Abstract
Intermittent hypoxia causes a persistent increase in sympathetic nerve activity (SNA), which progresses to hypertension in conditions such as obstructive sleep apnea. Orexins (A and B) are hypothalamic neurotransmitters with arousal-promoting and sympathoexcitatory effects. We investigated whether the sustained elevation of SNA, termed sympathetic long-term facilitation, after acute intermittent hypoxia (AIH) is caused by endogenous orexin acting on spinal sympathetic preganglionic neurons. The role of orexin in the increased SNA response to AIH was investigated in urethane-anesthetized, vagotomized, and artificially ventilated Sprague-Dawley rats (n = 58). A spinally infused subthreshold dose of orexin-A (intermittent; 10 pmol × 10) produced long-term enhancement in SNA (41.4% ± 6.9%) from baseline. This phenomenon was not produced by the same dose of orexin-A administered as a bolus intrathecal infusion (100 pmol; 7.3% ± 2.3%). The dual orexin receptor blocker, Almorexant, attenuated the effect of sympathetic long-term facilitation generated by intermittent orexin-A (20.7% ± 4.5% for Almorexant at 30 mg∙kg−1 and 18.5% ± 1.2% for 75 mg∙kg−1), but not in AIH. The peripheral chemoreflex sympathoexcitatory response to hypoxia was greatly enhanced by intermittent orexin-A and AIH. In both cases, the sympathetic chemoreflex sensitization was reduced by Almorexant. Taken together, spinally acting orexin-A is mechanistically sufficient to evoke sympathetic long-term facilitation. However, AIH-induced sympathetic long-term facilitation appears to rely on mechanisms that are independent of orexin neurotransmission. Our findings further reveal that the activation of spinal orexin receptors is critical to enhance peripheral chemoreceptor responses to hypoxia after AIH.
Introduction
Obstructive sleep apnea is a disorder characterized by episodes of repetitive apnea caused by intermittent collapse of upper airway muscles during sleep (Tilkian et al., 1976; Remmers et al., 1978). The apneic events give rise to frequent hypoxic episodes, and the stimulation of cardiorespiratory neurons in the brainstem and spinal cord (Sun et al., 2011). Consequently, a sequence of reflexes is activated, aimed at relieving the obstruction and promoting the restoration of normal breathing. These counterregulatory mechanisms maintain airway patency and are life-saving responses. However, persistence of intermittent hypoxia gives rise to detrimental changes in cardiorespiratory brain circuits. First, intermittent hypoxia induces a prolonged enhancement in sympathetic discharge from the cardioregulatory neurons, a phenomenon known as sympathetic long-term facilitation. In experimental models, acute intermittent hypoxia (AIH) (10 times 45-second bouts of 10% O2) causes an immediate augmentation of sympathetic activity for at least 60 minutes (Dick et al., 2007; Xing and Pilowsky, 2010). Prolongation of intermittent hypoxia from an acute to a chronic intermittent hypoxia protocol (intermittent hypoxia for days or weeks) severely exacerbates sympathetic activity, eventually leading to the development of neurogenic hypertension over time in animals (Fletcher et al., 1992a,b; Xing and Pilowsky, 2010) and humans (Caples et al., 2005). Second, AIH and chronic intermittent hypoxia also cause a gradual increase in peripheral chemoreceptor sensitivity to hypoxia (Poon and Siniaia, 2000), another pathophysiologic feature in neurogenic hypertension (Cutler et al., 2004a,b; Leuenberger et al., 2005; Imadojemu et al., 2007). It is now widely accepted that mechanistic understanding of the molecular targets that attenuate or block sympathetic hyperactivity is vital for preventing cardiovascular complications from sympathetic long-term facilitation.
Orexin is a hypothalamic neurotransmitter that promotes arousal (Sakurai et al., 2010; Sakurai and Mieda, 2011; Tsujino and Sakurai, 2013) and sympathoexcitation (Shahid et al., 2011, 2012a). Intermittent hypoxia increases the expression of prepro-orexin mRNA in the orexin neurons of the hypothalamus and orexin receptor (OXR) protein on the brainstem medullary neurons of rats (Liu et al., 2014). Orexin-A and -B enhance sympathetic activity after microinjection into the medullary pressor area or intrathecal infusion into the thoracic spinal region (Chen et al., 2000; Antunes et al., 2001; Machado et al., 2002; Shahid et al., 2011, 2012a). Orexin is extensively implicated in the formation of respiratory plasticity induced by AIH (Yamaguchi et al., 2015).
Respiratory and cardiovascular regulating regions share common sites in the hindbrain and the spinal cord (Pilowsky et al., 1985, 1990, 1993, 1994; Sun et al., 1997, 1998; Miyawaki et al., 2002; Nedoboy et al., 2016). This common morphologic relationship between respiratory and cardiovascular structures as well as common afferent inputs and different output pathways in many cases has led to the proposition that the two systems may interact. These observations indicate that the orexinergic system may also interact with the cardiovascular network, possibly having the potential to induce neuroplasticity in sympathetic neurons after AIH, similar in manner to that seen in the respiratory neurons (Kim et al., 2016). Therefore, orexin and its spinal receptor counterparts on sympathetic preganglionic neurons (SPN) may be an immediate therapeutic target for treating exacerbated sympathetic discharge and enhanced peripheral sympathetic chemoreflex that are robustly evident in AIH models (Xing and Pilowsky, 2010). Understanding the immediate targets that alter sympathetic discharge after AIH will serve to be an effective preventive measure before the hypoxia cycle progresses into a chronic phase (i.e., chronic intermittent hypoxia) with a manifestation of elevated arterial pressure from sympathetic hyperactivity (Lesske et al., 1997; Braga et al., 2006; Zoccal et al., 2008; Fisher and Paton, 2012).
Our study investigated whether endogenous orexin is mechanistically necessary for evoking immediate sympathetic long-term facilitation, and chemoreflex sensitization induced by AIH. For this purpose, we used a combination of pharmacologic and electrophysiologic approaches to confirm the mechanistic role of orexin-A on SPNs in urethane-anesthetized, vagotomized, paralyzed, and artificially ventilated rats. Orexin-A was intermittently infused into the spinal cord at a subthreshold dose (10 pmol × 10), which otherwise would not elicit sympathoexcitation as a single dose (100 pmol). Almorexant was intraperitoneally injected at either 30 or 75 mg∙kg−1 before intermittent orexin-A or AIH. Peripheral chemoreflex was activated by a single bout of hypoxia (10% O2) immediately before drug administration or AIH and immediately after all nerve recordings.
Materials and Methods
Animals.
All experimental procedures were executed in strict accordance with the guidelines set in the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, endorsed by the National Health and Medical Research Council of Australia. This study was approved by the Sydney Local Health District Animal Welfare Committee. The animals were housed in cages and were allowed to acclimatize for 7 days with environmental enrichment and access to food and water ad libitum for their basic behavioral and physiologic requirements.
Surgical Preparations.
Animal preparation was performed as previously described elsewhere (Farnham et al., 2008). Briefly, male Sprague Dawley rats (n = 58; 250–400 g) were anesthetized with urethane (1.2–1.4 g⋅kg−1, i.p., with additional doses of 30–40 mg in 10% solution, as required) to suppress nociceptive reflexes. Anesthetic depth was monitored throughout the experiment by observing for changes in blood pressure by greater than 10 mm Hg in response to a tail pinch. The animal was laid on an electric heating pad (Harvard Apparatus, Holliston, MA) for controlled thermoregulation. The body temperature was maintained at 37.0 ± 0.5°C.
General Surgical Procedure.
The right carotid artery and jugular vein were cannulated using polyethylene tubing (outer diameter: 0.90 mm × inner diameter: 0.50 mm; Microtube Extrusions, North Rocks, Australia) for the purpose of recording arterial blood pressure and infusing drugs and fluids, respectively. Electrocardiograms were recorded from leads connected to the forepaws of the rat, and heart rate (HR) was derived from it. Tracheal intubation was performed for artificial ventilation (rodent ventilator; Ugo Basile, Varese, Italy) and the recording of expired CO2 (CapStar 100 CO2 analyzer; CWE, Inc., Ardmore, PA). A tracheostomy was created, and a shortened 14 gauge cannula (length: 50 mm ϕo: 2.20 mm, Optiva I.V. Catheter Radiopaque; Smiths Medical Australasia, Bella Vista, Australia) was inserted. The rats were vagotomized, artificially ventilated with 100% oxygen-enriched room air, and paralyzed using pancuronium bromide (0.4 mg administered as a 0.2 ml bolus i.v. injection, followed by an infusion of 10% pancuronium diluted in 0.9% saline at a rate of 2 ml⋅hour−1; AstraZeneca, London, United Kingdom). Arterial blood gases were analyzed with an electrolyte and blood gas analyzer (VetStat; IDEXX Laboratories, Westbrook, ME). PaCO2 was maintained at 40.0 ± 5 mm Hg and pH between 7.35 and 7.45.
A retroperitoneal approach gives access to the left greater splanchnic nerve at a site proximal to the celiac ganglion and a dorsal approach allows for the left phrenic nerve to be isolated, dissected, and tied using 5/0 silk thread. Nerve activity was recorded using bipolar silver electrodes. The neurograms were amplified 10-fold by a very-low-noise preamplifier (CWE, Inc.), band-pass filtered (0.1–3 kHz), and amplified a further 1000 times for phrenic and 2000 times for splanchnic by a scaling amplifier (BMA-400 AC/DC Bioamplifier; CWE Inc.). The analog signal was then digitized (A/D converter 1401; Cambridge Electronic Design, Cambridge, United Kingdom), sampled at 5 kHz (1401 plus; Cambridge Electronic Design), and displayed using Spike 2 software (version 8; Cambridge Electronic Design).
Intrathecal Catheter Insertion.
The occipital musculature and connective tissue were removed from the occipital bone, which exposes the atlanto-occipital junction. A catheter (polyethylene, outer diameter: 0.50 mm × inner diameter: 0.20 mm; Microtube Extrusions) with a dead space of ∼6 µl was inserted into the intrathecal space through a slit in the dura and advanced caudally to the level of T5/6.
Acute Intermittent Hypoxia.
The experimental protocol in this study adopted the hypoxia protocol used in a previous study (Xing and Pilowsky, 2010). Intermittent bouts of hypoxia gas composed of 10% oxygen balanced with nitrogen (Coregas, Yennora, Australia) were given for 45 seconds 10 times. There were 5-minute intervals between each challenge. The gas tank was connected to the ventilator via a tube, and it was ensured that the normal 100% oxygen gas tap was turned off during each hypoxia challenge.
Intrathecal Drug Administration.
Depending on the variables being tested, control experiments were performed either by infusing 10 µl of 10 mM phosphate-buffered 0.9% saline (PBS), which was washed in with an additional 5 µl of PBS, 10 minutes before challenging the physiologic system by hypoxia or by infusing 10 µl of 10 mM PBS intermittently 10 times with 5-minute intervals between each infusion. Intrathecal drug administration comprised infusing 10 µl orexin-A (Sigma-Aldrich, Castle Hill, Australia) as a single 100 pmol 10 µl−1 (10 µM) infusion or 10 times repeated at 10 pmol 10 µl−1 (1 µM) per infusion, and the nerve response was recorded for a period of 60 minutes. Orexin-A was used because it targets both OX1Rs and OX2Rs whereas orexin-B is specific to OX2Rs (Shahid et al., 2012b). The solutions were infused using a 25-µl glass Hamilton microsyringe (AIS, Ringoes, NJ) over a time period of 10–15 seconds, as described previously elsewhere (Farnham et al., 2008), with 5-minute intervals between individual infusions.
Intraperitoneal Drug Injection.
Dimethylsulfoxide (DMSO) (Sigma-Aldrich) at 200 µl was injected using a 1-ml syringe (Terumo Australia, Macquarie Park, Australia) intraperitoneally as a vehicle control. Almorexant-HCl was dissolved in DMSO to make solutions at 30 mg⋅kg−1 or 75 mg⋅kg−1 (Selleck Chemicals, Houston, TX) which were injected intraperitoneally 10 minutes before performing either the intermittent orexin-A injection or the AIH protocol. Almorexant is capable of inducing a transient and reversible blockade of OXRs specifically in the central nervous system. Almorexant effectively moves through the blood–brain barrier and centrally promotes sleep at 30 mg⋅kg−1 when the drug is injected via the intraperitoneal route (Morairty et al., 2012).
Data Acquisition and Analysis.
Data were obtained using an ADC system (CED 1401; Cambridge Electronic Design) and Spike 2 acquisition/analysis software (version 8.04; Cambridge Electronic Design). The splanchnic sympathetic nerve activity (sSNA) raw data were rectified and smoothed (τ 1 second) and normalized to zero by subtracting the residual activity present 5 minutes after animal death.
Sympathetic nerve activity (SNA) was analyzed by obtaining the %sSNA range, where the activity level given as a mean value over a 1-minute interval at 60 minutes was subtracted by the mean at baseline. The nerve activity was recorded after treatment. The spike area under the curve (AUC) triggered by the very first hypoxic bout (before any drug treatment or AIH) and the final hypoxia (after the treatment and waiting period of 60 minutes) were subtracted, and compared against each treatment groups.
Phrenic nerve activity was rectified and smoothed (τ 0.05 seconds), and phrenic nerve frequency, and phrenic minute activity were all measured at the time points of 10 and 1 minutes before treatment and at the time points of 15, 30, 45, and 60 minutes after treatment. Mean arterial pressure (MAP) and HR (mean value across a 1-minute interval) were recorded at the 10- and 1-minute time points before the treatment (hypoxia, Almorexant, or intrathecal orexin-A). After the treatment, the activity was measured at the time points of 15, 30, 45, and 60 minutes to observe any changes. If the antagonist was used, the effect of the drug was taken as baseline. The temperature and CO2 were measured at time points of 10 and 1 minutes before treatment and at the time points of 15, 30, 45, and 60 minutes after treatment. However, the results for MAP, HR, and phrenic nerve activity are not provided in this study. These parameters were mainly used as indicators to check the vitality of the animal (phrenic, MAP, HR, and CO2 grouped data were not included).
Arterial blood gas levels (PaCO2 and pH) were measured immediately after the surgical procedure to check the status of the animal. Blood gas was measured again 5 minutes before the starting time to ensure optimal physiologic conditions of the animals, then at both 30 minutes and 60 minutes after treatment/AIH to maintain the condition of the animal.
Statistics.
Statistical analyses were performed using GraphPad Prism software (version 6.04; GraphPad Software, San Diego, CA). All grouped data are shown as mean ± S.E.M. Statistical significance of the treatment responses were measured by one-way analysis of variance, using multiple comparisons and Holm-Sidak correction. In all cases, the responses and differences in mean values were considered statistically significant if P < 0.05.
Results
Intermittent Spinally Administered Orexin-A Is Sufficient To Elicit Sympathetic Long-Term Facilitation
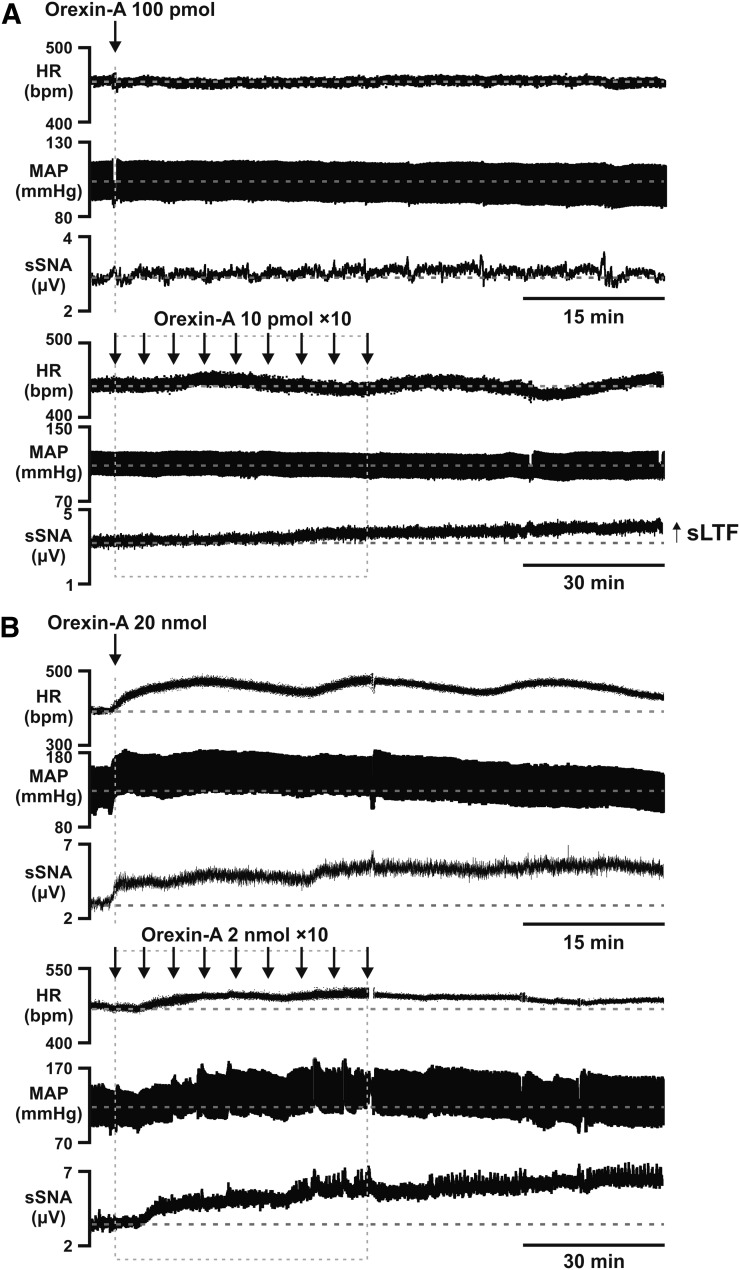
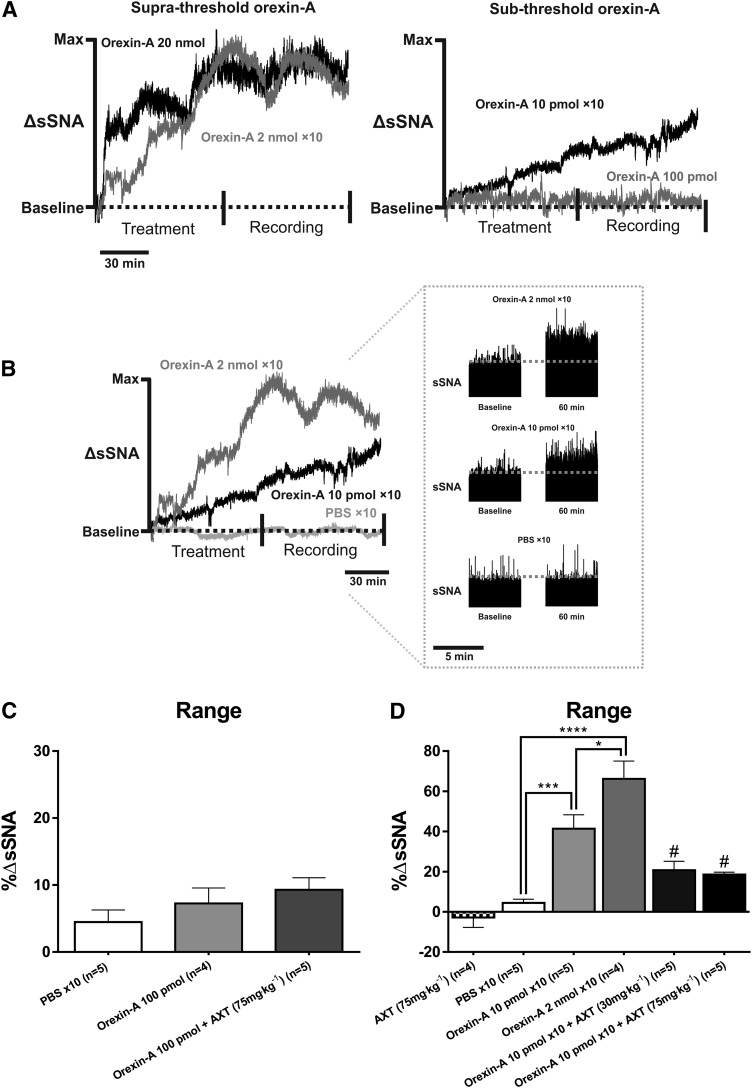
A subthreshold dose (100 pmol) of orexin-A was divided into 10 individual doses (10 pmol each) and infused intermittently into the intrathecal space. Similarly, an intermittent suprathreshold dose (2 nmol × 10) of orexin-A was infused into the intrathecal space. This dose was selected as a positive control (Shahid et al., 2011). Intermittent orexin-A enhances SNA during the 60-minute recording period (Fig. 1 and 2). The subthreshold dose does not elicit a sympathoexcitatory response when administered as a bolus infusion (Δ7.3% ± 2.3%; P > 0.05 versus control; Fig. 1A and Fig. 2). Pretreatment with Almorexant (75 mg⋅kg−1) before orexin-A (100 pmol) also had no effect on sSNA (Δ10.7% ± 2.5%; P > 0.05 versus control; Fig. 2C).
Fig. 1.
Effect of intrathecal orexin-A at (A) subthreshold bolus (100 pmol)/intermittent (10 pmol × 10) and (B) suprathreshold bolus (20 nmol)/intermittent (2 nmol × 10) doses in a urethane-anesthetized rat on (from the top) HR (beats per minute), MAP (mm Hg), and sSNA (µV). Time of administration of intrathecal orexin-A (both single and intermittent) are indicated by the arrow(s). Note that intermittent subthreshold (10 pmol) orexin-A causes the generation of sympathetic long-term facilitation.
Fig. 2.
A comparison of the sympathoexcitatory response elicited by bolus versus intermittent orexin-A at both (A) suprathreshold and (B) subthreshold doses. Single administration of orexin-A (100 pmol; n = 4) and orexin-A (100 pmol) + Almorexant (AXT) at 75 mg⋅kg−1 (n = 5) did not elevate sympathetic activity. (C) No significant changes were observed compared with PBS control (n = 5) when sSNA is measured as % range. Grouped data for the % range from every treatment group are compared. AXT at 75 mg⋅kg−1 (n = 4) produced no effect on baseline sympathetic activity. (D) AXT at 30 mg⋅kg−1 (n = 5) and 75 mg⋅kg−1 (n = 5) attenuated the effect of intermittent orexin-A (10 pmol × 10; n = 5) on sSNA. Statistical significance was determined using one-way analysis of variance followed by Holm-Sidak correction to compare the effects with the control. Data are expressed as mean ± S.E.M. ****P < 0.0001; ***P < 0.001; *P < 0.05. #P < 0.05 compared with intermittent orexin-A (10 pmol × 10).
Intraperitoneal injection of Almorexant at 75 mg⋅kg−1 alone did not affect the baseline sympathetic response (−Δ2.9% ± 4.9%) when compared with intermittent PBS control (Δ4.5% ± 1.8%; Fig. 2D). There was a statistically significant elevation in sSNA caused by both the intermittent subthreshold dose of orexin-A (10 pmol × 10; Δ41.4% ± 6.9%; P < 0.005) and the suprathreshold dose of orexin-A (2 nmol; Δ66.2 ± 8.8%; P < 0.001) compared with control (Δ4.5% ± 1.8%; Fig. 2D). Administering Almorexant before intermittent orexin-A (10 pmol) significantly attenuated these effects; the range was reduced to Δ20.7% ± 4.5% (P < 0.05) by Almorexant 30 mg⋅kg−1 and to Δ18.5% ± 1.2% (P < 0.05) by Almorexant 75 mg⋅kg−1 (Fig. 2D).
Sympathetic Long-Term Facilitation after Acute Intermittent Hypoxia Is Not Mediated by Orexin
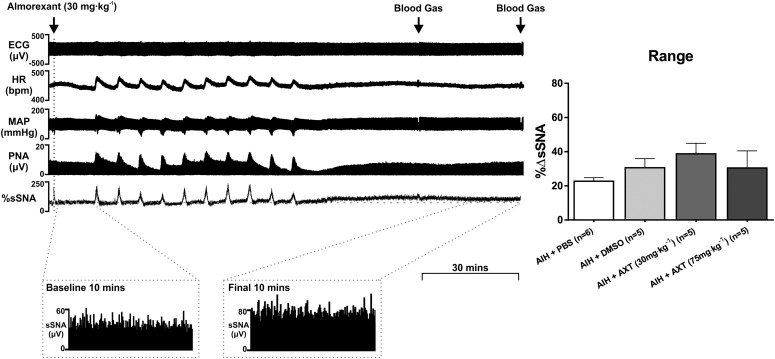
A robust increase in SNA is present after AIH (hypoxia administered for 45 seconds with 5-minute intervals, repeated 10 times), as previously described elsewhere (Xing and Pilowsky, 2010). Almorexant did not affect the sympathetic long-term facilitation evoked by AIH (Fig. 3). Acute intermittent hypoxia induced sympathetic long-term facilitation in all cases, regardless of prior injection of Almorexant at both 30 (Δ52.9% ± 9.9%; P > 0.05 versus DMSO +AIH) and 75 mg⋅kg−1 (Δ47.8% ± 12.7%; P > 0.05 versus DMSO + AIH; Fig. 3). To determine whether DMSO (solvent of Almorexant) alone affects nerve activity, studies were conducted on animals that had intrathecal PBS infusion and intraperitoneal administration of DMSO before AIH. The change in %sSNA, was Δ22.7% ± 2.0% for PBS control and Δ30.6% ± 5.3% for DMSO control after AIH (Fig. 3).
Fig. 3.
In vivo effects of acute intermittent hypoxia (AIH, 10 bouts of 10% oxygen interspersed by 5-minute intervals) and intraperitoneal injection of Almorexant (AXT). (A) Experimental trace displaying the effect of AXT 30 mg⋅kg−1 (n = 5) in AIH, recording the changes in electrocardiogram (ECG), HR, MAP, phrenic nerve activity (PNA), and sSNA. Blood gas was analyzed at 30 and 60 minutes after AIH as indicated by the arrows. A 10-minute recording of pre-AIH sSNA and post-AIH sSNA is referred by the expanded period as indicated. (B) Comparison between the change in sSNA between PBS-treated AIH (n = 6) and DMSO-treated AIH (n = 5). All AXT-treated groups are compared with the DMSO-treated AIH group, where both AXT 30 mg⋅kg−1 and 75 mg⋅kg−1 (n = 5) AXT injections were made 10 minutes before AIH. (C) Change in sSNA shown as % range. Statistical significance was determined using one-way analysis of variance followed by Holm-Sidak correction. Data are expressed as mean ± S.E.M.
Orexin Receptor Antagonism by Almorexant Desensitizes Chemoreflex Response
A hypoxic stimulus was given to animals for all treatment groups at their baseline nerve recordings and at the end of the 60-minute recording period. The AUC of the final and baseline sympathetic responses to hypoxia was subtracted to determine the effect of treatment on the peripheral chemoreflex.
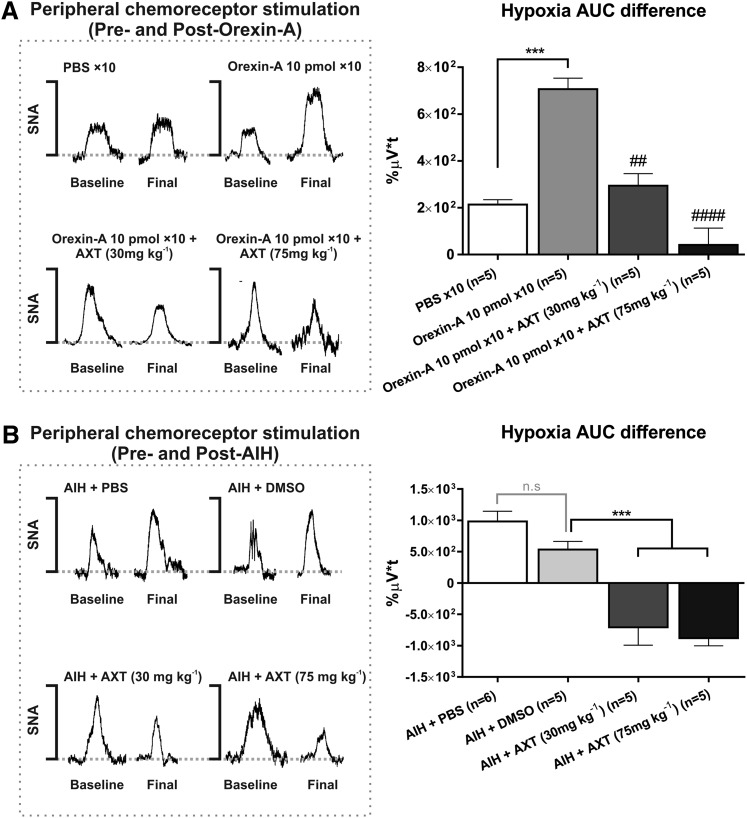
Orexin-A.
Intermittent intrathecal administration of orexin-A (10 pmol per infusion) sensitizes the sympathetic nerve response to hypoxia. The difference in AUC between the first hypoxia before intermittent treatment and the final hypoxia after intermittent orexin-A is significantly greater than intermittent PBS control (Δ707% ± 47% µV⋅t versus Δ213% ± 21% µV⋅t; P < 0.005; Fig. 4A). Almorexant, at both 30 mg⋅kg−1 (Δ294% ± 51% µV⋅t; P < 0.05 versus intermittent orexin-A) and 75 mg⋅kg−1 (Δ81% ± 90% µV⋅t; P < 0.005 versus intermittent orexin-A) doses, ablates the sympathetic response to the final hypoxia (Fig. 4A).
Fig. 4.
Difference in the AUC between the initial and final sympathetic responses to hypoxia-induced chemoreceptor activation. The net AUC difference in the sympathetic response to hypoxia was identified for the baseline activity and the response at 60 minutes after treatment: intermittent orexin-A or acute intermittent hypoxia (AIH). (A) The value for the net difference in AUC (final minus initial hypoxia) compared for the treatments groups involving PBS (n = 5), intermittent orexin-A 10 pmol × 10 (n = 5), and Almorexant (AXT) at 30 (n = 5) and 75 mg⋅kg−1 (n = 5). (B) Similarly, net difference in AUC between the baseline and final sympathetic response to hypoxia after AIH + PBS (n = 6), AIH + DMSO (n = 5), AIH + AXT 30 mg⋅kg−1 (n = 5), and AIH + AXT 75 mg⋅kg−1 (n = 5). For grouped data, statistical significance was determined using one-way analysis of variance followed by multiple comparisons with a Holm-Sidak correction to compare the effects with the control. All values are expressed as mean ± S.E.M. ***P < 0.005 for significance. ##P < 0.01 and ####P < 0.0001 compared with intermittent orexin-A (10 pmol × 10).
Acute Intermittent Hypoxia.
There is no significant difference between PBS and DMSO on the sympathetic chemoreflex response to a single burst of hypoxia. Almorexant at both doses significantly reduced the difference in AUC between the first and final hypoxia compared with the vehicle control group. A difference of −Δ708% ± 285% µV⋅t (P < 0.01 versus DMSO) for 30 mg⋅kg−1 and −Δ879% ± 124% µV⋅t (P < 0.001 versus DMSO) for 75 mg⋅kg−1 was observed (Fig. 4B).
Discussion
This is the first study to demonstrate that intermittent stimulation of spinal OXRs using subthreshold doses of orexin-A causes an immediate and prolonged increase in SNA. In this study we show that orexin neurotransmission is not essential for AIH-induced sympathetic long-term facilitation because the dual OXR antagonist Almorexant did not block the facilitatory response. Our study is also the first to show that intermittent orexin-A, like AIH, enhances the sensitivity of the peripheral chemoreflex. Sympathetic chemoreflex responses to brief hypoxia were intensified after spinal SPNs were intermittently stimulated by orexin-A, as we had previously shown for AIH (Xing and Pilowsky 2010). In our present study, heightened sensitivity to hypoxia required contributions from the orexinergic system because Almorexant completely abolished the sensitization of the sympathetic response to hypoxia.
Technical Considerations.
Orexin-A infusion into the thoracic (T5/6) subarachnoid space evokes sympathoexcitation, tachycardia, and pressor responses by activating OX1Rs and OX2Rs expressed on SPNs of the intermediolateral (Shahid et al., 2011). Spinal orexin-A also enhances the sympathetic baroreflex but blocks the somatosympathetic reflex (Shahid et al., 2011). Orexin-B, another endogenously released orexin, is also known to act on OX2Rs of the spinal SPNs to elicit sympathoexcitation (Antunes et al., 2001). The development of AIH-induced sympathetic long-term facilitation and heightened sympathetic chemoreflex sensitivity may have an endogenous orexin-B component that was not investigated in this study. OX2Rs that are stimulated specifically by orexin-B are coupled to either an excitatory phospholipase C–mediated intracellular pathway or an inhibitory G-protein that leads to the opening of K+ channels and hyperpolarization (Spinazzi et al., 2006; Shahid et al., 2012b). Although the principal effect of OX2Rs is excitatory (Antunes et al., 2001; van den Top et al., 2003), it is possible that inhibitory pathways activated by orexin-B may have important mechanistic contributions in regulating the sympathetic responses seen after intermittent hypoxia. Nevertheless, spinal SPNs are densely populated by OX1Rs (van den Top et al., 2003; Beig et al., 2015), and endogenous orexins are most likely to exert their effect primarily through OX1Rs.
Almorexant administered intraperitoneally is highly lipid soluble and acts at all OXRs. Morairty et al. (2012) demonstrated that intraperitoneal injection of Almorexant at 30 mg⋅kg−1 and 100 mg⋅kg−1 effectively antagonized but did not completely abolish central orexin receptor-mediated rapid eye movement (REM) and non-REM activity in rats without any dose-dependent differences. A recent study investigating the effect of stress on locomotor and cardiovascular activity (Beig et al., 2015) demonstrated that intraperitoneal Almorexant at 30 mg⋅kg−1 and 100 mg⋅kg−1 caused attenuations, but did not completely antagonize, behavioral responses related to orexin release with no significant dose-dependent changes. Centrally mediated sleep and stress are only partially blocked by Almorexant; thus Almorexant may have side effects in other central areas. The testes contain low levels of OXR mRNA expression (Jöhren et al., 2001), and orexin and OX2R mRNA is detectable in adrenal cortex (Randeva et al., 2001). Thus, it is possible that sympathetic long-term facilitation evoked by intermittent orexin-A was not completely blocked by Almorexant because OXRs on SPNs were not completely antagonized.
Another reason for choosing intraperitoneal injection over direct intrathecal injection is that the DMSO agent (the only available solvent for Almorexant) can be neurotoxic. DMSO can adversely affect astrocyte function (Yuan et al., 2014), which may lead to unknown off-target effects. For this reason, many neuropharmacologic studies requiring DMSO to dissolve their neuroprotectant or neurotoxic drugs dilute the DMSO concentration. However, Almorexant is only soluble in 100% DMSO, so the drug was injected intraperitoneally for all experiments.
The pharmacokinetic profile of Almorexant is characterized by rapidly decreasing concentrations to approximately 20% of the maximum concentration over 8 hours, with a terminal half-life of 32 hours in humans (Hoever et al., 2013). In male canines, oral administration of Almorexant at a dose of 100 mg⋅kg−1 induced clinical signs of somnolence for up to 6 hours (Brisbare-Roch et al., 2007). Stress-induced cardiovascular responses in Wistar rats were reduced by intraperitoneal Almorexant at 30 mg⋅kg−1 4 hours after the injection (Beig et al., 2015). The time course of our study does not exceed 3 hours after the administration of the drug, so systemic injection of Almorexant would manifest its full effect throughout the span of the experiments.
The baseline level for all cardiovascular parameters and sSNA in this study was established under hyperoxic and normocapnic conditions (PaO2 > 140 mm Hg, PaCO2 maintained at 40 ± 5 mm Hg) by adjusting the ventilation (tidal volume and pump frequency) without determination of apneic and recruitment thresholds. Olson et al. (2001) suggested that hypocapnia may restrain the development of facilitation in cardiorespiratory activity. A consistent monitoring of isocapnic and metabolic conditions in all groups tested ensures that no central chemoreceptor drive caused alterations to central presympathetic inputs to the spinal cord.
Potential Mechanisms Underlying the Formation of Sympathetic Long-Term Facilitation.
An earlier study demonstrated that exogenously applied orexin-A strengthens glutamatergic transmission in SPNs (Van Den Pol et al., 1998). Previous evidence has demonstrated that orexin neuron activation is necessary for the expression of phrenic facilitation after AIH (Toyama et al., 2009; Yamaguchi et al., 2015). Phrenic facilitation is evoked in the cervical phrenic motor nucleus by intracellular pathways activated by serotonergic G protein-coupled receptors coupled to protein kinase Cθ (PKCθ) and protein kinase A (PKA) effector proteins. PKCθ activates extracellular signal–regulated kinase/mitogen-activated protein kinase pathways (Devinney et al., 2015), whereas PKA phosphorylates phosphatidylinositol-3-kinase/protein kinase B pathways (Fields et al., 2015). Both mechanisms strengthen excitatory responses mediated by postsynaptic glutamatergic signals (Hoffman et al., 2012). Orexin-A activates OXRs 1 and 2 that are coupled to PKC and PKA proteins (Spinazzi et al., 2006; Shahid et al., 2012b). We speculate that the generation of sympathetic long-term facilitation in spinal thoracic SPNs may be similar in mechanism to phrenic facilitation.
Another potential mechanism for generating sympathetic long-term facilitation is via calcium-mediated mechanisms. Intracellular calcium stores in neurons regulate synaptic plasticity and signaling cascades that are important for long-term potentiation in memory (Baker et al., 2013). Intracellular ryanodine receptor activation leads to calcium-induced calcium release, causing calcium signal propagation that promotes the consolidation of labile memory into long-term memory (Raymond and Redman, 2002). Several studies have shown that ryanodine receptor antagonism, which prevents the release of Ca2+, inhibits the formation of long-term potentiation (Wang et al., 1996).
OX1R activation by orexin-A also induced calcium influx. OX1R up-regulates PLC proteins to hydrolyze phosphatidylinositol 4,5-biphosphate into inositol triphosphate and diacylglycerol (Shahid et al., 2012b), both of which increase intracellular Ca2+, thus evoking synaptic plasticity in SPNs.
Peripheral Chemoreceptor Sensitization Is Mediated by Orexin-A.
Our study is first to demonstrate that an increase in cardiorespiratory sensitivity to hypoxia after AIH is mediated by orexin-A. Enhanced chemoreflex sensitivity is pathogenic to neurogenic hypertension in obstructive sleep apnea (Leuenberger et al., 2005; Imadojemu et al., 2007; Xing and Pilowsky, 2010; Paton et al., 2013). Repetitive hypoxia causes a cumulative elevation in chemoreflex-mediated sympathoexcitatory response, gradually increasing the excitatory response for every succeeding stimulus during AIH or chronic intermittent hypoxia. Administration of Almorexant before intermittent orexin-A and AIH completely abolished the enhanced sensitivity to a brief hypoxic stimulus 60 minutes after the intermittent stimuli. These observations indicate two mechanistic features of the chemoreflex pathway: 1) AIH activates orexin neurons to exert excitatory effects on the sympathetic circuitry, and 2) the physiologic sympathoexcitatory responses to hypoxia require orexin neuronal inputs to the peripheral chemoreflex pathway.
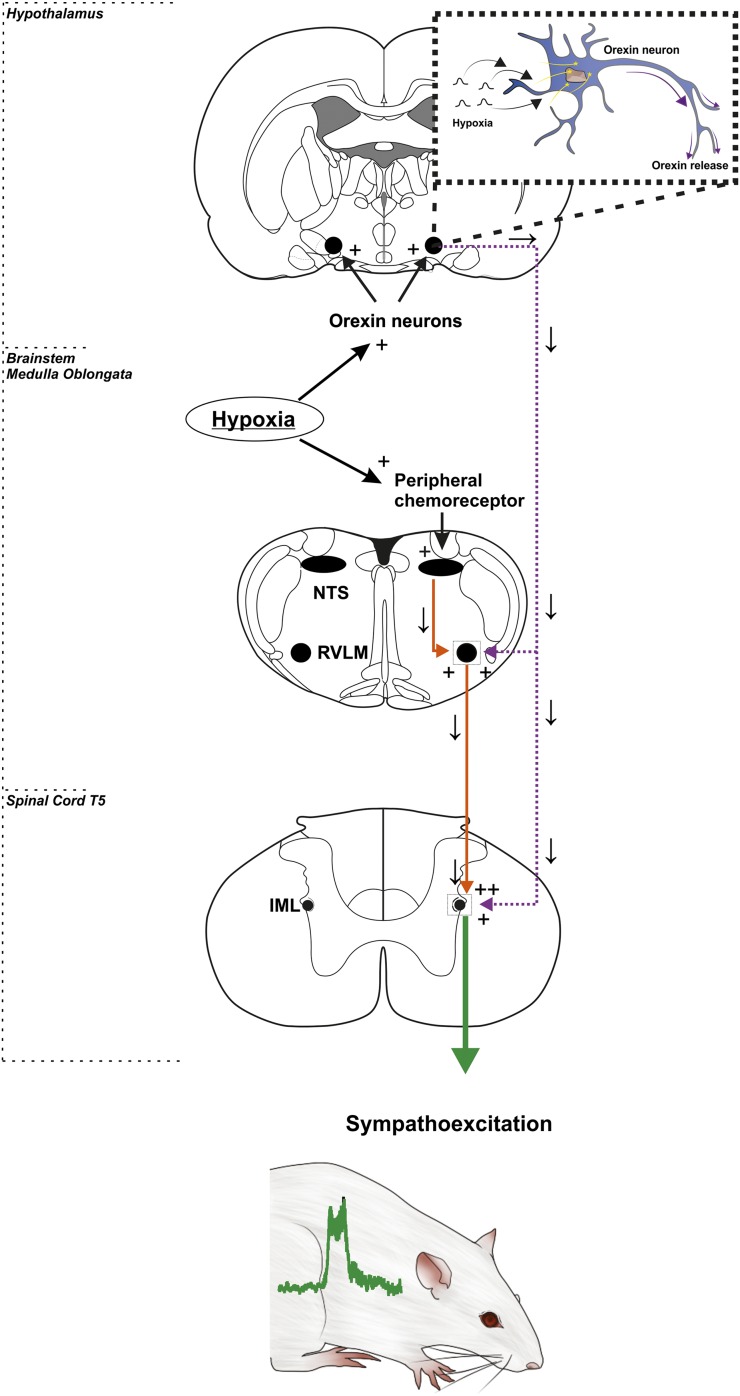
It remains unknown as to how orexin neurons are activated in response to hypoxia. One recent study demonstrated that C1 neurons of the rostral ventrolateral medulla, which are intrinsically hypoxia-sensitive neurons, have afferent inputs to the orexinergic neurons (Bochorishvili et al., 2014). C1 neurons respond to severe reductions in brain PaO2 (Sun and Reis, 1994). A large proportion of C1 neurons express Fos protein in conscious mammals exposed to hypoxia (Erickson and Millhorn, 1994), and sympathetic nerve activation elicited by carotid body stimulation is severely depressed after selective lesions of these cells (Schreihofer and Guyenet, 2000). The C1 neurons establish asymmetric synapses with orexin-immunoreactive cell bodies or dendrites (Bochorishvili et al., 2014). Anterograde tracing with viral vectors has revealed C1 cell projections to the lateral hypothalamus (Bochorishvili et al., 2014); the rostral ventrolateral medulla may be a key nucleus regulating both descending presympathetic neurons and afferents projections to orexin nuclei to activate state-dependent control of sympathetic responses to hypoxia (Fig. 5).
Fig. 5.
Sympathetic outflow from hypoxia-induced peripheral chemoreceptor activation requires a contribution from hypothalamic orexin neurons. Hypoxia directly activates the peripheral chemoreceptors of the carotid body, which sends afferent excitatory signals to the brainstem medulla. The nerves synapse to the nucleus tractus solitarii (NTS), which excite the rostral ventrolateral medulla (RVLM) nerves and further relay these signals to the intermediolateral (IML) of the spinal T5/6 level. Simultaneously, orexin neurons are excited by repetitive hypoxia. Both orexin receptor subtypes (OX1R and OX2R) are found on SPNs in the intermediolateral and are necessary to sensitize the sympathetic chemoreflex response to hypoxia.
Conclusion
In conclusion, our present study has determined a functional role for orexin in the cardiorespiratory regulation of SNA. Intermittent orexin-A infusion and AIH significantly enhance the activity of the sympathetic pathway and evoke sympathetic long-term facilitation. Peripheral chemoreflex sensitivity was also enhanced by intermittent orexin-A and AIH. This sympathetic response was completely abolished by OXRs antagonism using Almorexant. This is the first study to relate the significance of intermittent hypoxia-induced sympathetic elevation with orexin in the cardiorespiratory system. In future, targeting the OXRs may serve as an immediate option for preventing the acute symptoms of abnormal sympathetic hyperactivity that precede neurogenic hypertension.
Abbreviations
- AUC
area under the curve
- AIH
acute intermittent hypoxia
- DMSO
dimethylsulfoxide
- HR
heart rate
- MAP
mean arterial pressure
- OXR
orexin receptor
- PBS
phosphate-buffered 0.9% saline
- PKA
protein kinase A
- PKC
protein kinase C
- REM
rapid eye movement
- SNA
sympathetic nerve activity
- SPN
sympathetic preganglionic neuron
- sSNA
splanchnic sympathetic nerve activity
Authorship Contributions
Participated in research design: Kim, Pilowsky, Farnham.
Conducted experiments: Kim.
Contributed new reagents or analytic tools: Kim, Pilowsky, Farnham.
Performed data analysis: Kim, Pilowsky, Farnham.
Wrote or contributed to the writing of the manuscript: Kim, Pilowsky, Farnham.
Footnotes
This work was supported by the National Health and Medical Research Council of Australia Fellowship [Grant 1024489 (to P.M.P.), and NHMRC Project Grants 1065485, 1082215] and the Australian Research Council Discovery Early Career Researcher Award [Grant DE120100992] (to M.M.J.F.), and the Heart Research Institute. S.J.K. is supported by an Australian Postgraduate Award [APA SC0042] and a Heart Research Institute scholarship.
References
- Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. (2001) Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 281:R1801–R1807. [DOI] [PubMed] [Google Scholar]
- Baker KD, Edwards TM, Rickard NS. (2013) The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neurosci Biobehav Rev 37:1211–1239. [DOI] [PubMed] [Google Scholar]
- Beig MI, Dampney BW, Carrive P. (2015) Both Ox1r and Ox2r orexin receptors contribute to the cardiovascular and locomotor components of the novelty stress response in the rat. Neuropharmacology 89:146–156. [DOI] [PubMed] [Google Scholar]
- Bochorishvili G, Nguyen T, Coates MB, Viar KE, Stornetta RL, Guyenet PG. (2014) The orexinergic neurons receive synaptic input from C1 cells in rats. J Comp Neurol 522:3834–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VA, Soriano RN, Machado BH. (2006) Sympathoexcitatory response to peripheral chemoreflex activation is enhanced in juvenile rats exposed to chronic intermittent hypoxia. Exp Physiol 91:1025–1031. [DOI] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van Gerven J, de Haas SL, et al. (2007) Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med 13:150–155. [DOI] [PubMed] [Google Scholar]
- Caples SM, Gami AS, Somers VK. (2005) Obstructive sleep apnea. Ann Intern Med 142:187–197. [DOI] [PubMed] [Google Scholar]
- Chen CT, Hwang LL, Chang JK, Dun NJ. (2000) Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol 278:R692–R697. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Burk JR, Smith ML. (2004a) Periods of intermittent hypoxic apnea can alter chemoreflex control of sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol 287:H2054–H2060. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. (2004b) Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol (1985) 96:754–761. [DOI] [PubMed] [Google Scholar]
- Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS. (2015) Phrenic long-term facilitation requires PKCθ activity within phrenic motor neurons. J Neurosci 35:8107–8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Wang N, Prabhakar N. (2007) Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol 92:87–97. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. (1994) Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol 348:161–182. [DOI] [PubMed] [Google Scholar]
- Farnham MMJ, Li Q, Goodchild AK, Pilowsky PM. (2008) PACAP is expressed in sympathoexcitatory bulbospinal C1 neurons of the brain stem and increases sympathetic nerve activity in vivo. Am J Physiol Regul Integr Comp Physiol 294:R1304–R1311. [DOI] [PubMed] [Google Scholar]
- Fields DP, Springborn SR, Mitchell GS. (2015) Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol 114:2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Paton JFR. (2012) The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens 26:463–475. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. (1992a) Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 20:612–619. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. (1992b) Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19:555–561. [DOI] [PubMed] [Google Scholar]
- Hoever P, Hay J, Rad M, Cavallaro M, van Gerven JM, Dingemanse J. (2013) Tolerability, pharmacokinetics, and pharmacodynamics of single-dose almorexant, an orexin receptor antagonist, in healthy elderly subjects. J Clin Psychopharmacol 33:363–370. [DOI] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. (2012) Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol (1985) 113:1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. (2007) Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest 131:1406–1413. [DOI] [PubMed] [Google Scholar]
- Jöhren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. (2001) Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology 142:3324–3331. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YJ, Kakall Z, Farnham MMJ, Pilowsky PM. (2016) Intermittent hypoxia-induced cardiorespiratory long-term facilitation: a new role for microglia. Respir Physiol Neurobiol 226:30–38. [DOI] [PubMed] [Google Scholar]
- Lesske J, Fletcher EC, Bao G, Unger T. (1997) Hypertension caused by chronic intermittent hypoxia--influence of chemoreceptors and sympathetic nervous system. J Hypertens 15:1593–1603. [DOI] [PubMed] [Google Scholar]
- Leuenberger UA, Brubaker D, Quraishi SA, Hogeman CS, Imadojemu VA, Gray KS. (2005) Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci 121:87–93. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jiang L, Zhu F, Fu C, Lu S, Zhou J, Wu X, Bai C, Li S. (2014) Chronic intermittent hypoxia and the expression of orexin and its receptors in the brains of rats. Sleep Biol Rhythms 12:22–29. [Google Scholar]
- Machado BH, Bonagamba LGH, Dun SL, Kwok EH, Dun NJ. (2002) Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept 104:75–81. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Goodchild AK, Pilowsky PM. (2002) Evidence for a tonic GABA-ergic inhibition of excitatory respiratory-related afferents to presympathetic neurons in the rostral ventrolateral medulla. Brain Res 924:56–62. [DOI] [PubMed] [Google Scholar]
- Morairty SR, Revel FG, Malherbe P, Moreau JL, Valladao D, Wettstein JG, Kilduff TS, Borroni E. (2012) Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One 7:e39131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedoboy PE, Mohammed S, Kapoor K, Bhandare AM, Farnham MM, Pilowsky PM. (2016) pSer40 tyrosine hydroxylase immunohistochemistry identifies the anatomical location of C1 neurons in rat RVLM that are activated by hypotension. Neuroscience 317:162–172. [DOI] [PubMed] [Google Scholar]
- Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. (2001) Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol (1985) 91:709–716. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Sobotka PA, Fudim M, Engelman ZJ, Hart ECJ, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, et al. (2013) The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61:5–13. [DOI] [PubMed] [Google Scholar]
- Pilowsky P, Llewellyn-Smith IJ, Arnolda L, Lipski J, Minson J, Chalmers J, Zwillich C. (1993) Are the ventrally projecting dendrites of respiratory neurons a neuroanatomical basis for the chemosensitivity of the ventral medulla oblongata? Sleep 16(8, Suppl)S53–S55. [PubMed] [Google Scholar]
- Pilowsky P, Llewellyn-Smith IJ, Lipski J, Minson J, Arnolda L, Chalmers J. (1994) Projections from inspiratory neurons of the ventral respiratory group to the subretrofacial nucleus of the cat. Brain Res 633:63–71. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Jiang C, Lipski J. (1990) An intracellular study of respiratory neurons in the rostral ventrolateral medulla of the rat and their relationship to catecholamine-containing neurons. J Comp Neurol 301:604–617. [DOI] [PubMed] [Google Scholar]
- Pilowsky P, West M, Chalmers J. (1985) Renal sympathetic nerve responses to stimulation, inhibition and destruction of the ventrolateral medulla in the rabbit. Neurosci Lett 60:51–55. [DOI] [PubMed] [Google Scholar]
- Poon C-S, Siniaia MS. (2000) Plasticity of cardiorespiratory neural processing: classification and computational functions. Respir Physiol 122:83–109. [DOI] [PubMed] [Google Scholar]
- Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW. (2001) Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab 86:4808–4813. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Redman SJ. (2002) Different calcium sources are narrowly tuned to the induction of different forms of LTP. J Neurophysiol 88:249–255. [DOI] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. (1978) Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44:931–938. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M. (2011) Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci 32:451–462. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, Tsujino N. (2010) The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci 1200:149–161. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. (2000) Sympathetic reflexes after depletion of bulbospinal catecholaminergic neurons with anti-DβH-saporin. Am J Physiol Regul Integr Comp Physiol 279:R729–R742. [DOI] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA, Pilowsky PM. (2011) Intrathecal orexin A increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato-sympathetic reflex. Br J Pharmacol 162:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA, Pilowsky PM. (2012a) Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol 165:2292–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA, Pilowsky PM. (2012b) Orexin and central regulation of cardiorespiratory system. Vitam Horm 89:159–184. [DOI] [PubMed] [Google Scholar]
- Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG. (2006) Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacol Rev 58:46–57. [DOI] [PubMed] [Google Scholar]
- Sun MK, Reis DJ. (1994) Central neural mechanisms mediating excitation of sympathetic neurons by hypoxia. Prog Neurobiol 44:197–219. [DOI] [PubMed] [Google Scholar]
- Sun Q-J, Bautista TG, Berkowitz RG, Zhao W-J, Pilowsky PM. (2011) The temporal relationship between non-respiratory burst activity of expiratory laryngeal motoneurons and phrenic apnoea during stimulation of the superior laryngeal nerve in rat. J Physiol 589:1819–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q-J, Goodchild AK, Chalmers JP, Pilowsky PM. (1998) The pre-Bötzinger complex and phase-spanning neurons in the adult rat. Brain Res 809:204–213. [DOI] [PubMed] [Google Scholar]
- Sun Q-J, Minson J, Llewellyn-Smith IJ, Arnolda L, Chalmers J, Pilowsky P. (1997) Bötzinger neurons project towards bulbospinal neurons in the rostral ventrolateral medulla of the rat. J Comp Neurol 388:23–31. [DOI] [PubMed] [Google Scholar]
- Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. (1976) Hemodynamics in sleep-induced apnea. Studies during wakefulness and sleep. Ann Intern Med 85:714–719. [DOI] [PubMed] [Google Scholar]
- Toyama S, Sakurai T, Tatsumi K, Kuwaki T. (2009) Attenuated phrenic long-term facilitation in orexin neuron-ablated mice. Respir Physiol Neurobiol 168:295–302. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. (2013) Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. (1998) Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci 18:7962–7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Top M, Nolan MF, Lee K, Richardson PJ, Buijs RM, Davies CH, Spanswick D. (2003) Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol 549:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu J, Rowan MJ, Anwyl R. (1996) Ryanodine produces a low frequency stimulation-induced NMDA receptor-independent long-term potentiation in the rat dentate gyrus in vitro. J Physiol 495:755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Pilowsky PM. (2010) Acute intermittent hypoxia in rat in vivo elicits a robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J Physiol 588:3075–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Futatsuki T, Ushikai J, Kuroki C, Minami T, Kakihana Y, Kuwaki T. (2015) Intermittent but not sustained hypoxia activates orexin-containing neurons in mice. Respir Physiol Neurobiol 206:11–14. [DOI] [PubMed] [Google Scholar]
- Yuan C, Gao J, Guo J, Bai L, Marshall C, Cai Z, Wang L, Xiao M. (2014) Dimethyl sulfoxide damages mitochondrial integrity and membrane potential in cultured astrocytes. PLoS One 9:e107447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LGH, Braga VA, Pickering AE, Paton JFR, Machado BH. (2008) Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol 586:3253–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]