linked mutant) | |||||
| Zinc | 2XQ8 | Inhibition by pore blocker | +/− | Hilf et al., 2010 | |
| Xenon | 4ZZB | Inhibition (IC50 = 1.05 atm) | + | Weng et al., 2010; Sauguet et al., 2016 | |
| 4ZZC | |||||
| Cesium | 2XQ6 | Inhibition by pore blocker | WE | Hilf et al., 2010 | |
| 4ILA | Inhibition by pore blocker (GLIC A237F mutant) | Sauguet et al., 2013b | |||
| Ethanol | 4HFE | Potentiation by low ethanol ≥20 μM (F14ʹA ethanol-sensitive mutant) | + | Sauguet et al., 2013a | |
| GLIC wild type insensitive to ethanol | |||||
| ELIC | Isoflurane | 4Z90 | Resting state, in absence of agonists | + | Chen et al., 2015 |
| 4Z91 | Desensitized state – in presence of agonists Inhibition on ELIC (IC50 = 21.9 ± 1.5 μM) | Chen et al., 2015 | |||
| Bromo-flurazepam | 4A98 | Potentiation similar to flurazepam | + | Spurny et al., 2012 | |
| GABA and flurazepam | 2YOE | Potentiation flurazepam ≤50 μM and inhibition at higher concentrations (biphasic effects) | + | Spurny et al., 2012 | |
| Zopiclone | 4A97 | Inhibition ∼90% by 10 μM | NA | Spurny et al., 2012 | |
| Bromoform | 3ZKR | Inhibition (IC50 = 125 ± 10 μM) | + | Spurny et al., 2013 | |
| Barium | 2YN6 | Inhibition by decreasing agonist efficacy and blocking channel opening | WE | Zimmermann et al., 2012 | |
| Memantine | 4TWD | Inhibition by pore-blocking and affecting agonist binding site (IC50 = 118 μM) | WE | Ulens et al., 2014 | |
| Bromo-memantine | 4TWF | Inhibition similar to memantine | WE | Ulens et al., 2014 |
, Potentiation, +/−, biphasic effect; NA, not available; WE, without effect.
Refers to the most reported effect.
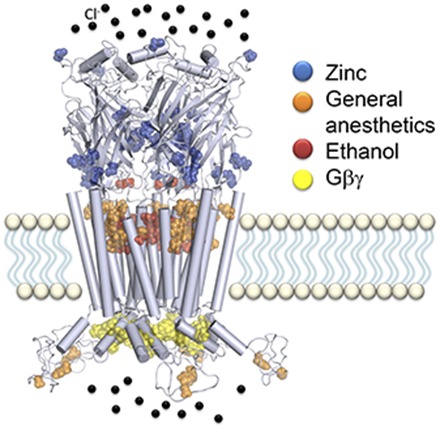
One chemically simple ligand able to modulate the activity of GlyR is Zn2+, which is stored in synaptic vesicles in glycinergic, GABAergic, and glutamatergic neurons. When this ion is released during neurotransmission, it can reach concentrations greater than 100 μM, which allows for the modulation of different receptors, including GlyR (Birinyi et al., 2001; Frederickson and Bush, 2001; Trombley et al., 2011). Zn2+ modulates α1 GlyR in a biphasic concentration-dependent manner. Potentiation of glycine-evoked currents occurs at low concentrations (<10 μM) whereas higher concentrations (>10 μM) produce inhibition (Bloomenthal et al., 1994; Laube et al., 1995).
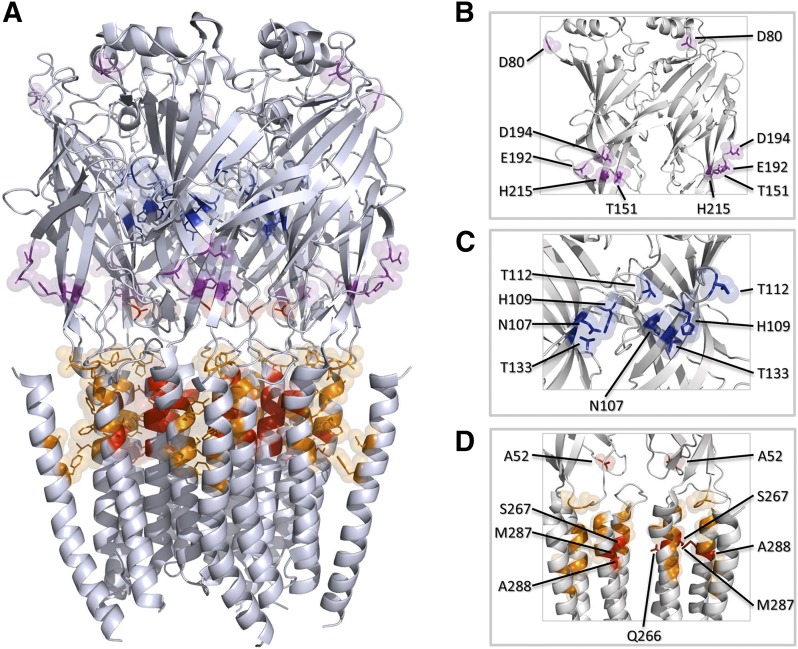
This biphasic modulation appears to be associated with different binding sites within the GlyR structure. The binding sites for Zn2+ associated with potentiation of glycinergic currents are located on the outer face of the N-terminal domain of α1 GlyR subunits and are composed of amino acids forming β-strands in the ECD (Fig. 2, A and B). These include the amino acids D80, E192, and D194 complemented by H215 and T151 (Laube et al., 1995; Laube et al., 2000; McCracken et al., 2013).
Fig. 2.
Putative binding sites for selected important modulators in the α1 GlyR structure. (A) Representation of a homopentameric α1 GlyR (PDB:3JAE) where the binding sites for low (magenta) and high (blue) Zn2+concentrations are shown. Amino acids forming the shared cavity for ethanol (red) and general anesthetics (orange) are also shown. (B) Detailed view of amino acids involved in Zn2+ potentiation by low concentrations (<10 μM), and (C) an internal pore view that show amino acids related to the inhibitory effects of Zn2+. (D) Ethanol and general anesthetics binding site; principal amino acids are colored red while the residues that complement the cavity are colored orange. All images were generated using PyMOL.
The binding of Zn2+ to GlyR produces an increase in channel open probability and burst duration, indicating an open state stabilization after agonist binding, even turning taurine, a partial agonist of GlyR, into a full agonist (Laube et al., 2000; Miller et al., 2008; Trombley et al., 2011; Farley and Mihic, 2015). On the other hand, the amino acids associated with the inhibitory effect of Zn2+ in α1 GlyR are located on the inner side of the ECD oriented toward the vestibule, and they include residues H107, H109, T112, and T133 (Fig. 2, A and C) (Miller et al., 2005b).
The inhibition of the glycine-evoked current is associated with reduced channel opening (gating) and a decrease in agonist efficacy (Miller et al., 2005a; Eto et al., 2007). Interestingly, in recent years it has been shown that modulation by Zn2+ is related to the effects of ethanol on GlyR, where Zn2+ increases the potentiation of glycinergic currents in presence of ethanol, which occurs in brain regions associated with the development of alcohol addiction (McCracken et al., 2010b, 2013; Morud et al., 2015).
Another important group of modulators are general anesthetics, which produce a marked depression in several CNS functions resulting in muscle relaxation, amnesia, and loss of consciousness (Rudolph and Antkowiak, 2004). Volatile anesthetics such as isoflurane, halothane, and enflurane potentiate glycine-evoked currents in α1 GlyR (Harrison et al., 1993; Downie et al., 1996; Mascia et al., 1996). The potentiation elicited by this group of pharmacologic agents is associated with a positive modulation on glycinergic synapses, which display increased decay time constant kinetics and frequency in glycinergic inhibitory postsynaptic potentials (Yamashita et al., 2001; Cheng and Kendig, 2002).
From mutagenesis and physiologic studies, it is believed that the binding site for volatile anesthetics on GlyR consists of amino acids located in TM2 and TM3 that form an intrasubunit cavity (Mihic et al., 1997; Roberts et al., 2006; Duret et al., 2011) and include residues S267 (TM2) and A288 (TM3) (Horani et al., 2015) (Fig. 2A,D). A similar cavity was described in GABAAR where amino acids S270 (TM2) and A291 (TM3) in the α1 subunit appeared to be important for the potentiation (Mascia et al., 2000; Jung et al., 2005; Jung and Harris, 2006). This binding site is shared with general anesthetics and alcohol and has been discussed previously elsewhere (Lobo and Harris, 2005; Howard et al., 2014; Olsen et al., 2014; Trudell et al., 2014).
Additionally, recent studies have reported that the endovenous anesthetic propofol binds to this cavity in a more superficial position compared with desflurane. However, this site is not the only one that is important for general anesthetics. For example, another study reported the presence of at least one residue (F380) outside this cavity located in the MA-stretch of the IL that is essential for propofol actions on α1 GlyR (Moraga-Cid et al., 2011). However, it has been reported that this putative binding site is exclusive for propofol because substitution of residue F380 by alanine attenuates the potentiation of glycinergic currents to propofol without altering the basal activity of the receptor or the modulation by other anesthetics such as isoflurane or etomidate (Moraga-Cid et al., 2011). More interestingly, this mutation does not affect ethanol actions on GlyR, supporting the notion that the pharmacologic actions of propofol and ethanol are possibly mediated by different sites in the protein.
The other pharmacologic compound that affects the regulation of GlyR is ethanol, one of the most popular drugs of abuse worldwide. The acute effects of alcohol in the CNS can vary from a decrease in sensorial reflexes, disinhibition in social behaviors, and euphoria, to depression, mental incoherence, coma, and death (Spanagel, 2009; Perkins et al., 2010). Despite the significant impact of ethanol on health and society, its mechanism of action has been difficult to elucidate, and several theories have been proposed.
One of the most accepted molecular targets to explain the complex effects of ethanol in mammals are the LGIC (Harris et al., 2008; Howard et al., 2014). Studies in cultured mammalian neurons and recombinant receptors have determined that several LGIC are modulated by clinically relevant ethanol concentrations (Lovinger et al., 1989; Aguayo, 1990; Lovinger, 1991; Aguayo and Pancetti, 1994; Cardoso et al., 1999; Moykkynen et al., 2003). Additional analysis found that low ethanol concentrations (≤50 mM) potentiate glycinergic currents by increasing the apparent affinity of GlyR with no change in efficacy (Aguayo et al., 1996; Crawford et al., 2007; Perkins et al., 2008), and showed that alcohol effects on glycinergic currents were developmentally regulated in cultured spinal neurons (Tapia et al., 1997; Tapia and Aguayo, 1998). Other studies have demonstrated that ethanol affects GlyR expressed in hypoglossal motoneurons (Eggers and Berger, 2004; Aguayo et al., 2014), the spinal cord (Celentano et al., 1988; Mariqueo et al., 2014), and ventral tegmental area neurons (Ye et al., 2001).
Two main hypotheses have been offered to explain the actions of ethanol on GlyR. The first and most studied and recognized mechanism proposes that the functional modulation of glycine-activated currents occurs by a direct interaction of alcohol with a group of amino acids that form a binding pocket, which is shared with other long-chain alcohols and general anesthetics (Lobo and Harris, 2005; Harris et al., 2008). This binding site for ethanol (Fig. 2D) comprises the S267 (TM2) and A288 residues (TM3) complemented by Q266 and M287. Besides this site, additional binding pockets for ethanol have been proposed in the TM4 domain (i.e., I409, Y410, and K411) (Lobo et al., 2006; McCracken et al., 2010a) and in the ECD (i.e., A52) (Crawford et al., 2007; Perkins et al., 2008). Thus, it is likely that several binding pockets for ethanol exist on GlyR, and they should become occupied as the concentration of the ligand increases.
Another hypothesis was proposed to explain the effects of ethanol at pharmacologic concentrations (≤100 mM) involving an intracellular mechanism and G protein modulation. These studies in recombinant and native GlyR determined that the potentiation of the receptor by ethanol is associated to an intracellular modulation by Gβγ (Yevenes et al., 2008, 2010). The binding of Gβγ to the IL of α1 subunits facilitates channel opening of GlyR, showing an increase in amplitude of glycine-evoked and GlyR open probability (nPo) (Yevenes et al., 2003). More detailed analysis determined that the physical contacts between two groups of basic amino acids in the IL of the α1 subunit (316RFRRK320 and 385KK386) are pivotal for the interaction between GlyR and Gβγ (Yevenes et al., 2006, 2008). Substitutions of these basic residues by alanines generated GlyR with low ethanol sensitivity in the pharmacologic range (≤100 mM) and did not display changes in ion channel function or the sensitivity to other agents such as isoflurane, propofol, zinc, alphaxolone, or longer n-alcohols, indicating a great degree of selectivity (Yevenes et al., 2008; Yevenes and Zeilhofer, 2011).
Future Directions for the Study of Regulatory GlyR Mechanisms
Although a considerable amount of information has been generated in the field of GlyR in the recent years, there are important issues that need to be addressed: 1) the composition of GlyR must be mapped throughout the CNS in terms of subunit composition and neuronal localization. 2) Selective agonists, antagonists, and modulators must be developed for specific GlyR subunits. Such compounds will shed light on the physiologic and pathologic roles of different GlyR subtypes in animals and humans. 3) Structural studies must be performed with pharmacologic concentrations of the ligands. It is likely that future technique refinements will show the existence of several binding sites for most of the modulators within GlyR.
Conclusion
In summary, GlyR offer a largely unexplored new dimension for the understanding of how inhibitory receptors play distinct roles in the CNS from the spinal cord to the cortex. Additional structural and functional data on different GlyR subtypes and key accessory proteins, together with the development of subunit selective ligands acting on synaptic and nonsynaptic receptors and genetically engineered animals, should increase our knowledge of these dimensions. One can expect that glycinergic-based pharmacotherapy will be developed for muscle tone, motor control, pain transmission, sedation, cognition, and reward.
Acknowledgments
The authors thank Lauren Aguayo for revising the manuscript and for expert technical assistance during the years. ASPET thanks Dr. Katie Strong for copyediting of this article.
Abbreviations
- AChBP
acetylcholine-binding protein
- CNS
central nervous system
- ECD
extracellular domain
- ELIC
Erwinia chrysanthemi ligand-gated ion channel
- 5HT-3
serotonin type 3 receptors
- GABAAR
GABAA receptor(s)
- Gβγ
G protein βγ subunits
- GLIC
Gloeobacter violaceus ligand-gated ion channel
- GluCl
glutamate-gated chloride channel
- GlyR
glycine receptor(s)
- IL
intracellular loop domain
- LGIC
ligand-gated ion channel
- nAChR
nicotinic acetylcholine receptor(s)
- TM
transmembrane domain
Authorship Contributions
Contributed to the writing of the manuscript: Burgos, Yévenes, Aguayo.
Footnotes
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant RO1 AA15150], Fondo Nacional de Desarrollo Científico y Tecnológico [Grant 1140515], and Comisión Nacional de Investigación Científica y Tecnológica [Grant DPI 20140008].
References
- Absalom NL, Lewis TM, Kaplan W, Pierce KD, Schofield PR. (2003) Role of charged residues in coupling ligand binding and channel activation in the extracellular domain of the glycine receptor. J Biol Chem 278:50151–50157. [DOI] [PubMed] [Google Scholar]
- Aguayo LG. (1990) Ethanol potentiates the GABAA-activated Cl− current in mouse hippocampal and cortical neurons. Eur J Pharmacol 187:127–130. [DOI] [PubMed] [Google Scholar]
- Aguayo LG, Castro P, Mariqueo T, Muñoz B, Xiong W, Zhang L, Lovinger DM, Homanics GE. (2014) Altered sedative effects of ethanol in mice with α1 glycine receptor subunits that are insensitive to Gβγ modulation. Neuropsychopharmacology 39:2538–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo LG, Pancetti FC. (1994) Ethanol modulation of the γ-aminobutyric acidA- and glycine-activated Cl− current in cultured mouse neurons. J Pharmacol Exp Ther 270:61–69. [PubMed] [Google Scholar]
- Aguayo LG, Tapia JC, Pancetti FC. (1996) Potentiation of the glycine-activated Cl− current by ethanol in cultured mouse spinal neurons. J Pharmacol Exp Ther 279:1116–1122. [PubMed] [Google Scholar]
- Aguayo LG, van Zundert B, Tapia JC, Carrasco MA, Alvarez FJ. (2004) Changes on the properties of glycine receptors during neuronal development. Brain Res Brain Res Rev 47:33–45. [DOI] [PubMed] [Google Scholar]
- Althoff T, Hibbs RE, Banerjee S, Gouaux E. (2014) X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature 512:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Benito-Gonzalez A, Siembab VC. (2013) Principles of interneuron development learned from Renshaw cells and the motoneuron recurrent inhibitory circuit. Ann N Y Acad Sci 1279:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila A, Nguyen L, Rigo JM. (2013) Glycine receptors and brain development. Front Cell Neurosci 7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaccini EJ, Trudell JR, Lindahl E. (2007) Normal-mode analysis of the glycine α1 receptor by three separate methods. J Chem Inf Model 47:1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birinyi A, Parker D, Antal M, Shupliakov O. (2001) Zinc co-localizes with GABA and glycine in synapses in the lamprey spinal cord. J Comp Neurol 433:208–221. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Leiter CR, Osterndorff-Kahanek E, Harris RA. (2015) Glycine receptors containing α2 or α3 subunits regulate specific ethanol-mediated behaviors. J Pharmacol Exp Ther 353:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomenthal AB, Goldwater E, Pritchett DB, Harrison NL. (1994) Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+. Mol Pharmacol 46:1156–1159. [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457:111–114. [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411:269–276. [DOI] [PubMed] [Google Scholar]
- Burgos CF, Castro PA, Mariqueo T, Bunster M, Guzmán L, Aguayo LG. (2015a) Evidence for α-helices in the large intracellular domain mediating modulation of the α1-glycine receptor by ethanol and Gβγ. J Pharmacol Exp Ther 352:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos CF, Munoz B, Guzman L, Aguayo LG. (2015b) Ethanol effects on glycinergic transmission: from molecular pharmacology to behavior responses. Pharmacol Res 101:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamai M, Specht CG, Heller J, Alcor D, Machado P, Vannier C, Triller A. (2009) Gephyrin oligomerization controls GlyR mobility and synaptic clustering. J Neurosci 29:7639–7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callister RJ, Graham BA. (2010) Early history of glycine receptor biology in mammalian spinal cord circuits. Front Mol Neurosci 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. (1999) Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 289:774–780. [PubMed] [Google Scholar]
- Carpenter EP, Beis K, Cameron AD, Iwata S. (2008) Overcoming the challenges of membrane protein crystallography. Curr Opin Struct Biol 18:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celentano JJ, Gibbs TT, Farb DH. (1988) Ethanol potentiates GABA- and glycine-induced chloride currents in chick spinal cord neurons. Brain Res 455:377–380. [DOI] [PubMed] [Google Scholar]
- Crawford DK, Trudell JR, Bertaccini EJ, Li K, Davies DL, Alkana RL. (2007) Evidence that ethanol acts on a target in loop 2 of the extracellular domain of α1 glycine receptors. J Neurochem 102:2097–2109. [DOI] [PubMed] [Google Scholar]
- Chau P, Höifödt-Lidö H, Löf E, Söderpalm B, Ericson M. (2010) Glycine receptors in the nucleus accumbens involved in the ethanol intake-reducing effect of acamprosate. Alcohol Clin Exp Res 34:39–45. [DOI] [PubMed] [Google Scholar]
- Chen Q, Kinde MN, Arjunan P, Wells MM, Cohen AE, Xu Y, Tang P. (2015) Direct pore binding as a mechanism for isoflurane inhibition of the pentameric ligand-gated ion channel ELIC. Sci Rep 5:13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Kendig JJ. (2002) Pre- and postsynaptic volatile anaesthetic actions on glycinergic transmission to spinal cord motor neurons. Br J Pharmacol 136:673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MH, Coalson RD, Cascio M. (2008) Molecular dynamics simulations of ethanol binding to the transmembrane domain of the glycine receptor: implications for the channel potentiation mechanism. Proteins 71:972–981. [DOI] [PubMed] [Google Scholar]
- Downie DL, Hall AC, Lieb WR, Franks NP. (1996) Effects of inhalational general anaesthetics on native glycine receptors in rat medullary neurones and recombinant glycine receptors in Xenopus oocytes. Br J Pharmacol 118:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Lü W, Wu S, Cheng Y, Gouaux E. (2015) Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 526:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret G, Van Renterghem C, Weng Y, Prevost M, Moraga-Cid G, Huon C, Sonner JM, Corringer PJ. (2011) Functional prokaryotic-eukaryotic chimera from the pentameric ligand-gated ion channel family. Proc Natl Acad Sci USA 108:12143–12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durisic N, Godin AG, Wever CM, Heyes CD, Lakadamyali M, Dent JA. (2012) Stoichiometry of the human glycine receptor revealed by direct subunit counting. J Neurosci 32:12915–12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre S, Drwal M, Laube B, Betz H. (2012) Probing the pharmacological properties of distinct subunit interfaces within heteromeric glycine receptors reveals a functional ββ agonist-binding site. J Neurochem 122:38–47. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Berger AJ. (2004) Mechanisms for the modulation of native glycine receptor channels by ethanol. J Neurophysiol 91:2685–2695. [DOI] [PubMed] [Google Scholar]
- Eto K, Arimura Y, Nabekura J, Noda M, Ishibashi H. (2007) The effect of zinc on glycinergic inhibitory postsynaptic currents in rat spinal dorsal horn neurons. Brain Res 1161:11–20. [DOI] [PubMed] [Google Scholar]
- Farley NM, Mihic SJ. (2015) Allosteric modulation of the glycine receptor activated by agonists differing in efficacy. Brain Res 1606:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ, Bush AI. (2001) Synaptically released zinc: physiological functions and pathological effects. Biometals 14:353–366. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gutierrez G, Cuello LG, Nair SK, Grosman C. (2013) Gating of the proton-gated ion channel from Gloeobacter violaceus at pH 4 as revealed by X-ray crystallography. Proc Natl Acad Sci USA 110:18716–18721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gutierrez G, Lukk T, Agarwal V, Papke D, Nair SK, Grosman C. (2012) Mutations that stabilize the open state of the Erwinia chrisanthemi ligand-gated ion channel fail to change the conformation of the pore domain in crystals. Proc Natl Acad Sci USA 109:6331–6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. (2005) The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 45:727–739. [DOI] [PubMed] [Google Scholar]
- Harris RA, Trudell JR, Mihic SJ. (2008) Ethanol’s molecular targets. Sci Signal 1:re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Kugler JL, Jones MV, Greenblatt EP, Pritchett DB. (1993) Positive modulation of human gamma-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Mol Pharmacol 44:628–632. [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wässle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schütz B, Abo-Salem OM, et al. (2004) GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304:884–887. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Schmieden V, Von Holst A, Laube B, Rohrer H, Betz H. (2000) Glycine receptors containing the α4 subunit in the embryonic sympathetic nervous system, spinal cord and male genital ridge. Eur J Neurosci 12:994–1001. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Topf M, Harvey K, Rees MI. (2008) The genetics of hyperekplexia: more than startle! Trends Genet 24:439–47. [DOI] [PubMed] [Google Scholar]
- Hassaine G, Deluz C, Grasso L, Wyss R, Tol MB, Hovius R, Graff A, Stahlberg H, Tomizaki T, Desmyter A, et al. (2014) X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 512:276–281. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf RJ, Bertozzi C, Zimmermann I, Reiter A, Trauner D, Dutzler R. (2010) Structural basis of open channel block in a prokaryotic pentameric ligand-gated ion channel. Nat Struct Mol Biol 17:1330–1336. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. (2008) X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452:375–379. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. (2009) Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457:115–118. [DOI] [PubMed] [Google Scholar]
- Horani S, Stater EP, Corringer PJ, Trudell JR, Harris RA, Howard RJ. (2015) Ethanol modulation is quantitatively determined by the transmembrane domain of human α1 glycine receptors. Alcohol Clin Exp Res 39:962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, Trudell JR, Harris RA. (2014) Seeking structural specificity: direct modulation of pentameric ligand-gated ion channels by alcohols and general anesthetics. Pharmacol Rev 66:396–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Chen H, Michelsen K, Schneider S, Shaffer PL. (2015) Crystal structure of human glycine receptor-α3 bound to antagonist strychnine. Nature 526:277–280. [DOI] [PubMed] [Google Scholar]
- Jung S, Akabas MH, Harris RA. (2005) Functional and structural analysis of the GABAA receptor alpha 1 subunit during channel gating and alcohol modulation. J Biol Chem 280:308–316. [DOI] [PubMed] [Google Scholar]
- Jung S, Harris RA. (2006) Sites in TM2 and 3 are critical for alcohol-induced conformational changes in GABA receptors. J Neurochem 96:885–892. [DOI] [PubMed] [Google Scholar]
- Kinde MN, Chen Q, Lawless MJ, Mowrey DD, Xu J, Saxena S, Xu Y, Tang P. (2015) Conformational changes underlying desensitization of the pentameric ligand-gated ion channel ELIC. Structure 23:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhse J, Kuryatov A, Maulet Y, Malosio ML, Schmieden V, Betz H. (1991) Alternative splicing generates two isoforms of the alpha 2 subunit of the inhibitory glycine receptor. FEBS Lett 283:73–77. [DOI] [PubMed] [Google Scholar]
- Langlhofer G, Janzen D, Meiselbach H, Villmann C. (2015) Length of the TM3-4 loop of the glycine receptor modulates receptor desensitization. Neurosci Lett 600:176–181. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. (2000) Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol 522:215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Rundström N, Kirsch J, Schmieden V, Betz H. (1995) Modulation by zinc ions of native rat and recombinant human inhibitory glycine receptors. J Physiol 483:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Maksay G, Schemm R, Betz H. (2002) Modulation of glycine receptor function: a novel approach for therapeutic intervention at inhibitory synapses? Trends Pharmacol Sci 23:519–527. [DOI] [PubMed] [Google Scholar]
- Laurent B, Murail S, Shahsavar A, Sauguet L, Delarue M, Baaden M. (2016) Sites of anesthetic inhibitory action on a cationic ligand-gated ion channel. Structure 24:595–605. [DOI] [PubMed] [Google Scholar]
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. (2004) Cys-loop receptors: new twists and turns. Trends Neurosci 27:329–336. [DOI] [PubMed] [Google Scholar]
- Lobo IA, Harris RA. (2005) Sites of alcohol and volatile anesthetic action on glycine receptors. Int Rev Neurobiol 65:53–87. [DOI] [PubMed] [Google Scholar]
- Lobo IA, Trudell JR, Harris RA. (2006) Accessibility to residues in transmembrane segment four of the glycine receptor. Neuropharmacology 50:174–181. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. (1991) Ethanol potentiation of 5-HT3 receptor-mediated ion current in NCB-20 neuroblastoma cells. Neurosci Lett 122:57–60. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. (1989) Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243:1721–1724. [DOI] [PubMed] [Google Scholar]
- Lynch JW. (2009) Native glycine receptor subtypes and their physiological roles. Neuropharmacology 56:303–309. [DOI] [PubMed] [Google Scholar]
- Ma D, Liu Z, Li L, Tang P, Xu Y. (2005) Structure and dynamics of the second and third transmembrane domains of human glycine receptor. Biochemistry 44:8790–8800. [DOI] [PubMed] [Google Scholar]
- Malosio ML, Marquèze-Pouey B, Kuhse J, Betz H. (1991) Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J 10:2401–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariqueo TA, Agurto A, Muñoz B, San Martin L, Coronado C, Fernández-Pérez EJ, Murath P, Sánchez A, Homanics GE, Aguayo LG. (2014) Effects of ethanol on glycinergic synaptic currents in mouse spinal cord neurons. J Neurophysiol 111:1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Machu TK, Harris RA. (1996) Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br J Pharmacol 119:1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. (2000) Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA 97:9305–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Blednov YA, Trudell JR, Benavidez JM, Betz H, Harris RA. (2013) Mutation of a zinc-binding residue in the glycine receptor α1 subunit changes ethanol sensitivity in vitro and alcohol consumption in vivo. J Pharmacol Exp Ther 344:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, McCracken ML, Gong DH, Trudell JR, Harris RA. (2010a) Linking of glycine receptor transmembrane segments three and four allows assignment of intrasubunit-facing residues. ACS Chem Neurosci 1:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Trudell JR, Goldstein BE, Harris RA, Mihic SJ. (2010b) Zinc enhances ethanol modulation of the α1 glycine receptor. Neuropharmacology 58:676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. (1997) Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389:385–389. [DOI] [PubMed] [Google Scholar]
- Miller PS, Aricescu AR. (2014) Crystal structure of a human GABAA receptor. Nature 512:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PS, Beato M, Harvey RJ, Smart TG. (2005a) Molecular determinants of glycine receptor alphabeta subunit sensitivities to Zn2+-mediated inhibition. J Physiol 566:657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PS, Da Silva HM, Smart TG. (2005b) Molecular basis for zinc potentiation at strychnine-sensitive glycine receptors. J Biol Chem 280:37877–37884. [DOI] [PubMed] [Google Scholar]
- Miller PS, Smart TG. (2010) Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol Sci 31:161–174. [DOI] [PubMed] [Google Scholar]
- Miller PS, Topf M, Smart TG. (2008) Mapping a molecular link between allosteric inhibition and activation of the glycine receptor. Nat Struct Mol Biol 15:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraga-Cid G, Sauguet L, Huon C, Malherbe L, Girard-Blanc C, Petres S, Murail S, Taly A, Baaden M, Delarue M, et al. (2015) Allosteric and hyperekplexic mutant phenotypes investigated on an α1 glycine receptor transmembrane structure. Proc Natl Acad Sci USA 112:2865–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraga-Cid G, Yevenes GE, Schmalzing G, Peoples RW, Aguayo LG. (2011) A Single phenylalanine residue in the main intracellular loop of α1 γ-aminobutyric acid type A and glycine receptors influences their sensitivity to propofol. Anesthesiology 115:464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morud J, Adermark L, Ericson M, Söderpalm B. (2015) Alterations in ethanol-induced accumbal transmission after acute and long-term zinc depletion. Addict Biol 20:170–181. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. (2001) Constructing inhibitory synapses. Nat Rev Neurosci 2:240–250. [DOI] [PubMed] [Google Scholar]
- Mowrey DD, Cui T, Jia Y, Ma D, Makhov AM, Zhang P, Tang P, Xu Y. (2013a) Open-channel structures of the human glycine receptor α1 full-length transmembrane domain. Structure 21:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrey DD, Liu Q, Bondarenko V, Chen Q, Seyoum E, Xu Y, Wu J, Tang P. (2013b) Insights into distinct modulation of α7 and α7β2 nicotinic acetylcholine receptors by the volatile anesthetic isoflurane. J Biol Chem 288:35793–35800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möykkynen T, Korpi ER, Lovinger DM. (2003) Ethanol inhibits alpha-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in central nervous system neurons by stabilizing desensitization. J Pharmacol Exp Ther 306:546–555. [DOI] [PubMed] [Google Scholar]
- Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, Changeux JP, Sonner JM, Delarue M, Corringer PJ. (2011) X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469:428–431. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Li GD, Wallner M, Trudell JR, Bertaccini EJ, Lindahl E, Miller KW, Alkana RL, Davies DL. (2014) Structural models of ligand-gated ion channels: sites of action for anesthetics and ethanol. Alcohol Clin Exp Res 38:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Chen Q, Willenbring D, Mowrey D, Kong XP, Cohen A, Divito CB, Xu Y, Tang P. (2012a) Structure of the pentameric ligand-gated ion channel GLIC bound with anesthetic ketamine. Structure 20:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Chen Q, Willenbring D, Yoshida K, Tillman T, Kashlan OB, Cohen A, Kong XP, Xu Y, Tang P. (2012b) Structure of the pentameric ligand-gated ion channel ELIC cocrystallized with its competitive antagonist acetylcholine. Nat Commun 3:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. (2008) Targets for ethanol action and antagonism in loop 2 of the extracellular domain of glycine receptors. J Neurochem 106:1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. (2010) Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol Ther 127:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless SA, Hanek AP, Price KL, Lynch JW, Lester HA, Dougherty DA, Lummis SC. (2011) A cation-π interaction at a phenylalanine residue in the glycine receptor binding site is conserved for different agonists. Mol Pharmacol 79:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost MS, Sauguet L, Nury H, Van Renterghem C, Huon C, Poitevin F, Baaden M, Delarue M, Corringer PJ. (2012) A locally closed conformation of a bacterial pentameric proton-gated ion channel. Nat Struct Mol Biol 19:642–649. [DOI] [PubMed] [Google Scholar]
- Rajendra S, Vandenberg RJ, Pierce KD, Cunningham AM, French PW, Barry PH, Schofield PR. (1995) The unique extracellular disulfide loop of the glycine receptor is a principal ligand binding element. EMBO J 14:2987–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MT, Phelan R, Erlichman BS, Pillai RN, Ma L, Lopreato GF, Mihic SJ. (2006) Occupancy of a single anesthetic binding pocket is sufficient to enhance glycine receptor function. J Biol Chem 281:3305–3311. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. (2004) Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci 5:709–720. [DOI] [PubMed] [Google Scholar]
- Sauguet L, Fourati Z, Prangé T, Delarue M, Colloc’h N. (2016) Structural basis for xenon inhibition in a cationic pentameric ligand-gated ion channel. PLoS One 11:e0149795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Howard RJ, Malherbe L, Lee US, Corringer PJ, Harris RA, Delarue M. (2013a) Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun 4:1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Poitevin F, Murail S, Van Renterghem C, Moraga-Cid G, Malherbe L, Thompson AW, Koehl P, Corringer PJ, Baaden M, et al. (2013b) Structural basis for ion permeation mechanism in pentameric ligand-gated ion channels. EMBO J 32:728–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Shahsavar A, Poitevin F, Huon C, Menny A, Nemecz À, Haouz A, Changeux JP, Corringer PJ, Delarue M. (2014) Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation. Proc Natl Acad Sci USA 111:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q, Han L, Lynch JW. (2012) Distinct properties of glycine receptor β+/α− interface: unambiguously characterizing heteromeric interface reconstituted in homomeric protein. J Biol Chem 287:21244–21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. (2004) Analysis of the set of GABA(A) receptor genes in the human genome. J Biol Chem 279:41422–41435. [DOI] [PubMed] [Google Scholar]
- Spanagel R. (2009) Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev 89:649–705. [DOI] [PubMed] [Google Scholar]
- Speranskiy K, Cascio M, Kurnikova M. (2007) Homology modeling and molecular dynamics simulations of the glycine receptor ligand binding domain. Proteins 67:950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurny R, Billen B, Howard RJ, Brams M, Debaveye S, Price KL, Weston DA, Strelkov SV, Tytgat J, Bertrand S, et al. (2013) Multisite binding of a general anesthetic to the prokaryotic pentameric Erwinia chrysanthemi ligand-gated ion channel (ELIC). J Biol Chem 288:8355–8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurny R, Ramerstorfer J, Price K, Brams M, Ernst M, Nury H, Verheij M, Legrand P, Bertrand D, Bertrand S, et al. (2012) Pentameric ligand-gated ion channel ELIC is activated by GABA and modulated by benzodiazepines. Proc Natl Acad Sci USA 109:E3028–E3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia JC, Aguayo LG. (1998) Changes in the properties of developing glycine receptors in cultured mouse spinal neurons. Synapse 28:185–194. [DOI] [PubMed] [Google Scholar]
- Tapia JC, Espinoza F, Aguayo LG. (1997) Differential intracellular regulation of cortical GABA(A) and spinal glycine receptors in cultured neurons. Brain Res 769:203–210. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Lester HA, Lummis SC. (2010) The structural basis of function in Cys-loop receptors. Q Rev Biophys 43:449–499. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Blakemore LJ, Hill BJ. (2011) Zinc modulation of glycine receptors. Neuroscience 186:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudell JR, Messing RO, Mayfield J, Harris RA. (2014) Alcohol dependence: molecular and behavioral evidence. Trends Pharmacol Sci 35:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulens C, Spurny R, Thompson AJ, Alqazzaz M, Debaveye S, Han L, Price K, Villalgordo JM, Tresadern G, Lynch JW, et al. (2014) The prokaryote ligand-gated ion channel ELIC captured in a pore blocker-bound conformation by the Alzheimer’s disease drug memantine. Structure 22:1399–1407. [DOI] [PubMed] [Google Scholar]
- Unwin N. (2005) Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol 346:967–989. [DOI] [PubMed] [Google Scholar]
- Unwin N, Fujiyoshi Y. (2012) Gating movement of acetylcholine receptor caught by plunge-freezing. J Mol Biol 422:617–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert B, Castro P, Aguayo LG. (2005) Glycinergic and GABAergic synaptic transmission are differentially affected by gephyrin in spinal neurons. Brain Res 1050:40–47. [DOI] [PubMed] [Google Scholar]
- Vijayan RS, Trivedi N, Roy SN, Bera I, Manoharan P, Payghan PV, Bhattacharyya D, Ghoshal N. (2012) Modeling the closed and open state conformations of the GABA(A) ion channel—plausible structural insights for channel gating. J Chem Inf Model 52:2958–2969. [DOI] [PubMed] [Google Scholar]
- Weng Y, Yang L, Corringer PJ, Sonner JM. (2010) Anesthetic sensitivity of the Gloeobacter violaceus proton-gated ion channel. Anesth Analg 110:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Ueno T, Akaike N, Ikemoto Y. (2001) Modulation of miniature inhibitory postsynaptic currents by isoflurane in rat dissociated neurons with glycinergic synaptic boutons. Eur J Pharmacol 431:269–276. [DOI] [PubMed] [Google Scholar]
- Ye JH, Tao L, Ren J, Schaefer R, Krnjevic K, Liu PL, Schiller DA, McArdle JJ. (2001) Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. J Pharmacol Exp Ther 296:77–83. [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Avila A, Guzmán L, Figueroa M, Peoples RW, Aguayo LG. (2010) Molecular requirements for ethanol differential allosteric modulation of glycine receptors based on selective Gβγ modulation. J Biol Chem 285:30203–30213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Guzmán L, Haeger S, Oliveira L, Olate J, Schmalzing G, Aguayo LG. (2006) Molecular determinants for G protein βγ modulation of ionotropic glycine receptors. J Biol Chem 281:39300–39307. [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Peoples RW, Schmalzing G, Aguayo LG. (2008) A selective G βγ-linked intracellular mechanism for modulation of a ligand-gated ion channel by ethanol. Proc Natl Acad Sci USA 105:20523–20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevenes GE, Peoples RW, Tapia JC, Parodi J, Soto X, Olate J, Aguayo LG. (2003) Modulation of glycine-activated ion channel function by G-protein βγ subunits. Nat Neurosci 6:819–824. [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Zeilhofer HU. (2011) Allosteric modulation of glycine receptors. Br J Pharmacol 164:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Hurdiss E, Greiner T, Lape R, Sivilotti L, Biggin PC. (2014) Agonist and antagonist binding in human glycine receptors. Biochemistry 53:6041–6051. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Wildner H, Yévenes GE. (2012) Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev 92:193–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann I, Dutzler R. (2011) Ligand activation of the prokaryotic pentameric ligand-gated ion channel ELIC. PLoS Biol 9:e1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann I, Marabelli A, Bertozzi C, Sivilotti LG, Dutzler R. (2012) Inhibition of the prokaryotic pentameric ligand-gated ion channel ELIC by divalent cations. PLoS Biol 10:e1001429. [DOI] [PMC free article] [PubMed] [Google Scholar]