Abstract
Morphine has been widely used as rescue treatment for heart attack and failure in humans for many decades. Relatively little has been known about the role of spinal opioid receptors in morphine cardioprotection. Recent studies have shown that intrathecal injection of morphine can reduce the heart injury caused by ischemia (I)/reperfusion (R) in rats. However, the molecular and cellular mechanisms underlying intrathecal morphine cardioprotection has not been determined. Here, we report that intrathecal morphine postconditioning (IMPOC) rescued mean artery pressure (MAP) and reduced myocardial injury in I/R. Pretreatment with either naloxone (NAL), a selective mu-opioid receptor antagonist, or nitric oxide synthase (NOS) inhibitors via intrathecal delivery completely abolished IMPOC cardioprotection, suggesting that the spinal mu-opioid receptor and its downstream NOS signaling pathway are involved in the mechanism of the morphine-induced effect. Consistent with this, IMPOC significantly enhanced spinal neural NOS phosphorylation, nitric oxide, and cGMP content in a similar time course. Intrathecal application of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, a specific inhibitor of guanylate cyclase, completely ablated IMPOC-induced enhancement of cardioprotection and spinal cGMP content. IMPOC rescue of MAP and ischemic injury is correlated with IMPOC enhancement of NOS signaling. Collectively, these findings strengthen the concept of spinal mu-opioid receptors as a therapeutic target that mediates morphine-induced cardioprotection. We also provide evidence suggesting that the activation of spinal NOS signaling is essential for morphine cardioprotection.
Introduction
Morphine has been widely used in the treatment of patients with heart attack and failure in the past century. This agent is thought to rescue diseased myocardium function by slowing respiratory rate, reducing anxiety, alieving pain, and dilating venous vessels (Pang et al., 2010). Recent evidence has also suggested that morphine and other opioids can produce a direct protective effect on the heart that is under ischemia (I) (Schultz and Gross, 2001; Pugsley, 2002; Ishii, 2014; Headrick et al., 2015). Systemic application of morphine can be given either before (preconditioning) or after (postconditioning) heart I induced by occlusion of the left coronary artery in experimental animals (Murry et al., 1986; Zhao et al., 2003). Although there is a debate about the therapeutic effect of morphine preconditioning on ischemic damage in the heart (Markiewicz et al., 1982; Shultz et al., 1996), morphine postconditioning has been widely reported to substantially reduce the infarct size (IS) induced by I/reperfusion (R) in rodents (Zhao and Vinten-Johansen, 2006) . There is strong evidence showing that the heart is not only the location expressing opioid receptors at high levels; it is also the site that synthesizes, stores, and releases endogenous opioids (Pugsley, 2002; Pradhan et al., 2012; Headrick et al., 2015). The primary opioid receptors expressed in cardiac cells are kappa and delta subtypes. Although mu-opioid receptors are expressed in the heart in the early developmental stage, the abundance of this receptor subtype seems very low in cardiac cells at the adult stage. A study from our laboratory (Zhang et al., 2005) has shown that kappa- and delta-opioid but not mu-opioid receptors are involved in opioid cardioprotection in an in vitro I/R model. This observation is in line with those from several studies showing that mu-opioid receptors are nearly absent in heart tissue during the adult stage (Headrick et al., 2015). Nevertheless, opioids and their receptors are especially abundant in spinal cord in which the activation of these receptors leads to the relief of pain (Pugsley, 2002). However, less is known about the therapeutic potential of spinal opioid receptors in morphine treatment of cardiac I/R. Several previous studies from our laboratory (Li et al., 2009; Ling Ling et al., 2010; Wong et al., 2010; Lu et al., 2015) have shown that intrathecal morphine preconditioning (IMPC) and intrathecal morphine postconditioning (IMPOC) can substantially reduce cardiac I injury in rats. There is strong evidence suggesting that preconditioning and postconditioning morphine cardioprotection are mediated via different mechanisms. Different subtypes of opioid receptors are likely involved in preconditioning and postconditioning morphine therapeutic effects in cardiac I (Headrick et al., 2015). Although IMPOC is thought to be more clinically significant than IMPC, the role of spinal opioid receptors and their molecular signaling have not been fully defined.
Nitric oxide (NO) contributes to many important brain functions. It has been demonstrated that morphine can promote the NO synthase (NOS) system and analgesic action (Rodriguez-Munoz and Garzon, 2013). NO is synthesized by the NOS from l-arginine and different cofactors. Neuronal NOS (nNOS) is the predominant form of NOS expressed in the brain and spinal cord (Cury et al., 2011). Intrathecal application of an opioid agonist, fentanyl, in preconditioning was found to reduce I/R injury in rats (Lu et al., 2014). This cardioprotection is likely mediated through a signaling pathway involving nNOS, but not endothelia or the inducible form of NOS (Lu et al., 2014). There are two key downstream players: NO-sensitive guanylyl cyclase [or soluble guanylyl cyclase (sGC)] and cGMP, which replays nNOS singling during the nociceptive process (Garry et al., 1994; Tao and Johns, 2002). An increase of cGMP by activation of NOS can ultimately lead to neuron hyperpolarization through promoting potassium channel opening. Such a signaling pathway is thought to play an important role in the antinociceptive effect (Cunha et al., 2010; Foletto et al., 2013).
Nevertheless, molecular and cellular pathways for IMPOC-induced cardioprotection have not been determined. To address this question, we used various approaches to explore the role of NOS signaling pathway in IMPOC-induced cardioprotection in I/R rats. Our data presented in this study have shown that there is an interrelationship between morphine cardioprotection and the morphine enhancement of spinal NOS signaling. The inhibition of spinal mu-opioid receptors and NOS signaling completely abolished any intrathecal morphine-induced therapeutic effect in the heart.
Materials and Methods
Animal Models.
All animal experiments were obtained and in-house bred in the Laboratory Animal Centre of Anhui Medical University. The experimental procedures used in this study were approved by Anhui Medical University animal ethics committee following the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (2011) (https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf). Male Sprague-Dawley rats weighing between 200 and 250 g were used for this study. The animals were housed in an air-conditioned room with controlled temperature (24 ± 2°C) and lights (lights on from 8:00 AM to 8:00 PM). The animals were allowed free access to food and water. Each animal was used only once in this study.
Drug Preparations.
The following drugs were used, morphine (0.3, 3, and 30 μg/kg); naloxone (NAL; 10 μg), Nω-nitro-l-arginine methyl ester (l-NAME; 30 nmol), a nonspecific NOS inhibitor; 7-nitroindazole (7NI; 100 nmol), a specific nNOS inhibitor; and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 11 nmol), a specific sGC inhibitor. l-NAME, 7NI, and ODQ were purchased from Sigma-Aldrich (St. Louis, MO). The doses of morphine used were based on those used in our previous study (Li et al., 2009), showing that they produced the optimum effect without causing significant side effects. The doses of l-NAME, 7NI, and ODQ were selected and justified following a previous study from our laboratory (Lu et al., 2014). Morphine, NAL, l-NAME, and ODQ were dissolved in a physiologic saline solution, and all drugs were administered intrathecally. 7NI was dissolved in a working solution containing 5% dimethylsulfoxide. Morphine was administered 5 minutes before R; 7NI, l-NAME, and ODQ were administered 10 minutes before morphine administration. The volume of the intrathecal injection of all drugs was 10 μl.
Surgical Procedure.
Rats were anesthetized with an intraperitoneal injection of pentobarbitone (50 mg/kg). After sterile preparation of the posterior neck, a small polyethylene-10 catheter (4 cm) (Smiths Medical, Ashford, UK) was inserted into the thoracic spinal cord through a puncture in the atlanto-occipital membrane, as mentioned before. The placement of the catheter was confirmed visually by backflow of cerebrospinal fluid through the lumen. After recovery, the rats were examined for the next 3 days for gross motor or sensory deficits. The animals demonstrating any deficit during this period were excluded from further experimentation. Three days after intrathecal catheter placement, the rats with intrathecal catheters were reanesthetized through intraperitoneal administration of pentobarbitone (50 mg/kg) followed by the repeated application of pentobarbitone at 25 mg/kg every 60–90 minutes as necessary. All of the animals underwent tracheotomy and tracheal intubation. The animals were provided with a mechanical ventilation using Harvard Apparatus Rodent Respirator at 70–80 breaths/min. The body temperature of experimental animals was monitored and maintained at 37 ± 1°C (mean ± S.D.) using a heating pad. The right femoral artery was cannulated for direct blood pressure monitoring via a pressure transducer, and the right femoral vein was cannulated for saline infusion. Subcutaneous stainless steel electrodes were connected to a PowerLab monitoring system to monitor the lead II ECG and heart rate. A left thoracotomy was performed to expose the heart at the fifth intercostal space. A 6-0 Prolene loop along with a snare occlude were placed at the origin of the left coronary artery. Regional I was induced by pulling the snare and securing the threads with a mosquito hemostat. I was confirmed by electrocardiographic changes, a substantial decrease in mean artery pressure (MAP), and cardiac cyanosis. Rats were omitted from further data analysis if severe hypotension (MAP < 30 mm Hg) or intractable ventricular fibrillation occurred. After surgical preparation, the rats were allowed to stabilize for 15 minutes.
Hemodynamic Measurement.
The MAP, ECG, and heart rate of experimental animals were recorded via a PowerLab monitoring system (ML750 PowerLab/4sp with MLT0380 Reusable BP Transducer; AD Instruments, Colorado Springs, CO). These parameters were recorded at the following time points corresponding to the basis of state (baseline), I for 25 minutes (I), I for 30 minutes (treatment), and R for 120 minutes (R).
IS Determination.
The hearts of experimental rats were excised and transferred to a Langendorff apparatus after 2 hours of R immediately perfused with normal saline solution for 1 minute at a pressure of 100 cm saline to flush out residual blood. The snare was retightened, and 0.25% Evan blue dye was injected to stain the normally perfused region of the heart. This procedure established the visualization of the non-I region and the area at risk (AAR). The hearts were then frozen and cut into 2-mm slices. Thereafter, the slices were incubated in 1% 2,3,5-triphenyltetrazolium in phosphate buffer at 37°C for 10 minutes. This was followed by immersion in 10% formalin for 20 minutes to enhance the contrast of the stain. The areas of infarct and the risk zone for each slice was traced and digitized using a computerized planimetry technique (SigmaScan 4.0; SYSTAT, Richmond, CA). The volumes of the left ventricles, IS, and AAR were calculated by multiplying each area with slice thickness and summing the product. The IS was plotted by the AAR. This ratio, expressed as a percentage of IS/AAR, represents the extent of infarction.
Western Blot Analysis of Phosphorylation of nNOS.
To separate the phosphorylated form of nNOS (pnNOS) from the nonphosphorylated form of NOS, we conducted the following experiments. Dorsal T2 to T6 segments of the collected spinal cord were homogenized in buffer containing 1 M Tris (pH 7.5), 1% NP40, 0.5 M EDTA (pH 7.5), 50 mM EGTA, 1 M dithiothreitol, 1 M benzanidine, and 0.1 M phenylmethylsulfonyl fluoride. The total amount of protein from each sample was determined using the Bradford dye assay prior to loading on polyacrylamide gels. Spinal cord homogenates (20 μg of protein) were separated using 8% SDS-PAGE and transferred to nitrocellulose membranes. These membranes were blocked with 5% skimmed milk for 1 hour followed by primary antibodies specific for β-actin (1:1000, loading control; Santa Cruz Biotechnology, Dallas, TX), for nNOS (1:2000; Cell Signaling Technology, Danvers, MA) or for pnNOS (1:1000; Abcam, Cambridge, MA; this antibody is specific for mouse nNOS phosphorylated on Ser1417). The membranes were washed and stained using goat anti-rabbit IgG conjugated to horseradish peroxidase. The bands were visualized with enhanced chemiluminescence (Amersham Pharmacia Biotech, Little Chalfont, UK). The positive pixel area of specific bands were measured with a computer-assisted image analysis system and normalized against the corresponding β-actin loading control bands. The ratio of pnNOS (Ser1417) to nNOS was calculated as the percentage of change by plotting the mean value of pnNOS against that of nNOS.
Nitrate plus Nitrite Measurements (Total NO).
Spinal tissue was homogenized in phosphate-buffered saline (pH 7.4) and centrifuged at 10,000g for 20 minutes. The supernatant was ultracentrifuged at 100,000g for 15 minutes and ultrafiltered using a 30-kDa molecular weight cutoff filter (EMD Millipore, Danvers, MA). The ultrafiltrate was used for the detection of NO concentration (Nitrate/Nitrite Colorimetric Assay Kit; Cayman Chemical, Ann Arbor, MI) expressed as micromoles of nitrate plus nitrite in milligrams of protein (Sedoris et al., 2012)
cGMP Measurement.
Spinal cord tissue samples (from T2 to T6 segments; 50 mg) were dissolved in 0.5 ml of 5% trichloroacetic acid. Then, they were then centrifuged to remove precipitated proteins, and the supernatant fractions were extracted with 5 ml of water-saturated ether three times and the residual ether was removed from the aqueous layer by heating the sample to 70°C for 5 minutes. The cGMP level was determined by enzyme immunoassay (Cyclic GMP EIA Kit; Cayman Chemical) according to the manufacturer instructions.
Statistical Analysis.
All data are presented as the mean ± S.E.M. The data were analyzed using two-way analysis of variance or one-way analysis of variance followed by Tukey’s multiple-comparisons test, Dunnett’s multiple-comparisons test, and Sidak’s multiple-comparisons test. Correlation analysis was carried out using linear regression (Prism 6; GraphPad Software, San Diego, CA). Statistical significance was established at the 95% confidence limit.
Results
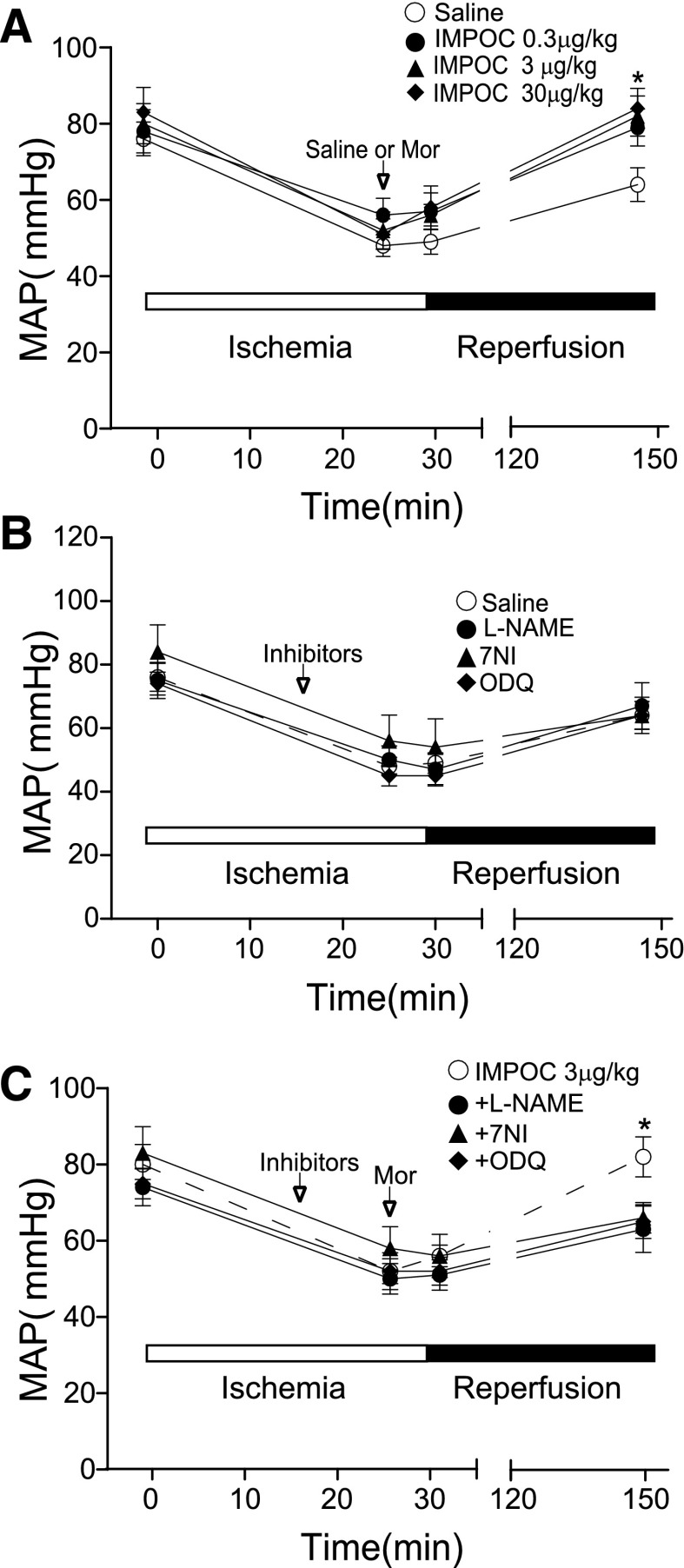
Occlusion of the coronary artery resulted in a substantial decrease in MAP (in millimeters of mercury) (Fig. 1A). Relief of the occlusion (R) slightly elevated MAP. Intrathecal application of morphine restored the deficiency of MAP induced by I in a dose-dependent manner. The levels of MAP after intrathecal morphine administration at 3 and 30 μg/kg were significantly elevated from 64 ± 4 mm Hg (control) to 82 ± 5 and 84 ± 5 mm Hg (P < 0.05, n = 6). To identify the role of the NOS signaling cascade in intrathecal morphine cardioprotection, we selected and tested the following three NOS signaling inhibitors: l-NAME for NOS, 7NI specific for nNOS, and ODQ for sGC. Intrathecal administration of these agents (l-NAME, 30 nmol; 7NI, 100 nmol; and ODQ, 11 nmol) alone did not significantly alter the course of I/R (Fig. 1B). However, preapplication of these inhibitors significantly reduced cardioprotection induced by morphine administration at 3 μg/kg intrathecally (Fig. 1C) (P < 0.05, n = 6).
Fig. 1.
The suppression of the rescue of MAP by IMPOC in I/R by intrathecal NOS and sGC inhibitors. (A) The graphs represent the dynamic change of MAP without and with morphine. The open and solid bars indicate the time courses of I, R, and IMPOC. Baseline: 15 minutes before I; I: 30 minutes; IMPOC: 5 minutes after 25 minutes of I; reperfusion: 120 minutes after I. Each data point represents the mean ± S.E.M. from six rats. The error bars invisible are smaller than the size of symbols. *P < 0.05, compared with control (saline). (B) The effects of intrathecal NOS and sGC inhibitors on MAP. Each data point represents the average from 6 rats. (C) The effect of NOS and sGC inhibitors on IMPOC rescue of MAP in I/R. Each data point represents the mean ± S.E.M. from six rats. *P < 0.05, comparison between IMPOC and IMPOC plus an inhibitor.
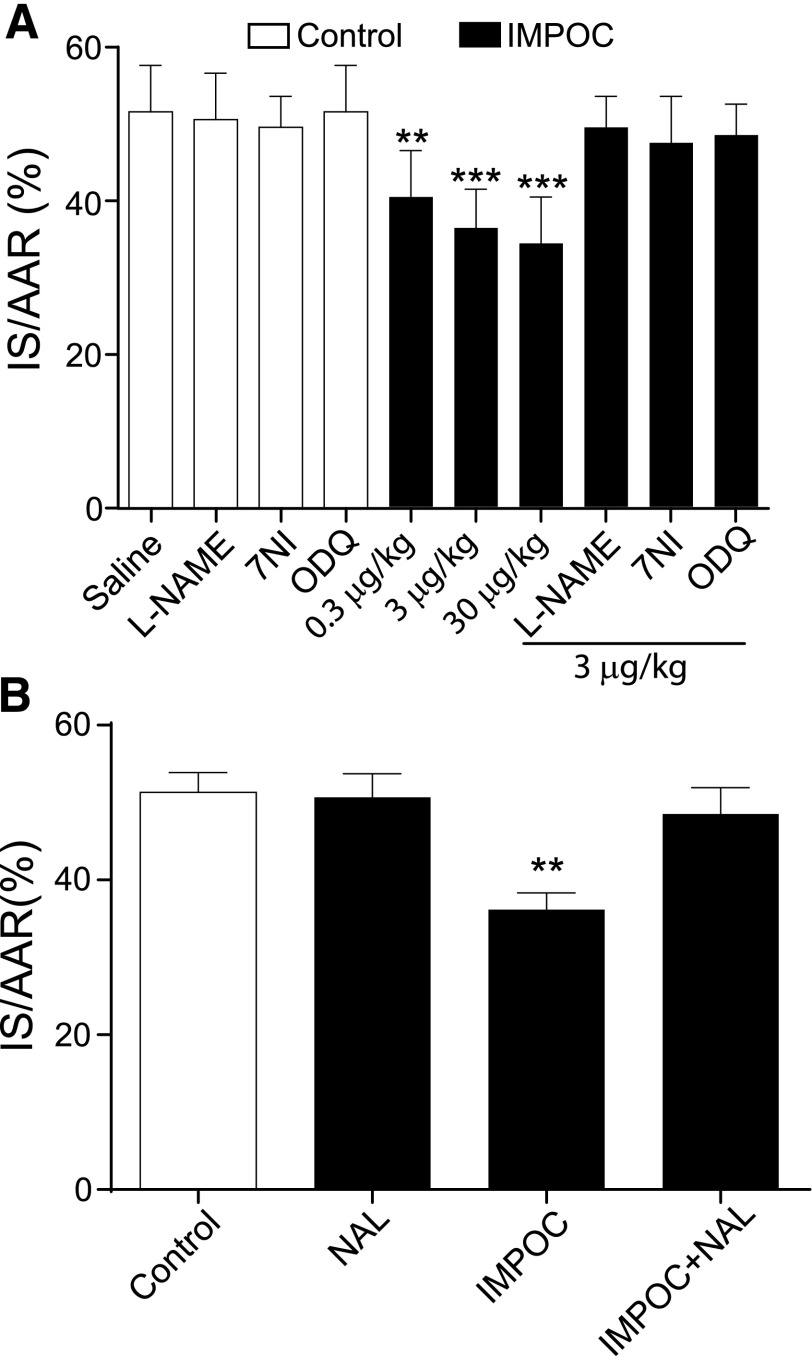
The percentage of IS/AAR has been widely used to indicate the severity of the heart injury due to either I or I/R. A previous study (Ling Ling et al., 2010) reported that IMPOC rescued IS/AAR (percentage) in the heart after I/R. We next ask whether NOS inhibitors can also affect IMPOC rescue of IS/AAR (percentage) following the identical experimental protocol described in Fig. 1. As a result, the control group IS/AAR was 51 ± 3% 120 minutes after I/R (Fig. 2). IMPOC at 0.3, 3, and 30 μg/kg significantly reduced myocardial IS/AAR (percentage) in a dose-dependent manner (from 51% ± 3% to 40% ± 3%, 36 ± 2%, and 34 ± 3%). Intrathecal application of l-NAME, 7 NI, or ODQ alone did not significantly affect the course of I/R (P > 0.05, n = 6). However, these agents completely abolished the IMPOC therapeutic effect on IS/AAR (percentage). To identify whether morphine reduces IS/AAR (percentage) through the activation of spinal mu-opioid receptors, we tested the effect of intrathecal NAL on IMPOC. Although NAL applied alone at 10 μg intrathecally did not significantly alter IS/AAR (percentage) in I/R rats, pretreatment with NAL prevented IMPOC-induced cardioprotection in these animals (Fig. 2B).
Fig. 2.
Suppression of IMPOC rescue of IS/AAR (percentage) by intrathecal NOS inhibitors and NAL. (A) The bar graphs represent the effects of intrathecal NOS and sGC inhibitors on IMPOC rescue of the percentage of IS/AAR (IS/AAR%). Each data point represents the mean ± S.E.M. from six rats. **P < 0.01, *** P < 0.001, compared with control (saline). (B) The bar graphs represent the blockage by intrathecal NAL of IMPOC rescue of IS/AAR%. Each data point represents the mean ± S.E.M. from six rats. **P < 0.01, compared with control.
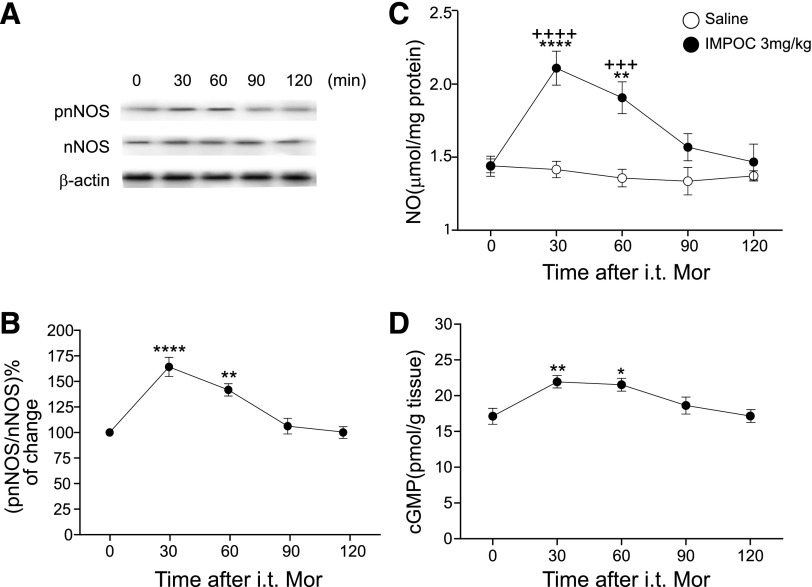
The above observations suggest that nNOS-cGMP signaling is critically involved in IMPOC rescue of MAP and ischemic heart injury. To examine the changes of thoracic spinal NOS signaling elements after IMPOC in I/R, we first asked whether IMPOC can promote the phosphorylation of nNOS, which is essential for the initiation of NOS signaling (Sanchez-Blazquez et al., 2010; Garzon et al., 2011). To address this question, we tested an antibody specifically against phosphorylation (Ser1417) of nNOS. IMPOC preferentially increased the level of pnNOS by 30 and 60 minutes after the treatment, although the basal level of nNOS was unchanged 120 minutes after IMPOC (Fig. 3, A and B). Consistent with the idea that phosphorylation of nNOS can promote NOS production, IMPOC significantly increased the protein expression of spinal NO from 1.44 ± 0.07 to 2.11 ± 0.12 pmol/g tissue in 30 minutes (P < 0.0001). The level of NO protein expression continued to increase for 60 minutes and returned to the basal level by 120 minutes. As an immediate downstream substrate of NOS, cGMP rises and relays NOS signaling pathway after NOS activation. Indeed, we observed that IMPOC significantly elevated the cGMP content in a time course similar to that of IMPOC enhancement of NO (Fig. 3D).
Fig. 3.
Enhancement of spinal NO and cGMP by IMPOC. (A) Enhancement of pnNOS by IMPOC in I/R. The top panel represents the Western blot images of the expression levels of pnNOS and nNOS proteins isolated from spinal cord after IMPC in I/R. (B) The graph shows the time course of the ratio change between pnNOS and nNOS proteins. Each data point represents the mean ± S.E.M from four rats. **P < 0.01, ****P < 0.0001, compared with the initial data point. (C) Time course of NO protein expression by IMPOC in I/R. Each data point represents the mean ± S.E.M from four rats. **P < 0.01, ****P < 0.0001, compared with the initial data point; +++P < 0.001, ++++P < 0.0001, compared with control (saline). (D) Time course of cGMP by IMPOC in I/R. Each data point represents the mean ± S.E.M. from four rats. *P < 0.05, **P < 0.01, compared with the initial data point. i.t., intrathecal; MOR, morphine.
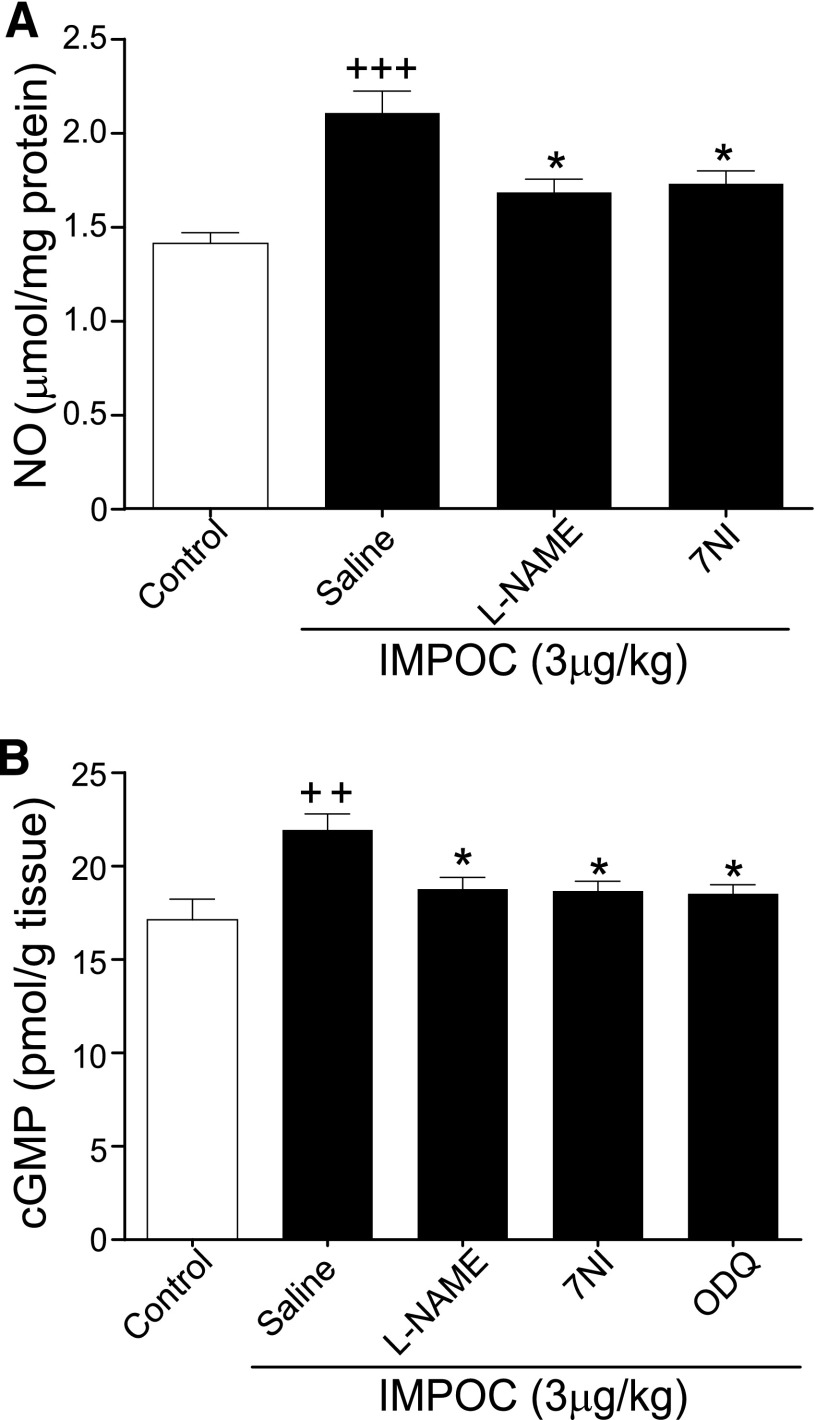
To further identify the role of NOS signaling in IMPOC-induced cardioprotection, we tested the effect of l-NAME and 7NI on IMPOC-induced changes in NO protein expression and cGMP contents. Intrathecal injection of l-NAME and 7NI significantly inhibited IMPOC-induced enhancement of NO protein expression (Fig. 4A). Similarly, both agents blocked IMPOC enhancement of cGMP content (Fig. 4B). sGC is the NOS downstream player and the key enzyme for cGMP production. In this regard, we tested the effect of intrathecal ODQ, an inhibitor of sGC, on IMPOC enhancement of cGMP content. Administration of ODG at 11 nmol intrathecally completely prevented any IMPOC-induced increase of cGMP content, suggesting that sGC is the key to promote the synthesis of NO.
Fig. 4.
The effect of intrathecal NOS and sCG inhibitors on IMPOC enhancement of spinal NO protein and cGMP content. (A) Quantitative analysis of the NO protein expression without and with IMPOC in I/R. Each bar graph represents the mean ± S.E.M. from four rats. +++P < 0.001, compared with control; *P < 0.05, compared with the saline group. (B) Quantitative analysis of cGMP contents without and with IMPOC in I/R. Each bar graph represents the average of the mean ± S.E.M. values from four rats. ++P < 0.01, compared with control; *P < 0.05, compared with the saline group.
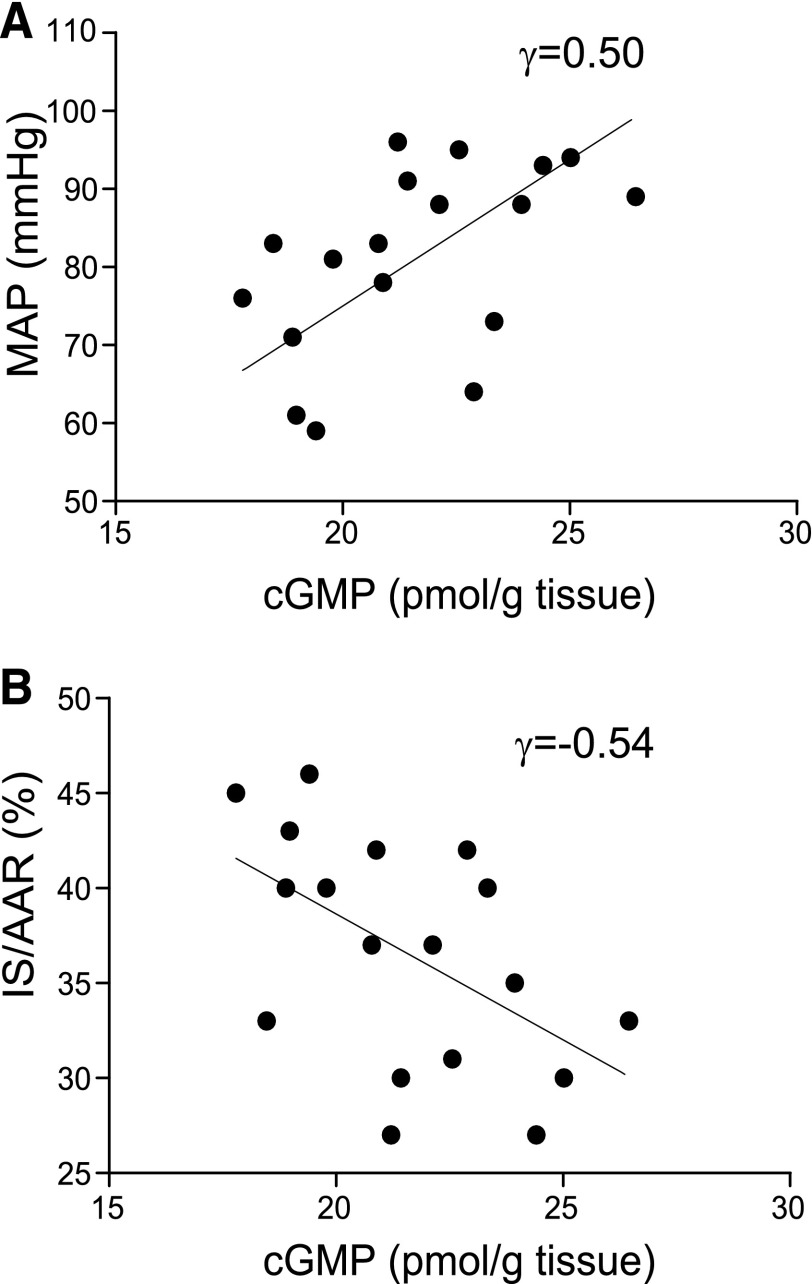
Our findings presented above reveal that IMPOC promotes phosphorylation (Ser1417) of spinal nNOS and subsequently activates the spinal NO/cGMP signaling pathway during I/R. To understand the interrelationship between IMPOC-induced neurochemical changes in thoracic spinal cord and the IMPOC therapeutic effect, we conducted a correlation analysis of MAP, IS/AAR (percentage), and cGMP. We observed that the levels of cGMP were inversely correlated with MAP (Fig. 5A, γ = 0.5, P < 0.05, n = 18). Similar, there was a strong correlation between cGMP content and IS/AAR (percentage) (Fig. 5B, γ = −0.54, P < 0.05, n = 18).
Fig. 5.
Correlation analysis of IMPOC therapeutic effect and IMPOC enhancement of spinal cGMP content. (A) Correlational analysis of IMPOC rescue of IS/AAR (percentage) and IMPOC enhancement of spinal cGMP content. Totally 18 data points are collected and analyzed. Each of them represents the data point of in vitro (cGMP content) and in vivo (IS/AAR) assays from the corresponding animal. There is a significant correlation between IS/AAR (percentage) and cGMP content (P < 0.05, linear regression, n = 18). (B) Correlational analysis of IMPOC rescue of MAP, and IMPOC enhancement of spinal cGMP content. There is a significant correlation between MAP and cGMP content (P < 0.05, linear regression, n = 18).
Discussion
Our data have provided the first evidence suggesting that spinal NOS signaling is critically involved in the IMPOC therapeutic effect on I-induced heart injury. This hypothesis is supported by the following observations described in this study. First, intrathecal injection of the inhibitors selective for NOS, nNOS, and sGC completely prevented IMPOC rescue of the deficiency in MAP and IS/AAR (percentage). Second, IMPOC promoted the phosphorylation level of nNOS isolated from the thoracic spinal cord. Moreover, IMPOC substantially increased NOS protein expression and cGMP content in a time course similar to that of IMPOC promotion of nNOS phosphorylation. Third, NOS inhibitors that prevented IMPOC cardioprotection in vivo when given intrathecally abolished IMPOC-induced enhancement of NO protein expression and cGMP content. Finally, there is a strong correlation between the IMPOC therapeutic effect on heart injury and the IMPOC enhancement of spinal cGMP content. In addition, we have provided evidence suggesting that spinal mu-opioid receptors likely mediate IMPOC-induced cardioprotection.
It is also interesting to note that intrathecal administration of morphine at a concentration as low as 0.3 μg/kg (0.06 μg intrathecally) can rescue both MAP and IS/AAR (percentage). This dose is significantly lower than that of the morphine used in the treatment of chronic inflammatory and neuropathic pain in rats. For instance, the minimal dose of morphine to suppress inflammatory and neuropathic pain ranges from 1 to 3 μg above that use in the treatment of rats (Parenti et al., 2016; Shinozaki et al., 2015). This strongly suggest that the thoracic spinal cord may be the essential site for clinical administration of morphine-induced cardioprotection. This idea is supported by the observation that pretreatment with intrathecal morphine prevented IMPOC-induced cardioprotection. This is also consistent with a previous study showing that intrathecally administered opioid receptor antagonists prevented IMPOC cardioprotection (Ling Ling et al., 2010). Morphine is likely to exert its cardioprotection by suppressing pain signal from I in the heart projecting to the central nervous system. It has been proposed that myocardial ischemic injury can serve as nociceptive stimulation, which can be projected to the central nervous system via cardiac sympathetic afferent neurons (Ardell, 2010). The relay of this noxious signal from the ischemic heart via cardiac sympathetic afferent neurons most likely involves releasing various nociceptive substances, such as peptides and glutamate, in the spinal cord (Steagall et al., 2012). Nevertheless, whether nociceptive signaling directly contributes to the extent of the heart injury remains to be clarified.
Accumulating evidence has indicated that NOS signaling plays an important role in the nociceptive process. NO reduces inflammation and edema by inhibiting nociceptive signaling formation in the spinal cord (Brock and Tonussi, 2008; Cury et al., 2011; Foletto et al., 2013). This is consistent with our observations that IMPOC promotes the activity of several molecular components important for the NOS signaling pathway, and intrathecal NOS inhibitors prevented the IMPOC therapeutic effect and IMPOC enhancement of NOS signaling.
It has been shown that phosphorylation of nNOS is important for nNOS activity (Zhou and Zhu, 2009). There are several sites that have been identified for the phosphorylation of nNOS (Rameau et al., 2004). Morphine is likely to increase nNOS activity by activating nNOS at Ser1417 (Sanchez-Blazquez et al., 2010; Garzon et al., 2011; Hinchee-Rodriguez et al., 2013). In line with this idea, IMPOC increases the phosphorylation of nNOS at Ser1417, which triggers NOS activation followed by an increase in cGMP.
The precise mechanism underlying nNOS-dependent IMPOC cardioprotection remains elusive. The activation of the NO/guanylate cyclase/cGMP pathway by intrathecal morphine stimulates sGC-cGMP, which in turn promotes a variety of downstream signaling. cGMP directly or indirectly acts at protein kinase G to trigger KATP channels (Han et al., 2001; Cunha et al., 2010). Such an increase in potassium channel activity leads to cell membrane hyperpolarization, thereby protecting neurons from over-response upon exposure to noxious stimuli (Cunha et al., 2010). Consistent with this idea, morphine was found to up-regulate KATP currents in primary nociceptive neurons through stimulation of the NO pathway (Lima et al., 2010). Based on the findings described in this study, we propose that IMPOC cardioprotection is likely mediated by a cGMP signal pathway.
It should be mentioned that intrathecal administration of morphine can provide direct access to both the spinal cord and dorsal root ganglia (DRGs). All three subtypes of opioid receptors are abundantly expressed in DRGs (Scherrer et al., 2009). These opioid receptors are critically involved in the morphine-induced analgesic effect on neuropathic and inflammatory pain (Obara et al., 2009). Although we cannot rule out the participation of DRG opioid receptors in IMPOC-induced cardiopretection, previous studies (Jiang et al., 2009; Zhang et al., 2011) from our laboratory showed that intracerebral ventricular injection of morphine in preconditioning and postconditioning significantly reduced the IS of I/R in rats. This suggests that opioid receptors in the central nervous system at least in part contribute to IMPOC-induced therapeutic action in the treatment of heart injury. Future studies should be carried out to determine whether peripheral opioid receptors are involved in morphine-induced cardioprotection.
In summary, our study provides evidence indicating that spinal NOS signaling plays a critical role in mediating the intrathecal morphine-induced therapeutic effect in ischemic heart injury. This finding enhances our understanding of molecular and cellular mechanisms underlying therapeutic mechanism of morphine cardioprotection.
Acknowledgments
The authors thank Jian Zhang (Anhui provincial Children’s hospital, Hefei, Anhui, People’s Republic of China) for data analysis.
Abbreviations
- AAR
area at risk
- DRG
dorsal root ganglion
- I
ischemia
- IMPC
intrathecal morphine preconditioning
- IMPOC
intrathecal morphine postconditioning
- IS
infarct size
- l-NAME
Nω-Nitro-l-arginine methyl ester
- MAP
mean artery pressure
- NAL
naloxone
- 7NI
7-nitroindazole
- NO
nitric oxide
- NOS
nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- pnNOS
phosphorylated form of nNOS
- R
reperfusion
- sGC
guanylate cyclase
Authorship Contributions
Participated in research design: Jiang, He, L. Zhang, and Y. Zhang
Conducted experiments: Jiang and Hu
Performed data analysis: Jiang, Hu, L. Zhang, and Y. Zhang
Wrote or contributed to the writing of the manuscript: Jiang, L. Zhang, and Y. Zhang
Footnotes
References
- Ardell JL. (2010) Sensory transduction of the ischemic myocardium. Am J Physiol Heart Circ Physiol 299:H1753–H1754. [DOI] [PubMed] [Google Scholar]
- Brock SC, Tonussi CR. (2008) Intrathecally injected morphine inhibits inflammatory paw edema: the involvement of nitric oxide and cyclic-guanosine monophosphate. Anesth Analg 106:965–971. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Roman-Campos D, Lotufo CM, Duarte HL, Souza GR, Verri WA, Jr, Funez MI, Dias QM, Schivo IR, Domingues AC, et al. (2010) Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc Natl Acad Sci U S A 107:4442–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury Y, Picolo G, Gutierrez VP, Ferreira SH. (2011) Pain and analgesia: the dual effect of nitric oxide in the nociceptive system. Nitric Oxide 25:243–254. [DOI] [PubMed] [Google Scholar]
- Foletto VR, Martins MA, Tonussi CR. (2013) The involvement of potassium channels in the peripheral antiedematogenic effect of intrathecally injected morphine in rats. Anesth Analg 116:232–238. [DOI] [PubMed] [Google Scholar]
- Garry MG, Richardson JD, Hargreaves KM. (1994) Sodium nitroprusside evokes the release of immunoreactive calcitonin gene-related peptide and substance P from dorsal horn slices via nitric oxide-dependent and nitric oxide-independent mechanisms. J Neurosci 14:4329–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzón J, Rodríguez-Muñoz M, Vicente-Sánchez A, Bailón C, Martínez-Murillo R, Sánchez-Blázquez P. (2011) RGSZ2 binds to the neural nitric oxide synthase PDZ domain to regulate mu-opioid receptor-mediated potentiation of the N-methyl-D-aspartate receptor-calmodulin-dependent protein kinase II pathway. Antioxid Redox Signal 15:873–887. [DOI] [PubMed] [Google Scholar]
- Han J, Kim N, Kim E, Ho WK, Earm YE. (2001) Modulation of ATP-sensitive potassium channels by cGMP-dependent protein kinase in rabbit ventricular myocytes. J Biol Chem 276:22140–22147. [DOI] [PubMed] [Google Scholar]
- Headrick JP, See Hoe LE, Du Toit EF, Peart JN. (2015) Opioid receptors and cardioprotection—“opioidergic conditioning” of the heart. Br J Pharmacol 172:2026–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchee-Rodriguez K, Garg N, Venkatakrishnan P, Roman MG, Adamo ML, Masters BS, Roman LJ. (2013) Neuronal nitric oxide synthase is phosphorylated in response to insulin stimulation in skeletal muscle. Biochem Biophys Res Commun 435:501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H. (2014) Cardioprotection with opioids—trusted old friends-clinical science. Curr Pharm Des 20:5794–5798. [DOI] [PubMed] [Google Scholar]
- Jiang LL, Zhang Y, Weng LJ, Li R, Cheng ZW. (2009) Cardioprotective effects of intracerebroventricular morphine postconditioning against ischemia-reperfusion injury in rat heart. Chin Pharmacol Bull 25:177–181. [Google Scholar]
- Li R, Wong GT, Wong TM, Zhang Y, Xia Z, Irwin MG. (2009) Intrathecal morphine preconditioning induces cardioprotection via activation of delta, kappa, and mu opioid receptors in rats. Anesth Analg 108:23–29. [DOI] [PubMed] [Google Scholar]
- Lima FO, Souza GR, Verri WA, Jr, Parada CA, Ferreira SH, Cunha FQ, Cunha TM. (2010) Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: involvement of the NO/cGMP/PKG/KATP signaling pathway. Pain 151:506–515. [DOI] [PubMed] [Google Scholar]
- Ling Ling J, Wong GT, Yao L, Xia Z, Irwin MG. (2010) Remote pharmacological post-conditioning by intrathecal morphine: cardiac protection from spinal opioid receptor activation. Acta Anaesthesiol Scand 54:1097–1104. [DOI] [PubMed] [Google Scholar]
- Lu Y, Hu J, Zhang Y, Dong C. (2014) Spinal neuronal NOS activation mediates intrathecal fentanyl preconditioning induced remote cardioprotection in rats. Int Immunopharmacol 19:127–131. [DOI] [PubMed] [Google Scholar]
- Lu Y, Hu J, Zhang Y, Dong CS, Wong GT. (2015) Remote intrathecal morphine preconditioning confers cardioprotection via spinal cord nitric oxide/cyclic guanosine monophosphate/protein kinase G pathway. J Surg Res 193:43–51. [DOI] [PubMed] [Google Scholar]
- Markiewicz W, Finberg JP, Lichtig C. (1982) Morphine increases myocardial infarction size in rats. Anesth Analg 61:843–846. [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136. [DOI] [PubMed] [Google Scholar]
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R. (2009) Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain 141:283–291. [DOI] [PubMed] [Google Scholar]
- Pang PS, Komajda M, Gheorghiade M. (2010) The current and future management of acute heart failure syndromes. Eur Heart J 31:784–793. [DOI] [PubMed] [Google Scholar]
- Parenti C, Arico G, Pennisi M, Venditti A, Scoto GM. (2016) Harpagophytum procumbens extract potentiates morphine antinociception in neuropathic rats. Nat Prod Res 30:1248–1255. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, Kieffer BL, Evans CJ. (2012) Ligand-directed signalling within the opioid receptor family. Br J Pharmacol 167:960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley MK. (2002) The diverse molecular mechanisms responsible for the actions of opioids on the cardiovascular system. Pharmacol Ther 93:51–75. [DOI] [PubMed] [Google Scholar]
- Rameau GA, Chiu LY, Ziff EB. (2004) Bidirectional regulation of neuronal nitric-oxide synthase phosphorylation at serine 847 by the N-methyl-D-aspartate receptor. J Biol Chem 279:14307–14314. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M, Garzón J. (2013) Nitric oxide and zinc-mediated protein assemblies involved in mu opioid receptor signaling. Mol Neurobiol 48:769–782. [DOI] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Rodríguez-Muñoz M, Garzón J. (2010) Mu-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling. PLoS One 5:e11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. (2009) Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JE, Gross GJ. (2001) Opioids and cardioprotection. Pharmacol Ther 89:123–137. [DOI] [PubMed] [Google Scholar]
- Sedoris KC, Gozal E, Ovechkin AV, Theile AR, Roberts AM. (2012) Interplay of endothelial and inducible nitric oxide synthases modulates the vascular response to ischaemia-reperfusion in the rabbit lung. Acta Physiol (Oxf) 204:331–343. [DOI] [PubMed] [Google Scholar]
- Shinozaki T, Yamada T, Nonaka T, Yamamoto T. (2015) Acetaminophen and non-steroidal anti-inflammatory drugs interact with morphine and tramadol analgesia for the treatment of neuropathic pain in rats. J Anesth 29:386–395. [DOI] [PubMed] [Google Scholar]
- Shultz JJ, Iskos D, Lurie KG. (1996) Alternative Mechanical Methods of Cardiopulmonary Resuscitation. Am J Ther 3:661–666. [DOI] [PubMed] [Google Scholar]
- Steagall RJ, Sipe AL, Williams CA, Joyner WL, Singh K. (2012) Substance P release in response to cardiac ischemia from rat thoracic spinal dorsal horn is mediated by TRPV1. Neuroscience 214:106–119. [DOI] [PubMed] [Google Scholar]
- Tao YX, Johns RA. (2002) Activation and up-regulation of spinal cord nitric oxide receptor, soluble guanylate cyclase, after formalin injection into the rat hind paw. Neuroscience 112:439–446. [DOI] [PubMed] [Google Scholar]
- Wong GT, Ling Ling J, Irwin MG. (2010) Activation of central opioid receptors induces cardioprotection against ischemia-reperfusion injury. Anesth Analg 111:24–28. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Irwin MG, Lu Y, Mei B, Zuo YM, Chen ZW, Wong TM. (2011) Intracerebroventricular administration of morphine confers remote cardioprotection--role of opioid receptors and calmodulin. Eur J Pharmacol 656:74–80. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Irwin MG, Wong TM, Chen M, Cao CM. (2005) Remifentanil preconditioning confers cardioprotection via cardiac kappa- and delta-opioid receptors. Anesthesiology 102:371–378. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Vinten-Johansen J. (2006) Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res 70:200–211. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285:H579–H588. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhu DY. (2009) Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20:223–230. [DOI] [PubMed] [Google Scholar]