Supplemental Digital Content is available in the text
Abstract
Degenerative disc disease and spinal stenosis lead to various symptoms. Degeneration of facet joints is added to this degenerative process with aging.
Seventy-four patients who were admitted to the Spinal Column Outpatient Clinic of the Neurosurgery Department with a diagnosis of degenerative narrow spinal canal and lumbar spondylolisthesis between 2011 and 2013 and who underwent surgery were included in the study.
Our study was conducted with 74 patients of whom 73.0% (n = 54) were female and 27.0% (n = 20) were male. Mean age was 54.86 ± 7.87 years (range 34–74).
Although we did not detect a difference between the two surgical methods with regard to clinical improvement, transforaminal lumbar interbody fusion (TLIF) is preferred due to radiological advantages observed one year later, ease of application, and the development of fewer complications.
INTRODUCTION
Degenerative disc disease and spinal stenosis lead to various symptoms. Spinal stenosis is defined as narrowing of the spinal canal and the intervertebral foramen below a critical value, and it frequently presents with claudication, leg pain, low back pain, paresthesia, and power loss due to ischemia as a result of neural or vascular structure compression.1,2 Degeneration in facet joints is also added to this degenerative process with aging. Facet joint degeneration leads to spinal instability.1–3
Surgical methods are applied in pathologies that develop degeneratively as a result of age and microtraumas and do not respond to medical therapies. These surgical treatments include simple decompression, posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF), posterolateral fusion (PLF), and posterior instrumentation. Frequently, pedicle screws are used for support due to nonunion development and increased instability in simple decompression methods.1,4–9 Although simple decompression methods have been used for many years, the PLIF procedure was first described by Cloward in 1940 and modified by Lin.10,11 Interbody fusion techniques provide better fusion and are very effective in the preservation of disc height.12 The PLIF procedure is considered the gold standard for the treatment of spinal instability; however, TLIF has been found superior to PLIF in some aspects.13 The advantages of PLIF include its being applied from the posterior and supporting three columns, not requiring an anterior opening, and having a relatively low cost. However, its main disadvantage is that it is only able to be applied between L3 and S1 due to conus medullaris compression.2 TLIF was introduced after modification by Harm et al in 1982.14,15
In this study, we aimed to compare the radiological and clinical outcomes of these two stabilization methods after a one year follow-up period.
MATERIAL AND METHOD
A total of 74 patients, who were admitted to the Spinal Column Outpatient Clinic of the Neurosurgery Department between 2011 and 2013, diagnosed with degenerative narrow spinal canal and lumbar spondylolisthesis, and underwent surgery were evaluated retrospectively. As this is a retrospective analysis, our ethic committee did not require patients’ approval.
Patients were divided to two groups according to surgical method. The multiaxial transpedicular screw system and polyetheretherketone (PEEK) interbody care were used for all patients. x-rays and lumbar spinal computed tomography (CT) were used to assess patients in the preoperative and postoperative periods. The same surgeon operated on all of the patients.
While Group 1 was composed of 41 patients from 40 to 73 years of age, who received a single-level PLIF and posterior lumbar transpedicular screw system, Group 2 was composed of 33 patients from 34 to 74 years of age who received a total laminectomy, a single-level TLIF, and a posterior lumbar transpedicular screw system.
All the patients were examined by the same senior physician. Documentation of the patients’ medical histories and examination records were made by the same physician. All patients were given the Owestry Disability Index (ODI) and the visual analog scale (VAS) in the preoperative period and postoperatively at 3 and 12 months.
Radiologic Technique
CT images were obtained postoperatively at hour 24 and at month 12. Analyses were done with the patient in the prone position with sections of 1 mm using a Toshiba Aquilion 64 BT device (Toshiba Medical Systems Corporation, Tochigi, Japan) (Figure 1). Multiplanar reconstruction (MPR) images were obtained on the sagittal and coronal planes from axial sections. Postoperative CT images at hour 24 and at month 12 were compared and measurements were taken (Figure 2). On the CT images of each patient, mean foraminal height loss, mean disc height change, disc angle difference, posterior displacement of the cage, mean height loss in the superior spina, mean height loss in the inferior spina, and lumbar lordosis angle change were measured in the spines at the level where the cage was placed. Mean foraminal height loss was estimated by subtracting the sum of foraminal height on sagittal MPR images obtained one year later by the CT from the sum on CT images taken a hour 24 and the dividing that value by two. The change in mean disc height was obtained from a MPR image on the midline sagittal plane. The disc was measured from the anterior, posterior, and medial parts. The total measurement on the CT image one year later was then subtracted from the CT image at hour 24, and the result was divided by three. The total lumbar lordosis angle was measured from the midline sagittal plane MPR CT image as the angle between the planes align with L1 spine superior plate and sacrum superior plate and the planes vertical to these planes (Figure 3A). Disc angle difference is the difference between two analyses of the angle between the inferior plate of the superior spine at cage level where the planes align with the superior plate of the inferior spine (Figure 3B). Posterior displacement of the cage is the posterior displacement measure of the cage. To calculate mean height loss in inferior and superior spines, the sum of anterior, posterior, medial heights of the spines in the midline sagittal plane on CT at one year and height in lateral margins of both spines in the midline coronal plane on the MPR image were subtracted from the CT measurements at postoperative hour 24, and the result was divided by 5 (Figure 3C).
FIGURE 1.
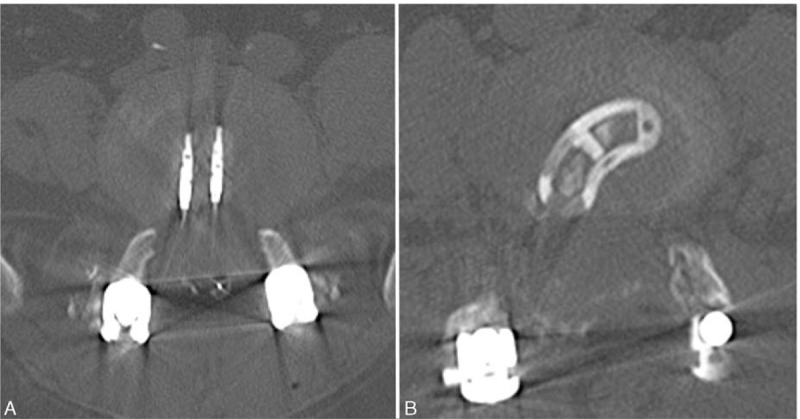
CT images of PLIF (1A) and TLIF (1B) on axial plane. PLIF posterior lumbar interbody fusion, TLIF transforaminal lumbar interbody fusion.
FIGURE 2.
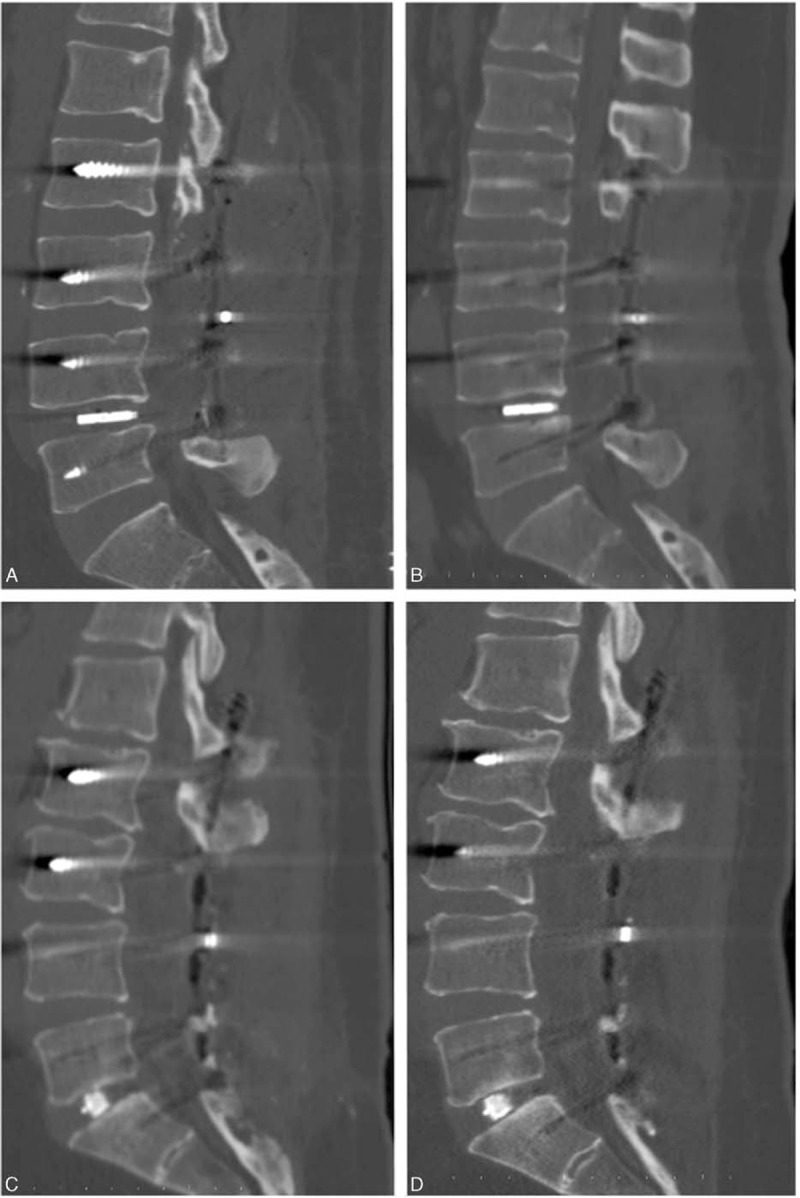
On CT MPR image on sagittal plane, early post-operative (A) and control image one year later (B) in the patient who underwent PLIF; early post-operative (C) and control image one year later (D) in the patient who underwent TLIF. CT = computed tomography; MPR = multiplanar reconstruction , PLIF = posterior lumbar interbody fusion, TLIF = = transforaminal lumbar interbody fusion.
FIGURE 3.
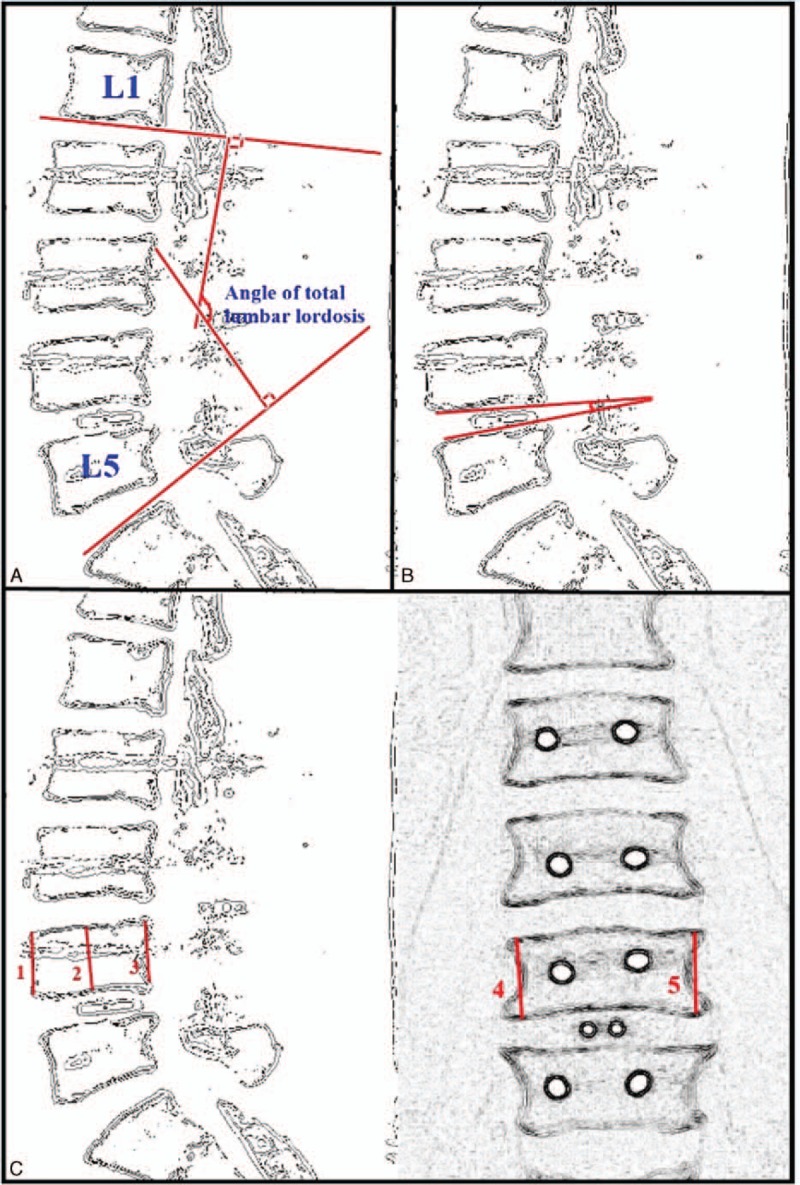
(A) Schematic appearance of total lumbar lordosis angle. (B) Schematic measurement of disc angle. (C) Schematic measurement of height loss in the superior spine according to the level of cage application.
Surgical Technique
Patients were placed in a semiflex prone position on the operating table. Skin and subcutaneous tissue were passed with a midline surgical incision. Paravertebral muscles were skimmed by subperiosteal blunt dissection. A total laminectomy and a medial facetectomy were performed using the PLIF method. A unilateral medial facetectomy and a hemilaminectomy were performed using the TLIF method. In PLIF and TLIF techniques, the dura and nerve roots were taken to medial in order to access the intervertebral discs after the dura had been exposed. A discectomy was performed. The cage filled with bone graft was placed in both end-plates in the intervertebral disc space. A transpedicular screw system was put in place to complete the posterior structure. The system was fixed with rods and transverse rod. The posterior fusion system was supported by the patient's own bones. All patients were followed-up with a hemovac drain for 24 hours.
RESULTS
Our study was conducted with 74 cases of whom 73.0% (n = 54) were female and 27.0% (n = 20) were male. The age of the patients varied from 34 to 74 years of age (mean 54.86 ± 7.87) (Table 1).
TABLE 1.
Age and Gender Distribution According to Groups
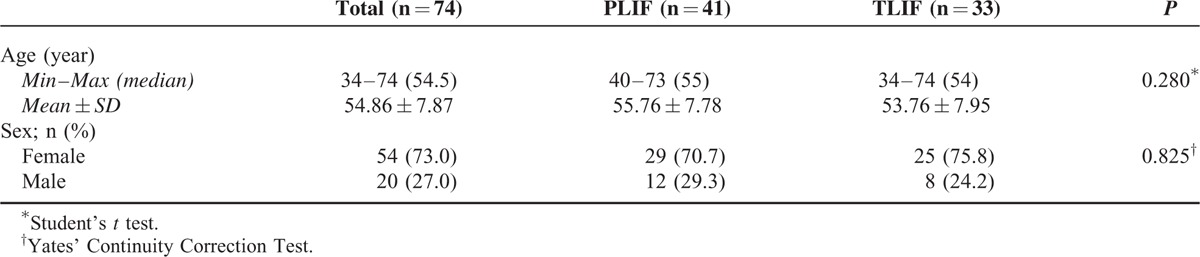
Age and gender distribution of the cases according to groups did not show a statistically significant difference (P > 0.05). Preoperative (P = 0.754) and postoperative (P = 0.517) neurogenic claudication (NC) measurements of the cases did not show a statistically significant difference between groups (P > 0.05). Preoperative (P = 0.554) and postoperative (P = 0.586) neurologic examination findings did not show a statistically significant difference between groups (P = 0.05). Preoperative (P = 0.258), postoperative at month 3 (P = 0.624) and postoperative at month 12 (P = 0.883) Owestry measurements of the cases did not show a statistically significant difference between groups (P > 0.05) (Graphic 1).
While preoperative (P = 0.157) and postoperative at month 3 (P = 0.271) VAS measurements of the cases did not show a statistically significant difference between groups (P > 0.05), postoperative month 12 VAS measurements of the patients who underwent PLIF were higher than those who underwent TLIF, and the difference was statistically significant (P = 0.009; P < 0.01). Preoperative (P = 0.249) and postoperative P = 0.234) hypoesthesia levels of the cases did not show a statistically significant difference between groups (P > 0.05). In the PLIF group, preoperative and postoperative hypoesthesia level change was found statistically significant (P = 0.005; P < 0.01). In the TLIF group, preoperative and postoperative hypoesthesia level change was found statistically significant (P = 0.001; P < 0.01). There was not a statistically significant difference between groups with regard to preoperative (P = 0.624) and postoperative (P = 1.000) Achilles reflex levels (P > 0.05). In the PLIF group, the change in postoperative Achilles reflex level was found statistically significant compared to preoperative Achilles reflex change (P = 0.004; P < 0.01). In the TLIF group, the change in postoperative Achilles reflex level was found statistically significant compared to preoperative Achilles reflex change (P = 0.006; P < 0.01) (Graphic 1) (Table 2).
TABLE 2.
Assessment of Hypoesthesia, Achilles Reflex, Patella Reflex Levels According to Groups
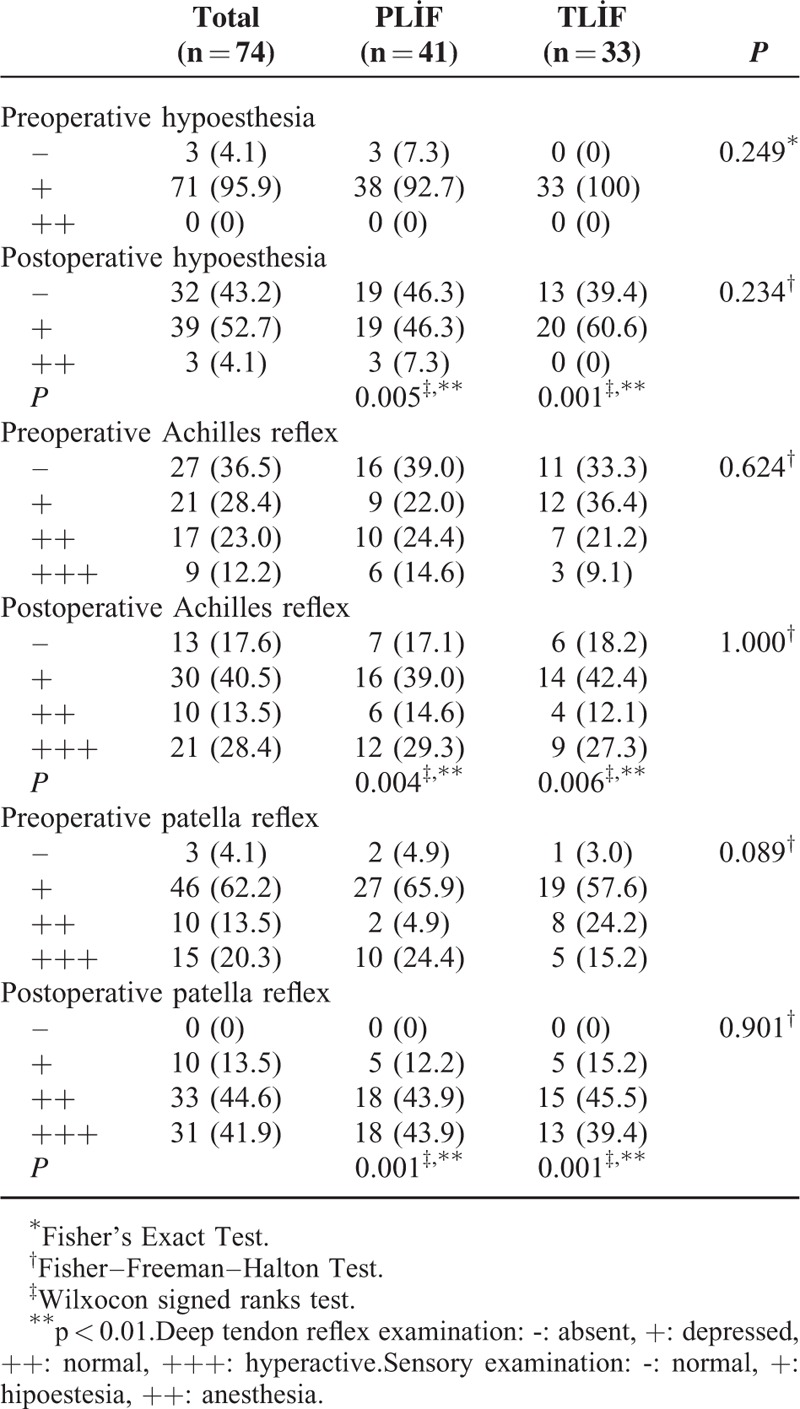
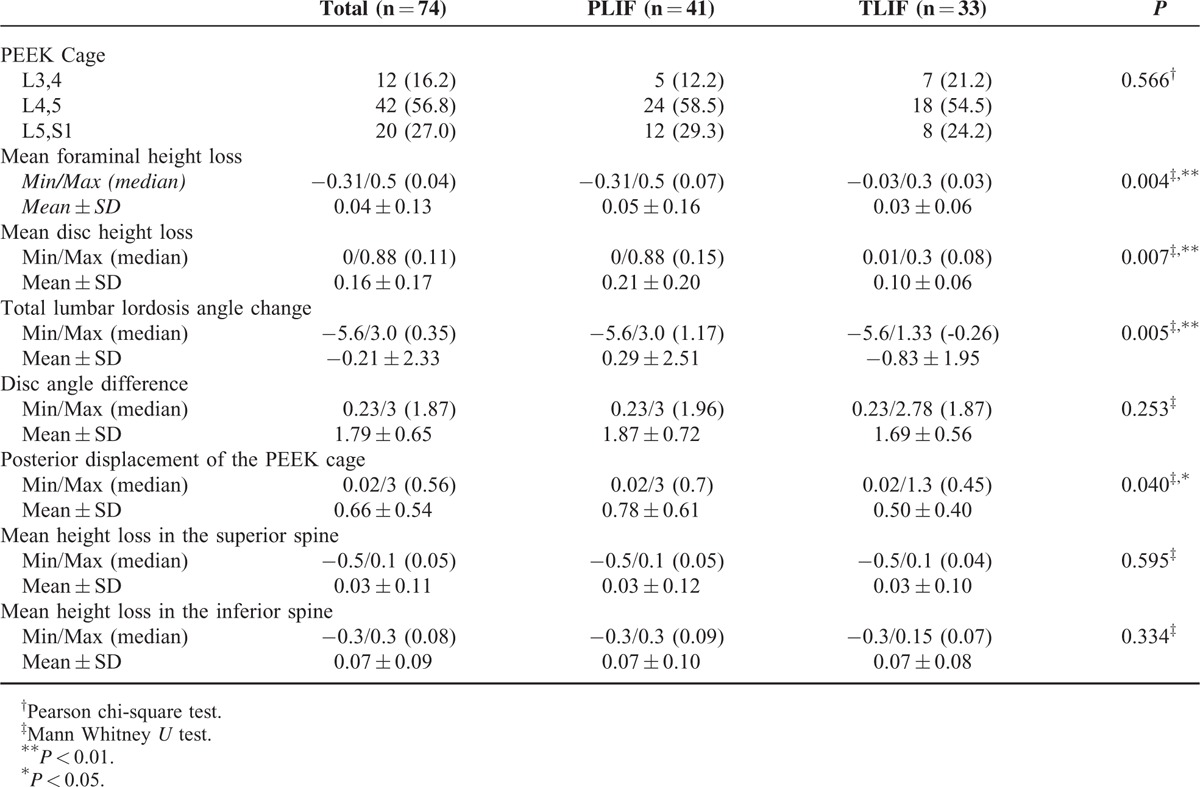
Mean foraminal height loss (P = 0.004), mean disc height loss (P = 0.007), total lumbar lordosis angle change (P = 0.005), and posterior displacement of cage (P = 0.040) levels were significantly higher in the PLIF group compared to the TLIF group (P < 0.01). There was no statistically significant difference between groups with regard to disc angle difference and mean height loss in the superior and inferior spines (P > 0.05). No significant difference was detected between groups with regard to the regions where it was placed (P > 0.05) (Table 3).
TABLE 3.
Assessment of Radiologic Measurements According to Groups
No major complications were observed in either of the groups. Dural injury was observed in two patients in the TLIF group and in five patients in the PLIF group, and the dural injuries were repaired with primary suturing. Fistula or meningitis was not observed in the postoperative period. Two patients in the TLIF group and one patient in the PLIF group were operated on again due to rot irritation of the transpedicular screw. Neurologic deficit did not develop in the postoperative period. Cage migration into the canal was observed in postoperative month one in two obese patients in the PLIF group. The cage was replaced during the new procedure. Simple cutaneous infection developed in five patients who all recovered with medical treatment.
Statistical Analysis
The Number Cruncher Statistical System 2007 (NCSS, Kaysville, UT) program was used for statistical analyses. The Student's t-test was used for intergroup comparison of normally distributed parameters in comparison of quantitative data, the Mann–Whitney U test was used for intergroup comparison of data not normally distributed beside descriptive statistical methods (mean, standard deviation, median, frequency, ratio, minimum, and maximum). The Pearson chi-square test, Fisher–Freeman–Halton test and Yates continuity correction test (Yates correction chi-square test) were used for comparison of qualitative data. The Friedman test was used in the in-group assessment of the variables which had three or more follow-ups, and the Wilcoxon Signed Rank Test was used for paired assessments. A P level of < 0.01 and 0.05 were accepted as statistically significant.
DISCUSSION
In this study, we evaluated 74 patents that underwent surgery due to degenerative disc, spinal stenosis, and lumbar spondylolisthesis. Two surgical techniques, PLIF and TLIF, were applied to the patients. Radiologic fusion values, ODI, VAS, and neurologic examination findings were compared at the end of one year. Nerve decompression, PLF, and simple decompression for symptomatic treatment are sufficient surgical methods; however, the addition of PLIF or TLIF to the surgical procedure provides a more durable fusion.2,4,6,16 Additionally, cages placed at the intervertebral disc preserve disc space, height, lumbar lordosis, and coronal and sagittal balance.3 In other studies, no difference was observed in flexion-extension, lateral bending, or axial rotation graphics obtained postoperatively at month 6 in patients who underwent a single-level PLIF or TLIF.17
The TLIF method is the modified form of the PLIF method defined by Clowart in 1940.12 It is not recommended to apply the TLIF and PLIF methods alone because they are applied without using the posterior lumbar fusion method which leads to cage migration and fusion problems.17 The PLIF and TLIF methods can easily be combined with the PLF system.12
The TLIF procedure reduces neuronal complication rates as it requires less dural retraction than the PLIF. Barns et al detected a 13.6% neurologic complication in the PLIF procedure. Nerve root injury was reported most frequently.12 In 2014, Goldstein et al.18 evaluated 1420 cases in 24 studies in systemic meta-analysis and found an overall complication rate of 19.4%. They found a dural injury rate of 5.4%, graft mal-position of 4.4%, screw mal-position of 2.6%, neurologic deficit and nerve injury of 3.8%, reoperation ratio of 3.3%, and reoperation for graft mal-position of 1.8% for PLIF and TLIF procedures. No major complications developed in the PLIF and TLIF procedures in our study. The overall complication rate was 23.9%, dural injury rate was 9.9%, graft mal-position rate was 2.82%, and the screw mal-position rate was 4.23%.
The primary goal of these surgical techniques is spinal fusion. Today, the best fusion is provided with 360° of stabilization. Increased surface area in the intervertebral disc space increases the likelihood of fusion. Increasing contact in the posterolateral region of end-plates also increases fusion.3,19 The TLIF is a method commonly used today. Hackenberg et al20 stated that accurate patient selection improved symptomatic achievement. This technique was reported to play an important role in the establishment of pelvis, lower extremity, and spinal biomechanical balance.2 The first paper in the literature about TLIF use was reported by Harms and Jeszenszky16 in 1998 and describes 191 cases of patients who had spondylolisthesis, recurrent disc surgery, and scoliosis. Lowe and Tahernia21 reported poor outcomes in two patients and good to excellent outcomes in the remaining patients. Audat et al2 reported that pain symptoms were relieved in 70% of 81 patients, and good outcomes were reported in 80% of the patients. Ames et al17 reported that anterior graft placement provided better stabilization compared to PLIF and they concluded that this resulted from bilateral facet and disc removal. Bilateral facet removal is not recommended.3 A statistically significant difference was not detected between the ODI rates of our patients, but a statistically significant difference was detected with regard to radiologic and VAS values.
In the literature, TLIF was reported to cause fewer complications than PLIF; provide a larger fusion area; preserve instability due to fewer injuries in the posterior spinous band; lead to fewer complications in contralateral surgeries because it is a unilateral procedure; lead to fewer paraspinal muscle injuries and epidural scar formation, fewer hemorrhages during the operation, and fewer dura and nerve injuries; and preserve lumbar lordosis better.2,12,22,23 Despite the absence of an acceptable methodology to compare these two surgical methods, posterior displacement of the cage, decrease in foraminal openings, and decreased height at disc level where the cage was placed were found to be significantly less in patients who underwent TLIF compared to patients who underwent PLIF. Also, the radiologic stabilization of TLIF may be interpreted better.
CONCLUSION
Although we did not detect a difference between the two surgical methods with regard to clinical improvement, TLIF is the preferred method due to radiologic advantages at one year follow-up, ease of application, and fewer complications. We believe that differences would be detected in clinical outcomes in long-term meta-analysis studies.
Supplementary Material
Footnotes
Abbreviations: CT = computed tomography, MPR = multiplanar reconstruction, ODI = Owestry Disability Index, PEEK = polyetheretherketone, PLF = posterolateral fusion, PLIF = posterior lumbar interbody fusion, TLIF = transforaminal lumbar interbody fusion, VAS = visual analog scale
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Yücesoy K, Özdemir N, Özer E, et al. Evaluation of 60 cases of surgically treated lumbar spinal stenosis. Nörobilim Dergisi 2003; 20:2. [Google Scholar]
- 2.Audat Z, Moutas O, Yousef K, et al. Comparison of clinical and radiological results of posterolateral fusion, posterior lumbar interbody fusion and tranforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singapore Med J 2012; 53:183–187. [PubMed] [Google Scholar]
- 3.Cole CD, McCall TD, Schmidt MH, et al. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2009; 2:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hee HT, Majd ME, Holt RT, et al. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J 2003; 12:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitecloud TS, 3rd, Roesch WW, Ricciardi JE. Transforaminal interbody fusion versus anterior–posterior interbody fusion of the lumbar spine: a financial analysis. J Spinal Disord 2001; 14:100–103. [DOI] [PubMed] [Google Scholar]
- 6.France JC, Yaszemski MJ, Lauerman WC, et al. A randomized prospective study of posterolateral lumbar fusion: outcomes with and without pedicle screw instrumentation. Spine 1999; 24:553–560. [DOI] [PubMed] [Google Scholar]
- 7.Möller H, Hedlund R. Surgery versus conservative management in adult isthmic spondylolisthesis—a prospective randomized study: part 1. Spine 2000; 25:1711–1715. [DOI] [PubMed] [Google Scholar]
- 8.Fritzell P, Hägg O, Wessberg P, et al. Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicentre randomized controlled trial from the Swedish Lumbar Spine Study group. Spine 2001; 26:2521–2534. [DOI] [PubMed] [Google Scholar]
- 9.Hallett A, Huntley JS, Gibson JN. Foraminal stenosis and single-level degenerative disc disease: a randomized controlled trial comparing decompression with decompression and instrumented fusion. Spine 2007; 32:1375–1380. [DOI] [PubMed] [Google Scholar]
- 10.Cloward RB. The treatment of ruptured lumbar intervertebral discs by ventral fusion: Indications, operative technique, after care. J Neurosurg 1953; 10:154–168. [DOI] [PubMed] [Google Scholar]
- 11.Lin PM. A technical modification of Cloward's posterior lumbar interbody fusion. Neurosurgery 1977; 1:118–124. [DOI] [PubMed] [Google Scholar]
- 12.Mura PP, Costaglioli M, Piredda M, et al. TLIF for symptomatic disc degeneration: a retrospective study of 100 patients. Eur Spine J 2011; 20:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakeb N, Ahsan K. Comparison of the early results of transforaminal lumbar interbody fusion and posterior lumbar interbody fusion in symptomatic lumbar instability. Indian J Orthop 2013; 47:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harms J, Jeszenszky D. The unilateral transforaminal approach for posterior lumbar interbody fusion. Orthop Traumatol 1998; 6:88–99. [Google Scholar]
- 15.Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion. Z Orthop Ihre Grenzgeb 1982; 120:343–347. [DOI] [PubMed] [Google Scholar]
- 16.Harms JG, Jeszenszky D. Die posteriore, lumbale, interkorporelle Fusion in unilateraler transforaminaler technik. Oper Orthop Traumatol (German) 1998; 10:90–102. [DOI] [PubMed] [Google Scholar]
- 17.Ames CP, Acosta FL, Jr, Chi J, et al. Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion performed at 1 and 2 levels. Spine 2005; 30:E562–E566. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein CL, Macwan K, Sundararajan K, et al. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res 2014; 472:1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mummaneni PV, Haid RW, Rodts GE. Lumbar interbody fusion: state-of-the-art technical advances. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves. J Neurosurg Spine 2004; 1:24–30. [DOI] [PubMed] [Google Scholar]
- 20.Hackenberg L, Halm H, Bullmann V, et al. Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine 2005; 14:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowe TG, Tahernia AD. Unilateral transforaminal posterior lumbar interbody fusion. Clin Orthop 2002; 394:64–72. [DOI] [PubMed] [Google Scholar]
- 22.Harms J, Rolinger H. A one-stage procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion. Z Orthop Ihre Grenzgeb (German) 1982; 120:343–347. [DOI] [PubMed] [Google Scholar]
- 23.Hey HW, Hee HT. Lumbar degenerative spinal deformity: surgical options of PLIF, TLIF and MI-TLIF. Indian J Orthop 2010; 44:159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


