Abstract
Histone deacetylase inhibitors (HDACIs) can disrupt the viability of prostate cancer (PCa) cells through modulation of the cytosolic androgen receptor (AR) chaperone protein heat shock protein 90 (HSP90). However, toxicities associated with their pleiotropic effects could contribute to the ineffectiveness of HDACIs in PCa treatment. We designed hybrid molecules containing partial chemical scaffolds of enzalutamide and suberoylanilide hydroxamic acid (SAHA), with weakened intrinsic pan-HDACI activities, to target HSP90 and AR in enzalutamide-resistant PCa cells. The potency of the new molecules, compounds 2-75 [4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-(7-(hydroxyamino)-7-oxoheptyl)benzamide] and 1005 [(E)-3-(4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluorophenyl)-N-hydroxyacrylamide], as inhibitors of nuclear and cytosolic histone deacetylases was substantially lower than that of SAHA in cell-free and in situ assays. Compounds 2-75 and 1005 antagonized gene activation by androgen without inducing chromatin association of AR. Enzalutamide had no effect on the levels of AR or HSP90, whereas the hybrid compounds induced degradation of both AR and HSP90, similar to (compound 1005) or more potently than (compound 2-75) SAHA. Similar to SAHA, compounds 2-75 and 1005 decreased the level of HSP90 and induced acetylation in a predicted approximately 55 kDa HSP90 fragment. Compared with SAHA, compound 2-75 induced greater hyperacetylation of the HDAC6 substrate α-tubulin. In contrast with SAHA, neither hybrid molecule caused substantial hyperacetylation of histones H3 and H4. Compounds 2-75 and 1005 induced p21 and caused loss of viability in the enzalutamide-resistant C4-2 cells, with efficacies that were comparable to or better than SAHA. The results suggest the potential of the new compounds as prototype antitumor drugs that would downregulate HSP90 and AR in enzalutamide-resistant PCa cells with weakened effects on nuclear HDACI targets.
Introduction
Prostate cancer (PCa) is the second leading cause of cancer-related deaths in men in the United States (Siegel et al., 2015). PCa is initially managed with surgery, radiation, androgen antagonists (e.g., bicalutamide), and surgical or chemical castration. However, relapsed or metastatic disease after castration [castration-recurrent prostate cancer (CRPC)] has a poor prognosis, with most patients dying within 2 years (Karantanos et al., 2015). Innovative approaches are urgently needed to treat patients with CRPC.
Androgen receptor (AR) signaling is a major driving force in all stages of PCa (Chen et al., 2009). CRPC cells evolve mechanisms to reactivate AR signaling under androgen deprivation conditions (Mitsiades, 2013); these mechanisms include overexpression and gain-of-function mutations of AR (Joseph et al., 2013; Korpal et al., 2013), overexpression of AR splice variants (Li et al., 2013), compensatory crosstalk between AR and other signaling pathways (Liu et al., 2014), and enhanced intratumoral androgen biosynthesis (Nakamura et al., 2005). Enzalutamide (Enz), an AR antagonist that prolongs survival of patients with CRPC, was recently approved by the U.S. Food and Drug Administration (Scher et al., 2012; Beer et al., 2014). Enz competitively binds to AR with 5- to 8-fold higher affinity than bicalutamide and, in contrast with bicalutamide, does not promote AR nuclear translocation (Tran et al., 2009). Nevertheless, acquired resistance to Enz typically develops within months and is associated with a relatively short-lived patient survival benefit. Indeed, in vivo generated CRPC cell-line models that vastly overexpress ARs (e.g., C4-2 cells), presumably in combination with changes in other cellular signaling pathways, are completely resistant to Enz in conditioned media while remaining addicted to AR (Patki et al., 2016).
A possible strategy to overcome resistance to androgen depletion is to induce destabilization and degradation of AR and its associated proteins in CRPC cells. AR is stabilized in the cytosol by its interaction with heat shock protein 90 (HSP90) and other chaperone proteins. HSP90 is commonly overexpressed in many types of cancer cells and has been explored as a drug target for cancer treatment, including PCa (Neckers and Workman, 2012; Bhat et al., 2014). HSP90 is an ATP-dependent molecular chaperone that aids the folding and stability of a number of client proteins, such as steroid receptors, protein kinases, transcription factors, and proteins involved in regulating cell survival. Therefore, inhibition of HSP90 leads to degradation of its client proteins via the ubiquitin-proteasome pathway. Association of the AR apoprotein with HSP90 is critical for stabilizing AR in a conformation that allows androgen binding (Veldscholte et al., 1992; Fang et al., 1996). In PCa cells, HSP90 inhibitors induce AR degradation and impair AR nuclear translocation while simultaneously reducing the levels of other oncogenic client proteins, such as p-AKT/AKT, epidermal growth factor receptor, insulin-like growth factor receptor, and survivin (Solit et al., 2002; Saporita et al., 2007; He et al., 2013; Liu et al., 2015). Simultaneous disruption of AR and other aberrant growth/survival networks via HSP90 inhibition is an advantageous treatment strategy for CRPC, because this would silence potentially mutually compensatory oncogenic signaling pathways. Despite this attractive scientific rationale, clinical development of HSP90 inhibitors for PCa treatment was disappointing (Heath et al., 2008; Oh et al., 2011; Pacey et al., 2011; Thakur et al., 2016) and was also limited by adverse toxicity to nontarget tissues.
One of the actions of histone deacetylase inhibitors (HDACIs) is to disrupt HSP90 activity. Histone deacetylases (HDACs) remove acetyl groups from lysine residues of histone and nonhistone proteins. Eighteen HDACs categorized into four classes have been identified in mammalian cells. Among them, HDAC6 is a zinc-dependent, class IIb HDAC and is localized in the cytoplasm (Krämer et al., 2014). HDAC6 deacetylates HSP90 (Bali et al., 2005; Kovacs et al., 2005). Inhibition of HDAC6 could result in hyperacetylation of HSP90, loss of ATP binding, and dissociation and degradation of its client proteins, including AR. The HDACIs LAQ-824 [(2E)-N-hydroxy-3-[4-[[(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide] (Chen et al., 2005), PDX-101 [belinostat, N-hydroxy-3-[3-](phenylamino)sulfonyl]phenyl]-2-propenamide] (Gravina et al., 2013), suberoylanilide hydroxamic acid (SAHA; vorinostat) (Marrocco et al., 2007; Sato et al., 2012), and natural products sulforaphane (Gibbs et al., 2009) and genistein (Basak et al., 2008) have all been reported to reduce AR protein levels in PCa cells by disrupting the HDAC6-HSP90 chaperone function.
Clinical use of HDACIs is now confined to hematologic malignancies. Although nuclear HDACs (e.g., HDAC1 and HDAC3) are indispensable for AR transcriptional activity (Welsbie et al., 2009) and increased HDAC levels have been reported in clinical CRPC samples and are positively correlated with Gleason scores (Weichert et al., 2008; Burdelski et al., 2015), they are not likely to serve as effective drug targets to treat PCa. HDACIs, such as vorinostat (SAHA) (Bradley et al., 2009), romidepsin [FK-228, cyclo[(2Z)-2-amino-2-butenoyl-l-valyl-(3S,4E)-3-hydroxy-7-mercapto-4-heptenoyl-d-valyl-d-cysteinyl],cyclic(3-5)disulfide] (Molife et al., 2010), panobinostat [LBH-589, (2E)-N-hydroxy-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]acrylamide] (Rathkopf et al., 2013), and pracinostat [SB-939, (E)-3-(2-butyl-1-(2-(diethylamino)ethyl)-1H-benzo[d]imidazol-5-yl)-N-hydroxyacrylamide] (Eigl et al., 2015) have been tested in patients with CRPC but have resulted in modest outcomes. Toxicities associated with their pleiotropic effects could contribute to the ineffectiveness of HDACIs in PCa treatment. Moreover, recent studies have associated the pleiotropic effects of HDACIs, particularly epigenetic modifications of chromatin-associated proteins, with induction of epithelial to mesenchymal transition in prostate, endometrial, and nasopharyngeal cancer cells (Kong et al., 2012; Uchida et al., 2012; Jiang et al., 2013; Tam and Weinberg, 2013). Therefore, clinical translation of the extensive and promising preclinical findings of the efficacies of HDACIs in treating solid tumors must address the issue of toxicities that prevent application of effective HDACI treatment regimens in the clinic.
We sought to develop Enz derivatives armed with HDACI activity to antagonize AR and HSP90 actions in AR-overexpressing and Enz-resistant CRPC cells with reduced pleiotropic effects typical of strong HDACIs. Accordingly, we have designed, synthesized, and tested the prototype compounds 2-75 [4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-(7-(hydroxyamino)-7-oxoheptyl)benzamide] and 1005 [(E)-3-(4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluorophenyl)-N-hydroxyacrylamide]. The 2-75 and 1005 chemical scaffolds are designed to retain AR binding affinity. The compounds are also designed to have lower intrinsic HDACI activity compared with SAHA, thus reducing the HDACI activity against nontarget proteins. Nevertheless, the HDACI activity is expected to produce effective disruption of AR as well as other HSP90 client proteins, resulting in loss of viability in Enz-resistant CRPC cells.
Materials and Methods
Compound Synthesis.
Detailed procedures for the synthesis of compounds 2-75, 1005, 3-52 [methyl 7-(4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluorobenzamido)heptanoate], and 1002 [(E)-ethyl 3-(4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluorophenyl)acrylate] are described the Supplemental Material. The chemical identities of the compounds were confirmed by using 1H and 13C-nuclear magnetic resonance and high-resolution mass spectrometry (Qin and Ratnam, 2016).
HDAC Activity Assay.
In vitro HDAC inhibition was measured by using the HDAC fluorometric assay/drug discovery kit (BML-AK500) and the HDAC6 fluorometric drug discovery kit (BML-AK516; both from Enzo Life Sciences, Farmingdale, NY) following the manufacturer’s protocols and instructions. IC50 values were calculated from nonlinear aggression plots using GraphPad Prism5 software (GraphPad Software Inc., La Jolla, CA).
Cell Culture and Reagents.
LNCaP and PC3 cell lines were from American Type Culture Collection (Manassas, VA). LNCaP and C4-2 cells were routinely grown at 37°C in 5% CO2 in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine mixture (Invitrogen), and 1 mM sodium pyruvate (Invitrogen). PC3 cells were grown in RPMI 1640 medium supplemented with 10% FBS (Invitrogen), 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine mixture (Invitrogen). Affinity-purified rabbit anti-human antibody to AR (sc-816), mouse anti-human antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sc-47724), and affinity-purified mouse anti-human antibody to α-tubulin (sc-8035) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Affinity-purified rabbit anti-human antibodies to HSP90 (C45G5) 4877, rabbit anti-human antibody to acetylated lysine 9441, and affinity-purified rabbit anti-human antibody to p21 Waf1/Cip1 2947S were purchased from Cell Signaling Technology (Danvers, MA). Affinity-purified rabbit anti-human antibodies to acetyl tubulin (ABT241), acetyl histone H4 (07-328), and acetyl histone H3 (ABE18) were purchased from Millipore (Billerica, MA). R1881 (17β-17-hydroxy-17-methyl-estra-4,9,11-trien-3-one) was kindly provided by Dr. Stephan Patrick (Barbara Ann Karmanos Cancer Institute, Wayne State University, Detroit, MI). Cycloheximide was from Sigma-Aldrich (St. Louis, MO). All experiments were conducted using phenol red–free growth media. For hormone depletion, cells were grown in phenol red–free RPMI 1640 medium supplemented with 10% charcoal stripped FBS (Sigma-Aldrich), which was heat inactivated at 56°C for 30 minutes, and a mixture of 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine for 96 hours.
Cell Viability Assay.
Cells were trypsinized and 6000 cells/well were seeded in 96-well plates coated with poly(d-lysine). The cells were seeded in phenol red–free medium supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine mixture, and 1 mM sodium pyruvate for C4-2 cells and phenol red–free medium supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine mixture for PC3 cells. The cells were grown at 37°C in 5% CO2. Twenty-four hours after seeding in the 96-well plates, the cells were treated with the indicated compound or dimethylsulfoxide (vehicle). The culture medium was not changed during the time course of the assay. On days 0 and 3, cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (10 µl, 5 mg/ml) was added to each well and incubated for 2 hours at 37°C. The formazan crystal sediments were dissolved in 100 µl dimethylsulfoxide, and the absorbance at 570 nm was measured using the BioTek Synergy 2 Microplate Reader (BioTek, Winooski, VT). The assay was conducted in sextuplicate wells and values were normalized to day 0 (Patki et al., 2014). IC50 values were calculated from nonlinear aggression plots using GraphPad Prism5 software.
Western Blot Analysis.
Cells were washed once with phosphate-buffered saline and then lysed with RIPA buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris with pH 8.0) containing a protease inhibitor cocktail (Pierce/Thermo Fisher Scientific, Waltham, MA). The cell lysates were then incubated on ice for 40 minutes. Total protein concentrations were estimated using the Bradford assay (Bio-Rad, Hercules, CA). Protein samples (10–40 μg) were heated at 95°C for 5 minutes and resolved by electrophoresis on 8% polyacrylamide-SDS gels and electrophoretically transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). The membranes were then probed overnight at 4°C with the appropriate primary antibody followed by the appropriate horseradish peroxidase–conjugated secondary antibody. The blots were then developed to visualize the protein bands using HyGLO Chemiluminescent HRP Antibody Detection Reagent (Denville Scientific, Metuchen, NJ) (Salazar et al., 2011).
RNA Isolation, Reverse Transcription, and Real-Time Polymerase Chain Reaction.
Total RNA was isolated from cells using the RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse-transcription polymerase chain reaction (PCR) was then performed using 500 ng total RNA with random primers and using the high-capacity complementary DNA Archive Kit (Applied Biosystems, Foster City, CA). The complementary DNA from this reaction was measured with quantitative real-time PCR using the StepONE Plus Real-Time PCR system (Life Technologies, Carlsbad, CA). All reactions were performed in triplicate and normalized to GAPDH values in the same samples. All primers and TaqMan probes were purchased from the Applied Biosystems inventory (Invitrogen) (Patki et al., 2015).
Chromatin Immunoprecipitation.
C4-2 cells were treated with either vehicle, R1881 (1 nM or 10 nM), or 10 μM of each indicated compound for 2 hours and then subjected to chromatin immunoprecipitation (ChIP) using anti-AR antibody (Santa Cruz Biotechnology). The ChIP assay was performed using the EX ChIP kit (Millipore, Temecula, CA) according to the vendor’s protocol. The ChIP signals were measured by quantitative real-time PCR analysis of the immunoprecipitated products. Each sample was tested in triplicate (Patki et al., 2013).
Statistical Analysis.
All experiments were performed in triplicate groups and repeated at least three times. The error bars in all graphs represent the standard deviation. Statistical analysis was performed using one-way analysis of variance with post hoc and least-squares differences and/or the t test (McFall et al., 2015).
Results
Design and Synthesis of Compounds 2-75 and 1005 with Partial Chemical Scaffolds of Enz and SAHA.
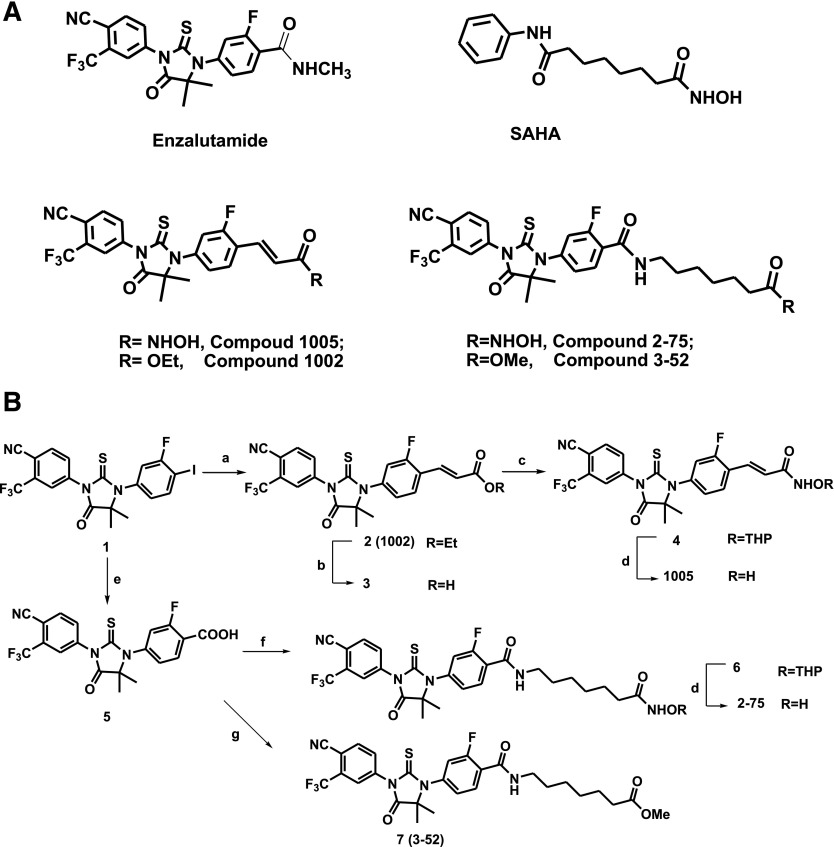
Compounds 2-75 and 1005 were designed to retain partial functional scaffolds of Enz and SAHA (Fig. 1A). Upon binding to AR, the cyano group of Enz/Enz derivatives forms a critical hydrogen bond with Arg752, and the conformationally restricted thiohydantoin ring in the middle forces the rest of the molecule to the “H11 pocket,” a region near the C terminus of helix 11 and the loop connecting helices 11 and 12 (Balbas et al., 2013). To design derivatives with both AR binding and HDACI activities, different linkers connecting Enz and a zinc binding group were introduced to retain the above structural features that are required for AR binding in an antagonist-related conformation. Compound 1005 is a cinnamyl hydroxamic acid derivative with a three-carbon linker. A relatively longer carbon chain in 2-75 was used to more closely mimic the chemical structure of SAHA. Compound 7 (3-52), a close structural analog of 2-75 using methyl ester to replace the zinc binding group, was synthesized as a control compound without an HDACI functional group (Fig. 1B). Compound 7 (3-52) and synthetic intermediate compound 2 (i.e., 1002, an ethyl ester analog of 1005; Fig. 1, A and B) were used to investigate AR antagonist properties of 2-75 and 1005 scaffolds, respectively.
Fig. 1.
Compound structures and synthetic schemes. (A) Chemical structures of Enz, SAHA, 1005, 2-75, 1002, and 3-52. (B) Synthesis of 1005, 2-75, 1002, and 3-52. Reagents and conditions are as follows: (a) ethyl acrylate, Pd(OAc)2, P(o-tolyl)3, DIPEA, DMF, 80°C, 75%; (b) 37% HCl (aq), acetonitrile, reflux, 91%; (c) NH2OTHP, BOP, DIPEA, DMF, 51%; (d) HCl (4 N in dioxane), MeOH, 60% for 1005, 32% for 2-75; (e) HCOOLi, Ac2O, Pd2(dba)3, LiCl, DMF, 80°C, 92%; (f) 7-amino-N-(tetrahydro-2H-pyran-2-yl)oxy heptanamide, HBTU, DIPEA, DMF, 30%; and (g) methyl 7-aminoheptanoate hydrochloride, HBTU, DIPEA, DMF, 47%. BOP, benzotriazol-l-yl-oxy-tris-(dimethylamino)phosphonium hexa-fluorophosphate; DIPEA, N,N-Diisopropylethylamine; DMF, N,N-Dimethylformamide; HBTU, 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate; THP, tetrahydropyranyl.
Both compounds 2-75 and 1005 were synthesized from a 4′-iodo–substituted intermediate (Fig. 1B) 1. An acrylate linker of 1005 was introduced via a Pd(OAc)2-catalyzed Heck reaction to afford compound 2, followed by hydrolysis of ethyl ester, coupling to tetrahydropyranyl acetal–protected hydroxylamine, and the final acidic deprotection. To synthesize 2-75, carboxylation of aryl iodine 1 was performed using a palladium-catalyzed carboxylation reaction (Cacchi et al., 2003), and the resulting carboxylic acid was then coupled with primary amines to attach the alkyl chain with protected hydroxamic acid (compound 6) or methyl ester (compound 7). Removal of the tetrahydropyranyl-protected group gave the final product 2-75. The detailed synthetic procedures are described in the Supplemental Material.
Compounds 2-75 and 1005 Possess Intrinsically Weak Inhibitor Activity Against Nuclear HDACs and Cytosolic HDAC6.
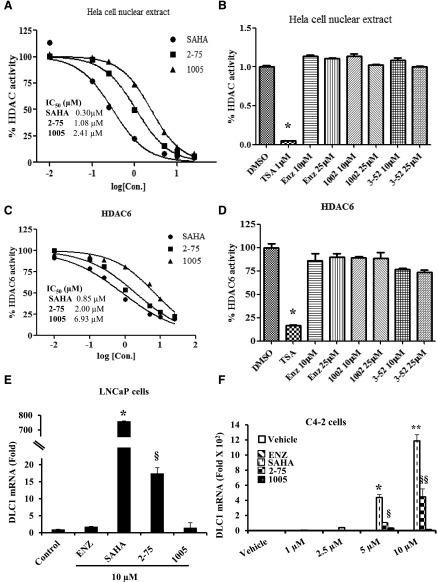
To measure HDAC inhibitory activities of 2-75 and 1005, cell-free enzymatic assays were performed against a nuclear extract of HeLa cells and also against human recombinant HDAC6. HDAC1 and HDAC2 are enriched in nuclear extracts, whereas HDAC6 is a cytosolic enzyme that is the principal modulator of the acetylation status of HSP90. The HeLa cell nuclear extract was used to evaluate inhibitory activity against nuclear HDACs. Compounds 2-75 and 1005 dose-dependently inhibited HDACs enriched in the HeLa nuclear extract, with IC50 values of 1.08 µM and 2.41 µM compared with a value of 0.30 µM for SAHA (Fig. 2A). In contrast, compounds 3-52 and 1002, which share the chemical scaffolds of 2-75 and 1005, respectively, but lack the HDACI functional group, did not inhibit nuclear HDAC activity even up to a concentration of 25 µM (Fig. 2B). As expected, Enz also lacked any HDACI activity, in contrast with a pan-HDACI, trichostatin A, which was used as a positive control (Fig. 2B). Compounds 2-75 and 1005 were also weaker inhibitors of recombinant HDAC6, with IC50 values of 2.0 µM and 6.93 µM, respectively, compared with the IC50 of 0.85 µM for SAHA (Fig. 2C). As expected, the negative control compounds 3-52 and 1002 as well as Enz did not show significant inhibitory activity against HDAC6 even at a concentration of 25 µM (Fig. 2D). Again, trichostatin A served as the positive control in Fig. 2D.
Fig. 2.
Measurement of intrinsic and in situ HDACI activities. (A) Dose-dependent inhibition of HDACs from HeLa cell nuclear extract by 2-75, 1005, and SAHA. HeLa cell nuclear extract was incubated with each drug at the indicated concentrations. IC50 values were calculated from nonlinear regression plots using GraphPad Prism5 software. (B) Enz, 1002, and 3-52 were tested against HeLa cell nuclear extract at 10 µM and 25 µM and TSA (1 µM) was used as a positive control. (C) Dose-dependent inhibition of recombinant HDAC6 by 2-75, 1005, and SAHA. Recombinant HDAC6 was incubated with each drug at the indicated concentrations. IC50 values were calculated from nonlinear regression plots using GraphPad Prism5 software. (D) Enz, 1002, and 3-52 were tested against recombinant HDAC6 at 10 µM and 25 µM and TSA (1 µM) was used as a positive control. (E) LNCaP cells were treated with the indicated compounds (10 µM) or vehicle (DMSO) for 48 hours. Cells were then harvested to quantify mRNA for DLC1 and values were normalized to the values for GAPDH mRNA. (F) C4-2 cells were treated with the indicated concentrations of Enz, SAHA, 2-75, or 1005 or vehicle (DMSO) for 48 hours. Cells were then harvested to quantify mRNA for DLC1 and values were normalized to the values for GAPDH mRNA. In all panels, the error bars represent the standard deviation of experimental triplicates. Where indicated *, **, §, §§, P < 0.05. DMSO, dimethylsulfoxide; TSA, trichostatin A.
The HDACI activities of 2-75 and 1005 were also compared with those of their parent compounds in situ in both LNCaP and C4-2 PCa cells using activation of the deleted in liver cancer 1 (DLC1) tumor suppressor gene as the readout. SAHA induces histone acetylation at the DLC1 promoter and effectively increases DLC1 mRNA expression in PCa cells (Zhou et al., 2012). Accordingly, DLC1 mRNA was strongly upregulated by SAHA in both LNCaP cells (Fig. 2E) and in C4-2 cells (Fig. 2F). As expected from the fact that DLC1 is not an AR-regulated gene, Enz had no effect on DLC1 mRNA expression. Consistent with the results of the cell-free HDACI assays above, compounds 2-75 and 1005 were poor inducers of DLC1 mRNA compared with SAHA, both in LNCaP cells and in C4-2 cells (Fig. 2, E and F). Furthermore, similar to the results from cell-free assays, 1005 was a much weaker HDACI than 2-75 in the in situ assays (Fig. 2, E and F). Taken together, the results indicate that compounds 2-75 and, to a greater degree, 1005 have much less potent HDACI activity in the cellular context, reflecting their intrinsically weak inhibitor activities compared with SAHA.
The Partial Enz Chemical Scaffold Confers AR-Targeted Antagonist Activity without Ligand-Induced Chromatin Association of AR.
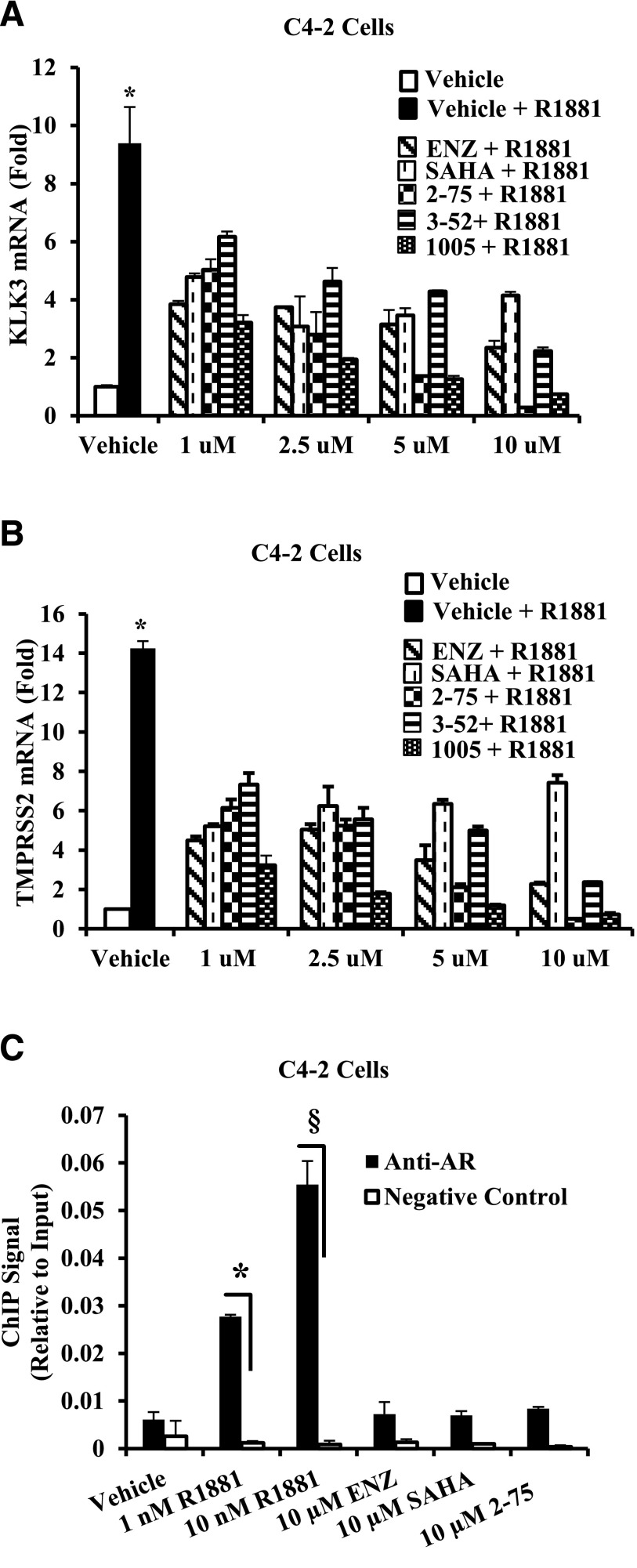
Although C4-2 cells are not growth inhibited by Enz because of hormone-independent actions of AR, the canonical androgen target genes kallikrein-related peptidase 3 (KLK3) and transmembrane protease, serine 2 (TMPRSS2) are activated by androgen and their activation is inhibited by androgen antagonists (Ratnam et al., 2013). HDACIs are also potent inhibitors of the androgen signaling axis because they cause degradation of AR in the cytosol in addition to other cellular effects (Chen et al., 2005; Gravina et al., 2013; Gryder et al., 2013). To test whether the Enz moiety could enable 2-75 and 1005 to target to AR, we tested the ability of compound 3-52 to inhibit activation of KLK3 and TMPRSS2 by androgen. Compound 3-52 shares the chemical scaffold of 2-75 but lacks the HDACI functional group; therefore, 3-52 should depend on the partial Enz chemical scaffold to antagonize gene activation by androgen. SAHA partially inhibited activation of KLK3 (Fig. 3A) and TMPRSS2 (Fig. 3B) by the synthetic androgen R1881 in C4-2 cells, whereas Enz showed progressive inhibition at higher doses. Compounds 2-75 and 1005 were both better inhibitors of gene activation by androgen compared with either Enz or SAHA (Fig. 3, A and B). On the other hand, compound 3-52 inhibited activation of KLK3 and TMPRSS2 to a degree that was comparable to Enz, suggesting that the partial Enz chemical scaffold in compounds 2-75 and 1005 retained an Enz-like AR binding property. The superior androgen antagonist activities of 2-75 and 1005 compared with Enz may be explained by their additional HDACI activities.
Fig. 3.
Testing the ability of compounds to interact with AR and to induce chromatin association of AR. (A and B) Data obtained using C4-2 cells are shown. After 96 hours of hormone depletion, cells were treated with R1881 (1 nM) and 1 µM, 2.5 µM, 5 µM, or 10 µM of the indicated compound or vehicle (DMSO) for 48 hours. Cells were then harvested to purify total RNA. The mRNAs for KLK3 and TMPRSS2 were quantified by normalizing to the values for GAPDH mRNA. In all panels, the error bars represent the standard deviation of experimental triplicates. (C) C4-2 cells plated in hormone-depleted medium were treated with vehicle, R1881, or the indicated compound for 2 hours. Cells were harvested and subjected to ChIP using AR antibody. TaqMan probes targeting androgen response element enhancer elements associated with the KLK3 gene were used to quantify the immunoprecipitated chromatin. In all panels, the error bars represent the standard deviation of experimental triplicates. Where indicated, * and §, P < 0.01.
AR ligands including agonists and classic androgen antagonists such as bicalutamide promote nuclear translocation of AR and the binding of AR to canonical hormone (androgen) response elements associated with androgen-regulated genes. In contrast, Enz does not stimulate AR nuclear translocation and DNA binding (Tran et al., 2009; Guerrero et al., 2013). To test whether the partial Enz chemical scaffold would mobilize AR to the chromatin, we employed ChIP using C4-2 cells treated with androgen, Enz, SAHA, and compound 2-75. As a target site for the ChIP assay, we chose the well established AR binding enhancer elements located 4 kb upstream of the transcription initiation site of the KLK3 gene. As seen in Fig. 3C, androgen treatment strongly stimulated chromatin association of AR, whereas Enz, SAHA, and compound 2-75 all gave the basal ChIP signal corresponding to the vehicle treatment control. These results suggest that the new compounds must antagonize AR in the cytosol rather than in the nuclear compartment.
Compounds 2-75 and 1005 Induce Enhanced Degradation of AR and HSP90 and Hyperacetylation in a Putative 55-kDa HSP90 Fragment.
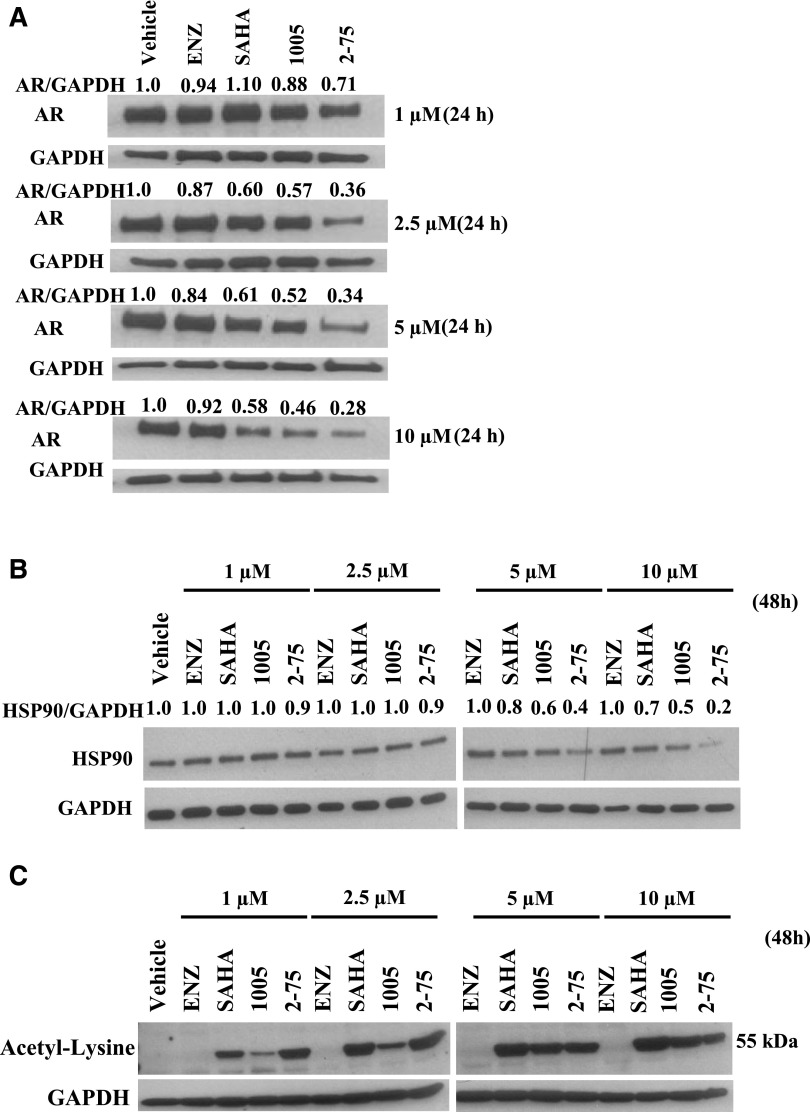
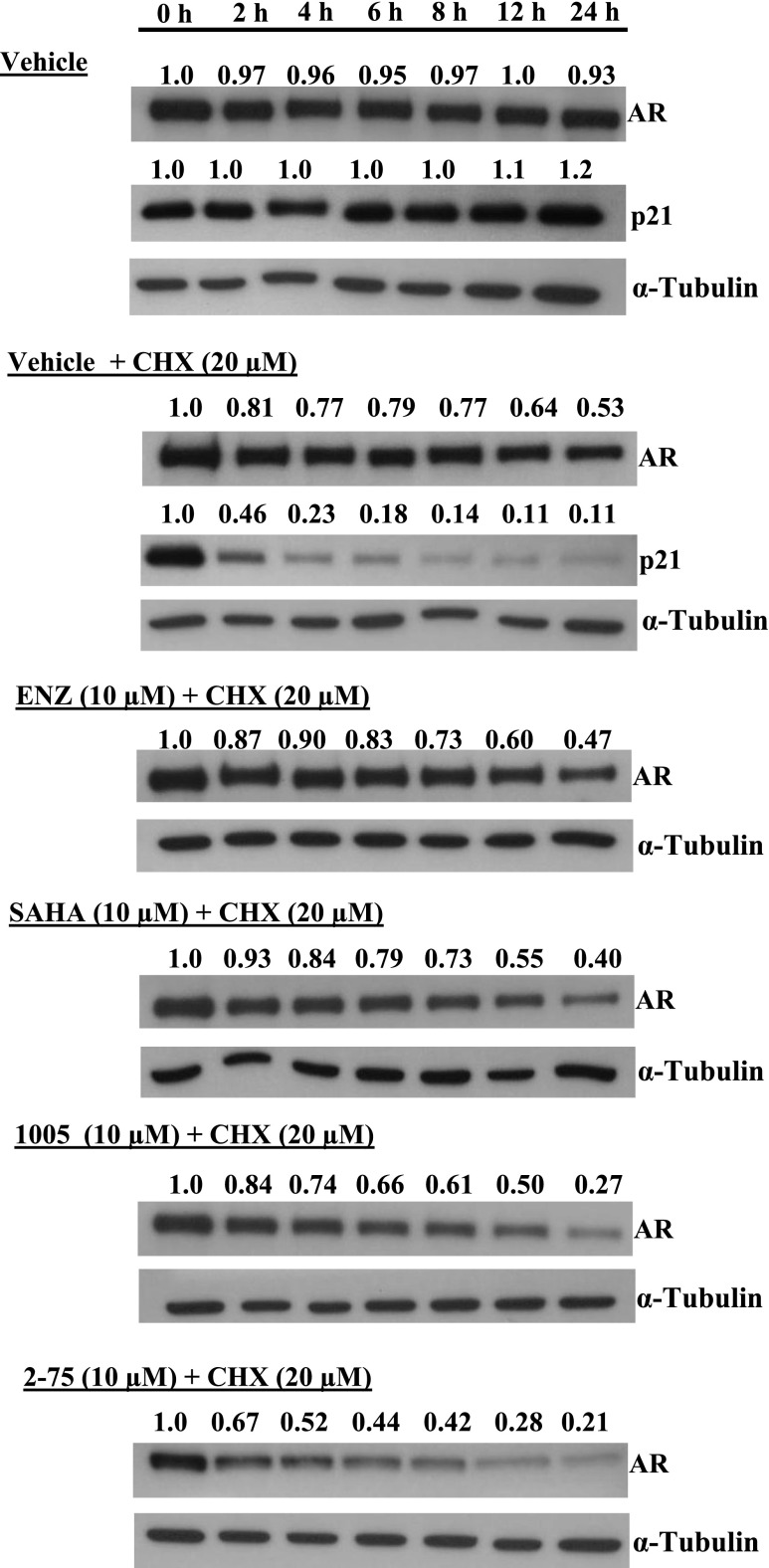
Previous observations using potent nontargeted HDACIs have shown that the compounds directly affect the AR signaling axis by hyperacetylation of the AR chaperone complex, through inhibition of HDAC6, leading to degradation of HSP90 as well as release and degradation of AR. We therefore hypothesized that despite their intrinsically weak HDACI activities, the Enz moiety may enable compounds 2-75 and 1005 to more effectively target AR in its chaperone complex, leading to relatively efficient degradation of AR. To test this possibility, we treated C4-2 cells with Enz, SAHA, 1005, and 2-75 at doses ranging from 1 µM to 10 µM for 24 hours. Western blots of the cell lysates were probed for AR and GAPDH (loading control) and the AR band intensities relative to GAPDH were quantified using ImageJ software (National Institutes of Health, Bethesda, MD) (Fig. 4A). Whereas Enz did not cause an appreciable change in the AR protein level, SAHA did cause a decrease in AR level in a dose-dependent manner (Fig. 4A). Compared with SAHA, both 2-75 and 1005 decreased the AR level to a greater extent, with 2-75 being more effective than 1005 at each dose (Fig. 4A). To determine whether the decrease in AR was attributable to an increase in the rate of AR degradation, we tested the effects of the compounds after blocking de novo protein synthesis using cycloheximide. We monitored degradation of p21 to confirm the activity of cycloheximide. As expected, there was a rapid decrease in p21 upon treatment with cycloheximide, confirming that the treatment efficiently blocked de novo protein synthesis (Fig. 5). In the presence of cycloheximide, the AR protein level was decreased by approximately one-half at the end of 24 hours, indicating a relatively slow turnover of the AR protein. Under these conditions, treatment with SAHA, 1005, and 2-75 all caused greater declines in the AR level, with 2-75 showing the strongest effect (Fig. 5). The results indicate that the decrease in AR caused by 1005 and 2-75 is due to increased degradation of AR. The extent of degradation of AR in C4-2 cells appeared adequate to offset the high level of overexpression of AR that is necessary to support growth in these cells.
Fig. 4.
Modulation of protein levels and hyperacetylation. C4-2 cells were treated with the indicated concentrations of Enz, SAHA, 1005, or 2-75 or with vehicle (dimethylsulfoxide) for the time indicated. Cells were then harvested for Western blot analysis using antibody to AR (A), HSP90 (B), acetyl lysine (C), or GAPDH (loading control). ImageJ software was used to determine the intensities of the bands relative to the vehicle control for each protein. The values were then divided by the values for GAPDH within the same samples.
Fig. 5.
Induction of AR degradation. C4-2 cells were pretreated with cycloheximide (20 µM) or with vehicle for 2 hours, followed by the introduction of Enz (10 µM), SAHA (10 µM), 1005 (10 µM), 2-75 (10 µM), or vehicle for the indicated durations. Cells were then harvested for Western blot analysis and probed with antibody to AR, p21, or α-tubulin (loading control). ImageJ software was used to determine the intensities of the bands relative to the 0-hour time point for each treatment. CHX, cycloheximide.
To explore a possible link between decreased AR levels and effects of the compounds on the AR chaperone complex, we examined whether compounds 2-75 and 1005 decreased the level of HSP90. Probing of the lysates from the treated cells (48-hour treatment) for HSP90 by Western blot and quantification of HSP90 was conducted by procedures similar to those used above for AR. Enz had no effect on the level of HSP90, whereas a decrease in HSP90 was evident at the higher doses in the SAHA-treated cells (5 µM and 10 µM) (Fig. 4B). On the other hand, cells treated with 1005 and 2-75 showed more marked reduction in HSP90, with 2-75 being more efficient than 1005. Probing identical Western blots with an antibody against acetylated lysine showed that SAHA as well as 1005 and 2-75, but not Enz, showed hyperacetylation of an approximately 55-kDa polypeptide (Fig. 4C), similar to one that has previously been identified as a fragment HSP90 produced by SAHA treatment (Park et al., 2015). Taken together, the above results are consistent with the view that the ability of the compounds to induce acetylation and reduction of HSP90, and consequently AR degradation, underlies the ability of the compounds to attenuate AR signaling.
The Hybrid Molecules Selectively Inhibit Cytosolic HDAC6 In Situ.
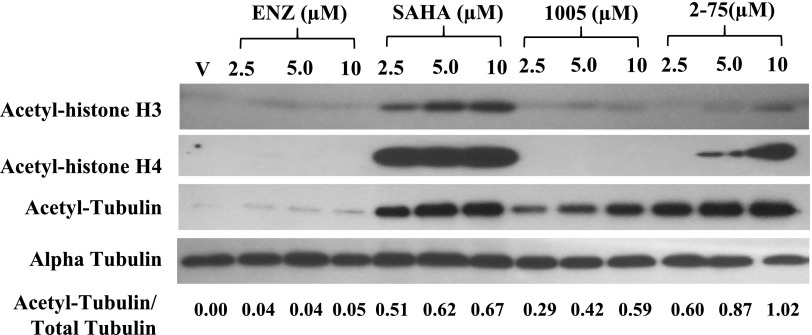
Because HSP90 in the AR chaperone complex is a target of the cytosolic HDAC6, the hyperacetylation of degradation of HSP90 induced by 2-75 and 1005 is likely to occur through inhibition of HDAC6. If this were the case, we may expect that 2-75 and 1005 would also induce hyperacetylation of α-tubulin which is diagnostic of HDAC6 inhibition. To test this possibility, we treated C4-2 cells with Enz, SAHA, 1005, and 2-75 at doses ranging from 2.5 µM to 10 µM for 24 hours. Western blots of the cell lysates were probed for acetyl tubulin, as well as total α-tubulin. The band intensities for acetyl tubulin relative to total α-tubulin were quantified using ImageJ software (Fig. 6). Compound 2-75 induced a greater degree of hyperacetylation of α-tubulin (relative to total tubulin) compared with SAHA, whereas 1005 produced a similar effect, albeit to a somewhat lesser degree than SAHA (Fig. 6). When the same cell lysates were probed using antibodies against acetylated histones H3 and H4, it was clear that SAHA alone induced a strong induction of histone acetylation (Fig. 6). The results clearly demonstrate strong and selective in situ activity of 2-75 and 1005 on cytosolic HDAC6.
Fig. 6.
Hyperacetylation of α-tubulin and histones H3 and H4. C4-2 cells were treated with Enz, SAHA, 1005, or 2-75 (2.5 µM, 5 µM, or 10 µM) or vehicle for 24 hours. Cells were then harvested for Western blot analysis and probed with antibody to acetyl histone H3, acetyl histone H4, acetyl tubulin, or α-tubulin. ImageJ software was used to determine the intensities of the bands of acetyl tubulin relative to the total amount of tubulin for each treatment. The ratio of acetyl tubulin to total tubulin in each sample is indicated.
Compounds 2-75 and 1005 Upregulate p21 and Inhibit Viability of Enz-Resistant PCa Cells.
HDACIs activate transcription of p21. However, because compounds 2-75 and 1005 exhibited weak intrinsic HDACI activity against nuclear HDACs and because their apparent major cellular HDACI activity was related to targeting of the AR axis within the cytosolic compartment, it was of interest to examine their ability to induce p21.
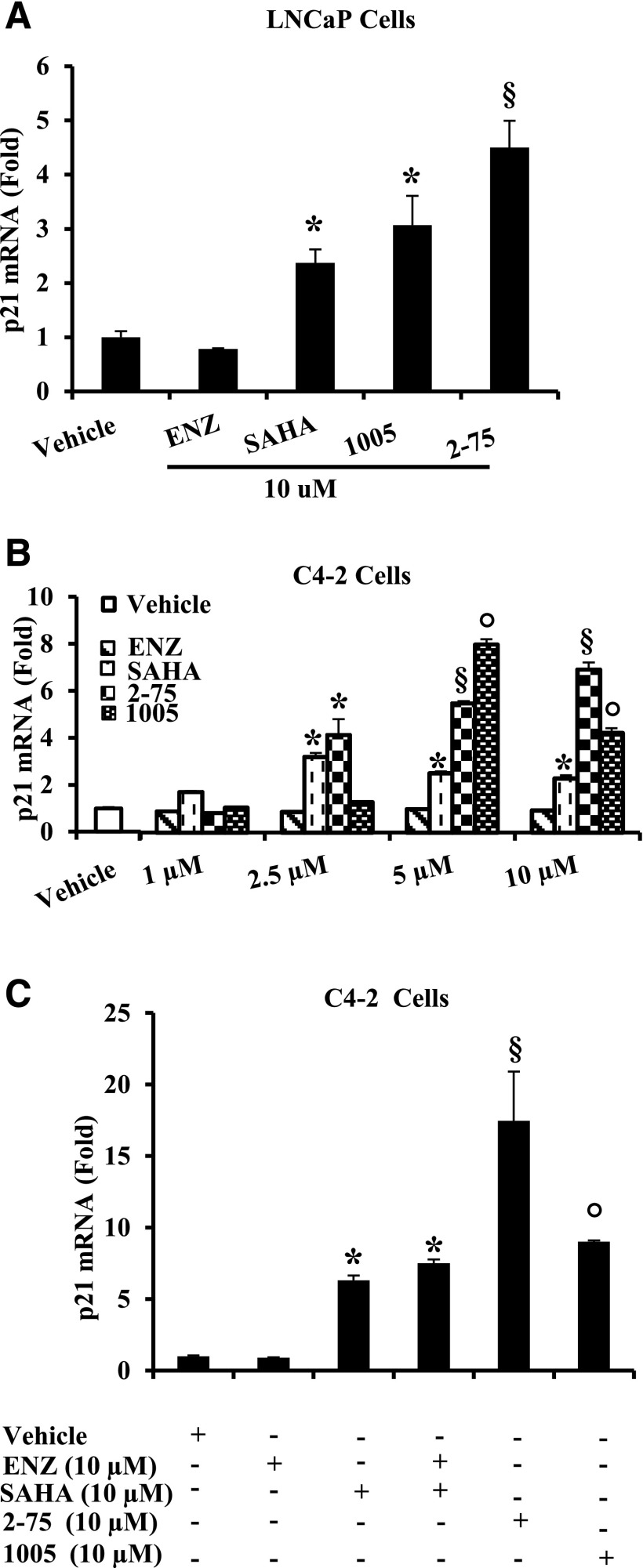
Enz had no effect on p21 mRNA expression in either the Enz-sensitive LNCaP cells (Fig. 7A) or in the Enz-insensitive C4-2 cells (Fig. 7B), whereas SAHA induced p21 mRNA in both cell lines (Fig. 7, A and B). Compounds 2-75 and 1005 both induced p21 to a greater extent than SAHA in the two cell lines (Fig. 7, A and B). Moreover, combined treatment with equimolar concentrations of Enz and SAHA did not induce p21 to a greater extent than SAHA alone, indicating the importance of the hybrid scaffold of 2-75 and 1005 (Fig. 7C).
Fig. 7.
Induction of p21 mRNA. (A) LNCaP cells were treated with Enz, SAHA, 1005, or 2-75 at a concentration of 10 µM or with vehicle (dimethylsulfoxide) for 48 hours. Cells were then harvested to quantify p21 mRNA and the values were normalized to those for GAPDH mRNA. (B) C4-2 cells were treated with Enz, SAHA, 1005, or 2-75 at the indicated concentrations or with vehicle (dimethylsulfoxide) for 48 hours. Cells were then harvested to quantify p21 mRNA and the values were normalized to those for GAPDH mRNA. (C) C4-2 cells were treated with either Enz (10 µM) or SAHA (10 µM), an equimolar (10 µM each) mixture of Enz and SAHA, 2-75 (10 µM), or 1005 (10 µM). Cells were then harvested to quantify p21 mRNA and the values were normalized to those for GAPDH mRNA. In all panels, the error bars represent the standard deviation of experimental triplicates. Where indicated, *, §, ○ P < 0.05.
To expect therapeutic effects from 2-75 and 1005, it is important to establish that, similar to SAHA, they can induce loss of viability in Enz-resistant CRPC cells, rather than mere growth inhibition. Therefore, the effects of 2-75, 1005, and SAHA on cell viability were assessed in the well established C4-2 model of Enz-resistant CRPC.
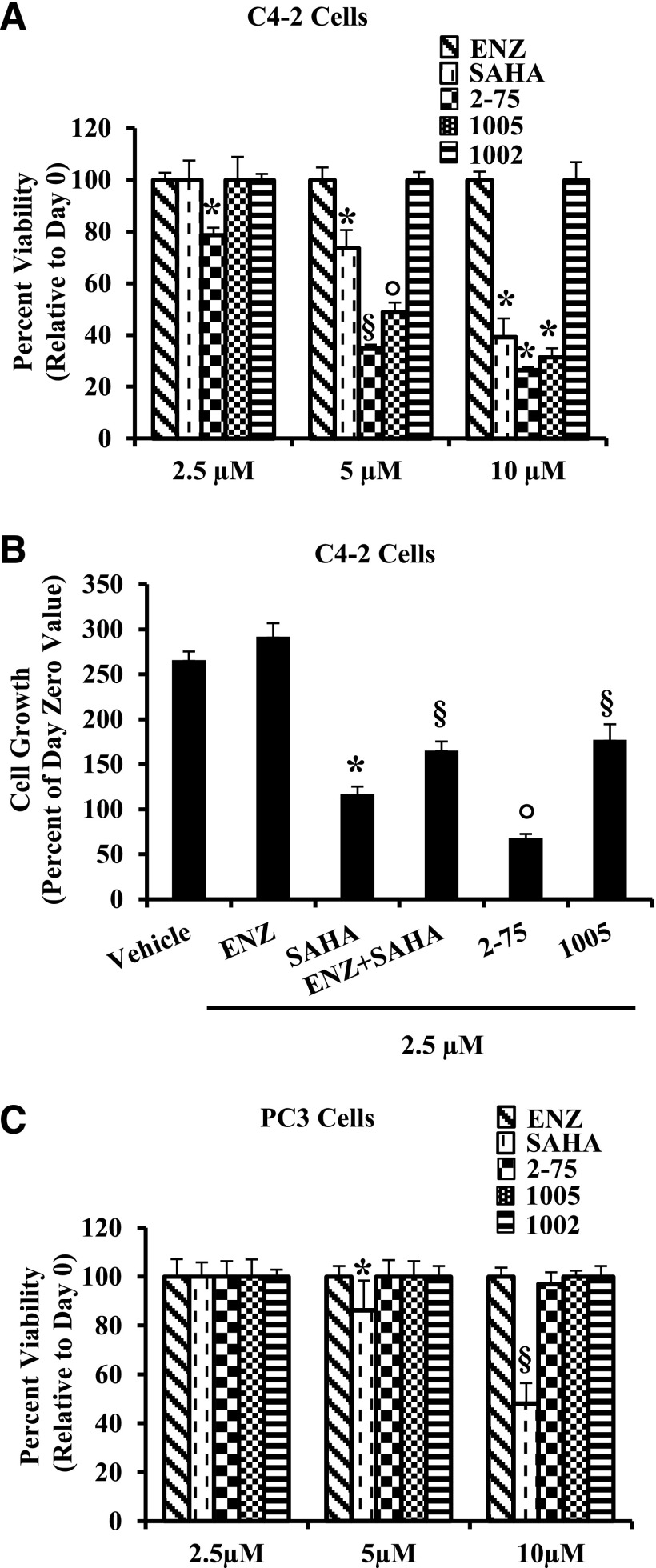
In C4-2 cells, Enz could not appreciably affect viability even at a concentration of 10 µM, whereas SAHA caused loss of viability in a dose-dependent manner (Fig. 8A). Compounds 2-75 and 1005 both caused greater loss of viability compared with SAHA, with compound 2-75 being more effective than 1005 (Fig. 8A). As a control, compound 1002, which has a chemical scaffold similar to 1005 but lacks the HDACI activity (Fig. 2, B and D), was unable to affect C4-2 cell viability (Fig. 8A). As another experimental control, although SAHA, 2-75, and 1005 caused growth inhibition, combining Enz with SAHA (each at 2.5 μM) did not enhance the ability of SAHA to inhibit cell growth at the lower drug concentration (2.5 μM) (Fig. 8B). In the AR-negative PC3 PCa cells, SAHA induced loss of viability in a dose-dependent manner (Fig. 8C). However, in contrast with C4-2 cells, neither 2-75 nor 1005 affected viability of PC3 cells within the duration of the assay (Fig. 8C). As expected, the AR-positive and hormone-dependent LNCaP cells were sensitive to SAHA, 2-75, and 1005 as well as Enz (Supplemental Fig. 1).
Fig. 8.
Effects on cell viability in Enz-resistant CRPC cells. (A) C4-2 cells were seeded in 96-well plates and 24 hours later, they were treated with the indicated compounds (2.5 µM, 5 µM, or 10 µM) or with vehicle (dimethylsulfoxide). Cell density was measured by the MTT assay on days 0 and 3 of treatment. Values equal to or above that on day 0 were considered to represent 100% viability. (B) C4-2 cells were seeded and treated 24 hours later with Enz (2.5 µM), SAHA (2.5 µM), an equimolar mixture of Enz and SAHA (each compound at 2.5 µM), 2-75 (2.5 µM), 1005 (2.5 µM), or vehicle (dimethylsulfoxide). Cell density was measured by the MTT assay on days 0 and 3 of treatment. The y-axis shows percent cell growth on day 3 relative to the cell density on day 0. (C) PC3 cells were seeded in 96-well plates and 24 hours later, they were treated with the indicated compounds (2.5 µM, 5 µM, or 10 µM) or with vehicle (dimethylsulfoxide). Cell density was measured by the MTT assay on days 0 and 3 of treatment. Values equal to or above that on day 0 were considered to represent 100% viability. In all panels, the error bars represent the standard deviation of experimental sextuplicate samples. Where indicated, *, §, ○ P < 0.05. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
The results indicate that despite the weaker inherent HDACI activities of 2-75 and 1005 compared with SAHA, the compounds could be as good or better at reducing viability of Enz-resistant and AR-overexpressing PCa cells.
Discussion
The success of clinical interventions in PCa, including surgical or chemical castration and treatment with androgen antagonists and androgen synthesis inhibitors, supports the view that the majority of prostate tumors are addicted to AR to support PCa growth and progression (Zegarra-Moro et al., 2002). Nevertheless, the current interventions that target androgen/AR signaling are circumvented by the tumors, most commonly through mechanisms that restore functional AR (Feldman and Feldman, 2001; Yuan and Balk, 2009), resulting in short-lived clinical benefit from the treatments. The goal of this study was to develop a class of compounds that may overcome this manner of resistance to the conventional treatments by efficiently disrupting both AR and HSP90 in the AR-HSP90 complex with minimal effects on most other cellular targets. To accomplish this, we synthesized compounds that would incorporate properties of two well known drugs: 1) an HDACI (SAHA) that efficiently modifies and disrupts the cytosolic AR chaperone complex and 2) an AR ligand (Enz) that is a high-affinity AR antagonist. We additionally sought to substantially weaken the intrinsic HDACI activity of the drug to minimize its ability to affect many targets. The studies described above suggest that compounds 2-75 and 1005 may be prototype molecules that fit this paradigm.
The HDACI functional groups in 2-75 and 1005 conferred only weak HDACI activity in cell-free assays using either nuclear HDACs or the cytosolic HDAC6 compared with SAHA; the shorter carbon chain in 1005 resulted in even weaker HDACI activity than 2-75. The relative potencies of HDACI inhibition of 2-75 and 1005 were clearly reflected in their relatively poor ability to induce DLC1, an established nuclear target gene of HDACIs (Guan et al., 2006; Zhou et al., 2010, 2012), which is strongly induced by SAHA. Compounds 2-75 and 1005 were also poor modulators of histone acetylation in situ compared with SAHA. Therefore, the new molecules may have less toxic effects than those associated with the potent pan-HDACI activity of SAHA (Bradley et al., 2009).
SAHA partially inhibited gene activation by androgen but did not produce a further dose-dependent inhibition between 1-µM and 10-µM concentrations. At this time, we do not have a clear explanation for why this effect of SAHA was only partial, except that it may be related to the pleiotropic cellular effects of SAHA, including its effects on crosstalking molecular pathways. More important, 2-75 and 1005 produced a dose-dependent inhibition of gene activation by androgen similar to Enz. The stronger inhibition observed for the compounds compared with Enz may be attributed to their HDACI moieties. However, the close parallel between the control compound 3-52 and Enz in their dose-dependent antagonism of gene activation by androgen, despite the lack of a HDACI functional group in 3-52, indicates that the Enz moiety in 2-75 and 1005 is functional in enabling binding to AR. In addition, ChIP analysis showed that the modified Enz scaffold retained the inability of Enz to mobilize AR to its chromatin binding sites in the nucleus in contrast with conventional androgen antagonists.
In the context of targeted delivery to AR via their Enz moiety, the weak intrinsic HDACI activities of 2-75 and 1005 were adequate to mimic or surpass the effects of SAHA on AR protein levels. The efficiency of degradation of AR by 1005 was comparable to SAHA but 2-75 clearly induced AR degradation to a greater degree at each dose. This difference between 2-75 and 1005 may be related to the fact that the HDACI activity of 1005 was less than that of 2-75, despite their common Enz moiety. To test the mechanism by which 2-75 and 1005 may cause AR degradation, we relied on literature reports that HDACIs destabilize and degrade AR by hyperacetylating and inducing degradation of the cytosolic AR chaperone protein, HSP90 (Chen et al., 2005; Gravina et al., 2013; Gryder et al., 2013). It has also been reported that the hyperacetylation and degradation of HSP90 coincides with the appearance of an approximately 55-kDa HSP90 polypeptide fragment. As a diagnostic test of this mechanism, we observed that similar to SAHA, 2-75 and 1005 did indeed cause a decrease in HSP90, with 2-75 being more efficient than either SAHA or 1005. We were able to observe the predicted approximately 55-kDa fragment in cells treated with SAHA, 2-75, or 1005 using an antibody against acetylated lysine; however, our antibody against HSP90 was unable to detect this fragment, possibly because the levels of the cleaved HSP90 fragment were too low to be in the detectable range of the antibody. Nevertheless, all indications point to the AR chaperone complex in the cytosol as mediating the action of 2-75 and 1005. Consistent with this view, HDAC6 (which is associated with the AR-HSP90 complex) was more strongly and selectively inhibited in situ by 2-75 compared with SAHA. Indeed, 1005 had weaker intrinsic HDACI activity than 2-75 and also strongly inhibited HDAC6 in situ with virtually no effect on histone acetylation.
An increase in the expression of the cyclin-dependent kinase inhibitor p21 (Gartel and Tyner, 2002) is a hallmark of the antiproliferative effects of HDACI (Gui et al., 2004; Marks, 2004). Potent inhibition of the nuclear HDAC1 at the promoter of the p21 gene is associated with induction of p21 by HDACI (Lagger et al., 2003). Inhibitors of HSP90 also increase p21 expression in PCa cells (Chen et al., 2005). Therefore, it is significant that p21 mRNA was induced by both 2-75 and 1005 more strongly than SAHA and that the combination with Enz did not further increase p21 induction by SAHA. The inability of Enz to induce p21 in the hormone-dependent LNCaP cells despite the sensitivity of the AR signaling in these cells to Enz suggests that induction of p21 by 2-75 and 1005 may not be directly related to disruption of AR; rather, it may be a result of their effects on additional HSP90 client proteins through AR-mediated targeting of HDACI activity to HSP90. Notably, AKT and glucocorticoid receptor are also HSP90 client proteins and upregulated AKT or glucocorticoid receptor signaling was reported to result in Enz resistance (Arora et al., 2013; Bitting and Armstrong, 2013; Montgomery et al., 2015; Toren et al., 2015). Therefore, these pathways could be involved in the loss of viability induced by the compounds.
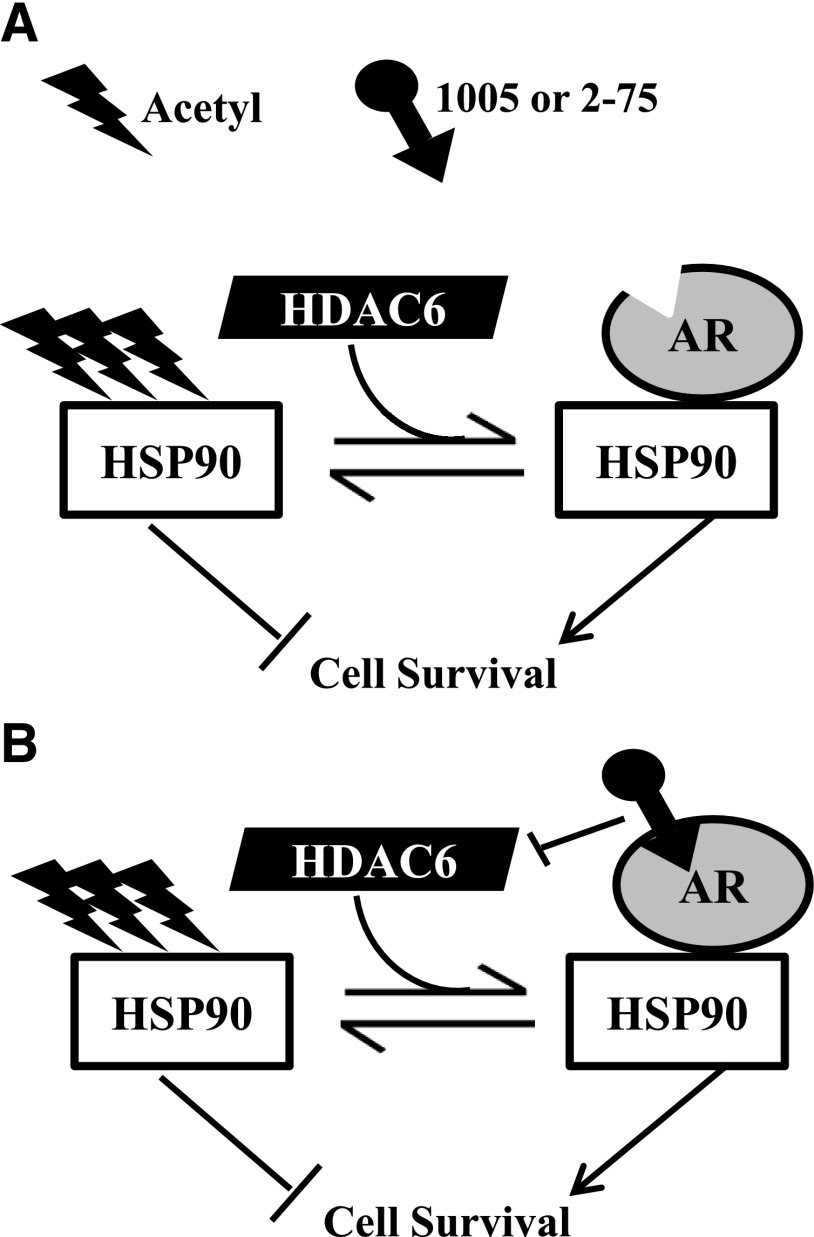
HDACI pharmacophores have previously been linked to a chemical scaffold of cyanonilutamide, which is another nonsteroidal AR antagonist (Gryder et al., 2013). However, the antiproliferative effects of cyanonilutamide-HDACIs were related to their ability to induce AR nuclear localization, enabling elevated local concentrations of HDACI activities in the nucleus. In contrast, our prototype drug molecules were designed to limit nuclear HDACI activities as an approach to limiting toxicity. Our working model that would need further testing is that in C4-2 cells, 2-75 and 1005 bind to cytosolic AR and inhibit HDAC6 associated with the AR chaperone complex, resulting in HSP90 acetylation and degradation, AR degradation, suppression of ligand-insensitive gene activation by AR, and inhibition HSP90 interactions with additional client proteins (schematic in Fig. 9). We propose that this mechanism may address some of the limitations of strong pan-HDACIs related to toxicity.
Fig. 9.
Mechanistic model for the actions of compounds 1005 and 2-75. (A) In the absence of bound ligand, AR is in a stable complex with HSP90, which is maintained in a hypoacetylated state by HDAC6. Hypoacetylated HSP90 stabilizes AR and also supports cell survival through regulation of its other client proteins. When 2-75 or 1005 bind to AR in the chaperone complex, their HDACI moieties inhibit HDAC6 that associates with the complex. Despite their relatively weak intrinsic HDACI activities, the efficiency of HDAC6 inhibition by the hybrid molecules is enhanced by their localized effect in the chaperone complex. (B) This results in hyperacetylation of HSP90, leading to destabilization of AR and also loss of cell survival through deregulation of other HSP90 client proteins.
Supplementary Material
Acknowledgments
The authors thank Yanfang Huang for technical support.
Abbreviations
- AR
androgen receptor
- ChIP
chromatin immunoprecipitation
- CRPC
castration-recurrent prostate cancer
- DLC1
deleted in liver cancer 1
- Enz
enzalutamide
- FBS
fetal bovine serum
- FK-228
cyclo[(2Z)-2-amino-2-butenoyl-l-valyl-(3S,4E)-3-hydroxy-7-mercapto-4-heptenoyl-d-valyl-d-cysteinyl],cyclic(3-5)disulfide
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HDAC
histone deacetylase
- HDACI
histone deacetylase inhibitor
- HSP90
heat shock protein 90
- KLK3
kallikrein-related peptidase 3
- LAQ-824
(2E)-N-hydroxy-3-[4-[[(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide
- LBH-589
(2E)-N-hydroxy-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]acrylamide
- 1002
(E)-ethyl 3-(4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluorophenyl)acrylate
- 1005
(E)-3-(4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluorophenyl)-N-hydroxyacrylamide
- PCa
prostate cancer
- PCR
polymerase chain reaction
- PDX-101
N-hydroxy-3-[3-](phenylamino)sulfonyl]phenyl]-2-propenamide
- R1881
17β-17-hydroxy-17-methyl-estra-4,9,11-trien-3-one
- SAHA
suberoylanilide hydroxamic acid
- SB-939
(E)-3-(2-butyl-1-(2-(diethylamino)ethyl)-1H-benzo[d]imidazol-5-yl)-N-hydroxyacrylamide
- 3-52
methyl 7-(4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluorobenzamido)heptanoate
- TMPRSS2
transmembrane protease, serine 2
- 2-75
4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-(7-(hydroxyamino)-7-oxoheptyl)benzamide
Authorship Contributions
Participated in research design: Rosati, Chen, Patki, McFall, Ou, Ratnam, Qin.
Conducted experiments: Rosati, Chen, Patki, Ou, Qin.
Contributed new reagents or analytic tools: Rosati, Chen, McFall, Ou, Heath, Ratnam, Qin.
Performed data analysis: Rosati, Chen, McFall, Ou, Ratnam, Qin.
Wrote or contributed to the writing of the manuscript: Rosati, Chen, Ratnam, Qin.
Footnotes
This research was supported by the U.S. Department of Defense [Prostate Cancer Research Program Idea Development Award W81XWH-12-1-0340 (to Z.Q.)], Barbara Ann Karmanos Cancer Institute [Strategic Research Initiative Grant KCI-2014-1 (to Z.Q. and M.R.)], and Wayne State University School of Medicine [Cancer Biology Program NRSA-T32 Training Grant Fellowship 1T32 CA009531 (to R.R.)]. This study used the Wayne State University School of Medicine Pharmacology Core, which is supported, in part, by the National Institutes of Health National Cancer Institute [Grant P30-CA022453 (to the Barbara Ann Karmanos Cancer Institute at Wayne State University)].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis C, et al. (2013) Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155:1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbas MD, Evans MJ, Hosfield DJ, Wongvipat J, Arora VK, Watson PA, Chen Y, Greene GL, Shen Y, Sawyers CL. (2013) Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife 2:e00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, et al. (2005) Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem 280:26729–26734. [DOI] [PubMed] [Google Scholar]
- Basak S, Pookot D, Noonan EJ, Dahiya R. (2008) Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol Cancer Ther 7:3195–3202. [DOI] [PubMed] [Google Scholar]
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, et al. PREVAIL Investigators (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Tummalapalli SR, Rotella DP. (2014) Progress in the discovery and development of heat shock protein 90 (Hsp90) inhibitors. J Med Chem 57:8718–8728. [DOI] [PubMed] [Google Scholar]
- Bitting RL, Armstrong AJ. (2013) Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr Relat Cancer 20:R83–R99. [DOI] [PubMed] [Google Scholar]
- Bradley D, Rathkopf D, Dunn R, Stadler WM, Liu G, Smith DC, Pili R, Zwiebel J, Scher H, Hussain M. (2009) Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862): trial results and interleukin-6 analysis: a study by the Department of Defense Prostate Cancer Clinical Trial Consortium and University of Chicago Phase 2 Consortium. Cancer 115:5541–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdelski C, Ruge OM, Melling N, Koop C, Simon R, Steurer S, Sauter G, Kluth M, Hube-Magg C, Minner S, et al. (2015) HDAC1 overexpression independently predicts biochemical recurrence and is associated with rapid tumor cell proliferation and genomic instability in prostate cancer. Exp Mol Pathol 98:419–426. [DOI] [PubMed] [Google Scholar]
- Cacchi S, Fabrizi G, Goggiamani A. (2003) Palladium-catalyzed hydroxycarbonylation of aryl and vinyl halides or triflates by acetic anhydride and formate anions. Org Lett 5:4269–4272. [DOI] [PubMed] [Google Scholar]
- Chen L, Meng S, Wang H, Bali P, Bai W, Li B, Atadja P, Bhalla KN, Wu J. (2005) Chemical ablation of androgen receptor in prostate cancer cells by the histone deacetylase inhibitor LAQ824. Mol Cancer Ther 4:1311–1319. [DOI] [PubMed] [Google Scholar]
- Chen Y, Clegg NJ, Scher HI. (2009) Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target. Lancet Oncol 10:981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigl BJ, North S, Winquist E, Finch D, Wood L, Sridhar SS, Powers J, Good J, Sharma M, Squire JA, et al. (2015) A phase II study of the HDAC inhibitor SB939 in patients with castration resistant prostate cancer: NCIC clinical trials group study IND195. Invest New Drugs 33:969–976. [DOI] [PubMed] [Google Scholar]
- Fang Y, Fliss AE, Robins DM, Caplan AJ. (1996) Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem 271:28697–28702. [DOI] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D. (2001) The development of androgen-independent prostate cancer. Nat Rev Cancer 1:34–45. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Tyner AL. (2002) The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther 1:639–649. [PubMed] [Google Scholar]
- Gibbs A, Schwartzman J, Deng V, Alumkal J. (2009) Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc Natl Acad Sci USA 106:16663–16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravina GL, Marampon F, Muzi P, Mancini A, Piccolella M, Negri-Cesi P, Motta M, Lenzi A, Di Cesare E, Tombolini V, et al. (2013) PXD101 potentiates hormonal therapy and prevents the onset of castration-resistant phenotype modulating androgen receptor, HSP90, and CRM1 in preclinical models of prostate cancer. Endocr Relat Cancer 20:321–337. [DOI] [PubMed] [Google Scholar]
- Gryder BE, Akbashev MJ, Rood MK, Raftery ED, Meyers WM, Dillard P, Khan S, Oyelere AK. (2013) Selectively targeting prostate cancer with antiandrogen equipped histone deacetylase inhibitors. ACS Chem Biol 8:2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan M, Zhou X, Soulitzis N, Spandidos DA, Popescu NC. (2006) Aberrant methylation and deacetylation of deleted in liver cancer-1 gene in prostate cancer: potential clinical applications. Clin Cancer Res 12:1412–1419. [DOI] [PubMed] [Google Scholar]
- Guerrero J, Alfaro IE, Gómez F, Protter AA, Bernales S. (2013) Enzalutamide, an androgen receptor signaling inhibitor, induces tumor regression in a mouse model of castration-resistant prostate cancer. Prostate 73:1291–1305. [DOI] [PubMed] [Google Scholar]
- Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. (2004) Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci USA 101:1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Zhang C, Shafi AA, Sequeira M, Acquaviva J, Friedland JC, Sang J, Smith DL, Weigel NL, Wada Y, et al. (2013) Potent activity of the Hsp90 inhibitor ganetespib in prostate cancer cells irrespective of androgen receptor status or variant receptor expression. Int J Oncol 42:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath EI, Hillman DW, Vaishampayan U, Sheng S, Sarkar F, Harper F, Gaskins M, Pitot HC, Tan W, Ivy SP, et al. (2008) A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin Cancer Res 14:7940–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang GM, Wang HS, Zhang F, Zhang KS, Liu ZC, Fang R, Wang H, Cai SH, Du J. (2013) Histone deacetylase inhibitor induction of epithelial-mesenchymal transitions via up-regulation of Snail facilitates cancer progression. Biochim Biophys Acta 1833:663–671. [DOI] [PubMed] [Google Scholar]
- Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, Moon M, Maneval EC, Chen I, Darimont B, et al. (2013) A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov 3:1020–1029. [DOI] [PubMed] [Google Scholar]
- Karantanos T, Evans CP, Tombal B, Thompson TC, Montironi R, Isaacs WB. (2015) Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol 67:470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Ahmad A, Bao B, Li Y, Banerjee S, Sarkar FH. (2012) Histone deacetylase inhibitors induce epithelial-to-mesenchymal transition in prostate cancer cells. PLoS One 7:e45045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, Yuan J, Kovats SG, Kim S, Cooke VG, et al. (2013) An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov 3:1030–1043. [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. (2005) HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18:601–607. [DOI] [PubMed] [Google Scholar]
- Krämer OH, Mahboobi S, Sellmer A. (2014) Drugging the HDAC6-HSP90 interplay in malignant cells. Trends Pharmacol Sci 35:501–509. [DOI] [PubMed] [Google Scholar]
- Lagger G, Doetzlhofer A, Schuettengruber B, Haidweger E, Simboeck E, Tischler J, Chiocca S, Suske G, Rotheneder H, Wintersberger E, et al. (2003) The tumor suppressor p53 and histone deacetylase 1 are antagonistic regulators of the cyclin-dependent kinase inhibitor p21/WAF1/CIP1 gene. Mol Cell Biol 23:2669–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. (2013) Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res 73:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhang Z, Tang H, Jiang Z, You L, Liao Y. (2014) Crosstalk between IGF-1R and other tumor promoting pathways. Curr Pharm Des 20:2912–2921. [DOI] [PubMed] [Google Scholar]
- Liu W, Vielhauer GA, Holzbeierlein JM, Zhao H, Ghosh S, Brown D, Lee E, Blagg BS. (2015) KU675, a concomitant heat-shock protein inhibitor of Hsp90 and Hsc70 that manifests isoform selectivity for Hsp90α in prostate cancer cells. Mol Pharmacol 88:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PA. (2004) The mechanism of the anti-tumor activity of the histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA). Cell Cycle 3:534–535. [DOI] [PubMed] [Google Scholar]
- Marrocco DL, Tilley WD, Bianco-Miotto T, Evdokiou A, Scher HI, Rifkind RA, Marks PA, Richon VM, Butler LM. (2007) Suberoylanilide hydroxamic acid (vorinostat) represses androgen receptor expression and acts synergistically with an androgen receptor antagonist to inhibit prostate cancer cell proliferation. Mol Cancer Ther 6:51–60. [DOI] [PubMed] [Google Scholar]
- McFall T, Patki M, Rosati R, Ratnam M. (2015) Role of the short isoform of the progesterone receptor in breast cancer cell invasiveness at estrogen and progesterone levels in the pre- and post-menopausal ranges. Oncotarget 6:33146–33164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades N. (2013) A road map to comprehensive androgen receptor axis targeting for castration-resistant prostate cancer. Cancer Res 73:4599–4605. [DOI] [PubMed] [Google Scholar]
- Molife LR, Attard G, Fong PC, Karavasilis V, Reid AH, Patterson S, Riggs CE, Jr, Higano C, Stadler WM, McCulloch W, et al. (2010) Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC). Ann Oncol 21:109–113. [DOI] [PubMed] [Google Scholar]
- Montgomery B, Kheoh T, Molina A, Li J, Bellmunt J, Tran N, Loriot Y, Efstathiou E, Ryan CJ, Scher HI, et al. (2015) Impact of baseline corticosteroids on survival and steroid androgens in metastatic castration-resistant prostate cancer: exploratory analysis from COU-AA-301. Eur Urol 67:866–873. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Suzuki T, Nakabayashi M, Endoh M, Sakamoto K, Mikami Y, Moriya T, Ito A, Takahashi S, Yamada S, et al. (2005) In situ androgen producing enzymes in human prostate cancer. Endocr Relat Cancer 12:101–107. [DOI] [PubMed] [Google Scholar]
- Neckers L, Workman P. (2012) Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WK, Galsky MD, Stadler WM, Srinivas S, Chu F, Bubley G, Goddard J, Dunbar J, Ross RW. (2011) Multicenter phase II trial of the heat shock protein 90 inhibitor, retaspimycin hydrochloride (IPI-504), in patients with castration-resistant prostate cancer. Urology 78:626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, Arkenau HT, Moreno-Farre J, Banerji U, Roels B, et al. (2011) A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res 17:1561–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JA, Kim YE, Song S, Kwon HJ, Lee Y. (2015) Suberoylanilide hydroxamic acid induces ROS-mediated cleavage of HSP90 in leukemia cells. Cell Stress Chaperones 20:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki M, Chari V, Sivakumaran S, Gonit M, Trumbly R, Ratnam M. (2013) The ETS domain transcription factor ELK1 directs a critical component of growth signaling by the androgen receptor in prostate cancer cells. J Biol Chem 288:11047–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki M, Gadgeel S, Huang Y, McFall T, Shields AF, Matherly LH, Bepler G, Ratnam M. (2014) Glucocorticoid receptor status is a principal determinant of variability in the sensitivity of non-small-cell lung cancer cells to pemetrexed. J Thorac Oncol 9:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki M, Huang Y, Ratnam M. (2016) Restoration of the cellular secretory milieu overrides androgen dependence of in vivo generated castration resistant prostate cancer cells overexpressing the androgen receptor. Biochem Biophys Res Commun 476:69–74. [DOI] [PubMed] [Google Scholar]
- Patki M, Salazar Md, Trumbly R, Ratnam M. (2015) Differential effects of estrogen-dependent transactivation vs. transrepression by the estrogen receptor on invasiveness of HER2 overexpressing breast cancer cells. Biochem Biophys Res Commun 457:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Ratnam M. (2016) inventors, Wayne State University, assignee. First-in-Class Targeted Androgen Receptor Down-Regulators (TARDs). U.S. patent 62/288,810. 2016 Jan 29. [Google Scholar]
- Rathkopf DE, Picus J, Hussain A, Ellard S, Chi KN, Nydam T, Allen-Freda E, Mishra KK, Porro MG, Scher HI, et al. (2013) A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol 72:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam M, Patki M, Gonit M, Trumbly R. (2013) Mechanisms of ARE-independent gene activation by the androgen receptor in prostate cancer cells: potential targets for better intervention strategies, in Androgen-Responsive Genes in Prostate Cancer (Wang Z. editor) pp 85–100, Springer, New York. [Google Scholar]
- Salazar MD, Ratnam M, Patki M, Kisovic I, Trumbly R, Iman M, Ratnam M. (2011) During hormone depletion or tamoxifen treatment of breast cancer cells the estrogen receptor apoprotein supports cell cycling through the retinoic acid receptor α1 apoprotein. Breast Cancer Res 13:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporita AJ, Ai J, Wang Z. (2007) The Hsp90 inhibitor, 17-AAG, prevents the ligand-independent nuclear localization of androgen receptor in refractory prostate cancer cells. Prostate 67:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Asano T, Ito K, Asano T. (2012) Vorinostat and bortezomib synergistically cause ubiquitinated protein accumulation in prostate cancer cells. J Urol 188:2410–2418. [DOI] [PubMed] [Google Scholar]
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et al. AFFIRM Investigators (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187–1197. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29. [DOI] [PubMed] [Google Scholar]
- Solit DB, Zheng FF, Drobnjak M, Münster PN, Higgins B, Verbel D, Heller G, Tong W, Cordon-Cardo C, Agus DB, et al. (2002) 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res 8:986–993. [PubMed] [Google Scholar]
- Tam WL, Weinberg RA. (2013) The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 19:1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur MK, Heilbrun LK, Sheng S, Stein M, Liu G, Antonarakis ES, Vaishampayan U, Dzinic SH, Li X, Freeman S, et al. (2016) A phase II trial of ganetespib, a heat shock protein 90 Hsp90) inhibitor, in patients with docetaxel-pretreated metastatic castrate-resistant prostate cancer (CRPC)-a prostate cancer clinical trials consortium (PCCTC) study. Invest New Drugs 34:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toren P, Kim S, Cordonnier T, Crafter C, Davies BR, Fazli L, Gleave ME, Zoubeidi A. (2015) Combination AZD5363 with enzalutamide significantly delays enzalutamide-resistant prostate cancer in preclinical models. Eur Urol 67:986–990. [DOI] [PubMed] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. (2009) Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324:787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Maruyama T, Nishikawa-Uchida S, Oda H, Miyazaki K, Yamasaki A, Yoshimura Y. (2012) Studies using an in vitro model show evidence of involvement of epithelial-mesenchymal transition of human endometrial epithelial cells in human embryo implantation. J Biol Chem 287:4441–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldscholte J, Berrevoets CA, Zegers ND, van der Kwast TH, Grootegoed JA, Mulder E. (1992) Hormone-induced dissociation of the androgen receptor-heat-shock protein complex: use of a new monoclonal antibody to distinguish transformed from nontransformed receptors. Biochemistry 31:7422–7430. [DOI] [PubMed] [Google Scholar]
- Weichert W, Röske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, et al. (2008) Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer 98:604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsbie DS, Xu J, Chen Y, Borsu L, Scher HI, Rosen N, Sawyers CL. (2009) Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res 69:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Balk SP. (2009) Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol 27:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. (2002) Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res 62:1008–1013. [PubMed] [Google Scholar]
- Zhou X, Yang XY, Popescu NC. (2010) Synergistic antineoplastic effect of DLC1 tumor suppressor protein and histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), on prostate and liver cancer cells: perspectives for therapeutics. Int J Oncol 36:999–1005. [DOI] [PubMed] [Google Scholar]
- Zhou X, Yang XY, Popescu NC. (2012) Preclinical evaluation of combined antineoplastic effect of DLC1 tumor suppressor protein and suberoylanilide hydroxamic acid on prostate cancer cells. Biochem Biophys Res Commun 420:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.