Abstract
We evaluated seizure profile, Toll-like receptor (TLR)-4 polymorphisms, and serum matrix metalloproteinases (MMPs) in patients with calcified neurocysticercosis.
One-hundred nine patients with calcified neurocysticercosis with newly diagnosed seizures and 109 control subjects were enrolled. TLR-4 Asp299Gly and Thr399Ile polymorphisms and serum MMP-9 levels were evaluated. The patients were followed for 1 year.
Asp/Gly (P = 0.012) and Thr/Ile (P = 0.002), Gly (Asp/Gly plus Gly/Gly) (P = 0.008) and Ile (Thr/Ile plus Ile/Ile) (P = 0.003) genotypes were significantly associated with calcified neurocysticercosis compared with controls. Gly/Gly and Ile/Ile genotypes were not significantly associated (P = 0.529 for Gly/Gly, P = 0.798 for Ile/Ile) with either group. The levels of MMP-9 were higher in calcified neurocysticercosis (P = < 0.001). The levels of MMP-9 were higher in patients with multiple calcified neurocysticercosis compared with single calcified neurocysticercosis (P = < 0.001).
Headache (P = 0.031), status epilepticus (P = 0.029), Todd paralysis (P = 0.039), lesion size >10 mm (P = 0.001), and perilesional edema (P = < 0.001) were significantly associated with seizure recurrence. Heterozygous form Asp/Gly (P = < 0.001) and heterozygous form Thr/Ile (P = < 0.001) were significantly associated with seizure recurrence. The Gly (Asp/Gly plus Gly/Gly) (P = < 0.001) and Ile (Thr/Ile plus Ile/Ile) (P = < 0.001) genotypes were also significantly associated with seizure recurrence. Higher serum MMP-9 levels were significantly associated with seizure recurrence (P = < 0.001).
The TLR-4 gene abnormalities may trigger inflammation around calcified neurocysticercosis leading to an increase in perilesional edema and provocation of seizures.
INTRODUCTION
Neurocysticercosis—a central nervous system disease caused by a parasite Taenia solium—is a major cause of epilepsy in endemic regions. Seizures occur in nearly 80% of people with neurocysticercosis.1 In endemic regions, studies suggest that up to one-third of active epilepsy is due to neurocysticercosis. Calcified lesions usually represent an end stage of neurocysticercosis in its natural evolution in the brain parenchyma. Although calcified stage is considered an inactive stage, it is associated with seizures in a large population in neurocysticercosis endemic countries.2
Several factors contribute to epileptogenicity of calcified lesions. These include blood–brain barrier dysfunction, perilesional inflammation, perilesional gliosis, and remote hippocampal sclerosis.3–7 Calcified neurocysticercosis lesions have been associated with drug-resistant and surgically remediable epilepsy as well.8
What provokes an inactive calcified lesion to become epileptogenic is largely unanswered. It is widely presumed that perilesional inflammation around a calcification occurs spontaneously and is often associated with disruption of blood–brain barrier, making the lesion epileptogenic.3,9,10 The exact mechanism for blood–brain dysfunction causing development of seizures/epilepsy, lesional or nonlesional, is unknown. The proposed hypothesis is that inflammation around a calcified neurocysticercosis occurs due to host response against the parasite antigen leading to blood–brain barrier dysfunction and increased vascular permeability, along with neuronal dysfunction, culminating in increased cortical excitability and seizure. An animal model, addressing the issue of generation of an epileptic focus, also suggests that blood–brain barrier dysfunction with leakage of antigenic components into the brain can cause cortical reorganization and induce seizures.10
Possibly, a genetic factor may be responsible for initiating inflammation around calcified lesions and subsequent seizures. Innate immune system consists of Toll-like receptors (TLRs) out of which TLR-4, in particular, plays a distinct role in central nervous system immune response against neurocysticercosis.11 The exact functional significance of TLRs and their polymorphisms in central nervous system infection are largely not defined. It had been shown in several studies that TLR polymorphisms evoke T-helper cell type 1 of immune response, which is responsible for inducing pro-inflammatory cytokines, leading to the development of symptomatic neurocysticercosis. A study, in recent past, demonstrated that TLR-4 Asp299Gly and Thr399Ile gene polymorphisms were significantly associated with the occurrence of neurocysticercosis and progression to symptomatic neurocysticercosis, compared with control subjects or those with asymptomatic neurocysticercosis.12 Another study indicated that higher levels of TLR-4 expression in symptomatic patients with neurocysticercosis correlated with enhanced pro-inflammatory cytokine production.13
Matrix metalloproteinases (MMPs) are Zn2+ -dependent endopeptidases and they can degrade various components of blood–brain barrier and other important central nervous system matrix proteins.14 In patients with neurocysticercosis, MMPs have been shown to play a role in the pathogenesis. In the central nervous system, during the phase of inflammation, MMP-9 causes degradation of extracellular matrix proteins due to their proteolytic activity, leading to blood–brain barrier disruption and cellular infiltration. This leads to enhanced inflammation and blood–brain barrier permeability in many central nervous system diseases including neurocysticercosis.15 Probably, enhanced inflammation and increased blood–brain barrier permeability are responsible for symptomatic neurocysticercosis. Level of MMP-9 expression closely represents the degree of blood–brain barrier breakdown in various stages of neurocysticercosis. Gupta et al4 observed that this correlation between MMP-9 expression and degree of blood–brain barrier disruption was highest in the colloidal stage and lowest in the calcified stage.
In the present study, we selectively included newly recognized patients of seizures and calcified neurocysticercosis, and evaluated the role of TLR-4 gene polymorphisms (Asp299Gly and Thr399Ile) and serum MMP-9 levels in seizure provocation.
METHODS
This was a case-control study with prospective follow up. Patients included were of single and multiple calcified neurocysticercosis. The study was carried out in a tertiary care hospital in north-central part of India. The study was started after taking ethical approval from the Institutional Ethics Committee. A written, informed, and valid consent was taken from each subject participating in the study before their inclusion. If the patient was a minor, then the consent was taken from the parents. The study was conducted over a period extending from August 2013 to December 2015.
Inclusion Criteria
Neurocysticercosis was diagnosed on the basis of presence of punctate round calcified parenchymal lesions in patients, belonging to endemic zone, presenting with seizures (a symptom consistent with neurocysticercosis).5,16 Only those patients who presented with newly diagnosed seizures were enrolled (Figure 1).
FIGURE 1.
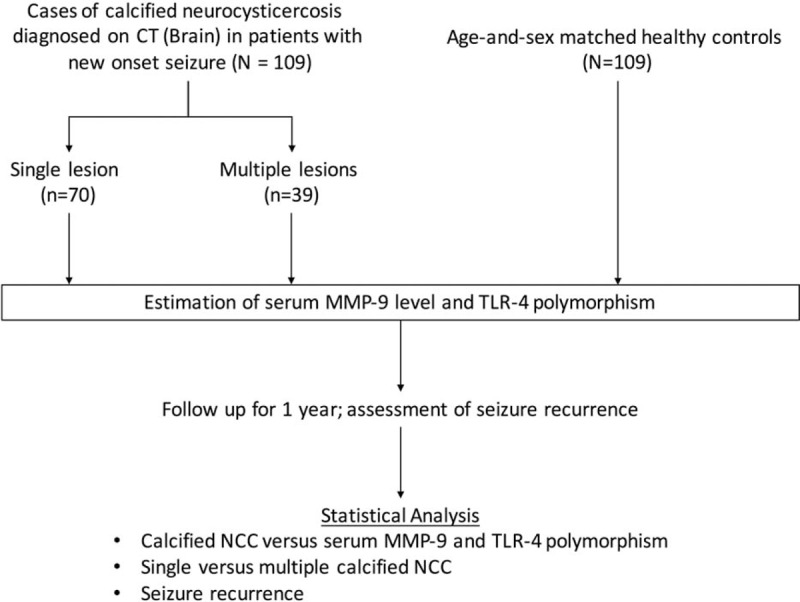
Flow diagram of the study. MMP = matrix metalloproteinase, NCC = neurocysticercosis, TLR = Toll-like receptor.
Exclusion Criteria
Patients with different stages of neurocysticercosis were excluded from the study.
Controls
A total of 109 age and sex-matched healthy controls were also enrolled for the purpose of genetic assessment.
Patient Evaluation
All enrolments were subjected to a detailed neurological evaluation. A battery of laboratory tests, like complete hemogram, fasting blood sugar, erythrocyte sedimentation rate, liver function tests, renal function tests, and enzyme-linked immunosorbent assay for human immunodeficiency virus, was performed in all enrolled subjects. Serological evaluation for neurocysticercosis was not performed.
Electroencephalography was done initially as a part of seizure evaluation using 21-channel machine with 10 to 20 system of electrode placement. Electroencephalograms were classified either as normal or abnormal. Electroencephalography was considered abnormal if it showed any epileptiform activity, like spikes, spike wave, sharp waves, sharp-slow wave, and polyspike waves.
All the patients with seizures were subjected to a contrast-enhanced computed tomography within 7 days of last seizure. The site and size of the calcified neurocysticercosis along with perilesional edema were noted. Perilesional edema was labeled on computed tomography if calcified lesion was surrounded by distinct perilesional hypodensity.
Treatment
All enrolled patients were given antiepileptic drugs. Oxcarbazepine was used as the first-line antiepileptic medication in a dose of 10 to 15 mg/kg/day given in 2 divided doses. Clobazam was added if either the first-line drug was not tolerated or if the seizure control was not achieved. None of the patients received either albendazole or corticosteroids.
Genetic Evaluation
For genetic analysis, peripheral blood of patients and controls was collected in ethylenediaminetetraacetic acid vials. Deoxyribonucleic acid (DNA) was extracted using salting-out method and then purified. Polymerase chain reaction-sequencing by chain termination (polymerase chain reaction-sequencing) was used to identify TLR-4 genotype. A set of primer sequences for the TLR-4 gene polymorphisms (Asp299Gly and Thr399Ile) was designed: forward 5′-AGTCCATCGTTTGGTTCTGG-3′ and reverse 5′-TCAAATTGGAATGCTGGAAA-3′ (Integrated DNA Technology). Polymerase chain reaction amplifications were done in all enrolled subjects and controls in 25-μL volume that contained 10× assay buffer; 200 μM each of dATP, dCTP, dGTP, dTTP; 0.1 μM of each primer; and 1.0 U of Taq DNA polymerase (Finzymes, Thermo Scientific). Polymerase chain reaction conditions were as follows: an initial denaturation was done at 94°C temperature for 10 minutes that was followed by 35 cycles of denaturing at 94°C temperature for 30 seconds, annealing was done at 54.4°C temperature for 30 seconds, and an extension at 72°C temperature for 40 seconds, and thereafter final extension at 72°C temperature for 7 minutes followed by cooling to 4°C. Template-free water was used as a negative control. After amplification of the genetic material, the products were then subjected to exo-sap purification; finally, 1 to 2 μL of purified polymerase chain reaction products were subjected to sequencing polymerase chain reaction by BigDye Terminator cycle sequencing kit v3.1 and a single primer. The initially amplified sequencing polymerase chain reaction genetic products were again purified by ethanol, ethylenediaminetetraacetic acid, and sodium acetate precipitation. All the above experiments were also done with the second primer.
Serum Enzyme-linked Immunosorbent Assay for MMP-9
The serum MMP-9 concentration was estimated by using commercial enzyme-linked immunosorbent assay kits (Abcam, Cambridge, UK).
Follow-up
Enrolled subjects were followed for any seizure recurrence. All data were recorded at baseline, 3 months, 6 months, and 1 year of follow-up, along with visits resulting from breakthrough seizures. Seizure recurrence was defined as recurrence of a seizure episode at least 1 week after the initiation of the antiepileptic drugs. Status epilepticus in patients was defined as a continuous seizure or repetitive seizures without regaining consciousness in 30-minute period. Serial seizures were defined as multiple seizures occurring within 24 hours.
Statistical Analysis
Statistical analysis was done using SPSS version 16.0 (Chicago, LA). Qualitative variables were expressed as percentages. Qualitative variables were compared using chi-square/Fisher exact test as applicable. Normality distribution of continuous data was tested using Shapiro–Wilk test. Continuous data, which were not normally distributed, were analyzed using Mann–Whitney U test. Correlation between number of lesion and serum MMP-9 level was done using Spearman rho. Logistic regression model was used to test the association between genotypes and occurrence of calcified granulomas. Case-control status was taken as a dependent variable, whereas genotype, age, and sex were taken as independent variables. Age and sex-adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated using logistic regression. Allele and haplotype frequencies were compared using SNPstats.17
The sample size was calculated using Quanto version 1.2.4 (web-based open source sample size calculator for genetic studies).18 Power of the study was assumed to be 80 and the significance level was taken as 0.05 (2-sided). Matched case-control model was selected for calculation of the sample size. Based upon a previous study done in our region, minor allele frequency in controls was taken as 0.80.12,19 OR was taken as 2.5, and a recessive model was selected. As per these calculations, a minimum sample size of 101 in each arm was calculated.
RESULTS
A total of 198 consecutive patients presenting with newly diagnosed seizures were screened for the presence of calcified neurocysticercosis. Eighty-nine patients were not included because either they had single solitary cystic granuloma or they had multiple neurocysticercosis in varying stages. One-hundred nine patients were finally enrolled as per the study protocol. Seventy patients had single calcified neurocysticercosis and 39 patients had multiple calcified neurocysticercosis (Figure 2).
FIGURE 2.
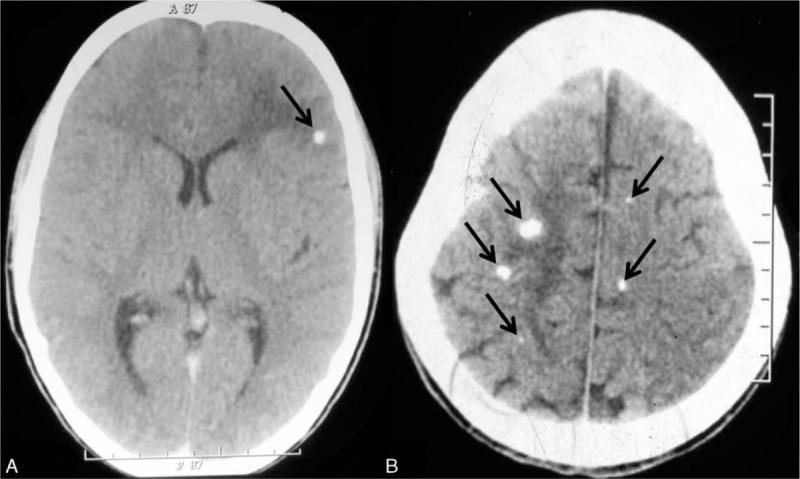
Noncontrast computed tomography of the brain showing single calcified neurocysticercosis with perilesional edema (A, arrow) and multiple calcified neurocysticercosis with perilesional edema (B, arrows).
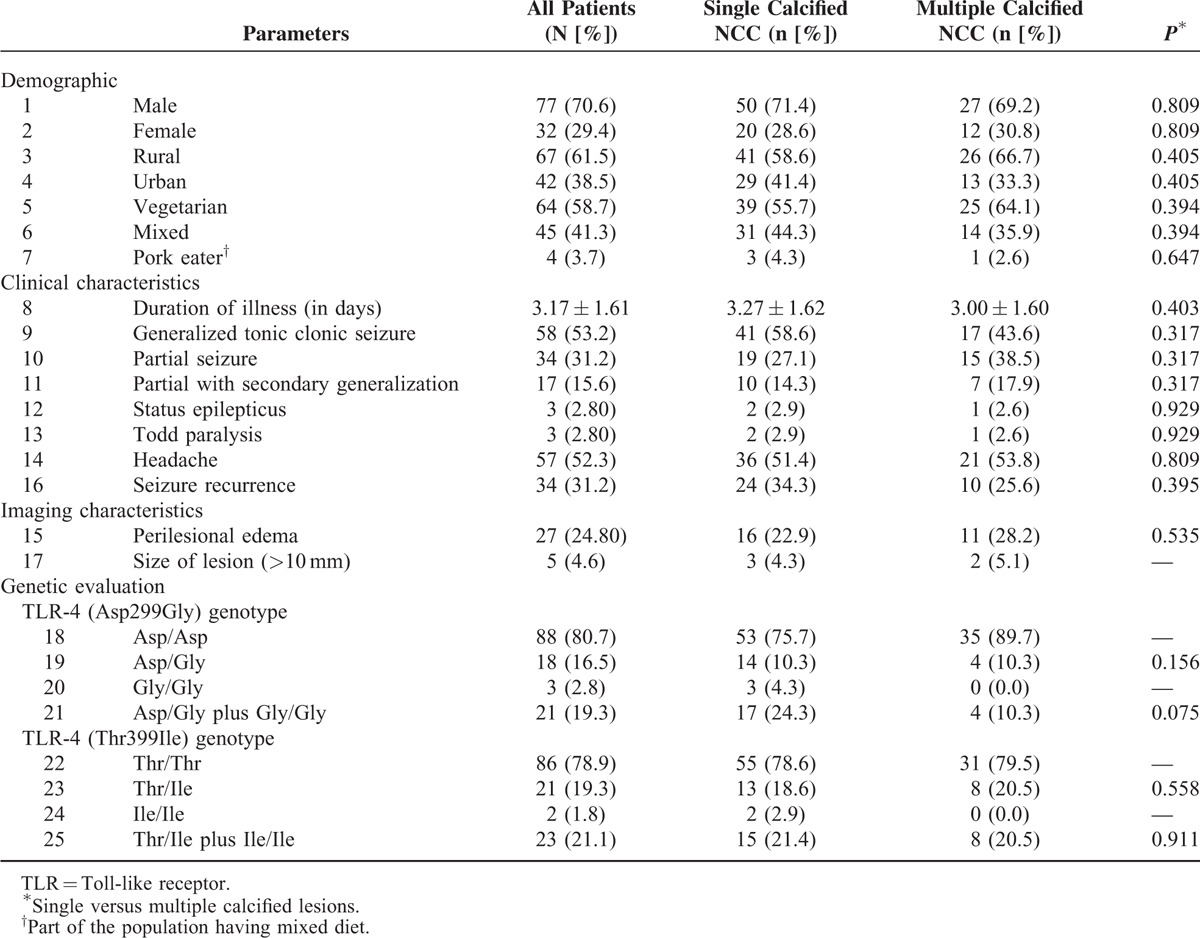
Mean age of patients with calcified neurocysticercosis was 19.44 ± 10.50. Most common seizure type seen in these patients was generalized tonic-clonic seizure (53.2%), followed by partial seizure in 31.2% cases. The baseline clinical and neuroimaging characteristics of enrolled patients have been provided in Table 1.
TABLE 1.
Baseline Characteristics of Patients With Calcified Neurocysticercosis (NCC)
TLR-4 Polymorphism
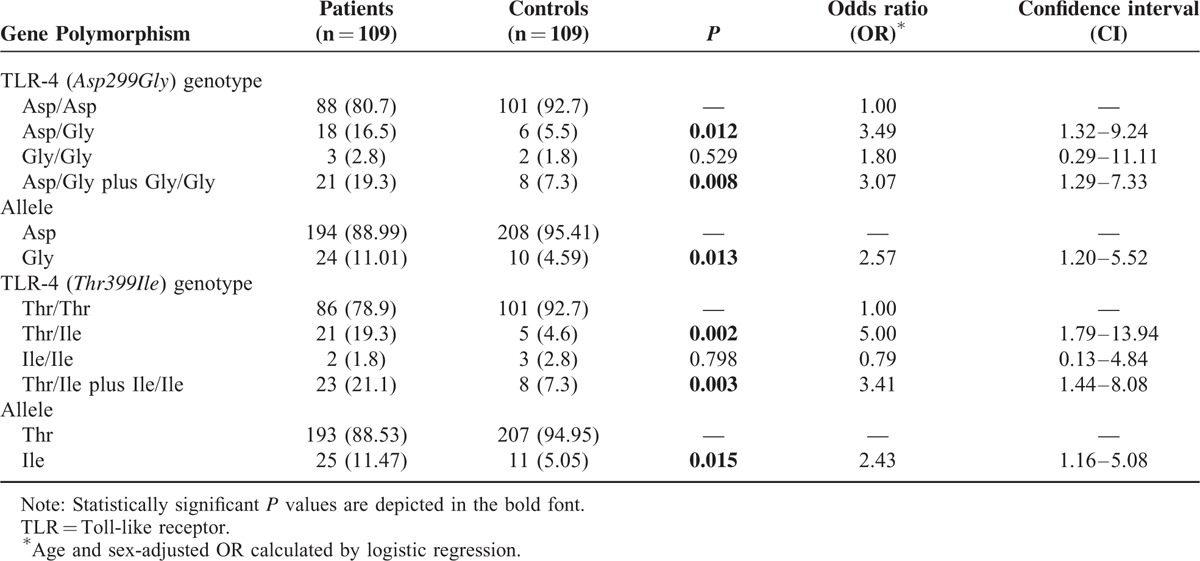
We observed that Asp/Gly and Thr/Ile genotypes were more frequent in cases with calcified neurocysticercosis as compared with controls (P = 0.012, OR 3.49, 95% CI 1.32–9.24; and P = 0.002, OR 5.00, 95% CI 1.79–13.94, respectively).
The Gly (Asp/Gly plus Gly/Gly) and Ile (Thr/Ile plus Ile/Ile) genotypes were also significantly associated with patients having calcified neurocysticercosis. The Gly/Gly and Ile/Ile genotypes, however, were not found to be associated with calcified neurocysticercosis (Table 2). Significantly higher prevalence of the Gly (11.01% vs 4.59%; P = 0.013) and the Ile alleles (11.47% vs 5.05%; P = 0.015) was found in patients with calcified neurocysticercosis compared with the control group. (Table 2)
TABLE 2.
Distribution of TLR-4 Asp299Gly and TLR-4 Thr399Ile Polymorphisms in Patients With Calcified Neurocysticercosis and Controls
An analysis for the additive effect of both of the polymorphisms and haplotype analysis was performed. The frequency of haplotypes containing Gly/Thr was 0.7 in the control group and 8.1 in the neurocysticercosis group. We found a positive association of haplotypes containing Gly/Thr with calcified neurocysticercosis (P = 0.011, OR 9.77, 95% CI 1.72–55.55).
Serum MMP-9
The levels of MMP-9 were significantly higher in cases (median = 6596.469) as compared with healthy controls (median = 5974.536) (P < 0.001; Mann–Whitney U = 754.00).
Single Versus Multiple Calcified Neurocysticercosis
Assessment of TLR-4 genotypes and haplotypes did not reveal any statistically significant difference between the single and multiple calcified lesions group. A significant positive correlation was found between the number of lesions and MMP-9 levels (rho = 0.586, P < 0.001).
Seizure Recurrence
A total of 34 patients developed a recurrence of seizure during follow-up (Table 3). Among the clinical characteristics, headache (P = 0.031), status epilepticus (P = 0.029), and Todd paralysis (P = 0.029) were significantly associated with seizure recurrence. Similarly, lesion size of greater than 10 mm (P = 0.001) and perilesional edema (P < 0.001) were significantly associated with seizure recurrence.
TABLE 3.
Characteristics of Patients With Calcified Neurocysticercosis Having Seizure Recurrence
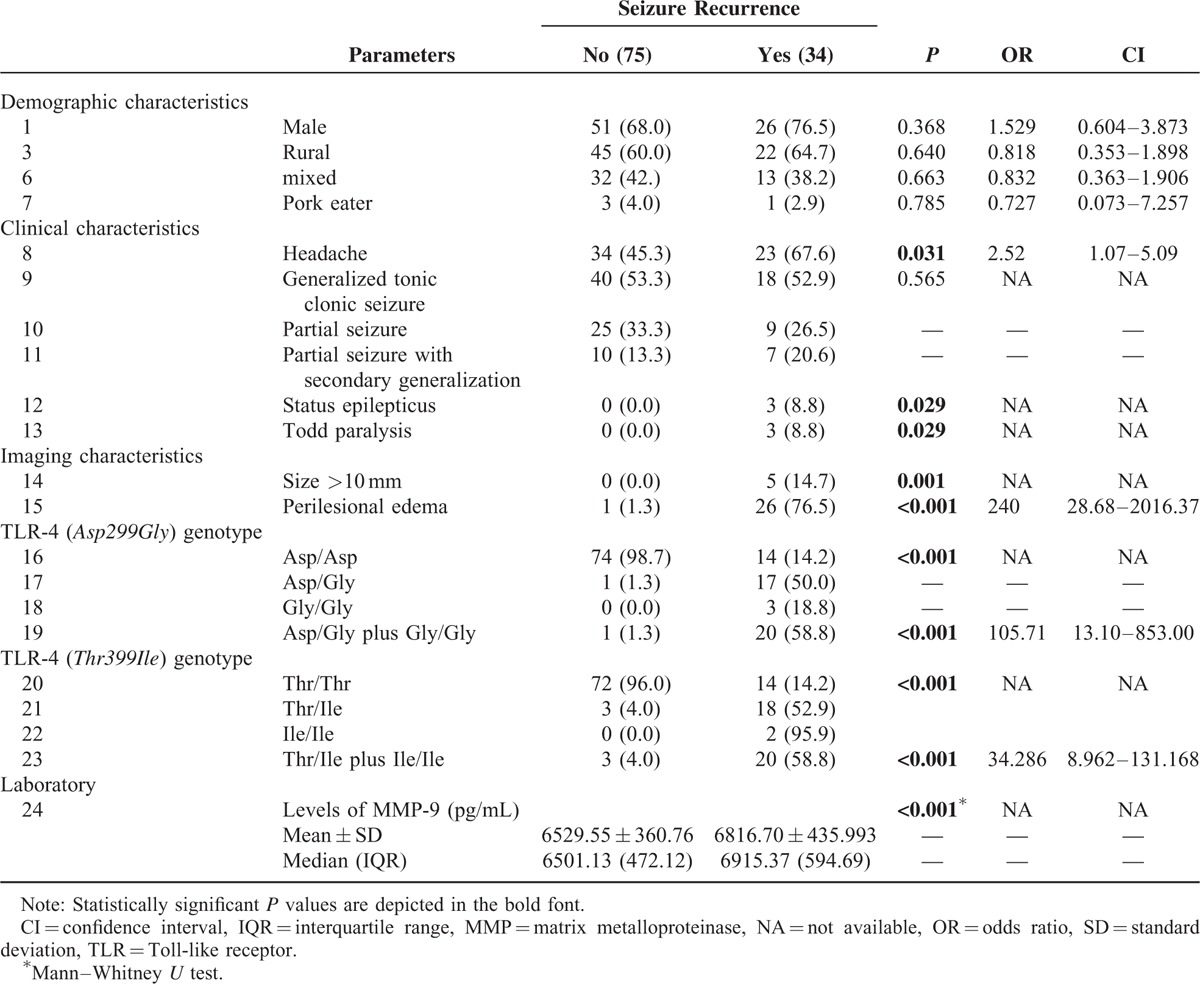
We observed that heterozygous and mutant forms of TLR-4 (Asp299Gly) genotype were significantly associated with seizure recurrence (P < 0.001, OR 105.71, 95% CI 13.10–853.00); also heterozygous and mutant forms of TLR-4 (Thr399Ile) genotype were also significantly associated with seizure recurrence (P < 0.001, OR 34.286, 95% CI 8.962–131.168).
Higher serum MMP-9 levels were found to be associated with seizure recurrence (P < 0.001) (Table 3).
DISCUSSION
Calcified neurocysticercosis represents the healed end stage of natural evolution of cysticercus cellulose (a larval form of T solium) in the brain parenchyma and is considered to have a causal relationship with epilepsy. However, in a proportion of population, in endemic countries, it may remain asymptomatic and often such lesions are discovered accidentally. It is likely that some genetic or environmental factors are responsible for provoking seizures.
We observed that TLR-4 gene polymorphisms (Asp299Gly and Thr399Ile) were significantly associated with calcified neurocysticercosis and seizures. Earlier, Verma et al12 observed similar findings in 140 patients with neurocysticercosis (82 symptomatic with active epilepsy and 58 asymptomatic). They observed that TLR-4 Asp299Gly and Thr399Ile polymorphisms were associated with the development of symptomatic neurocysticercosis. According to Verma et al, genetic variations in the TLR-4 gene modulated the inflammatory response in the human brain against neurocysticercosis, leading to occurrence of seizures, the most frequent symptom in these patients.13 In contrast to our subjects, the population enrolled in both these studies was heterogeneous and included patients representing all the stages of neurocysticercosis.
Perilesional edema in calcified neurocysticercosis is considered to be an indicator of active inflammation around the lesion.20 The exact pathophysiology of perilesional edema in patients with calcified neurocysticercosis is largely unknown. It is presumed that there is an episodic host inflammatory reaction against neurocysticercal antigen that is intermittently released. 3,6,7 Possibly, this inflammatory reaction is genetically mediated. In our study, seizure recurrences were significantly associated with perilesional edema. Our findings suggest that TLR-4 gene abnormalities might be responsible for activating inflammation around calcified lesions making it subsequently symptomatic.
Another important observation that we made was that serum MMP-9 levels were significantly elevated in patients with calcified neurocysticercosis. Additionally, serum MMP-9 levels were significantly associated with seizure recurrence. Verma et al15 noted almost similar observations and found that that serum MMP-9 activity was significantly associated with symptomatic neurocysticercosis (different stages) compared with healthy controls and asymptomatic neurocysticercosis (different stages). Our study extends the results of the study in estimating the levels in a refined set of population. Gupta et al demonstrated that perilesional inflammation can be quantified using dynamic contrast-enhanced magnetic resonance imaging in calcified neurocysticercosis and may help distinguishing between symptomatic and asymptomatic subjects. Dynamic contrast-enhanced magnetic resonance imaging indices may be used as a noninvasive image biomarker of blood–brain barrier breakdown in different stages of neurocysticercosis. It was further noted that MMP-9 levels may serve as a biomarker of blood–brain barrier destruction.4,21 A significant increase in the MMP-9 (R279Q) gene polymorphism has been observed in symptomatic subjects with calcified neurocysticercosis when compared with asymptomatic patients and control subjects.3
There were certain limitations in our study. Because our study was outpatient department based, we did not include asymptomatic patients with calcified neurocysticercosis as controls, which would have helped us to conclude whether genetic abnormities predispose calcified neurocysticercosis or symptoms (seizure) in calcified neurocysticercosis. Estimation of cysticercal antibody could have been done for the definitive diagnosis of neurocysticercosis.
We conclude that TLR-4 gene abnormalities may trigger inflammation around calcified neurocysticercosis leading to production of perilesional edema, and provoke seizures.
Footnotes
Abbreviations: DNA = deoxyribonucleic acid, MMP = matrix metalloproteinase, TLR = Toll-like receptor
GL, RKG, and AJ contributed equally.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Carabin H, Ndimubanzi PC, Budke CM, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis 2011; 5:e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh G, Sachdev MS, Tirath A, et al. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia 2000; 41:718–726. [DOI] [PubMed] [Google Scholar]
- 3.Nash TE, Mahanty S, Loeb JA, et al. Neurocysticercosis: a natural human model of epileptogenesis. Epilepsia 2015; 56:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta RK, Awasthi R, Rathore RK, et al. Understanding epileptogenesis in calcified neurocysticercosis with perfusion MRI. Neurology 2012; 78:618–625. [DOI] [PubMed] [Google Scholar]
- 5.Del Brutto OH, Salgado P, Lama J, et al. Calcified neurocysticercosis associates with hippocampal atrophy: a population-based study. Am J Trop Med Hyg 2015; 92:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash TE, Del Brutto OH, Butman JA, et al. Calcific neurocysticercosis and epileptogenesis. Neurology 2004; 62:1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nash TE, Pretell EJ, Lescano AG, et al. Cysticercosis Working Group in Peru. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol 2008; 7:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal M, Chand P, Modi M, et al. neurocysticercosis: an uncommon cause of drug-refractory epilepsy in North Indian population. Epilepsia 2015; 56:1747–1752. [DOI] [PubMed] [Google Scholar]
- 9.Nash TE, Patronas NJ. Edema associated with calcified lesions in neurocysticercosis. Neurology 1999; 53:777–781. [DOI] [PubMed] [Google Scholar]
- 10.Seiffert E, Dreier JP, Ivens S, et al. Lasting blood–brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci 2004; 24:7829–7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra BB, Mishra PK, Teale JM. Expression and distribution of Toll Like receptors in the brain during murine neurocysticercosis. J Neuroimmunol 2006; 181:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma A, Prasad KN, Gupta RK, et al. Toll-like receptor 4 polymorphism and its association with symptomatic neurocysticercosis. J Infect Dis 2010; 202:1219–1225. [DOI] [PubMed] [Google Scholar]
- 13.Verma A, Prasad KN, Cheekatla SS, et al. Immune response in symptomatic and asymptomatic neurocysticercosis. Med Microbiol Immunol 2011; 200:255–261. [DOI] [PubMed] [Google Scholar]
- 14.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011; 41:271–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma A, Prasad KN, Nyati KK, et al. Association of MMP-2 and MMP-9 with clinical outcome of neurocysticercosis. Parasitology 2011; 138:1423–1428. [DOI] [PubMed] [Google Scholar]
- 16.Del Brutto OR, Rajshekhar V, White AC, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology 2001; 57:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solé X, Guinó E, Valls J, et al. SNPStats: a web tool for the analysis of association studies. Bioinformatics 2006; 22:1928–1929. [DOI] [PubMed] [Google Scholar]
- 18.Gauderman WJ. Sample size requirements for association studies of gene-gene interaction. Am J Epidemiol 2002; 155:478–484. [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Garg RK, Jain A, et al. Toll like receptor-4 gene polymorphisms in patients with solitary cysticercus granuloma. J Neurol Sci 2015; 355:180–185. [DOI] [PubMed] [Google Scholar]
- 20.Fujita M, Mahanty S, Zoghbi SS, et al. PET reveals inflammation around calcified Taenia solium granulomas with perilesional edema. PLoS One 2013; 8:e74052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta RK, Awasthi R, Garg RK, et al. T1-weighted dynamic contrast-enhanced MR evaluation of different stages of neurocysticercosis and its relationship with serum MMP-9 expression. AJNR Am J Neuroradiol 2013; 34:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]


