Abstract
Obesity has emerged as a principal cause of mortality worldwide, reflecting comorbidities including cancer risk, particularly in colorectum. Although this relationship is established epidemiologically, molecular mechanisms linking colorectal cancer and obesity continue to be refined. Guanylyl cyclase C (GUCY2C), a membrane-bound guanylyl cyclase expressed in intestinal epithelial cells, binds the paracrine hormones guanylin and uroguanylin, inducing cGMP signaling in colorectum and small intestine, respectively. Guanylin is the most commonly lost gene product in sporadic colorectal cancer, and its universal loss early in transformation silences GUCY2C, a tumor suppressor, disrupting epithelial homeostasis underlying tumorigenesis. In small intestine, eating induces endocrine secretion of uroguanylin, the afferent limb of a novel gut-brain axis that activates hypothalamic GUCY2C-cGMP signaling mediating satiety opposing obesity. Recent studies revealed that diet-induced obesity suppressed guanylin and uroguanylin expression in mice and humans. Hormone loss reflects reversible calorie-induced endoplasmic reticulum stress and the associated unfolded protein response, rather than the endocrine, adipokine, or inflammatory milieu of obesity. Loss of intestinal uroguanylin secretion silences the hypothalamic GUCY2C endocrine axis, creating a feed-forward loop contributing to hyperphagia in obesity. Importantly, calorie-induced guanylin loss silences the GUCY2C-cGMP paracrine axis underlying obesity-induced epithelial dysfunction and colorectal tumorigenesis. Indeed, genetically enforced guanylin replacement eliminated diet-induced intestinal tumorigenesis in mice. Taken together, these observations suggest that GUCY2C hormone axes are at the intersection of obesity and colorectal cancer. Moreover, they suggest that hormone replacement that restores GUCY2C signaling may be a novel therapeutic paradigm to prevent both hyperphagia and intestinal tumorigenesis in obesity.
Introduction
Obesity is poised to become the leading cause of morbidity and mortality in the United States and world (Yang and Colditz, 2015). Two-thirds of the US adult population is overweight [body mass index (BMI) >25 kg/m2], and half of that overweight population is obese (BMI >30 kg/m2) (Yang and Colditz, 2015; Nagendran et al., 2016). This pandemic reflects the convergence of reinforcing lifestyle issues, including decreased energy demands coupled with increased accessibility to calorie-dense foods, resulting in dysregulation of endogenous mechanisms of metabolic regulation. Despite these insights, the molecular mechanisms that underlie aberrant energy balance and feeding behavior in obesity, as well as effective therapeutic strategies to combat these defects, remain elusive.
Obesity is associated with comorbidities that reduce life expectancy and incur substantial treatment costs, including cancer, depression, diabetes, cardiovascular disease, hypertension, infertility, liver disease, sleep apnea, osteoarthritis, and stroke (Astrup et al., 2015). Mechanistic relationships that underlie some comorbidities, such as that between obesity and cardiovascular disease, are well characterized. In contrast, molecular mechanisms linking obesity to cancer continue to be refined.
This review highlights recent insights that causally link the pathophysiology of overfeeding in obesity with mechanisms underlying colorectal tumorigenesis through guanylyl cyclase C (GUCY2C)-hormone signaling axes. This emerging pathophysiological paradigm suggests that consumption of excess calories suppresses GUCY2C hormone expression in intestine, disrupting hypothalamic endocrine regulation of satiety promoting hyperphagia and paracrine regulation of colorectal epithelial homeostasis underlying tumorigenesis. The correlative therapeutic paradigm suggests that hyperphagia and colorectal cancer contributing to the morbidity of obesity both can be prevented by hormone replacement that restores GUCY2C signaling.
GUCY2C Signaling and the Paracrine Hormone Hypothesis of Colorectal Cancer
GUCY2C is a membrane-associated guanylyl cyclase receptor expressed primarily in intestinal epithelium (Kuhn, 2016) and in brain (Gong et al., 2011; Valentino et al., 2011; Begg et al., 2014; Kim et al., 2016). Cognate ligands are structurally similar peptides and include the hormones guanylin and uroguanylin produced in small and large intestine, respectively, and the bacterial heat-stable enterotoxins (STs) produced by diarrheagenic bacteria (Canani et al., 2015; Kuhn, 2016). In intestine, GUCY2C resides in apical brush border membranes, where it has a role in fluid homeostasis. Indeed, one of the best-characterized functions of GUCY2C is driving intestinal secretion and ST-induced enterotoxigenic diarrhea (Fiskerstrand et al., 2012; Kuhn, 2016). Association of ST with the extracellular ligand binding domain of GUCY2C activates the cytoplasmic catalytic domain, which converts GTP to cGMP. In small intestine, this second messenger activates cGMP-dependent protein kinase PKGII (Vaandrager et al., 2000). In contrast, in colorectum cGMP inhibits phosphodiesterase type 3, raising intracellular concentrations of cAMP (Chao et al., 1994; Poppe et al., 2008). These signaling events lead to phosphorylation of the cystic fibrosis transmembrane conductance regulator, a chloride channel, which mediates the concentration-dependent efflux of chloride and bicarbonate ions from intestinal cells (Fiskerstrand et al., 2012; Dekkers et al., 2013; Kuhn, 2016). This flow of anions drives electrogenic sodium efflux, producing an osmotic gradient that ultimately results in fluid accumulation in the intestinal lumen manifesting as diarrhea (Dekkers et al., 2013). Consistent with these findings, families with activating or inactivating mutations in GUCY2C exhibit changes in fluid secretion that are consistent with this secretory function (Fiskerstrand et al., 2012; Romi et al., 2012; Muller et al., 2015; Smith et al., 2015).
The intestinal epithelium is a dynamic structure, continuously regenerating throughout life. Stem cells in the crypt give rise to proliferating daughter cells that migrate up the crypt-villus axis and terminally differentiate into the mature cell types of the intestine (Barker, 2014). This continuous regeneration requires tight integration of homeostatic processes, such as proliferation, migration, metabolic reprogramming, differentiation, and genomic integrity (Barker, 2014). In turn, these homeostatic processes are the canonical pathways that are universally defective in all cancers (Hanahan and Weinberg, 2011). It is perhaps not surprising that regulators of these pathways are frequently involved in cancer initiation and progression.
In that context, the GUCY2C signaling axis regulates these regenerative processes, functioning as a tumor suppressor to maintain the integrity of the intestinal epithelium. GUCY2C-deficient (Gucy2c−/−) mice exhibit epithelial dysfunction that recapitulates discreet attributes of tumorigenesis, including an expanded proliferating crypt compartment, increased DNA damage, metabolic reprogramming to a Warburg glycolytic phenotype, restricted differentiation and lineage commitment, and amplified oncogenic signaling through changes in p21, Akt, and other pathways (Li et al., 2007b; Rinaldi et al., 2010; Han et al., 2011; Okabayashi et al., 2012). Consistent with its role as a tumor suppressor, intestinal neoplasia in Gucy2c−/− mice was substantially increased, compared with wild-type mice, by the chemical carcinogen azoxymethane or by mutations in adenomatous polyposis coli (Li et al., 2007b; Rinaldi et al., 2010; Basu et al., 2014).
The GUCY2C paracrine hormones guanylin and uroguanylin are the most commonly lost gene products in colorectal cancer (Zhang et al., 1997; Cohen et al., 1998; Steinbrecher et al., 2000; Notterman et al., 2001), and this loss produces epithelial dysfunction recapitulating the phenotype in Gucy2c−/− mice. In preliminary studies, guanylin mRNA was lost early in the pathophysiological continuum of tumorigenesis, and adenomas, as well as adenocarcinomas, were devoid of guanylin expression in both mice and humans (Zhang et al., 1997; Cohen et al., 1998; Steinbrecher et al., 2000; Notterman et al., 2001). In a cohort of ∼300 human patients, guanylin mRNA and protein were lost by >85% of colorectal tumors compared with matched normal adjacent tissue (Wilson et al., 2014). Together, these observations reveal that guanylin loss silencing GUCY2C is a universal mechanism that occurs at the earliest stages of transformation through a process that is conserved across species. In turn, they suggest that guanylin loss silencing the GUCY2C tumor suppressor plays a fundamental role in the pathogenesis of colorectal cancer initiation. The ability to translate these findings to effective cancer prevention is underscored by the approval of the oral GUCY2C ligand linaclotide (Linzess) (Ironwood Pharmaceuticals, Cambridge, MA) to treat constipation-type irritable bowel syndrome and chronic constipation, using a clinically tractable paradigm to reactivate the dormant paracrine GUCY2C-cGMP signaling axis to prevent intestinal tumorigenesis (Brenner, 2013; Scarpignato and Blandizzi, 2014). These observations suggest a novel pathophysiological paradigm for sporadic colorectal cancer in which a disease generally considered to reflect irreversible genetic mutations in adenomatous polyposis coli and β-catenin (Kinzler and Vogelstein, 1996; Zeuner et al., 2014) is transformed into a potentially preventable and reversible disease of paracrine hormone deficiency that can be treated with oral GUCY2C ligand replacement therapy.
The GUCY2C Endocrine Hormone Axis in Appetite and Satiety
Although GUCY2C has been characterized primarily as an intestinal paracrine hormone receptor, it is also expressed in the hypothalamus of the central nervous system (Gong et al., 2011; Valentino et al., 2011; Begg et al., 2014; Folgueira et al., 2016a; Kim et al., 2016). This specialized region of the brain is responsible for integrating neural and endocrine signaling to maintain homeostasis, utilizing discreet nuclei to regulate processes such as appetite, sleep, aggression, and body temperature (Jego et al., 2013; Falkner et al., 2016; Fischer et al., 2016; Xu and Xie, 2016). Moreover, GUCY2C is enriched in the arcuate nucleus, a collection of neurons positioned ventrolateral to the third ventricle of the brain, which serves as the central site for energy balance by regulating appetite to ensure that food intake matches energy expenditure (Kim et al., 2016; Xu and Xie, 2016). This periventricular organ receives its blood supply through fenestrated capillaries, allowing it unique access to endocrine hormone signals from distant sites outside the nervous system (Zhang et al., 2015). Receptors to these endocrine hormones on arcuate nucleus neurons establish signaling axes that allow distant organs to relay information to the brain about nutritional status and energy balance. These peptide ligands include ghrelin, the lone circulating orexigenic (appetite-stimulating) hormone released primarily by the stomach (Zigman et al., 2016), and anorexigenic (appetite-suppressing) hormones such as glucagon-like peptide 1, cholecystokinin, leptin, and insulin (Efeyan et al., 2015).
Recent studies have established a novel gut-brain axis that signals through the GUCY2C endocrine ligand uroguanylin as an additional anorexigenic pathway (Valentino et al., 2011; Begg et al., 2014; Folgueira et al., 2016a; Kim et al., 2016). Although the precise mechanisms mediating uroguanylin-induced satiety continue to be defined (Valentino et al., 2011; Begg et al., 2014; Folgueira et al., 2016a; Kim et al., 2016), systemic uroguanylin levels increase postprandially in both mice and humans similarly to other anorexigenic hormones such as insulin, glucagon-like peptide 1, and cholecystokinin, suggesting a similar role for uroguanylin. GUCY2C expression in neurons expressing the leptin receptor (Allison et al., 2015), and activation-induced transcription of the anorexigenic second-messenger pro-opiomelanocortin, provide additional evidence supporting the role of the GUCY2C-uroguanylin gut-brain axis (Valentino et al., 2011; Kim et al., 2016). Indeed, Gucy2c−/− mice are hyperphagic and obese, suggesting that this regulatory pathway may play an important role in weight maintenance and the pathophysiology of obesity (Valentino et al., 2011).
Diet-Induced Suppression of GUCY2C Hormones and the Pathophysiology of Obesity
Obesity is exemplified by the dysfunction of endogenous neuroendocrine axes designed to limit overfeeding. Unfortunately, failure of many of these homeostatic mechanisms is mediated by central resistance to anorexigenic hormones, precluding their utility in the pharmacologic treatment of obesity. Indeed, insulin and leptin circulate at consistently elevated levels in obesity, resulting in hypothalamic desensitization to these hormones and ultimately impaired appetite suppression (Williams et al., 2014).
Like the endocrine axes for insulin and leptin, the uroguanylin-GUCY2C gut-brain axis also is impaired in obesity. However, the mechanism underlying this pathology is fundamentally different from that of the leptin and insulin axes. In contrast to leptin and insulin, serum uroguanylin levels are reduced in obesity, resulting in a hormone deficiency that silences GUCY2C signaling in brain (Kim et al., 2016). Consequently, hypothalamic GUCY2C is not desensitized but, rather, remains responsive to hormone stimulation and actually is overexpressed in hypothalamus in diet-induced obesity (Kim et al., 2016). This mechanistic distinction suggests that GUCY2C may be amenable to therapeutic targeting for the treatment of obesity. Indeed, conditional transgenic expression of uroguanylin in brain reduced appetite, food consumption, and weight gain over months in the context of chronic exposure to an obesogenic diet.
Although uroguanylin has emerged as the afferent limb of a novel gut-brain axis regulating body weight, mechanisms mediating those effects continue to be refined (Kim et al., 2016). Thus, in one study, GUCY2C did not modulate body weight (Begg et al., 2014). However, this study used male mice, a model that is particularly insensitive to central regulation of body weight by the uroguanylin-GUCY2C signaling axis (Valentino et al., 2011). Also, a recent study suggested that uroguanylin expression in intestine was elevated, rather than reduced, by diet-induced obesity (Folgueira et al., 2016b). However, this study assessed uroguanylin expression in duodenum, which is the lowest source of hormone in small intestine (Whitaker et al., 1997; Nakazato et al., 1998; Qian et al., 2000). Indeed, the greatest uroguanylin expression, and the largest source of circulating hormone, is jejunum (Whitaker et al., 1997; Nakazato et al., 1998; Qian et al., 2000, 2008), the tissue analyzed in the most recent study (Kim et al., 2016). These inconsistencies notwithstanding, the uroguanylin-GUCY2C axis has emerged as a promising new target for obesity pharmacotherapy (Valentino et al., 2011; Begg et al., 2014; Folgueira et al., 2016a; Kim et al., 2016).
GUCY2C and Obesity-Associated Colorectal Cancer
Potential mechanisms linking obesity and colorectal cancer risk traditionally have included endocrine, adipokine, and inflammatory changes associated with the adipose mass (Renehan et al., 2015). Although there is some evidence to support each of these links, definitive proof establishing their contribution in humans has been inconsistent. Thus, the underlying mechanisms that link obesity to colorectal tumorigenesis continue to be refined. In that regard, GUCY2C has emerged as a novel regulator of both intestinal tumorigenesis through paracrine hormone signaling in the gut and obesity through endocrine hormone signaling in hypothalamus. These observations suggest a novel pathophysiological hypothesis in which silencing GUCY2C signaling resides at the intersection of obesity and colorectal cancer. The correlative therapeutic hypothesis suggests that both obesity and colorectal tumorigenesis might be prevented or reversed by preserving the GUCY2C-cGMP signaling axis.
Obesity Silences the GUCY2C Paracrine Axis, Leading to Tumorigenesis
Previous studies highlighted the role of guanylin loss silencing GUCY2C in the development of sporadic colorectal cancer, offering a plausible mechanism whereby disruption of GUCY2C-cGMP signaling mediates tumorigenesis (Zhang et al., 1997; Cohen et al., 1998; Shailubhai et al., 2000; Steinbrecher et al., 2000; Notterman et al., 2001; Li et al., 2007b; Rinaldi et al., 2010; Wilson et al., 2014). Unexpectedly, hypercaloric diets eliminated guanylin expression in mouse intestine, silencing GUCY2C associated with epithelial dysfunction amplifying tumorigenesis, similar to that observed in Gucy2c−/− mice (Lin et al., 2016). Moreover, obesity-induced guanylin loss also was observed in humans, in which there is an inverse relationship between guanylin mRNA expression and BMI in normal colonic epithelium, with morbidly obese patients (BMI ≥35 kg/m2) exhibiting an 80% decrease in guanylin mRNA expression compared with lean individuals (Lin et al., 2016).
Downstream of guanylin loss, obesity also silenced the GUCY2C-cGMP signaling axis. Obese mice exhibited decreased intestinal epithelial phospho-vasodilator-stimulated phosphoprotein an established downstream target of cGMP (Erdmann et al., 2013; Lin et al., 2016). Loss of downstream GUCY2C-cGMP signaling resulted in epithelial dysfunction, recapitulating the phenotype of Gucy2c−/− mice. This phenotype was characterized by increased DNA damage, hyperproliferation, metabolic reprogramming to a Warburg glycolytic phenotype, and aberrant oncogenic signaling (Li et al., 2007a,b; Rinaldi et al., 2010; Lin et al., 2016). Finally, these pathophysiological changes specifically reflected guanylin loss. Indeed, conditional transgenic guanylin expression in intestine reactivated GUCY2C and reversed epithelial dysfunction in vivo (Lin et al., 2016). These GUCY2C-dependent changes in intestinal epithelium in obesity reflecting guanylin loss silencing GUCY2C precisely recapitulate known risk factors for colorectal cancer (Li et al., 2007a,b; Rinaldi et al., 2010; Han et al., 2011; Okabayashi et al., 2012).
In contrast to the paradigm that links cancer risk to obesity through endocrine, adipokine, or inflammatory changes reflecting adiposity, dietary manipulation revealed that loss of guanylin expression in obesity is independent of body mass (Lin et al., 2016), consistent with another recent report of a calorie-modulated, obesity-independent colorectal cancer mechanism (Schulz et al., 2014). Indeed, whereas a high-fat diet is associated with both hypercaloric intake and weight gain, mice on a high-carbohydrate diet maintain lean body weights despite consuming ∼40% excess calories (Dogan et al., 2007). Similarly, there is an established polymorphism in C57BL/6 mice in which some (∼5%) consume excess calories from fat but maintain lean body mass (Fearnside et al., 2008). Moreover, BALB/c mice resist obesogenic diets and maintain lean body mass while consuming excess calories from fat (Fearnside et al., 2008). Surprisingly, intestinal guanylin expression was lost in all of these models, dissociating the impact of calories consumed from changes in lean body mass on hormone expression (Lin et al., 2016). Conversely, guanylin expression was restored in mice that were switched from a high- to a low-calorie diet, even though these mice remained persistently obese (Lin et al., 2016).
Endoplasmic Reticulum Stress Suppresses GUCY2C Hormone Expression in Diet-Induced Obesity
Obesity is associated with endoplasmic reticulum (ER) stress in intestine that, in part, mediates the pathophysiological effects of hyperphagia and overnutrition (Hayashi et al., 2014; Lin et al., 2016), including suppression of GUCY2C hormone expression. Hypercaloric diets induced canonical markers of ER stress in intestine, including the chaperone GRP78 (binding immunoglobulin protein) and the proapoptotic CCAAT/-enhancer-binding protein homologous protein (Lin et al., 2016). Diet-induced induction of ER stress was associated with loss of guanylin in colorectum and uroguanylin in small intestine of obese mice (Lin et al., 2016). Furthermore, induction of ER stress pharmacologically by thapsigargan or tunicamycin (Keestra-Gounder et al., 2016), or genetically by eliminating XBP1 expression (van Galen et al., 2014; Keestra-Gounder et al., 2016), in lean mice recapitulated diet-induced obesity and produced hormone loss (Lin et al., 2016). ER stress induces a tripartite unfolded protein response directed at preserving ER homeostasis, mediated by inositol-requiring enzyme 1 and activating transcription factor 6, which both increase transcription of chaperones, and protein kinase R-like ER-localized eIF2α kinase, which inhibits protein translation (Wang and Kaufman, 2016). Indeed, pharmacologic or genetic inhibition of protein kinase R-like ER-localized eIF2α kinase signaling prevents hormone loss produced by ER stress (Lin et al., 2016). Conversely, administration of the chemical chaperone tauroursodeoxycholic acid, which relieves ER stress (Keestra-Gounder et al., 2016), restored hormone expression in obese mice (Kim et al., 2016; Lin et al., 2016).
These observations suggest the novel pathophysiological hypothesis that colorectal cancer in obesity recapitulates molecular mechanisms underlying sporadic intestinal tumorigenesis involving calorie-induced guanylin loss silencing the GUCY2C tumor suppressor, producing epithelial dysfunction underlying transformation (Zhang et al., 1997; Cohen et al., 1998; Steinbrecher et al., 2000; Notterman et al., 2001; Wilson et al., 2014). In that context, this hypothesis expands the paradigm of colorectal cancer in obesity from a disease reflecting dysregulation of the adipokine, endocrine, and inflammatory milieu reflecting body mass, to one of calorie-induced local paracrine hormone insufficiency silencing a tumor suppressor. Beyond pathophysiology, the correlative therapeutic hypothesis suggests that GUCY2C ligand replacement could be a strategy to prevent obesity-associated colorectal cancer. To test this hypothesis, mice were generated with an inducible intestine-specific guanylin transgene whose expression cannot be silenced (Kim et al., 2016; Lin et al., 2016). Conditional expression of this guanylin transgene eliminated tumorigenesis in obese animals on a high-fat diet (Kim et al., 2016; Lin et al., 2016). These observations provide proof of principle for the utility of GUCY2C ligand replacement therapy to prevent colorectal cancer in the context of diet-induced obesity.
Translation of GUCY2C-Targeted Chemoprevention for Colorectal Cancer
These observations support the suggestion that one key step in intestinal tumorigenesis in obesity is diet-induced guanylin loss silencing GUCY2C. Moreover, they establish the paradigm of paracrine hormone replacement to prevent intestinal tumor initiation and progression in obesity (Alvarez, 2016). The immediate tractability of this novel chemoprevention approach can best be appreciated by considering that the oral GUCY2C agonist linaclotide (Linzess) is approved for the treatment of constipation-type irritable bowel syndrome (Brenner, 2013). In that regard, linaclotide is activated in the acidic environment of the stomach and has its primary effect on secretion in the duodenum. Indeed, <3% of oral linaclotide can be recovered in evacuated stool (Busby et al., 2013), suggesting that exposure in the colorectum might be limited. Thus, proof-of-concept trials have been initiated to define the ability of once daily oral linaclotide to activate GUCY2C, induce cGMP signaling, and regulate homeostatic pathways, including epithelial cell proliferation, across the rostral-caudal axis of the colorectum in normal healthy volunteers (ClinicalTrials.gov Identifier: NCT01950403). The ultimate goal of this clinical program is to expand this paradigm to evaluate the utility of linaclotide for chemoprevention of intestinal transformation in populations at risk, including obese individuals.
Discussion
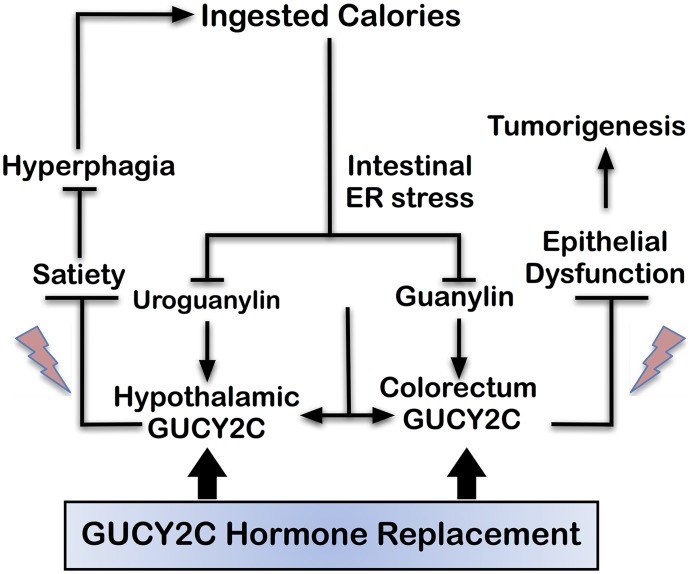
Recent insights into the role of the GUCY2C signaling axis in obesity and cancer suggest a novel paradigm whereby ingested calories induce intestinal ER stress, producing paracrine (guanylin) and endocrine (uroguanylin) hormone loss that silences GUCY2C signaling in colorectum and hypothalamus, respectively. In colorectum, loss of GUCY2C paracrine signaling produces epithelial dysfunction contributing to tumor initiation (Kim et al., 2016; Lin et al., 2016). In hypothalamus, loss of endocrine GUCY2C signaling leads to suppression of satiety circuits, producing hyperphagia contributing to obesity. Taken together, these observations suggest a novel pathophysiological model in which hormone loss silencing GUCY2C in obesity comprises a positive feed-forward loop (Fig. 1). Thus, overnutrition suppresses uroguanylin regulation of satiety, amplifying hyperphagia by silencing the GUCY2C gut-brain endocrine axis. In turn, increased hyperphagia amplifies colorectal cancer risk through loss of guanylin and GUCY2C paracrine hormone signaling, associated with epithelial dysfunction. This pathophysiological model uniquely positions GUCY2C-hormone axes at the intersection of pathophysiological mechanisms underlying obesity and colorectal cancer. In that context, paracrine and endocrine limbs of these axes can be repaired by GUCY2C hormone replacement, preventing tumor initiation in the intestine and improving satiety responses regulating feeding in obesity (Kim et al., 2016; Lin et al., 2016). The feasibility of these novel therapeutic approaches is underscored by the regulatory approval and clinical application of the GUCY2C ligand linaclotide for use in patients with constipation-type irritable bowel syndrome or chronic constipation (Brenner, 2013). Importantly, a research program is currently underway to evaluate the utility of linaclotide as a chemopreventive agent for colorectal tumorigenesis in mice and humans.
Fig. 1.
GUCY2C signaling at the intersection of obesity and colorectal cancer. Hyperphagia and overingestion of calories induce intestinal ER stress, which suppresses expression of uroguanylin in small intestine and guanylin in colorectum. Loss of uroguanylin in small intestine prevents endocrine secretion of that hormone following the ingestion of food. Loss of endocrine secretion disrupts the afferent limb of a novel gut-brain axis that silences hypothalamic GUCY2C, reducing satiety signals (lightning bolt symbols) limiting food intake. In turn, this amplifies the ingestion of excess calories, representing a positive feed-forward loop contributing to hyperphagia, which is central to the pathophysiology of obesity. Moreover, loss of the paracrine hormone guanylin silences the GUCY2C tumor suppressor in colorectum disrupting homeostasis (lightning bolt symbols), resulting in epithelial dysfunction underlying tumorigenesis. Importantly, hormone replacement with GUCY2C ligands, such as linaclotide, reverses these pathophysiological mechanisms, opposing both obesity and colorectal tumorigenesis.
Abbreviations
- BMI
body mass index
- ER
endoplasmic reticulum
- GUCY2C
guanylyl cyclase C
- ST
bacterial heat-stable enterotoxin
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Blomain, Merlino, Pattison, Snook, Waldman.
Footnotes
This work was supported by the National Institutes of Health National Cancer Institute [Grants CA170533 and CA180500]; National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK103492-01A1]; a Pharmaceutical Research and Manufacturers of America Foundation Predoctoral Fellowship Award in Pharmacology; and Targeted Diagnostic and Therapeutics.
Conflict of interest: S.A.W. is the Chair (uncompensated) of the Scientific Advisory Board of Targeted Diagnostics & Therapeutics, which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
References
- Allison MB, Patterson CM, Krashes MJ, Lowell BB, Myers MG, Jr, Olson DP. (2015) TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Mol Metab 4:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J. (2016) Silence of the GUCY2C tumor suppressor. Sci Transl Med 8:322–325. [Google Scholar]
- Astrup A, Raben A, Geiker N. (2015) The role of higher protein diets in weight control and obesity-related comorbidities. Int J Obes 39:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. (2014) Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 15:19–33. [DOI] [PubMed] [Google Scholar]
- Basu N, Saha S, Khan I, Ramachandra SG, Visweswariah SS. (2014) Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21. J Biol Chem 289:581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg DP, Steinbrecher KA, Mul JD, Chambers AP, Kohli R, Haller A, Cohen MB, Woods SC, Seeley RJ. (2014) Effect of guanylate cyclase-C activity on energy and glucose homeostasis. Diabetes 63:3798–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DM. (2013) Linaclotide for the treatment of irritable bowel syndrome with constipation: is it time to reshuffle the deck? Gastroenterology 145:476–478. [DOI] [PubMed] [Google Scholar]
- Busby RW, Kessler MM, Bartolini WP, Bryant AP, Hannig G, Higgins CS, Solinga RM, Tobin JV, Wakefield JD, Kurtz CB, et al. (2013) Pharmacologic properties, metabolism, and disposition of linaclotide, a novel therapeutic peptide approved for the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation. J Pharmacol Exp Ther 344:196–206. [DOI] [PubMed] [Google Scholar]
- Canani RB, Castaldo G, Bacchetta R, Martín MG, Goulet O. (2015) Congenital diarrhoeal disorders: advances in this evolving web of inherited enteropathies. Nat Rev Gastroenterol Hepatol 12:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AC, de Sauvage FJ, Dong YJ, Wagner JA, Goeddel DV, Gardner P. (1994) Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J 13:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MB, Hawkins JA, Witte DP. (1998) Guanylin mRNA expression in human intestine and colorectal adenocarcinoma. Lab Invest 78:101–108. [PubMed] [Google Scholar]
- Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, et al. (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19:939–945. [DOI] [PubMed] [Google Scholar]
- Dogan S, Hu X, Zhang Y, Maihle NJ, Grande JP, Cleary MP. (2007) Effects of high-fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-alpha mice. Breast Cancer Res 9:R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Comb WC, Sabatini DM. (2015) Nutrient-sensing mechanisms and pathways. Nature 517:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann J, Stark K, Esslinger UB, Rumpf PM, Koesling D, de Wit C, Kaiser FJ, Braunholz D, Medack A, Fischer M, et al. CARDIoGRAM (2013) Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature 504:432–436. [DOI] [PubMed] [Google Scholar]
- Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D. (2016) Hypothalamic control of male aggression-seeking behavior. Nat Neurosci 19:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnside JF, Dumas ME, Rothwell AR, Wilder SP, Cloarec O, Toye A, Blancher C, Holmes E, Tatoud R, Barton RH, et al. (2008) Phylometabonomic patterns of adaptation to high fat diet feeding in inbred mice. PLoS One 3:e1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AW, Hoefig CS, Abreu-Vieira G, de Jong JM, Petrovic N, Mittag J, Cannon B, Nedergaard J. (2016) Leptin raises defended body temperature without activating thermogenesis. Cell Reports 14:1621–1631. [DOI] [PubMed] [Google Scholar]
- Fiskerstrand T, Arshad N, Haukanes BI, Tronstad RR, Pham KD, Johansson S, Håvik B, Tønder SL, Levy SE, Brackman D, et al. (2012) Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med 366:1586–1595. [DOI] [PubMed] [Google Scholar]
- Folgueira C, Beiroa D, Callon A, Al-Massadi O, Barja-Fernandez S, Senra A, Fernø J, López M, Dieguez C, Casanueva FF, et al. (2016a) Uroguanylin action in the brain reduces weight gain in obese mice via different efferent autonomic pathways. Diabetes 65:421–432. [DOI] [PubMed] [Google Scholar]
- Folgueira C, Sanchez-Rebordelo E, Barja-Fernandez S, Leis R, Tovar S, Casanueva FF, Dieguez C, Nogueiras R, Seoane LM. (2016b) Uroguanylin levels in intestine and plasma are regulated by nutritional status in a leptin-dependent manner. Eur J Nutr 55:529–536. [DOI] [PubMed] [Google Scholar]
- Gong R, Ding C, Hu J, Lu Y, Liu F, Mann E, Xu F, Cohen MB, Luo M. (2011) Role for the membrane receptor guanylyl cyclase-C in attention deficiency and hyperactive behavior. Science 333:1642–1646. [DOI] [PubMed] [Google Scholar]
- Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, Cohen MB. (2011) Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One 6:e16139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Yamada R, Das SS, Sato T, Takahashi A, Hiratsuka M, Hirasawa N. (2014) Glucagon-like peptide-1 production in the GLUTag cell line is impaired by free fatty acids via endoplasmic reticulum stress. Metabolism 63:800–811. [DOI] [PubMed] [Google Scholar]
- Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D, Adamantidis AR. (2013) Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci 16:1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra-Gounder AM, Byndloss MX, Seyffert N, Young BM, Chávez-Arroyo A, Tsai AY, Cevallos SA, Winter MG, Pham OH, Tiffany CR, et al. (2016) NOD1 and NOD2 signalling links ER stress with inflammation. Nature 532:394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GW, Lin JE, Snook AE, Aing AS, Merlino DJ, Li P, Waldman SA. (2016) Calorie-induced ER stress suppresses uroguanylin satiety signaling in diet-induced obesity. Nutr Diabetes 6:e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. (1996) Lessons from hereditary colorectal cancer. Cell 87:159–170. [DOI] [PubMed] [Google Scholar]
- Kuhn M. (2016) Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev 96:751–804. [DOI] [PubMed] [Google Scholar]
- Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. (2007a) Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am J Pathol 171:1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, Baran AA, Siracusa LD, Pitari GM, Waldman SA. (2007b) Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology 133:599–607. [DOI] [PubMed] [Google Scholar]
- Lin JE, Colon-Gonzalez F, Blomain ES, Kim GW, Aing AS, Stoecker BA, Rock JM, Snook AE, Zhan T, Hyslop TM, et al. (2016) Obesity-induced colorectal cancer is driven by caloric silencing of the guanylin-GUCY2C paracrine signaling axis. Cancer Res 76:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Rasool I, Heinz-Erian P, Mildenberger E, Hulstrunk C, Muller A, Michaud L, Koot BG, Ballauff A, Vodopiutz J, et al. (2015) Congenital secretory diarrhoea caused by activating germline mutations in GUCY2C. Gut DOI: 10.1136/gutjnl-2015-309441 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagendran J, Moore MD, Norris CM, Khani-Hanjani A, Graham MM, Freed DH, Nagendran J. (2016) The varying effects of obesity and morbid obesity on outcomes following cardiac transplantation. Int J Obes 40:721–724. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Yamaguchi H, Date Y, Miyazato M, Kangawa K, Goy MF, Chino N, Matsukura S. (1998) Tissue distribution, cellular source, and structural analysis of rat immunoreactive uroguanylin. Endocrinology 139:5247–5254. [DOI] [PubMed] [Google Scholar]
- Notterman DA, Alon U, Sierk AJ, Levine AJ. (2001) Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res 61:3124–3130. [PubMed] [Google Scholar]
- Okabayashi K, Ashrafian H, Hasegawa H, Yoo JH, Patel VM, Harling L, Rowland SP, Ali M, Kitagawa Y, Darzi A, et al. (2012) Body mass index category as a risk factor for colorectal adenomas: a systematic review and meta-analysis. Am J Gastroenterol 107:1175–1185, quiz 1186. [DOI] [PubMed] [Google Scholar]
- Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, et al. (2008) Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods 5:277–278. [DOI] [PubMed] [Google Scholar]
- Qian X, Moss NG, Fellner RC, Goy MF. (2008) Circulating prouroguanylin is processed to its active natriuretic form exclusively within the renal tubules. Endocrinology 149:4499–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Prabhakar S, Nandi A, Visweswariah SS, Goy MF. (2000) Expression of GC-C, a receptor-guanylate cyclase, and its endogenous ligands uroguanylin and guanylin along the rostrocaudal axis of the intestine. Endocrinology 141:3210–3224. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Zwahlen M, Egger M. (2015) Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 15:484–498. [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Cleveland R, Norat T, Biessy C, Rohrmann S, Linseisen J, Boeing H, Pischon T, Panico S, Agnoli C, et al. (2010) Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer 126:1702–1715. [DOI] [PubMed] [Google Scholar]
- Romi H, Cohen I, Landau D, Alkrinawi S, Yerushalmi B, Hershkovitz R, Newman-Heiman N, Cutting GR, Ofir R, Sivan S, et al. (2012) Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C. Am J Hum Genet 90:893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpignato C, Blandizzi C. (2014) Editorial: adequate management may reduce the colorectal cancer risk associated with constipation. Aliment Pharmacol Ther 40:562–564. [DOI] [PubMed] [Google Scholar]
- Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C, et al. (2014) High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 514:508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ, et al. (2000) Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res 60:5151–5157. [PubMed] [Google Scholar]
- Smith A, Bulman DE, Goldsmith C, Bareke E, Majewski J, Boycott KM, Nikkel SM, Nikkel SM, FORGE Canada Consortium (2015) Meconium ileus in a Lebanese family secondary to mutations in the GUCY2C gene. Eur J Hum Genet 23:990–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, Cohen MB. (2000) Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem Biophys Res Commun 273:225–230. [DOI] [PubMed] [Google Scholar]
- Vaandrager AB, Bot AG, Ruth P, Pfeifer A, Hofmann F, De Jonge HR. (2000) Differential role of cyclic GMP-dependent protein kinase II in ion transport in murine small intestine and colon. Gastroenterology 118:108–114. [DOI] [PubMed] [Google Scholar]
- Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G, Magee MS, Hyslop T, Schulz S, Waldman SA. (2011) A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest 121:3578–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Galen P, Kreso A, Mbong N, Kent DG, Fitzmaurice T, Chambers JE, Xie S, Laurenti E, Hermans K, Eppert K, et al. (2014) The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature 510:268–272. [DOI] [PubMed] [Google Scholar]
- Wang M, Kaufman RJ. (2016) Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529:326–335. [DOI] [PubMed] [Google Scholar]
- Whitaker TL, Witte DP, Scott MC, Cohen MB. (1997) Uroguanylin and guanylin: distinct but overlapping patterns of messenger RNA expression in mouse intestine. Gastroenterology 113:1000–1006. [DOI] [PubMed] [Google Scholar]
- Williams KW, Liu T, Kong X, Fukuda M, Deng Y, Berglund ED, Deng Z, Gao Y, Liu T, Sohn JW, et al. (2014) Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab 20:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Lin JE, Li P, Snook AE, Gong J, Sato T, Liu C, Girondo MA, Rui H, Hyslop T, et al. (2014) The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer. Cancer Epidemiol Biomarkers Prev 23:2328–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Xie X. (2016) Neurotrophic factor control of satiety and body weight. Nat Rev Neurosci 17:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Colditz GA. (2015) Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med 175:1412–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuner A, Todaro M, Stassi G, De Maria R. (2014) Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell 15:692–705. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. (1997) Gene expression profiles in normal and cancer cells. Science 276:1268–1272. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Dodd GT, Tiganis T. (2015) Protein tyrosine phosphatases in hypothalamic insulin and leptin signaling. Trends Pharmacol Sci 36:661–674. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Bouret SG, Andrews ZB. (2016) Obesity impairs the action of the neuroendocrine ghrelin system. Trends Endocrinol Metab 27:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]