Abstract
Anticonvulsants can increase the risk of developing neurotoxicity in infants; however, the underlying mechanism has not been elucidated to date. Thyroxine [3,5,3′,5′-l-tetraiodothyronine (T4)] plays crucial roles in the development of the central nervous system. In this study, we hypothesized that induction of UDP-glucuronosyltransferase 1A1 (UGT1A1)—an enzyme involved in the metabolism of T4—by anticonvulsants would reduce serum T4 levels and cause neurodevelopmental toxicity. Exposure of mice to phenytoin during both the prenatal and postnatal periods significantly induced UGT1A1 and decreased serum T4 levels on postnatal day 14. In the phenytoin-treated mice, the mRNA levels of synaptophysin and synapsin I in the hippocampus were lower than those in the control mice. The thickness of the external granule cell layer was greater in phenytoin-treated mice, indicating that induction of UGT1A1 during the perinatal period caused neurodevelopmental disorders. Exposure to phenytoin during only the postnatal period also caused these neurodevelopmental disorders. A T4 replacement attenuated the increase in thickness of the external granule cell layer, indicating that the reduced T4 was specifically associated with the phenytoin-induced neurodevelopmental disorder. In addition, these neurodevelopmental disorders were also found in the carbamazepine- and pregnenolone-16-α-carbonitrile–treated mice. Our study is the first to indicate that UGT1A1 can control neurodevelopment by regulating serum T4 levels.
Introduction
Hypothyroidism during the perinatal period results in irreversible damage and severe mental and physical retardation, which is known as cretinism in humans (Koibuchi and Chin, 2000). In rodents, perinatal hypothyroidism induced by propylthiouracil (PTU), methimazole, and thyroidectomy leads to impaired performance on a variety of motor and behavioral learning tasks (Eayrs and Levine, 1963; Davenport and Dorcey, 1972; Hasebe et al., 2008). It has been reported that, in the cerebellum, perinatal hypothyroidism induced various anatomic alterations, including delayed migration of granule cells, reduction of growth and branching of dendritic arborization of Purkinje cells, reduction of synaptogenesis between Purkinje cells and granule cell axons, delayed myelination, and changes in the synaptic connection among cerebellar neurons and afferent neuronal fibers in the cerebellum in rodents (Nicholson and Altman, 1972a,b,c; Legrand, 1979, 1980). These neurotoxicities in the cerebellum can further cause impaired performance on motor tasks. In the hippocampus, hypothyroidism decreased the neurogenesis and synaptogenesis and changed a morphology of pyramidal cells in rats (Desouza et al., 2005; Koromilas et al., 2010). These neurotoxicities in the hippocampus have been regarded as the cause of impaired learning ability. These data indicate that thyroid hormones play crucial roles in the development of the central nervous system.
Thyroid hormones consist of 3,5,3′-l-triiodothyronine (T3) and 3,5,3′,5′-l-tetraiodothyronine (T4). As T4 solely enters the developing brain more readily than T3, where thyroid hormone receptors (TRs) exist, the serum T4 level is tightly associated with brain development during the perinatal period (Calvo et al., 1990). The liver is a major organ of T4 metabolism (Ohnhaus and Studer, 1983; Malik and Hodgson, 2002). Glucuronidation by UDP-glucuronosyltransferase (UGT) 1A1 is one of the main pathways of T4 metabolism (Richardson et al., 2014). UGT1A1 can be induced by various drugs, foods, and environmental pollutants through nuclear receptors, such as aryl hydrocarbon receptor, constitutive androstane receptor (CAR), pregnane X receptor (PXR), and peroxisome proliferator–activated receptor α (Jemnitz et al., 2000; Xu et al., 2005). Polychlorinated biphenyl (PCB) is a potent activator of aryl hydrocarbon receptor (Sanderson et al., 1996), whereas phenytoin and carbamazepine are activators of CAR (Faucette et al., 2007; Kachaylo et al., 2011). The UGT enzyme is not directly involved in T4 function. However, induction of UGT1A1 has been shown to decrease serum T4, which does indicate that UGT1A1 plays a key role in modulating T4 levels (Franklyn et al., 1984; Koopman-Esseboom et al., 1994).
A case-control study showed that children exposed to phenytoin during the fetal period had significantly lower intelligence quotient at 4–8 years of age (Vanoverloop et al., 1992). Another case-control study showed that children exposed to carbamazepine during the fetal period also had a significantly lower intelligence quotient at 6 months to 6 years of age (Ornoy and Cohen, 1996). Several cohort studies reported that exposure to PCB during the fetal and neonatal periods impaired visual recognition memory and cognitive abilities (Jacobson and Jacobson, 1996). As phenytoin and carbamazepine can pharmacologically reduce the blood pressure of pregnant women, the lowered transfer of oxygen to fetus was originally hypothesized as a cause of mental retardation in neonates (Imosemi and Osinubi, 2011). However, it has been reported that children exposed to phenytoin during only the neonatal period exhibited cognitive and behavioral impairments (Bacon et al., 1981). Furthermore, PCB has been reported not to reduce blood pressure (Goncharov et al., 2011). These inconsistent findings indicate that the actual mechanism of neurodevelopmental disorder in children exposed to phenytoin, carbamazepine, and PCB remains unclear.
T4 is extremely essential for brain development during the perinatal period. As phenytoin, carbamazepine, and PCB can commonly induce UGT1A1 (Soars et al., 2004; Smith et al., 2005; Shelby and Klaassen, 2006), the lowered T4 level caused by UGT1A1 induction might be the cause of phenytoin-, carbamazepine-, and PCB-induced neurodevelopmental disorders. Therefore, in this study, we investigated the effect of UGT1A1 induction on neurodevelopment during the perinatal period in mice. We analyzed histologic development of the cerebellum and synaptogenesis in the hippocampus to understand the effect of phenytoin on neurodevelopment during the perinatal period. Synaptogenesis in the hippocampus was examined by determining the expression levels of synaptophysin and synapsin I, marker genes of synaptic density (Thiel, 1993). Development of the cerebellum was examined by measuring the migration amount of granule cell in the cerebellum (Rakic, 1971).
Materials and Methods
Chemicals and Reagents.
UDP-glucuronic acid (UDPGA), l-thyroxine, estradiol, carbamazepine, pregnenolone-16-α-carbonitrile (PCN), and alamethicin were purchased from Sigma-Aldrich (St. Louis, MO). PTU, phenytoin, and phenylhydrazine were purchased from Wako Pure Chemical (Osaka, Japan). Free-T4 AccuBind enzyme-linked immunosorbent assay kit was purchased from Monobind (Lake Forest, CA). All other chemicals and solvents were of analytical grade or the highest grade commercially available.
Animals, Treatments, and Tissue Collection.
Tg (UGT1A1*28) Ugt1−/− [humanized UGT1 (hUGT1)] mice were developed previously in a C57BL/6 background (Fujiwara et al., 2010). Wild-type mice (C57BL/6NCrSlc) were obtained from SLC Japan (Shizuoka, Japan). All animals received food and water ad libitum, and mouse handling and experimental procedures were conducted in accordance with the animal care protocol approved by Kitasato University (Tokyo, Japan).
To investigate the neurodevelopment of mice exposed to phenytoin during the fetal and neonatal period, dams and their pups were treated with phenytoin daily from gestation day 12, 13, or 14 to postnatal day 0 (p.o. 80 mg/kg) and from postnatal day 1 to day 14 (s.c. 35 mg/kg). To investigate the neurodevelopment of mice exposed to phenytoin during the neonatal period only, phenytoin (s.c. 35 mg/kg), carbamazepine (s.c. 35 mg/kg), and PCN (s.c. 10 mg/kg) were administered to pups daily from postnatal day 1 or day 11 to day 14. To investigate the effect of T4 replacement on neurodevelopment in phenytoin-treated mice, phenytoin (s.c. 35 mg/kg) and T4 (s.c. 100 μg/kg) were administered to pups daily from postnatal day 1 to day 14. To develop hypothyroid models, mice were given PTU (50 ppm) in drinking water after the 14 days beginning of mating to postnatal day 14. To investigate the effect of bilirubin on serum T4 level, phenylhydrazine was administered to hUGT1 mice intraperitoneally on postnatal days 15 and 16 (20 mg/kg).
Mice were anesthetized by diethyl ether inhalation, and the liver was perfused with ice-cold 1.15% KCl on postnatal day 14. The liver was removed and rinsed in cold 1.15% KCl and stored at −80°C. Brains were removed and rinsed in cold phosphate-buffered saline (PBS). The hippocampus was dissected on ice immediately and stored at −80°C. For histologic analysis, mice were anesthetized by diethyl ether inhalation and perfused transcardially with 4% paraformaldehyde in PBS (pH 7.4) on postnatal day 14. Brains were removed, postfixed overnight in the same fixative, and dehydrated using gradual ethanol and embedded in paraffin.
Determination of Serum T4 and Bilirubin Levels.
Blood was obtained from the submandibular vein on postnatal day 14 and incubated at 4°C for 60 minutes to clot. Then, the blood was centrifuged at 3000g for 5 minutes. The supernatant was used as a serum sample. Free thyroxine (T4) levels were quantified using a Free-T4 AccuBind enzyme-linked immunosorbent assay kit (Monobind). Procedures were performed according to the manufacturer’s instructions. Total serum bilirubin levels were quantified using a Bilirubinometer (B-105N; Erma, Tokyo, Japan).
Quantitative Reverse-Transcription Polymerase Chain Reaction.
Total RNA of hUGT1 mouse liver and hippocampus was extracted with TRIzol reagent (Life Technologies, Carlsbad, CA). The cDNA was synthesized from 1 µg of total RNA of hUGT1 mouse liver and hippocampus using ReverTra Ace (Toyobo, Osaka, Japan). After the reverse-transcription reaction at 37°C for 15 minutes and at 50°C for 5 minutes, the reaction mixture was incubated at 95°C for 5 minutes to deactivate the reverse transcriptase. Quantitative reverse-transcription polymerase chain reaction (PCR) was performed with THUNDERBIRD SYBR qPCR Mix (Toyobo), and the reactions were run in a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Primer pairs listed in Table 1 were used to detect cyclophilin B, UGT1A1, Sulfotransferase 1b1 (Sult1b1), type 1 iodothyronine deiodinase, type 2 iodothyronine deiodinase, type 3 iodothyronine deiodinase, synapsin I, and synaptophysin. After an initial denaturation at 95°C for 30 seconds, the amplification was performed by denaturation at 95°C for 5 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds for 45 cycles. Expression levels were normalized with mouse cyclophilin mRNA level.
TABLE 1.
Sequence of primers used for quantitative reverse-transcription PCR
| Isoforms and Primers |
Sequence |
|---|---|
| CPH | |
| CPH-S | 5′-CAG ACG CCA CTG TCG CTT T-3′ |
| CPH-AS | 5′-TGT CTT TGG AAC TTT GTC TGC AA-3′ |
| UGT1A1 | |
| UGT1A1-S | 5′-CCT TGC CTC AGA ATT CCT TC-3′ |
| UGT1A1-AS | 5′-ATT GAT CCC AAA GAG AAA ACC AC-3′ |
| Sult1b1 | |
| Sult1b1-S | 5′-TCC ATC TCA GGT CAC CAC CA-3′ |
| Sult1b1-AS | 5′-TCT CCA AAC GTC TTC TGA GGC-3′ |
| D1 | |
| D1-S | 5′-GTT TAG CAC AAG CAA GAG GCA-3′ |
| D1-AS | 5′-GCG CCT GCG ATT TGG TTT AG-3′ |
| D2 | |
| D2-S | 5′-GAA TCC CAT TGC CTC ACC GA-3′ |
| D2-AS | 5′-AGG CTG CAA CAG GGT TTC TT-3′ |
| D3 | |
| D3-S | 5′-ATT GCT GTG GCT CGA ACT GA-3′ |
| D3-AS | 5′-GAA ATG CTG GGG ACT TTC GC-3′ |
| Synapsin I | |
| Synapsin I-S | 5′-TGC CAA CAA GAC GGA GAG TG-3′ |
| Synapsin I-AS | 5′-TAG TGC CCC CTT TAA CGC AG -3′ |
| Synaptophysin | |
| Synaptophysin-S | 5′-TTT GCC ATC TTC GCC TTT GC-3′ |
| Synaptophysin-AS | 5′-GTG CAG CCT GAA TGG GTA CT-3′ |
AS, antisense; CPH, cyclophilin; D1, type 1 iodothyronine deiodinase; D2, type 2 iodothyronine deiodinase; D3, type 3 iodothyronine deiodinase; S, sense.
Enzyme Assays.
Perfused liver with 1.15% KCl was homogenized in 3 volumes of homogenization buffer (1.15% KCl/10 mM potassium phosphate buffer, pH 7.4). The homogenate was centrifuged at 10,000g for 30 minutes at 4°C, and the supernatant was collected. The supernatant was centrifuged at 105,000g for 60 minutes at 4°C, and the pellet was suspended in the same buffer and used as the microsomal fraction. Protein concentrations of microsomal fractions were measured by the Bradford method using bovine serum albumin as a standard (Bradford, 1976). Thyroxine-glucuronide formation was determined according to the method of Kato et al. (2008) with slight modifications. In brief, a typical incubation mixture (200 μl of total volume) contained 100 mM Tris-HCl buffer (pH 7.4), 5 mM MgCl2, 25 μg/ml alamethicin, 100 μM thyroxine, and 1 mg/ml liver microsomes of humanized UGT1 mouse or 0.4 mg/ml human liver microsomes. The reaction was initiated by the addition of 5 mM UDPGA after a 3-minute preincubation at 37°C. After incubation at 37°C for 240 minutes, the reaction was terminated by the addition of 200 μl of cold acetonitrile. Although the incubation time (4 hours) was slightly longer than the optimal condition, it was necessary to obtain a detectable peak area of T4-glucuronide in the liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analysis. After removal of the protein by centrifugation at 12,000g for 5 minutes, a 10-μl portion of the sample was injected into the LC-MS/MS system. Quantification of T4 glucuronidation activity was performed by comparing the peak areas of T4 glucuronide in hUGT1 mouse liver microsomes to that in human liver microsomes and calculating by reference to the previous study (Yamanaka et al., 2007). Estradiol glucuronide formation was determined according to the previous method (Kutsuno et al., 2015), with slight modifications. In brief, a typical incubation mixture (200 μl of total volume) contained 100 mM Tris-HCl buffer (pH 7.4), 5 mM MgCl2, 25 μg/ml alamethicin, 50 μg/ml estradiol, and 0.4 mg/ml mouse liver microsomes or 0.2 mg/ml human liver microsomes. The reaction was initiated by the addition of 2.5 mM UDPGA (final concentration) after a 3-minute preincubation at 37°C. Incubation was performed for 30 minutes. The reaction was terminated by the addition of 200 μl of cold methanol. After removal of the protein by centrifugation at 12,000g for 5 minutes, a 50-μl portion of the sample was subjected to high-performance liquid chromatography (HPLC).
T4-Binding Assay.
A typical assay mixture (500 μl of total volume) contained 0.15 M phosphate buffer (pH 7.4), 2.5% serum from hUGT1 mice, and 100 μM phenytoin or 100 μM salicylic acid. Phenytoin and salicylic acid were dissolved in ethanol, and the final concentration of ethanol in the assay tube was 0.5%. The assay mixture was incubated at 37°C for 1 hour. The ultrafiltration device was rinsed with 0.15 M phosphate buffer and spun for at least 10 minutes at 1500g after rinsing to avoid dilution errors due to the presence of any remaining rinse solution in the device. The ultrafiltration experiments were carried out by placing a 500-μl aliquot of each test mixture into a Centrifree micropartition device (Millipore, Bedford, MA) and spinning this mixture at 1500g for 10 minutes at 37°C. A 10-μl portion of filtrate was injected into the LC-MS/MS system for analysis of thyroxine content.
LC-MS/MS Analysis for Thyroxine Glucuronides.
LC-MS/MS was performed using an Acquity UPLC H-Class system including a binary pump, an automatic sampler, and a column oven (Waters Corp., Milford, MA), which was equipped with a 50 × 2.1–mm Acquity 1.6-mm C18 column (Waters). The column temperature was 35°C. The mobile phase was 0.1% formic acid (A) and acetonitrile including 0.1% formic acid (B). The conditions for elution were as follows: 25% B (0–1 minute), 25–70% B (1–4 minutes), 70% B (4–10 minutes), 70–25% B (10–11 minutes). Linear gradients were used for all solvent changes. The flow rate was 0.5 ml/min. The eluent was introduced by electrospray ionization into the mass spectrometer (Xevo TQD; Waters) operating in positive ionization mode. Multiple reaction monitoring mode, using specific precursor/product ion transition, was used for quantification. The capillary and sampling cone voltages were set to 3500 and 35 V, respectively. Source and desolvation temperatures were set to 150 and 500°C, respectively, and the cone and desolvation gas flows were set to 50 and 600 l/h, respectively. The collision energy was set to 35 V. Two mass/charge (m/z) ion transitions were recorded in the multiple reaction monitoring mode: m/z 778 and 732 for thyroxine, and m/z 954 and 778 for thyroxine glucuronide. The retention times of thyroxine glucuronide and thyroxine were 7.1 and 8.9 minutes, respectively.
HPLC Conditions.
Estradiol 3-O-glucuronide was determined by the HPLC system with an LC-10AD pump (Shimadzu, Kyoto, Japan), an FP-2020 fluorescence detector (JASCO, Tokyo, Japan), an SIL-10A autosampler (Shimadzu), an SLC-10A system controller (Shimadzu), and a Mightysil RP-18 GP column (4.6 × 150 mm, 5 μm; Kanto Chemical, Tokyo, Japan). The mobile phases were 10 mM H3PO4-methanol (45:55, v/v). The flow rate was 1.0 ml/min. Estradiol 3-O-glucuronide was detected with a fluorescence detector at 280-nm excitation and 310-nm emission. The retention time was 6.4 minutes.
Western Blotting Analysis.
One hundred fifty micrograms of liver microsomes was subjected to neutral polyacrylamide gel electrophoresis 4–12% Bis-Tris Gel (Life Technologies) and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore) following the manufacturer’s protocol. The membrane was blocked for 3 hours with 50 mg/ml skimmed milk in PBS and then incubated with anti-UGT1A1 antibody (sc-27415; Santa Cruz Biotechnology Inc., Santa Cruz, CA) diluted with PBS (1:200) as a primary antibody overnight. The membrane was washed with PBS three times and incubated with anti-goat secondary antibody (A5420; Sigma-Aldrich) diluted with PBS (1:10,000) for 1 hour. The bands were detected using Chemi-Lumi One Super western blotting detection reagents (Nacalai Tesque, Kyoto, Japan).
Histologic Analysis.
The paraffin blocks of brains were sliced at a thickness of 8 μm. To maintain consistency of analysis, only the cerebellar vermis region was used for histologic analysis. The brain sections were deparaffinized, hydrated with xylene and gradual ethanol, and then stained with H&E stain. External granule cell area was measured within 50 μm in the horizontal direction of the external granule cell layer from randomly selected fields using an image analysis software (ImageJ; Texelcraft, Tokyo, Japan).
Rotarod Study.
Motor coordination and motor learning were assessed on an accelerating rotarod test. Mice treated with phenytoin during the perinatal period, control mice, and PTU-induced hypothyroidism mice were trained on a rotarod at a fixed speed of 6 rpm for 5 minutes. After 24 hours of training, mice were placed on a stationary rod, and acceleration was initiated. The speed of the rotarod accelerated from 6 to 20 over 5 minutes. The time to fall off the rod was measured.
Statistical Analysis.
All data were presented as means (± S.D.). The differences in serum T4 concentration between hUGT1 mice and wild-type mice and the effect of phenytoin through breast milk during the perinatal period and neonatal period were analyzed by Student’s t test. The effect of T4 replacement and carbamazepine and PCN treatment during the neonatal period and UGT1A1 inducibility of various UGT inducers were analyzed by analysis of variance and Dunnett's procedure for multiple comparisons. Induction of UGT1A1 mRNA by carbamazepine and PCN was analyzed by Student’s t test with a Bonferroni correction for multiple comparisons. The difference in rotarod performance between control mice, phenytoin-treated mice, and PTU-treated mice was analyzed by the Kaplan-Meier method and the log-rank test for trend. P < 0.05 was considered significant.
Results
UGT1A1 Activity Controls the Serum T4 Level.
There is a species difference in UGT1A1 activity between wild-type mice and humans (Cai et al., 2010). To overcome the species difference in UGT1A1 activity, hUGT1 mice in which the original Ugt1 locus was disrupted and replaced with the human UGT1 locus have been developed (Fujiwara et al., 2010). Bilirubin is a specific substrate of UGT1A1. It was reported that serum bilirubin was 0.4 mg/dl in 2-day-old wild-type mice (Bortolussi et al., 2012), whereas serum bilirubin in 2-day-old hUGT1 mice was 2.5–3.0 mg/dl (Fujiwara et al., 2010). The bilirubin level in hUGT1 mice is more than 6-fold higher than the level in wild-type mice, suggesting that UGT1A1 activity in hUGT1 mice is lower than that in wild-type mice. In this study, serum free and total T4 levels in hUGT1 mice were statistically higher than those in wild-type mice (Table 2). Phenylhydrazine lyses erythrocytes, leading to a significant increase of serum bilirubin levels (Rice and Shapiro, 2008). Phenylhydrazine increased the serum bilirubin levels in hUGT1 mice from 3.8 to 20.1 mg/dl (Table 3). The serum T4 levels were similar between phenylhydrazine-treated and control mice, indicating that the difference in UGT1A1 activity, but not serum bilirubin, might be mainly controlling serum T4 level.
TABLE 2.
Serum T4 levels in neonatal wild-type (WT) and hUGT1 mice
| Free T4 |
Total T4 |
|
|---|---|---|
| ng/dl | μg/dl | |
| WT (n = 18) | 2.89 ± 0.12 | 6.0 ± 0.4 |
| hUGT1 (n = 15) | 3.04 ± 0.03* | 8.1 ± 1.4* |
P < 0.05.
TABLE 3.
Bilirubin and serum-free T4 levels in phenylhydrazine-treated hUGT1 mice
| Bilirubin |
Free T4 |
|
|---|---|---|
| mg/dl | ng/dl | |
| Control (n = 3) | 3.8 ± 4.4 | 1.9 ± 0.3 |
| Phenylhydrazine (n = 3) | 20.1 ± 4.8* | 1.7 ± 0.1 |
P < 0.05.
Effect of Phenytoin Treatments during Perinatal Period on Serum T4 Levels and T4 Metabolism-Related Gene Expression.
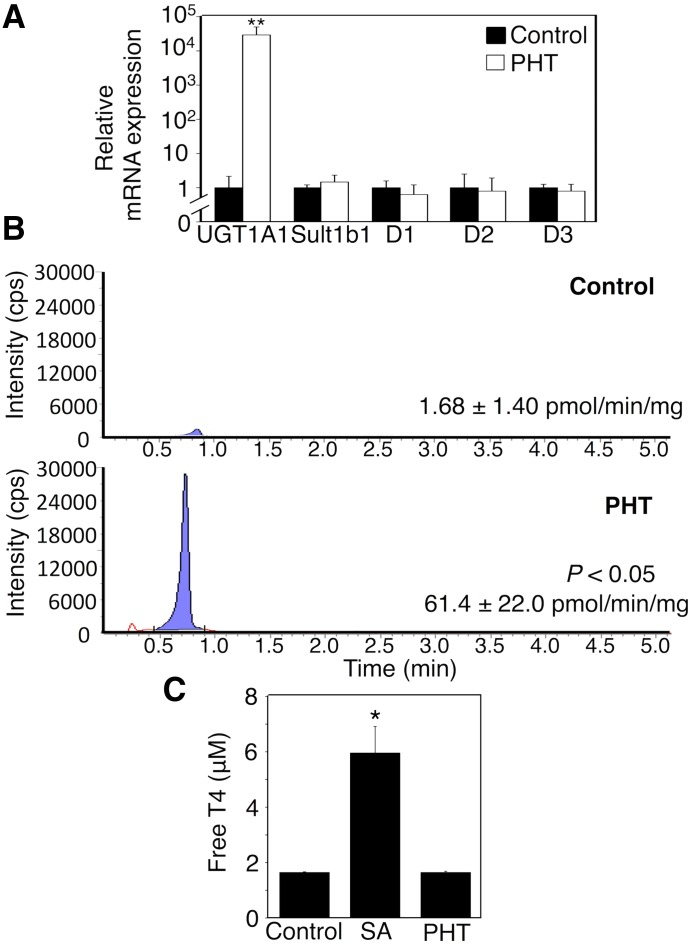
In this study, phenytoin decreased serum T4 levels in 14-day-old hUGT1 mice (Table 4). Decreased protein binding of T4 can result in a reduced serum T4 level (Wang et al., 1998). Phenytoin significantly increased mRNA levels of UGT1A1, whereas mRNA levels of other T4-metabolizing enzymes, such as type 1 iodothyronine deiodinase, type 2 iodothyronine deiodinase, type 3 iodothyronine deiodinase, and sult1b1, did not statistically change by phenytoin (Fig. 1A). T4 glucuronidation activity toward T4 in liver microsomes of phenytoin-treated mice was higher than that in liver microsomes of control mice (Fig. 1B). Quantification of T4 glucuronidation activity in liver microsomes revealed that the exposure to phenytoin during the perinatal period increased T4 glucuronidation activity 38-fold. Salicylic acid, which is an inhibitor of T4 binding to serum proteins (Wang et al., 1999), increased the free T4 level in vitro (Fig. 1C). In contrast, phenytoin did not increase the free T4 level in vitro (Fig. 1C). These data indicated that phenytoin did not inhibit T4 binding to serum proteins but induced hepatic UGT1A1, causing the accelerated T4 metabolism.
TABLE 4.
Effects of perinatal or postnatal chemical treatments on serum T4 levels
| Number of Animals |
Free T4 |
|
|---|---|---|
| μM | ||
| Control | 15 | 3.0 ± 0.0 |
| Phenytoin | 8 | 1.4 ± 0.4** |
| PTU | 4 | 0.6 ± 0.0** |
| Phenytoin (postnatal) | 7 | 1.6 ± 0.8* |
| Phenytoin (postnatal) + T4 | 6 | 3.3 ± 0.3 |
| Carbamazepine (postnatal) | 4 | 2.4 ± 0.1* |
| PCN (postnatal) | 4 | 1.5 ± 0.2** |
| PHT (through breast milk) | 5 | 2.5 ± 0.2* |
PHT, phenytoin.
P < 0.05; **P < 0.01.
Fig. 1.
Effect of phenytoin treatments during the perinatal period on serum T4 levels and T4 metabolism-related gene expression. Dams and their pups were treated with phenytoin daily from gestation day 12, 13, or 14 to postnatal day 0 (p.o.) and from postnatal day 1 to day 14 (s.c.). (A) Total RNA was prepared from the livers, and mRNA levels of T4-metabolizing enzymes were determined in control mice (closed columns, n = 3) and phenytoin (PHT)-treated mice (open columns, n = 3). Liver microsomes were prepared, and UGT1A1 activity toward T4 was measured. (B) Tandem mass spectrometric chromatograms for T4 glucuronidation in control mice and phenytoin-treated mice are shown. (C) The effect of salicylic acid (SA, n = 3) and phenytoin (n = 3) on T4 binding was determined in vitro. Each column is the mean ± S.D. of biologic replicates. *P < 0.05; **P < 0.01 compared with control. PHT, phenytoin.
mRNA Levels of Neurodevelopment-Related Genes in Brains of hUGT1 Mice Treated with Phenytoin during Perinatal Period.
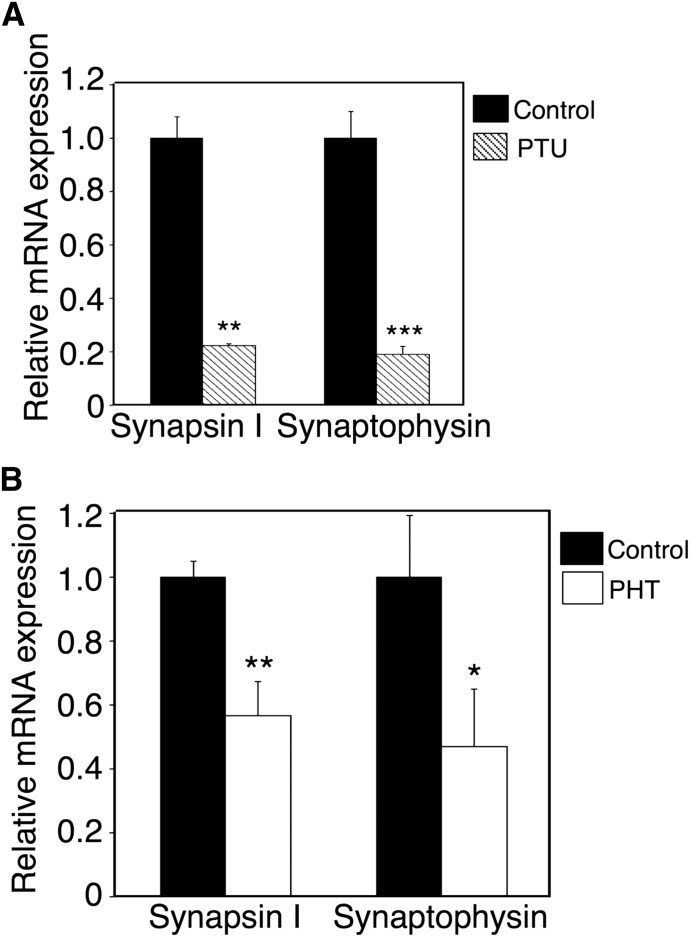
In humans and rodents, hypothyroidism impairs hippocampal function, leading to defective performance on a variety of behavioral learning tasks (Koromilas et al., 2010). Learning ability is strongly associated with hippocampus synaptogenesis (Shors, 2004). In the present study, we assessed the expression levels of synapsin I and synaptophysin, molecular markers of synaptic density (Thiel, 1993), to investigate the effect of phenytoin on synaptogenesis in the hippocampus. In PTU-induced hypothyroidism (Table 4), the mRNA expression levels of synapsin I and synaptophysin were significantly lower than those in control mice (Fig. 2A). In hUGT1 mice, phenytoin lowered mRNA expression levels of synapsin I and synaptophysin (Fig. 2B). These data indicated that the exposure to phenytoin during the perinatal period decreased the synaptogenesis in the hippocampus at the gene expression level in hUGT1 mice.
Fig. 2.
mRNA levels of neurodevelopment-related genes in brains of hUGT1 mice treated with phenytoin during the perinatal period. PTU was given to dams from gestation day 14 to postnatal day 14. (A) The mRNA levels of synapsin I and synaptophysin in the hippocampus were measured in control (closed column, n = 3) and PTU-treated mice (shaded column, n = 3). Dams and their pups were treated with phenytoin daily from gestation day 12, 13, or 14 to postnatal day 0 (p.o.) and from postnatal day 1 to day 14 (s.c.). (B) On postnatal day 14, mRNA levels of synapsin I and synaptophysin in the hippocampus were determined in control (closed column, n = 3) and phenytoin (PHT)-treated mice (open column, n = 3). Error bars show the S.D. of biologic replicates. *P < 0.05; **P < 0.01; ***P < 0.001 compared with control.
Effect of Exposure to Phenytoin during Perinatal Period on Migration of Granule Cells in hUGT1 Mice.
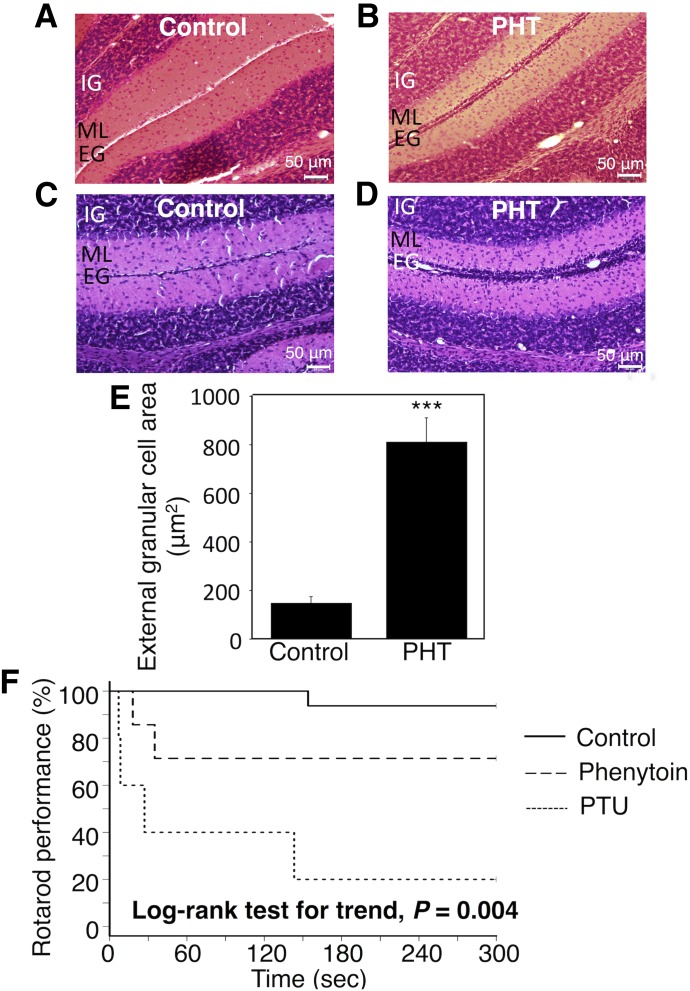
Cerebellar lesions cause impairment of visual-motor coordination ability (Becker et al., 1990). Children exposed to phenytoin during pregnancy had a significantly lower score on a visual-motor integration test (Vanoverloop et al., 1992), suggesting that phenytoin led to impairments in the cerebellum. In the present study, to investigate the effect of phenytoin exposure during the perinatal period on cerebellum development, area of cerebellar granule cell migration was evaluated in control and phenytoin-treated mice. In the control mice, cerebellar granule cells completely migrated from the external granule layer to the internal granule layer (Fig. 3A). In contrast, cerebellar granule cells remained at the external granule layer in the phenytoin-treated mice (Fig. 3B). The phenytoin-induced delay in the migration of cerebellar granule cells was reproducible (n = 6) (Fig. 3, C and D). The quantification of external granule cell area at the external granule layer showed that the external granule cell area in the phenytoin-treated mice increased 5.4-fold compared with that in the control mice (Fig. 3E). These results suggest that the exposure to phenytoin during the perinatal period delays cerebellum development at the morphology level.
Fig. 3.
Effect of exposure to phenytoin during the perinatal period on migration of granule cells and rotarod performance in hUGT1 mice. Dams and their pups were treated with phenytoin daily from gestation day 12, 13, or 14 to postnatal day 0 (p.o.) and from postnatal day 1 to day 14 (s.c.). On postnatal days 14, the midsagittal sections of the cerebellum vermis in control mice (A and C) and phenytoin (PHT)-treated mice (B and D) were stained with hematoxylin and eosin. (E) External granule cell area was measured within 50 μm in the horizontal direction of the external granule cell layer from randomly selected fields (n = 6). Prior to the rotarod study, control mice, phenytoin-treated mice, and PTU-induced hypothyroidism mice were trained on a rotarod at a fixed speed of 6 rpm for 5 minutes. In the rotarod study, the speed of the rotarod was accelerated from 6 to 20 over 5 minutes. The time to fall off the rod was measured. (F) The difference in rotarod performance between control mice (solid line, n = 15), phenytoin-treated mice (dashed line, n = 7), and PTU-treated mice (dotted line, n = 5) was analyzed by the Kaplan-Meier method and the log-rank test for trend. Error bars show the S.D. of biologic replicates. ***P < 0.001 compared with control. EG, external granule layer; IG, internal granule layer; ML, molecular layer.
Effect of Exposure to Phenytoin during Perinatal Period on Rotarod Performance in hUGT1 Mice.
In humans and rodents, hypothyroidism led to impaired performance on motor coordination and behavioral learning tasks (Koibuchi and Chin, 2000; Koromilas et al., 2010). In the present study, the effect of exposure to phenytoin during the perinatal period on behavioral phenotypes was analyzed on a rotarod using Kaplan-Meier analysis (Fig. 3F). None of the control mice fell off the rod for the first 60 seconds. One of the 16 tested control mice fell off the rotarod during the next 60 seconds. In contrast, two of the seven phenytoin-treated mice fell off the rotarod in the first 60 seconds. Three of five PTU-treated mice fell off the rotarod in the first 60 seconds. A log-rank test for trend revealed that the rotarod performance of PTU-treated mice and phenytoin-treated mice was lower than that in control mice (P = 0.004), indicating that exposure to phenytoin during the perinatal period impaired motor activity and motor learning ability, similar to hypothyroidism.
Effects of Postnatal Phenytoin Treatment on UGT1A1 Activity and Rotarod Performance in Wild-Type Mice.
To investigate the effect of phenytoin treatment during the neonatal period in wild-type mice, we subcutaneously administered phenytoin to wild-type mice from postnatal day 1 to day 14. Microsomal UGT1A1 activity was increased 2-fold by phenytoin in wild-type mice (Supplemental Fig. 1A). All of the phenytoin-treated wild-type mice fell off the rotarod within 270 seconds (Supplemental Fig. 1B). Meanwhile, two out of 10 untreated wild-type mice did not fall off the rotarod for more than 300 seconds. Although phenytoin induced UGT1A1 activity and neurodevelopmental toxicity in both hUGT1 mice and wild-type mice, the effect was more significant in hUGT1 mice (Fig. 3F).
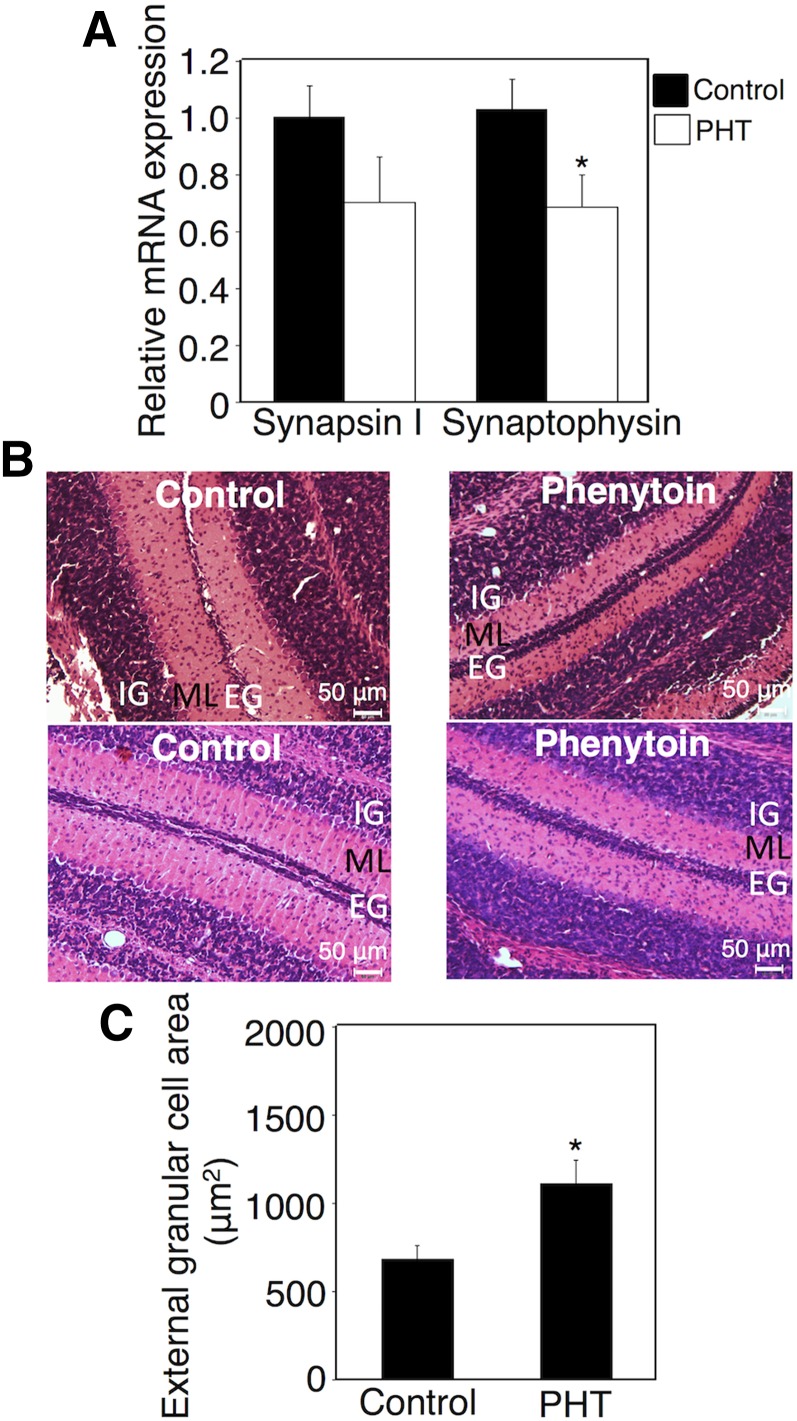
Effect of Exposure to Phenytoin during Postnatal Period on Serum Free T4 Levels and Neurodevelopment in hUGT1 Mice.
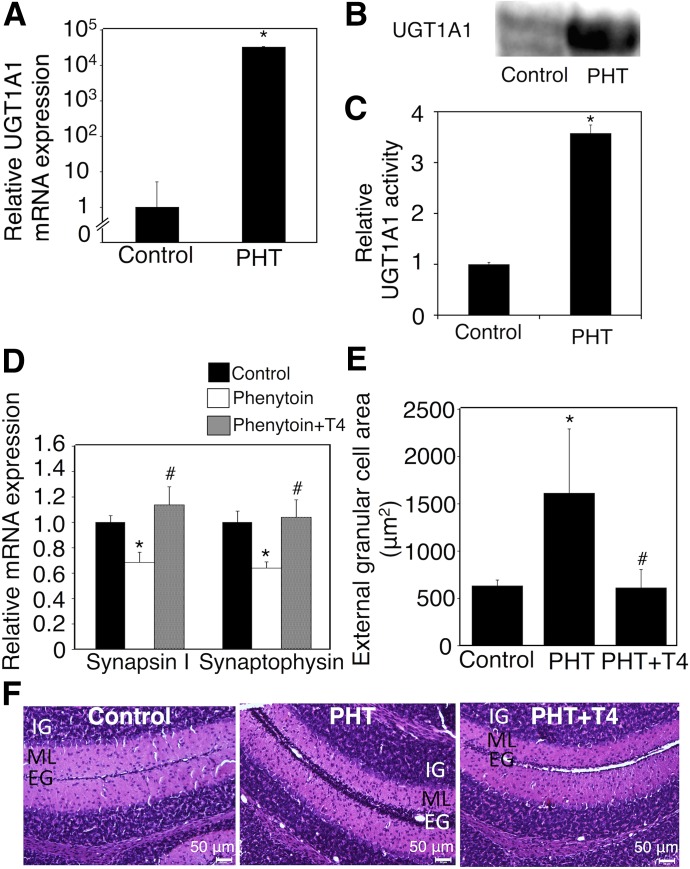
To investigate the effect of the exposure of neonates to phenytoin during only the neonatal period on neurodevelopment, we subcutaneously administered phenytoin to hUGT1 mice from postnatal day 1 to day 14 and assessed the synaptogenesis in the hippocampus and migration of cerebellar granule cells in these mice. The exposure of hUGT1 mice to phenytoin during the postnatal period decreased serum T4 levels (Table 4). hUGT1 mice exposed to phenytoin during only the postnatal period showed an induction of UGT1A1 mRNA (Fig. 4A) similar to the mice exposed to phenytoin during the perinatal period (Fig. 1A). It was reported that mRNA levels for UGT1A1 might not correlate with protein levels (Ohtsuki et al., 2012). However, western blot analysis of liver microsomes showed that phenytoin treatment during the neonatal period induced UGT1A1 protein in the liver (Fig. 4B), which was in agreement with the finding that UGT1A1 mRNA level was increased by phenytoin treatment (Fig. 4A). It was further found that UGT1A1 activity was increased 3.5-fold by phenytoin treatment (Fig. 4C). The expression levels of synapsin I and synaptophysin in the hippocampus were decreased by phenytoin (Fig. 4D). Furthermore, the delay in the migration of external granule cells was observed in hUGT1 mice exposed to phenytoin during the postnatal period (Fig. 4, E and F). The quantification of the external granule cell area showed that exposure to phenytoin during the postnatal period increased the external granule cell area 2.5-fold. These data showed that exposure to phenytoin, especially during the postnatal period, delayed neurodevelopment in hUGT1 mice. In terms of brain development, the postnatal period in mice corresponds to the third trimester and postnatal period in humans (Zoeller and Rovet, 2004), suggesting that the administration of phenytoin during the third trimester and postnatal period can increase the risk for neurodevelopmental disorder in children of humans.
Fig. 4.
Effect of exposure to phenytoin and T4 replacement during the postnatal period on serum free T4 levels and neurodevelopment in hUGT1 mice. Phenytoin (PHT; s.c. 35 mg/kg) was administered to pups daily from postnatal day 1 to day 14 (PHT group). Phenytoin (s.c. 35 mg/kg) and T4 (s.c. 100 μg/kg) were simultaneously administered to pups daily from postnatal day 1 to day 14 (PHT+T4 group). (A) Total RNA was prepared from the livers of control (n = 3) and phenytoin-treated mice (n = 3), and mRNA levels of UGT1A1 were determined. (B) Pooled liver microsomes were prepared from control (n = 3) and phenytoin-treated (n = 2) hUGT1 mice, and western blotting against UGT1A1 was performed. (C) UGT1A1 activity toward estradiol was measured. (D) Total RNA was prepared from the hippocampus, and mRNA levels of synapsin I and synaptophysin were determined in the control group (closed columns, n = 3), PHT group (open columns, n = 3), and PHT+T4 group (gray columns, n = 3). (E) External granule cell area was measured within 50 μm in the horizontal direction of external granule cell layer from randomly selected fields (n = 3). (F) The midsagittal sections of the cerebellum vermis in the control, PHT, and PHT+T4 groups were stained with hematoxylin and eosin. Error bars show the S.D. of biologic replicates. *P < 0.05 compared with control group; #P < 0.05 compared with the PHT group. EG, external granule layer; IG, internal granule layer; ML, molecular layer.
Effect of T4 Replacement on Phenytoin-Induced Neurodevelopmental Toxicity in hUGT1 Mice.
As phenytoin functions in the brain as an anticonvulsant drug, phenytoin might have directly inhibited neurodevelopment without T4 reduction. We next investigated whether T4 replacement could attenuate neurodevelopmental disorder induced by phenytoin exposure during the postnatal period in hUGT1 mice. Phenytoin and T4 were simultaneously administered to hUGT1 mice daily from postnatal day 1 to day 14. The T4 replacement (100 μg/kg/day) increased the serum T4 level to the same level as control (Table 4). T4 replacement increased the mRNA expression levels of synapsin I and synaptophysin to the same levels as those in control mice (Fig. 4D). T4 replacement accelerated the migration of the external granule cells (Fig. 4, E and F). T4 replacement attenuated the neurodevelopmental disorder induced by phenytoin exposure during the postnatal period in hUGT1 mice, indicating that reduced T4 was tightly associated with the neurodevelopmental disorder induced by phenytoin.
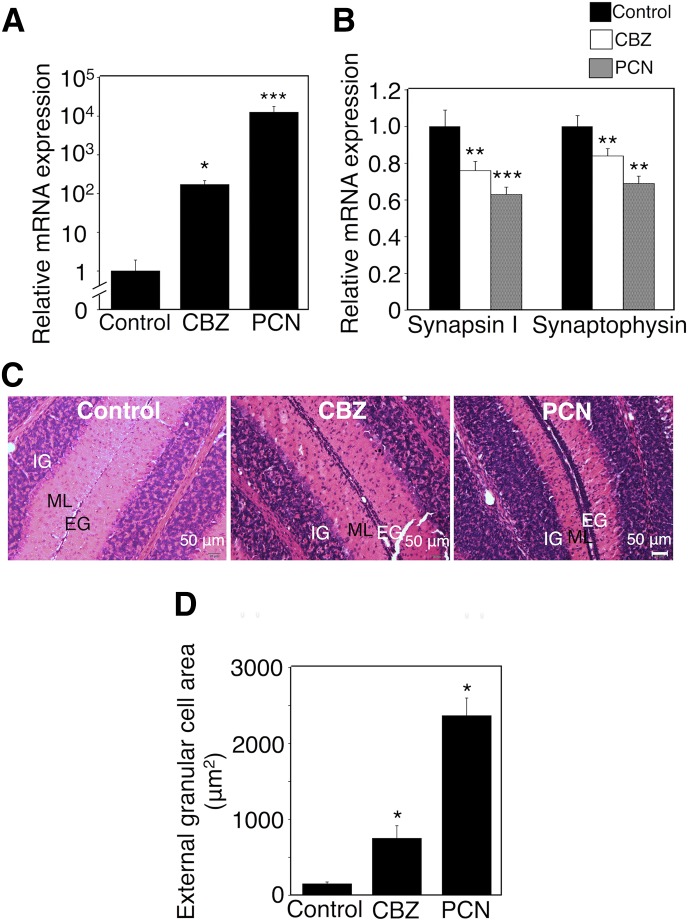
Effect of Carbamazepine and PCN on Serum Free T4 Level and Neurodevelopmental Toxicity in hUGT1 Mice.
Phenytoin induces UGT1A1 through CAR (Smith et al., 2005). Activation of CAR during the postnatal period by phenytoin might have induced the neurodevelopmental disorder. Treatments of hUGT1 mice with carbamazepine, another CAR activator, decreased serum T4 levels (Table 4). The mRNA level of UGT1A1 was induced 200-fold by the carbamazepine treatment (Fig. 5A). UGT1A1 glucuronidation activity was increased 2-fold by carbamazepine (Supplemental Fig. 2). Carbamazepine significantly decreased the expression levels of synapsin I and synaptophysin in the hippocampus (Fig. 5B). The delay in the migration of external granule cells was observed in hUGT1 mice exposed to carbamazepine (Fig. 5C). PCN, a PXR ligand, is an inducer of UGT1A1. Although Usui et al. (2006) reported that PCN did not induce UGT1A1 mRNA in HepG2 cells, Buckley and Klaassen (2009) and Wagner et al. (2005) showed that hepatic Ugt1a1 mRNA was induced 1.5-fold by PCN in mice. Treatments of hUGT1 mice with PCN decreased serum T4 levels (Table 4). In the present study, mRNA level of UGT1A1 was induced 10,000-fold by postnatal PCN treatment (Fig. 5A). UGT1A1 glucuronidation activity was increased 2.5-fold by PCN (Supplemental Fig. 2). PCN significantly decreased the expression levels of synapsin I and synaptophysin in the hippocampus (Fig. 5B). The delay in the migration of external granule cells was observed in hUGT1 mice exposed to PCN (Fig. 5C). The quantification of external granule cell area showed that carbamazepine and PCN increased the external granule cell area 5.1-fold and 16-fold, respectively (Fig. 5D). Not only CAR activation but also PXR activation led to neurodevelopmental disorder, indicating that UGT1A1 induction was the factor causing neurodevelopmental disorder.
Fig. 5.
Effect of exposure to carbamazepine and PCN during the postnatal period on serum free T4 levels and neurodevelopment in hUGT1 mice. Carbamazepine (CBZ; s.c. 35 mg/kg) and PCN (s.c. 10 mg/kg) were administered to pups daily from postnatal day 1 to day 14. (A) Total RNA was prepared from livers of three hUGT1 mice each, and mRNA levels of UGT1A1 were determined. (B) Total RNA was prepared from the hippocampus, and mRNA levels of synapsin I and synaptophysin in the hippocampus were determined in control mice (closed columns, n = 3), CBZ-treated mice (open columns, n = 3), and PCN-treated mice (gray columns, n = 3). (C) The midsagittal sections of the cerebellum vermis in control mice, CBZ-treated mice, and PCN-treated mice were stained with hematoxylin and eosin. (D) External granule cell area was measured within 50 μm in the horizontal direction of the external granule cell layer from randomly selected fields (n = 3). Error bars show the S.D. of biologic replicates. *P < 0.05; **P < 0.01; ***P < 0.001 compared with control. EG, external granule layer; IG, internal granule layer; ML, molecular layer.
Effect of Phenytoin Treatment during the Postnatal Period through Breast Milk on Serum T4 Levels and Neurodevelopment in hUGT1 Mice.
Phenytoin treatment has no contraindication in breast-feeding women (Bar-Oz et al., 2000). As phenytoin can be slightly secreted to breast milk, children might be exposed to up to 5% of the therapeutic doses of phenytoin by breast-feeding (Steen et al., 1982). We investigated the effect of phenytoin exposure at low concentrations through breast milk on neurodevelopment in hUGT1 mice by administering a therapeutic dose of phenytoin (80 mg/kg) to lactating hUGT1 mice. Exposure to phenytoin through breast milk in hUGT1 mice decreased serum T4 levels (Table 4). In hUGT1 mice exposed to phenytoin via breast milk, the expression levels of synapsin I and synaptophysin were lower than those in control mice (Fig. 6A). Furthermore, a delay in the migration of external granule cells was observed in hUGT1 mice exposed to phenytoin via breast milk (Fig. 6B, upper panels). In the control mice, cerebellar granule cells completely migrated from the external granule layer to the internal granule layer, whereas cerebellar granule cells remained at the external granule layer in the phenytoin-treated mice (Fig. 6B, upper panels). The phenytoin-induced delay in the migration of cerebellar granule cells was reproducible (n = 6) (Fig. 6B, lower panels). The quantification of external granule cell area showed that the external granule cell area in the phenytoin-treated mice increased 1.7-fold compared with those in the control mice (Fig. 6C). These data suggest that the exposure to phenytoin at low concentrations through breast milk still caused the neurodevelopmental disorder in neonatal mice.
Fig. 6.
Effect of phenytoin treatment during the postnatal period through breast milk on serum free T4 levels and neurodevelopment in hUGT1 mice. Phenytoin (PHT; s.c. 35 mg/kg) was administered to dams daily from postnatal day 1 to day 14. (A) Total RNA was prepared, and mRNA levels of synapsin I and synaptophysin in the hippocampus were determined in control (closed columns, n = 3) and phenytoin-treated mice (open columns, n = 3). (B) On postnatal day 14, the midsagittal sections of the cerebellum vermis in control mice and phenytoin-treated mice were stained with hematoxylin and eosin. (C) External granule cell area was measured within 50 μm in the horizontal direction of the external granule cell layer from randomly selected fields (n = 6). Error bars show the S.D. of biologic replicates. *P < 0.05 compared with control. EG, external granule layer; IG, internal granule layer; ML, molecular layer.
Discussion
In the present study, total and free serum thyroxine levels in hUGT1 mice were higher than those in wild-type mice on postnatal day 14 (Table 2). The UGT1A1 activity in hUGT1 mice was lower than that of wild-type mice (Fujiwara et al., 2010), indicating that the low activity of UGT1A1 was the cause for higher T4 level in hUGT1 mice than in wild-type mice. The Ugt1 knockout mice were, therefore, expected to have even higher T4 levels than wild-type mice. In contrast to our expectation, a previous study reported that total serum T4 levels were similar between Ugt1 knockout mice and wild-type mice at 5 days after birth (Nguyen et al., 2008). As it has been reported that there are age differences in regulation of serum T4 levels (Ingbar, 1976), these inconsistent findings can be explained by the difference in age of mice. In fact, free T4 levels in 2-month-old hUGT1 mice were lower than those in 2-month-old wild-type mice, whereas free T4 levels in 6-month-old hUGT1 mice were similar to those in wild-type mice (Supplemental Fig. 3). As the serum T4 level is controlled by multiple factors, such as T4-metabolizing enzymes, T4-binding proteins, hypothalamic-pituitary-thyroid negative feedback regulation, and age, the process of controlling serum T4 levels is highly complex. Whereas lower UGT1A1 activities slightly affected the serum T4 levels (Table 2), increased UGT1A1 activities dramatically reduced the T4 levels (Fig. 1A; Table 4). Therefore, UGT1A1 can be the determining factor of serum T4 levels, especially when strongly induced.
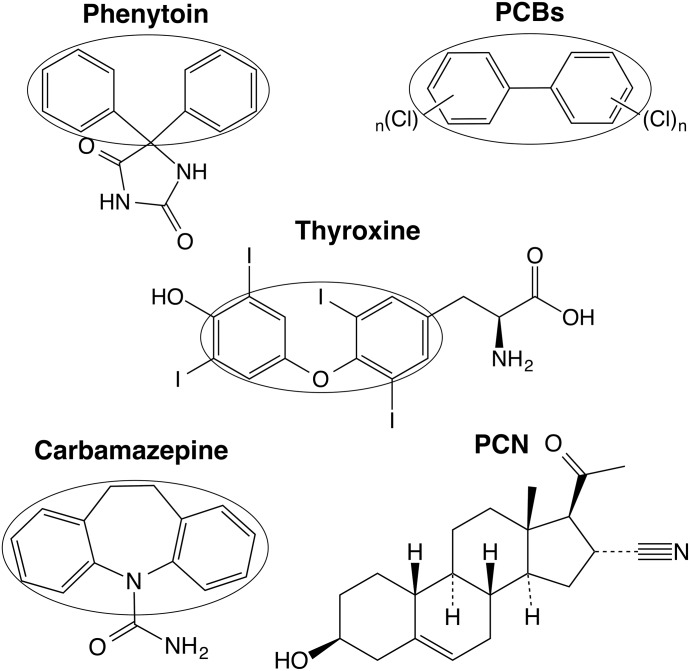
Since exposure to phenytoin during the postnatal period caused a reduction of T4 and neurodevelopmental disorder in hUGT1 mice (Figs. 2B and 3, E and F; Table 4), lowered transfer of oxygen to the fetus is not a primary cause of neurodevelopmental disorder in children exposed to phenytoin during the perinatal period. As phenytoin functions in the brain as an anticonvulsant drug, phenytoin might have directly induced neurodevelopmental toxicity without T4 reduction. Due to the similarity of the chemical structure between PCBs and thyroxine (Fig. 7), it has been proposed that PCBs inhibit the binding of thyroxine to TRs (McKinney, 1989). As the structures of phenytoin and carbamazepine are also similar to that of thyroxine, phenytoin- and carbamazepine-induced neurodevelopmental disorder might have been caused by disruption of T4 binding to TRs. In addition, as phenytoin activates the nuclear receptor CAR (Smith et al., 2005), activation of CAR during the postnatal period might have induced neurodevelopmental disorder. The present study showed that T4 replacement attenuated neurodevelopmental disorder induced by phenytoin exposure during the postnatal period in hUGT1 mice (Fig. 4, D–F), indicating that the reduced T4 was specifically associated with neurodevelopmental disorder induced by phenytoin. Not only phenytoin but also the PXR activator PCN caused neurodevelopmental disorder (Fig. 5, B–D); therefore, CAR activation was not associated with phenytoin-induced neurodevelopmental disorder. In addition, PCN is not structurally similar to thyroxine (Fig. 7), indicating that the disruption of T4 binding to TRs might not be associated with neurodevelopmental disorder. The present study showed that phenytoin neither inhibited binding of T4 to T4-binding proteins nor induced T4-metabolizing enzymes except for UGT1A1 (Fig. 1A), supporting the fact that induction of UGT1A1 caused neurodevelopmental disorder in hUGT1 mice. Further studies demonstrated that phenytoin-induced neurodevelopmental disorder resulted from the reduced T4 caused by the induction of UGT1A1.
Fig. 7.
Chemical structures of thyroxine, phenytoin, PCBs, carbamazepine, and PCN. Chemical structures of phenytoin, PCBs, carbamazepine, and PCN were compared with that of thyroxine. Structural similarities among these compounds are indicated by ovals.
An important question was whether UGT1A1 induction by phenytoin could actually induce neurodevelopmental disorder in humans. First, it was unclear whether a therapeutic dose of phenytoin could reach a concentration sufficient to induce UGT1A1 and reduce serum T4 levels in humans. In this study, the maximum plasma concentration of phenytoin in 13-day-old hUGT1 mice treated with phenytoin (s.c. 35 mg/kg) was about 100 μM (data not shown). The effective therapeutic range of phenytoin is from 50 to 100 μM in humans (Bochner et al., 1972), indicating that the plasma concentration of phenytoin can reach a concentration sufficient to induce UGT1A1 and reduce the serum T4 level by the administration of a therapeutic dose of phenytoin. In fact, a clinical study reported that exposure to phenytoin resulted in about 20% decrease of serum free and total T4 levels in humans (Franklyn et al., 1984). Second, it remained unclear whether the reduced serum T4 level could induce neurodevelopmental disorder in humans. In human infants, a normal level of serum free T4 level ranges from 0.9 to 2.6 ng/dl (Singer et al., 2013). A case report demonstrated that an approximately 30% decrease of serum free T4 level in infants caused neurodevelopmental disorder (Namba et al., 2008). These data suggest that the present results in hUGT1 mice are translatable to humans.
In the present study, exposure to phenytoin through breast milk at therapeutic doses caused neurodevelopmental disorder in neonatal mice (Fig. 6, A and B). These data suggest that pregnant women who take UGT1A1 inducers such as phenytoin should avoid breast-feeding after delivery so that the children do not develop neurodevelopmental disorder. Furthermore, to avoid the potential development of neurodevelopmental disorder in children, UGT1A1 inducers should not be administered to pregnant women during the third trimester. However, as withdrawal of anticonvulsant drugs and a switch to other anticonvulsant drugs can cause recurrences (Lowenstein and Alldredge, 1993; Wang et al., 2013), certain pregnant women still require phenytoin and carbamazepine. In our study, T4 replacement attenuated neurodevelopmental disorder in hUGT1 mice exposed to phenytoin (Fig. 4, D and E). Therefore, T4 replacement therapy would attenuate neurodevelopmental disorder induced by UGT1A1 inducers during pregnancy in humans. Withdrawal of UGT1A1 inducers, T4 replacement, and bottle-feeding can lead to a decrease in the prevalence rate of neurodevelopmental disorder in human children.
In the present study, we demonstrated that UGT1A1 induction decreased serum T4 levels, causing neurodevelopmental disorder in hUGT1 mice. Neurodevelopmental disorder induced by exposure to UGT1A1 inducers during the perinatal period was attenuated by a T4 replacement therapy. Not only anticonvulsants but also other potential UGT1A1 inducers through breast milk during the postnatal period might present a risk of developing neurodevelopmental disorder. Withdrawal of UGT1A1 inducers, T4 replacement, and bottle-feeding can decrease the risk for neurodevelopmental disorder in humans.
Supplementary Material
Acknowledgments
The authors thank Yuki Kutsuno for excellent editorial and technical assistance, and Dr. Naoki Itoh and Dr. Mari Endo for technical support.
Abbreviations
- CAR
constitutive androstane receptor
- HPLC
high-performance liquid chromatography
- hUGT1
humanized UGT1
- LC-MS/MS
liquid chromatography coupled with tandem mass spectrometry
- PBS
phosphate-buffered saline
- PCB
polychlorinated biphenyl
- PCN
pregnenolone-16-α-carbonitrile
- PCR
polymerase chain reaction
- PTU
propylthiouracil
- PXR
pregnane X receptor
- Sult
sulfotransferase
- T3
3,5,3′-l-triiodothyronine
- T4
3,5,3′,5′-l-tetraiodothyronine
- TR
thyroid hormone receptor
- UDPGA
UDP-glucuronic acid
- UGT
UDP-glucuronosyltransferase
Authorship Contributions
Participated in research design: Kobayashi, Itoh, Tukey, Fujiwara.
Conducted experiments: Hirashima, Takemoto, Sasaki, Fujiwara.
Contributed new reagents or analytic tools: Takemoto.
Performed data analysis: Hirashima, Michimae, Fujiwara.
Wrote or contributed to the writing of the manuscript: Hirashima, Tukey, Fujiwara.
Footnotes
This work was supported by a Grant-in-Aid for Encouragement of Young Scientists B to R.F. [Grant 26870562]. This work was also supported in part by the National Institutes of Health National Institute of Environmental Health Sciences [Grant P42-ES010337] and National Institute of General Medical Sciences [Grant R01-GM100481 and GM086713].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Bacon CJ, Cranage JD, Hierons AM, Rawlins MD, Webb JK. (1981) Behavioural effects of phenobarbitone and phenytoin in small children. Arch Dis Child 56:836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Oz B, Nulman I, Koren G, Ito S. (2000) Anticonvulsants and breast feeding: a critical review. Paediatr Drugs 2:113–126. [DOI] [PubMed] [Google Scholar]
- Becker WJ, Kunesch E, Freund HJ. (1990) Coordination of a multi-joint movement in normal humans and in patients with cerebellar dysfunction. Can J Neurol Sci 17:264–274. [DOI] [PubMed] [Google Scholar]
- Bochner F, Hooper WD, Tyrer JH, Eadie MJ. (1972) Effect of dosage increments on blood phenytoin concentrations. J Neurol Neurosurg Psychiatry 35:873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolussi G, Zentilin L, Baj G, Giraudi P, Bellarosa C, Giacca M, Tiribelli C, Muro AF. (2012) Rescue of bilirubin-induced neonatal lethality in a mouse model of Crigler-Najjar syndrome type I by AAV9-mediated gene transfer. FASEB J 26:1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. [DOI] [PubMed] [Google Scholar]
- Buckley DB, Klaassen CD. (2009) Induction of mouse UDP-glucuronosyltransferase mRNA expression in liver and intestine by activators of aryl-hydrocarbon receptor, constitutive androstane receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and nuclear factor erythroid 2-related factor 2. Drug Metab Dispos 37:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Nguyen N, Peterkin V, Yang YS, Hotz K, La Placa DB, Chen S, Tukey RH, Stevens JC. (2010) A humanized UGT1 mouse model expressing the UGT1A1*28 allele for assessing drug clearance by UGT1A1-dependent glucuronidation. Drug Metab Dispos 38:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo R, Obregón MJ, Ruiz de Oña C, Escobar del Rey F, Morreale de Escobar G. (1990) Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J Clin Invest 86:889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport JW, Dorcey TP. (1972) Hypothyroidism: learning deficit induced in rats by early exposure to thiouracil. Horm Behav 3:97–112. [DOI] [PubMed] [Google Scholar]
- Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. (2005) Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci 29:414–426. [DOI] [PubMed] [Google Scholar]
- Eayrs JT, Levine S. (1963) Influence of thyroidectomy and subsequent replacement therapy upon conditioned avoidance learning in the rat. J Endocrinol 25:505–513. [Google Scholar]
- Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, Negishi M, Wang H. (2007) Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther 320:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklyn JA, Sheppard MC, Ramsden DB. (1984) Measurement of free thyroid hormones in patients on long-term phenytoin therapy. Eur J Clin Pharmacol 26:633–634. [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Nguyen N, Chen S, Tukey RH. (2010) Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc Natl Acad Sci USA 107:5024–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharov A, Pavuk M, Foushee HR, Carpenter DO, Anniston Environmental Health Reseach Consortium (2011) Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environ Health Perspect 119:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe M, Matsumoto I, Imagawa T, Uehara M. (2008) Effects of an anti-thyroid drug, methimazole, administration to rat dams on the cerebellar cortex development in their pups. Int J Dev Neurosci 26:409–414. [DOI] [PubMed] [Google Scholar]
- Imosemi IO, Osinubi AA. (2011) Phenytoin-induced toxicity in the postnatal developing cerebellum of Wistar rats, effect of Calotropis procera on Histomorphometric parameters. Int J Morphol 29:331–338. [Google Scholar]
- Ingbar SH. (1976) Effect of aging on thyroid economy in man. J Am Geriatr Soc 24:49–53. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. (1996) Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med 335:783–789. [DOI] [PubMed] [Google Scholar]
- Jemnitz K, Veres Z, Monostory K, Vereczkey L. (2000) Glucuronidation of thyroxine in primary monolayer cultures of rat hepatocytes: in vitro induction of UDP-glucuronosyltranferases by methylcholanthrene, clofibrate, and dexamethasone alone and in combination. Drug Metab Dispos 28:34–37. [PubMed] [Google Scholar]
- Kachaylo EM, Pustylnyak VO, Lyakhovich VV, Gulyaeva LF. (2011) Constitutive androstane receptor (CAR) is a xenosensor and target for therapy. Biochemistry (Mosc) 76:1087–1097. [DOI] [PubMed] [Google Scholar]
- Kato Y, Ikushiro S, Emi Y, Tamaki S, Suzuki H, Sakaki T, Yamada S, Degawa M. (2008) Hepatic UDP-glucuronosyltransferases responsible for glucuronidation of thyroxine in humans. Drug Metab Dispos 36:51–55. [DOI] [PubMed] [Google Scholar]
- Koibuchi N, Chin WW. (2000) Thyroid hormone action and brain development. Trends Endocrinol Metab 11:123–128. [DOI] [PubMed] [Google Scholar]
- Koopman-Esseboom C, Morse DC, Weisglas-Kuperus N, Lutkeschipholt IJ, Van der Paauw CG, Tuinstra LG, Brouwer A, Sauer PJ. (1994) Effects of dioxins and polychlorinated biphenyls on thyroid hormone status of pregnant women and their infants. Pediatr Res 36:468–473. [DOI] [PubMed] [Google Scholar]
- Koromilas C, Liapi C, Schulpis KH, Kalafatakis K, Zarros A, Tsakiris S. (2010) Structural and functional alterations in the hippocampus due to hypothyroidism. Metab Brain Dis 25:339–354. [DOI] [PubMed] [Google Scholar]
- Kutsuno Y, Hirashima R, Sakamoto M, Ushikubo H, Michimae H, Itoh T, Tukey RH, Fujiwara R. (2015) Expression of UDP-Glucuronosyltransferase 1 (UGT1) and Glucuronidation Activity toward Endogenous Substances in Humanized UGT1 Mouse Brain. Drug Metab Dispos 43:1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand J. (1979) Morphogenetic actions of thyroid hormones. Trends Neurosci 2:234–236. [Google Scholar]
- Legrand, J (1980) Effects of thyroid hormone on brain development, with particular emphasis on glial cells and myelination, in Multidisciplinary Approach to Brain Development pp 279–292, Elsevier/North-Holland Biomedical Press, Amsterdam.
- Lowenstein DH, Alldredge BK. (1993) Status epilepticus at an urban public hospital in the 1980s. Neurology 43:483–488. [DOI] [PubMed] [Google Scholar]
- Malik R, Hodgson H. (2002) The relationship between the thyroid gland and the liver. QJM 95:559–569. [DOI] [PubMed] [Google Scholar]
- McKinney JD. (1989) Multifunctional receptor model for dioxin and related compound toxic action: possible thyroid hormone-responsive effector-linked site. Environ Health Perspect 82:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba N, Etani Y, Kitaoka T, Nakamoto Y, Nakacho M, Bessho K, Miyoshi Y, Mushiake S, Mohri I, Arai H, et al. (2008) Clinical phenotype and endocrinological investigations in a patient with a mutation in the MCT8 thyroid hormone transporter. Eur J Pediatr 167:785–791. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Bonzo JA, Chen S, Chouinard S, Kelner MJ, Hardiman G, Bélanger A, Tukey RH. (2008) Disruption of the ugt1 locus in mice resembles human Crigler-Najjar type I disease. J Biol Chem 283:7901–7911. [DOI] [PubMed] [Google Scholar]
- Nicholson JL, Altman J. (1972a) Synaptogenesis in the rat cerebellum: effects of early hypo- and hyperthyroidism. Science 176:530–532. [DOI] [PubMed] [Google Scholar]
- Nicholson JL, Altman J. (1972b) The effects of early hypo- and hyperthyroidism on the development of rat cerebellar cortex. I. Cell proliferation and differentiation. Brain Res 44:13–23. [DOI] [PubMed] [Google Scholar]
- Nicholson JL, Altman J. (1972c) The effects of early hypo- and hyperthyroidism on the development of the rat cerebellar cortex. II. Synaptogenesis in the molecular layer. Brain Res 44:25–36. [DOI] [PubMed] [Google Scholar]
- Ohnhaus EE, Studer H. (1983) A link between liver microsomal enzyme activity and thyroid hormone metabolism in man. Br J Clin Pharmacol 15:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Schaefer O, Kawakami H, Inoue T, Liehner S, Saito A, Ishiguro N, Kishimoto W, Ludwig-Schwellinger E, Ebner T, et al. (2012) Simultaneous absolute protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases as a novel approach for the characterization of individual human liver: comparison with mRNA levels and activities. Drug Metab Dispos 40:83–92. [DOI] [PubMed] [Google Scholar]
- Ornoy A, Cohen E. (1996) Outcome of children born to epileptic mothers treated with carbamazepine during pregnancy. Arch Dis Child 75:517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. (1971) Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol 141:283–312. [DOI] [PubMed] [Google Scholar]
- Rice AC, Shapiro SM. (2008) A new animal model of hemolytic hyperbilirubinemia-induced bilirubin encephalopathy (kernicterus). Pediatr Res 64:265–269. [DOI] [PubMed] [Google Scholar]
- Richardson VM, Ferguson SS, Sey YM, Devito MJ. (2014) In vitro metabolism of thyroxine by rat and human hepatocytes. Xenobiotica 44:391–403. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Aarts JM, Brouwer A, Froese KL, Denison MS, Giesy JP. (1996) Comparison of Ah receptor-mediated luciferase and ethoxyresorufin-O-deethylase induction in H4IIE cells: implications for their use as bioanalytical tools for the detection of polyhalogenated aromatic hydrocarbons. Toxicol Appl Pharmacol 137:316–325. [DOI] [PubMed] [Google Scholar]
- Shelby MK, Klaassen CD. (2006) Induction of rat UDP-glucuronosyltransferases in liver and duodenum by microsomal enzyme inducers that activate various transcriptional pathways. Drug Metab Dispos 34:1772–1778. [DOI] [PubMed] [Google Scholar]
- Shors TJ. (2004) Memory traces of trace memories: neurogenesis, synaptogenesis and awareness. Trends Neurosci 27:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer K, Menon RK, Lesperance MM, McHugh JB, Gebarski SS, Avram AM. (2013) Residual thyroid tissue after thyroidectomy in a patient with TSH receptor-activating mutation presenting as a neck mass. J Clin Endocrinol Metab 98:448–452. [DOI] [PubMed] [Google Scholar]
- Smith CM, Faucette SR, Wang H, LeCluyse EL. (2005) Modulation of UDP-glucuronosyltransferase 1A1 in primary human hepatocytes by prototypical inducers. J Biochem Mol Toxicol 19:96–108. [DOI] [PubMed] [Google Scholar]
- Soars MG, Petullo DM, Eckstein JA, Kasper SC, Wrighton SA. (2004) An assessment of udp-glucuronosyltransferase induction using primary human hepatocytes. Drug Metab Dispos 32:140–148. [DOI] [PubMed] [Google Scholar]
- Steen B, Rane A, Lönnerholm G, Falk O, Elwin CE, Sjöqvist F. (1982) Phenytoin excretion in human breast milk and plasma levels in nursed infants. Ther Drug Monit 4:331–334. [DOI] [PubMed] [Google Scholar]
- Thiel G. (1993) Synapsin I, synapsin II, and synaptophysin: marker proteins of synaptic vesicles. Brain Pathol 3:87–95. [DOI] [PubMed] [Google Scholar]
- Usui T, Kuno T, Mizutani T. (2006) Induction of human UDP-glucuronosyltransferase 1A1 by cortisol-GR. Mol Biol Rep 33:91–96. [DOI] [PubMed] [Google Scholar]
- Vanoverloop D, Schnell RR, Harvey EA, Holmes LB. (1992) The effects of prenatal exposure to phenytoin and other anticonvulsants on intellectual function at 4 to 8 years of age. Neurotoxicol Teratol 14:329–335. [DOI] [PubMed] [Google Scholar]
- Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, Denk H, Trauner M. (2005) CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42:420–430. [DOI] [PubMed] [Google Scholar]
- Wang R, Nelson JC, Wilcox RB. (1998) Salsalate administration--a potential pharmacological model of the sick euthyroid syndrome. J Clin Endocrinol Metab 83:3095–3099. [DOI] [PubMed] [Google Scholar]
- Wang R, Nelson JC, Wilcox RB. (1999) Salsalate and salicylate binding to and their displacement of thyroxine from thyroxine-binding globulin, transthyrin, and albumin. Thyroid 9:359–364. [DOI] [PubMed] [Google Scholar]
- Wang SP, Mintzer S, Skidmore CT, Zhan T, Stuckert E, Nei M, Sperling MR. (2013) Seizure recurrence and remission after switching antiepileptic drugs. Epilepsia 54:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN. (2005) Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res 28:249–268. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Nakajima M, Katoh M, Yokoi T. (2007) Glucuronidation of thyroxine in human liver, jejunum, and kidney microsomes. Drug Metab Dispos 35:1642–1648. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J. (2004) Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol 16:809–818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.