Supplemental Digital Content is available in the text
Abstract
More than half of all known proteins, and almost all membrane and extra-cellular proteins have oligosaccharide structures or glycans attached to them. Defects in glycosylation pathways are directly involved in at least 30 severe human diseases.
A multiple center cross-sectional study (China, Croatia, and Scotland) was carried out to investigate the possible association between hypertension and IgG glycosylation. A hydrophilic interaction chromatography of fluorescently labeled glycans was used to analyze N-glycans attached to IgG in plasma samples from a total of 4757 individuals of Chinese Han, Croatian, and Scottish ethnicity.
Five glycans (IgG with digalactosylated glycans) significantly differed in participants with prehypertension or hypertension compared to those with normal blood pressure, while additional 17 glycan traits were only significantly differed in participants with hypertension compared to those of normal blood pressure. These glycans were also significant correlated with systolic blood pressure (SBP) or diastolic blood pressure (DBP).
The present study demonstrated for the 1st time an association between hypertension and IgG glycome composition. These findings suggest that the individual variation in N-glycosylation of IgG contributes to pathogenesis of hypertension, presumably via its effect on pro- and/or anti-inflammatory pathways.
INTRODUCTION
In year 2000, 26.4% of the global adult population were diagnosed with hypertension (26.6% of men and 26.1% of women), and 29.2% are estimated to have this condition by 2025 (29.0% of men and 29.5% of women), resulting in 972 million of affected adults in 2000 and estimation of 1.56 billion affected by 2025.1 Hypertension ranks the 1st in chronic medical problems in those who visited primary health care providers in USA. According to The American Heart Association, treatment of hypertension was estimated to cost both directly and indirectly $76.6 billion in 2010.2
Incidence of hypertension rises with ageing, and it is reported that 90% of middle-aged, and older adults are likely to develop elevated blood pressure over their remaining lifetime.3 Hypertension is a complex disease that is influenced by both heritable and environmental factors. Genome wide association studies have identified numerous common genetic variants with small effects, as well as some rare genetic variants with large effects on blood pressure,4 but the investigation of the molecular genetics of hypertension still remains in its infancy.5 The examples of environmental factors influencing blood pressure include: lifestyle factors such as dietary salt intake,6,7 consumption of fruits and low fat products, exercise,8 weight loss,9 alcohol intake,10 caffeine consumption,11 and vitamin D deficiency;12 early life events, such as low birth weight, maternal smoking, and lack of breast feeding;13 and stress factor, such as physical and psychological stress.14
Carbohydrates are important in many biological processes including those underlying disease, and full significance of their distribution and function is the focus of cutting edge research. Glycosylation, which includes the addition of sugar chains to proteins and lipids, is in fact the most common and complex posttranslational modification.15 More than half of all known proteins, and almost all membrane and extra-cellular proteins have oligosaccharide structures or glycans attached to them.16 Defects in glycosylation pathways are directly involved in at least 30 severe human diseases, and complete absence of N-glycosylation is embryonically lethal.17 Glycosylation pathways are altered in numerous diseases, giving rise to potential disease markers and a better understanding of disease pathogenesis. In autoimmune diseases, the glycosylation of serum antibodies changes and in rheumatoid arthritis, for example, has been correlated with disease progression and severity.18 Glycans were also shown to have multiple roles in the initiation and progression of coronary artery disease, stroke, and peripheral vascular disease.19 Glycans are thus a key group of intermediate phenotypes critical to many cell functions and therefore disease processes.
Immunoglobulin (Ig) is a cluster of proteins with antibody activity (including IgA, IgD, IgE, immunoglobulin G [IgG], IgM), accounting for approximately 20% of plasma proteins. Among immunoglobulins, IgG is the most abundant (more than 80%), the major antibody in the secondary humoral response of immunity.20 IgG exists in the form of a glycoprotein, with average 2.8 N-linked sugar chains per molecule of IgG, 2 of which are linked to the conservative region of fragment crystallizable region (Fc) Asn297,18 and others are linked to the nonconservative glycosylation sites of fragment antigen-binding (Fab) and the hinge region.21,22 Level and structure of IgG glycosylation are associated with the incidence and development of many inflammatory diseases, such as systemic lupus erythematosus, glomerulonephritis, inflammatory bowel disease, Alzheimer disease, and progressive mild cognitive impairment.18,23–27 Kaneshige (1987)28 investigated IgG glycosylation of 35 patients with type II diabetes and 14 cases of age-matched healthy controls and found that IgG glycosylation level is elevated in patients with diabetes. Pucić et al (2011)29 carried out the 1st population study of IgG glycome, showing that there is a very high variability in the composition of the IgG glycome. In a large-scale association study, Nikolac Perkovic et al30 reported the association of body mass index (BMI) and changes in IgG galactosylation, suggesting the relation of IgG galactosylation with chronic inflammation that accompanies the development of obesity.
Since hypertension is a worldwide public health problem which is identified as the leading cause of cardiovascular mortality by the World Health Organization, the present study aimed to determine to what extent the IgG N-glycome correlates with the occurrence of prehypertension and hypertension. Recently, we reported associations between plasma glycans and blood pressures in both Chinese and Croatian populations.31 In this study, we focused on IgG glycans to eliminate the effects of varying concentrations of plasma proteins, which might complicate the interpretation of the changes observed in the total plasma glycome.
MATERIALS AND METHODS
Study Design
In order to determine to whether and what extent the IgG N-glycome correlates with the occurrence of prehypertension and hypertension, we carried out a population-based cross-sectional study in Chinese, Croatian, and Scottish.
Participants and Settings
Chinese Han participants were recruited from a community-based cohort in Xicheng District, Beijing, from January to April of 2012. All of the participants had to meet the following inclusion criteria: over 18 years of age, no history of somatic and psychiatric abnormalities as registered in their medical records, and no history of taking any medicine in the past 2 weeks. Individuals with a diagnosis of specific severe diseases concerning the cardiovascular system, respiratory system, genitourinary system, digestive system, and hematic system were excluded.
Examinees from the Croatia and Scotland were recruited within a large genetic epidemiology program32–34 on 2 Croatian Adriatic islands Korčula and Vis, and the Northern Scottish Orkney Islands.35 Sampling of the subjects from Croatia was based on the information from the voting register of Croatia, and adult inhabitants were included in the program.
Volunteers from the Northern Scottish Orkney Islands were recruited within the Orkney Complex Disease Study.35 The sample recruiting protocols were described in details in the previous publications.32–35
In total, 4757 participants, including 913 Chinese Han from Beijing, 985 Croatian Korčula and 896 Croatian Vis, and 1963 Scottish Orkney were covered in the study.
The studies were approved by the local Ethics Committees in China, Croatia, and Scotland, respectively. Written informed consent was obtained from all participants. All studies have been executed with the full cooperation of examinees, adequate understanding, and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Variables
After participants filled in the questionnaire regarding their demographic characteristics (gender and age), the physical examinations and interview were carried out by trained nurses and physicians. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice on the right arm by trained nurses using a standard mercury sphygmomanometer with the subjects resting at least 5 minutes in a sitting position. Hypertension and prehypertension were defined according to the recommendations of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.36 The classification of BP for adults aged 18 years or older is as follows: normal, SDP lower than 120 mm Hg, DBP lower than 80 mm Hg; prehypertension, SDP 120 to 139 mm Hg, DBP 80 to 89 mm Hg; stage 1 of hypertension, SDP 140 to 159 mm Hg, DBP 90 to 99 mm Hg; and stage 2 of hypertension, SDP 160 mm Hg or greater, DBP 100 mm Hg or greater. In this study, we combined the stage 1 and stage 2 of hypertension in the final analysis.
Fasting blood samples were collected in the morning after an overnight fasting by venipuncture for laboratory measurements and glycomic analysis. The samples were processed immediately and blood plasma was separated from whole blood to be stored at −80 °C until further analysis.
IgG Isolation and Glycans Analysis
Glycan release and labeling of IgG of Chinese and Orkney samples were done by reference to the “in solution” method as described by Ruhaak et al.37 In short, IgG was denatured by incubation with 20 μL of 2% SDS at 60 °C for 10 minutes. Subsequently, 10 μL 4% NP-40 and 1.25 mU of N-glycosidase F (ProZvme) in 10 μL 5 × PBS were added to the samples and incubated overnight at 37 °C. Glycans were labeled with 2-aminobenzamide using 2-picoline-borane as reductant. Labeled glycans were purified with hydrophilic interaction liquid chromatography solid phase extraction using microcrystalline cellulose.38
Glycan release and labeling of Vis and Korčula samples were performed by the “in gel” method as reported by Royle et al.39 Briefly, IgG was immobilized in a block of sodium dodecyl sulfate-polyacrylamide gel in a 96-well microtiter plate, and N-glycans were released by digestion with recombinant N-glycosidase F (ProZyme, San Leandro, CA). Released glycans were labeled with 2-aminobenzamide and purified using solid-phase extraction with Whatman 3MM chromatography paper.
IgG N-glycans were separated by hydrophilic interaction liquid chromatography on a Waters Acquity UPLC instrument (Walters Corporation, Milford, MA) into 24 peaks.40
Statistical Analysis
Based on our previously publication, in which plasma N-Glycan G0 is associated with SBP in Chinese Han and Croatian populations (r = 0.194 and 0.314, respectively),31 the sample size of each group was estimated to be 538 using StatsToDo (http://www.statstodo.com/SSizCorr_Pgm.php) at the significance level of 0.05/23/2 and power of 90%.
UPLC measured glycans are subject to high experimental variability that results in nonbiological, technical variability in data. One part of technical variability comes from the global properties of measurements which can be controlled with normalization method to adjust individual measurements, balance them across all samples, and make them comparable. Raw glycan intensities were therefore normalized by total area, that is, peak area of every glycan in each sample was divided by the total chromatographic area of the sample. Resulting glycan values were percentages of a whole and all glycans of every sample sum to a 100.
Samples were analyzed on 96-well plates, which made that each cohort was run in 10 or more batches. Batch correction was performed by applying empirical Bayes method ComBat with parametric priors41 from “sva” R package42 to control the batch effect, a source of nonbiological variability in the data. Model matrix was created without covariates, and batch variable presenting membership to 96-well plate was passed as a separate argument (batch argument in ComBat function). Prior to batch correction, each glycan was log-transformed to account for right-skewness of glycan distributions.
Both Croatian and Orkney ethnic groups are cohorts of genetically isolated populations. Even though such populations are homogeneous and lack population substructure, they are well known for a larger proportion of related individuals, resulting in inflated type I error in association studies.43 Individual genotype data were obtained from larger genetic epidemiology programs (Croatia – 10001 Dalmatians,34 Orkney - Orkney Complex Disease Study),35 and quality control was performed as described in Lauc et al 2013.44 To account for family relatedness in the data, environmental residuals from polygenic function of GenABEL package for R45 were computed on batch corrected glycans. Polygenic function was adjusted for relatedness in phenotypes by modeling polygenic relationship (estimated from kinship matrix) between individuals as a random effect. Residuals from this analysis were corrected for relatedness and were used for association analyses with blood pressure variables. Because of the lack of family relationships in China cohort, this population was only corrected for batch effects.
From 23 directly measured glycans an additional 54 derived traits describing the percentage of galactosylation, sialylation, bisecting N-GlcNAc, and fucosylation were calculated (Supplementary Table S6). Since correction for relatedness was based on genotypes of individuals, samples that were not genotyped were removed prior to any statistical analysis. In total, 4102 individuals were both successfully genotyped and had their glycans analyzed, including 589 in Chinese, 829 in Korčula, 799 in Vis, and 1885 in Orkney. Prior to statistical analyses, all glycans were then standardized to mean 0 and standard deviation of 1.
Participants with complete data of blood pressures and IgG N-glycans were then selected for the analysis of descriptive statistics, Mann–Whitney U test and bivariate correlation coefficients. From this subset we selected participants with complete information on gender and age for analysis of partial correlation and multiple linear regression (1 person was excluded from the Chinese population, which is showed in Supplementary Figure S1).
Normality distributions of all analysis results were tested by the Kolmogorov–Smirnov test, and of which P < 0.10 was considered statistically significant. For majority of the variables that could not be assumed to be normally distributed (Table S1), median together with 25th percentile and 75th percentile were used in descriptive statistics, and Mann–Whitney U test was used to test the differences between groups. Multiple linear regression was applied to test associations between glycans and blood pressures adjusted for gender and age. Bivariate correlation coefficients of IgG N-glycans and blood pressures were assessed by Spearman rank correlation method, and partial correlation coefficients were also calculated to adjust for gender and age. Pooling analysis was carried out by introducing dummy variables representing the different populations. All reported P values were 2-sided, and P < 0.05 was considered statistically significant (if not otherwise stated). Bonferroni correction in multiple comparisons was referred to our previous publication,30,44 in which the significance level was divided by the 23 (number of directly measured glycans) instead of 77 (total of 23 directly measured and 54 glycans derived traits). Statistical analyses were done with the SPSS software, version 13.0 (SPSS Inc., Chicago, IL) and R version 3.1.0.46
RESULTS
Demographic and Biochemical Characteristics Comparisons Among the Normal Blood Pressure, Prehypertension, and Hypertension
Among the 4757 participants, 589 (64.5% of 913) Chinese Han, 829 (84.2% of 985) Croatian Korčula, 799 (89.2% of 896) Croatian Vis, and 1885 (96.0% of 1963) from Scottish Orkney had complete data on blood pressures and glycan levels, and were included in the further analyses. We further excluded 2 participants from Orkney because of low blood pressures (SDP < 90 mm Hg and DBP < 60 mm Hg). In total, 4100 samples were included in the final statistical analysis (see Supplementary Figure S1).
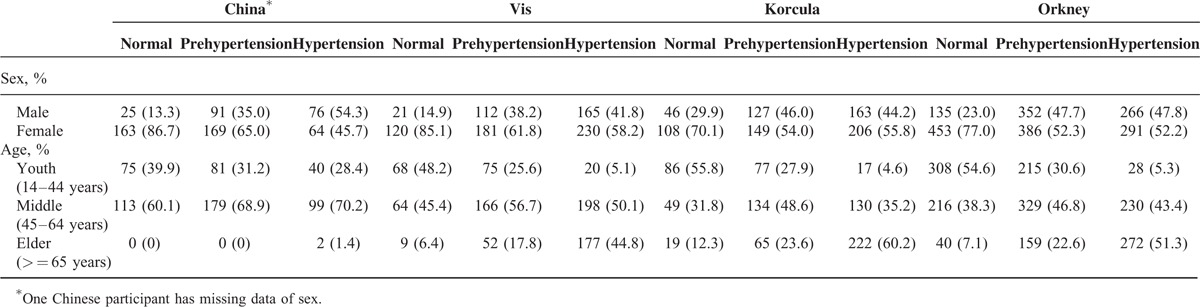
The gender and age distributions and the incidence of hypertension of the 4100 participants are listed in Table 1. The incidences of hypertension were 23.9%, 47.6%, 46.2%, and 29.6% in the recruited Chinese Han, Croatian Korčula and Vis, and Scottish Orkney, respectively.
TABLE 1.
The Sex and Age Distribution in 4 Populations: Chinese Han, Croatian's Vis and Korčula, and Scottish Orkney
The IgG N-Glycome Composition in Individuals With Normal Blood Pressure, Prehypertension, and Hypertension
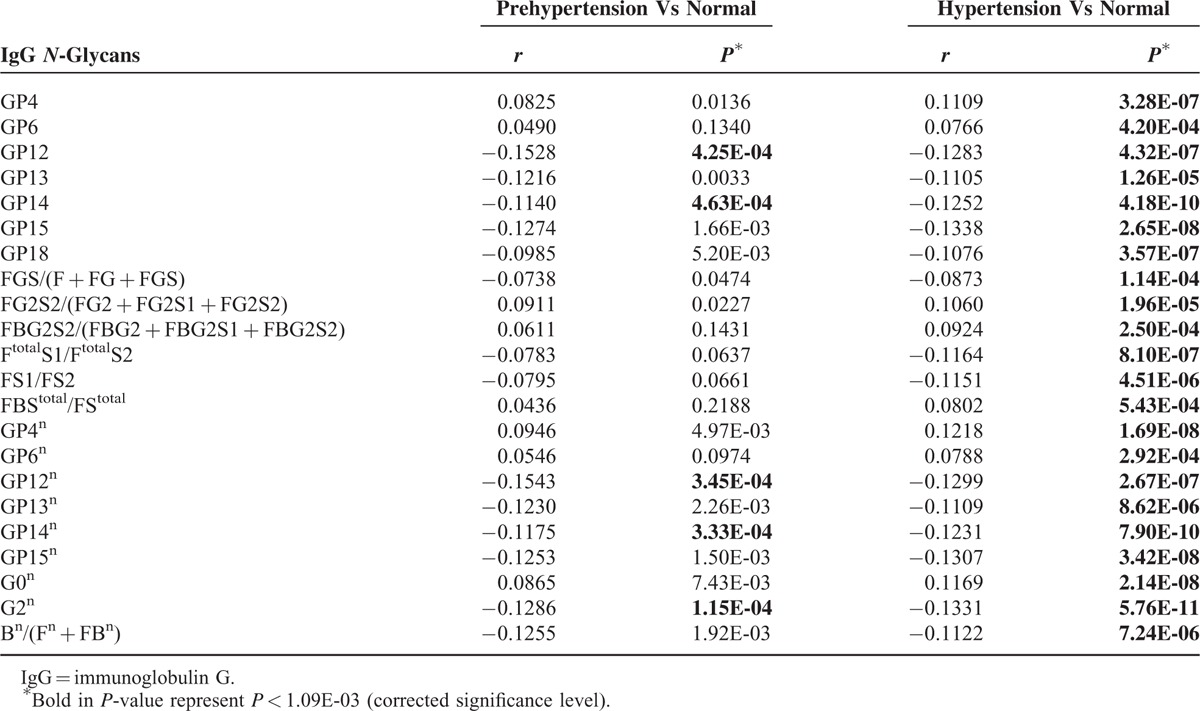
The results from descriptive analysis are listed in Supplementary Table S2, showing the median together with 25th and 75th percentile of individual IgG N-glycan traits by populations and blood pressures category (normal, prehypertension, or hypertension). The comparisons of IgG N-glycans between participants with hypertension, prehypertension, or normal blood pressure in each population and the pooling of 4 populations are shown in Table S3 (prehypertension vs normal) and Table S4 (hypertension vs normal), and part of those in the pooling of 4 populations (remaining statistically significant after controlling for age and gender) are summarized in Table 2. The corrected significance level was set to 1.09 × 10–3, that is, the significance level 0.05 was divided by 2 (prehypertension vs normal, and hypertension vs normal), and then by 23 (number of direct measured IgG N-glycan traits), referred to our previous publications.30,44
TABLE 2.
IgG N-Glycans Associated With Prehypertension or Hypertension in the Pooling of 4 Populations
In comparisons between participants with prehypertension and normal blood pressures, all of IgG N-glycans were not of statistical significances in each individual population, although G2n was lower in participant with prehypertension than those with normal blood pressure in each populations after controlling for age and gender (P = 0.1891, 0.0248, 0.2632, and 0.0113 in Chinese, Korčula, Vis, and Orkney population, respectively) (Figure 1, and Table S3).
FIGURE 1.
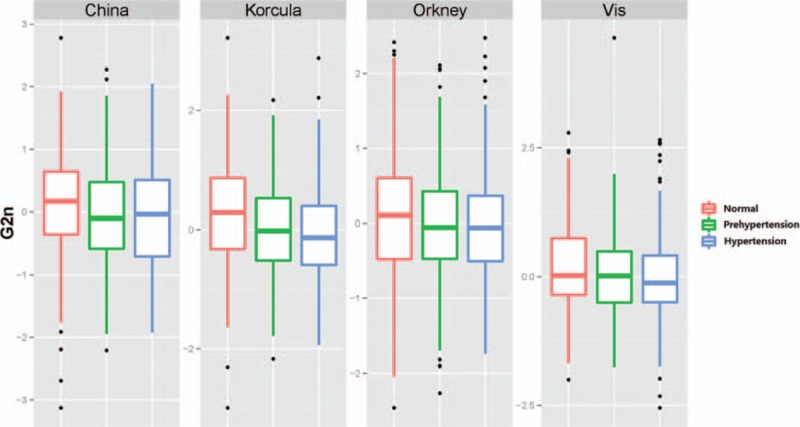
The boxplot of G2n in participants with normal, prehypertension, and hypertension.
Therefore, the pooling analysis of the 4 populations was performed. Five glycans were of significant differences in participants with prehypertension compared to those of normal in the pooling of the 4 populations, after controlling for age and sex (P = 1.15E-04, 4.25E-04, 4.63E-04, 3.45E-04, and 3.33E-04 for G2n, GP12, GP14, GP12n, and GP14n, respectively) (Table 2). Although not of statistical significances in each individual population, the sign of all coefficients are consistent in each individual population and the pooling.
After controlling for age and sex, 22 of 77 IgG N-glycan traits were significantly different between participants with hypertension and those of normal blood pressure in the pooling of the 4 populations, after controlling for age and sex (Table 2). Thirteen, 2, and 5 statistically significant correlations were also observed in Korčula, Vis, and Orkney populations, but not in Chinese, after controlling for age and sex (Supplementary Table S4). Majority of glycans with no statistical significances had the same directions in comparisons of each population with those in the pooling, except for FG2S2/(FG2 + FG2S1 + FG2S2) and FBStotal/FStotal (Table S4). The level of FG2S2/(FG2 + FG2S1 + FG2S2) was statistically the same (b = 0.00, P = 1.00) in participants with hypertension or normal in Chinese population, whereas it was higher in those with hypertension than in those of normal blood pressures in other 3 populations and the pooling of the 4 populations (b = 0.1762, P = 0.0022; b = 0.0966, P = 0.0879; b = 0.1278, P = 7.33E-04; b = 0.1060, P = 1.96E-05 in Korčula, Vis, and Orkney populations, respectively), might accounting for the ethnic difference between Chinese and European (Table S4). The level of FBStotal/FStotal was lower in participants with hypertension than those of normal blood pressures in Chinese and Vis populations (b = −0.0084, P = 0.8768 and b = −0.0231, P = 0.6715 in Chinese and Vis populations, respectively), while higher in Korčula and Orkney populations, with no statistical significance in all the comparisons (b = 0.1730, P = 1.98E-03 and b = 0.0856, P = 0.0102 in Korčula and Orkney populations, respectively), might according for the limited samples (Table S4).
The Correlations of IgG N-Glycan Traits and Systolic or Diastolic Blood Pressures
The correlations of IgG N-glycan traits and SBP or DBP in each population and the pooling of the 4 populations are shown in Table S5, and those of statistical significance are summarized in Table 3 (SBP) and Table 4 (DBP), with corrected significance level of 1.09E-03 (the significance level 0.05 was divided by 2 [SBP and DBP], and then by 23 [number of direct measured IgG N-glycan traits]).
TABLE 3.
The Correlation of IgG N-Glycans With SBP, Controlling for Age and Sex
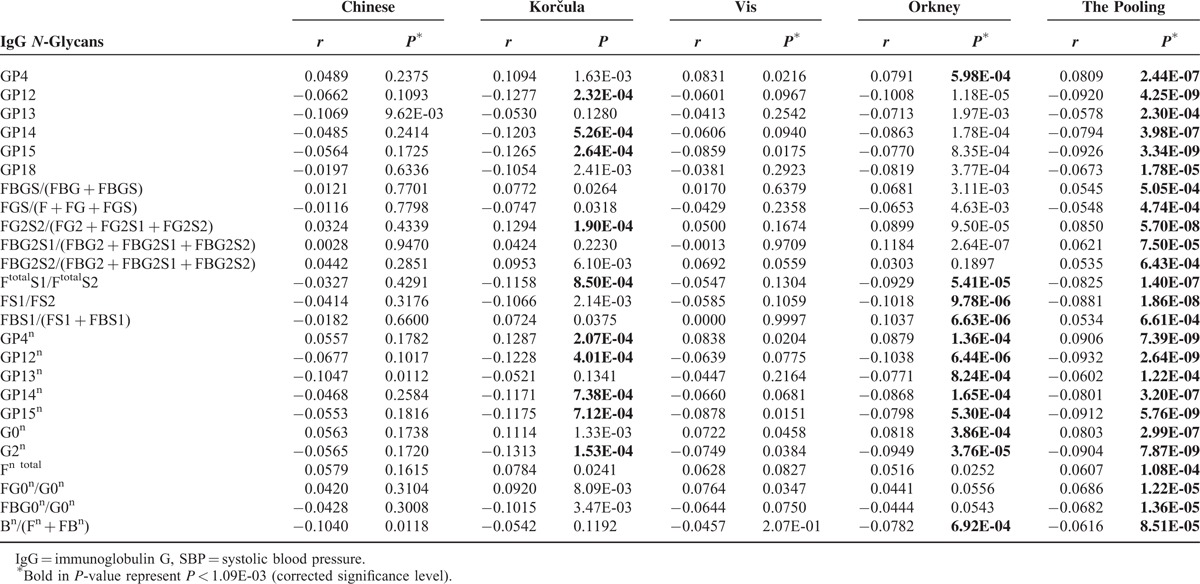
TABLE 4.
The Correlation of IgG N-Glycans With DBP, Controlling for Age and Sex
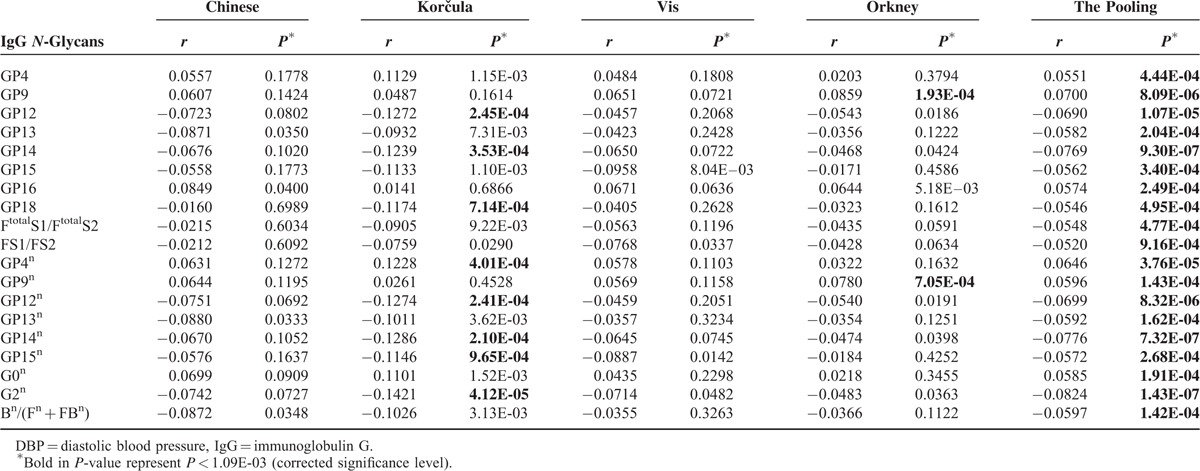
In the pooling analysis of 4099 individuals, 25 IgG N-glycan traits were correlated (10 positively and 15 negatively) with SBP after adjustment for sex and age, among which 10 were of significance in Korčula population, 18 in Orkney population, and there was no significant correlations in the other 2 populations (Table 3). The biggest coefficient was observed for GP12n (b = 0.0932, P = 2.64E-09). Even though not all of them were statistically significant, all but FBS1/(FS1 + FBS1) showed the same trend, suggesting that more samples are needed in each cohort to reach the statistical power. This was further hinted by results of the pooled analysis.
Nineteen IgG N-glycans trait were correlated (6 positively and 13 negatively) with DBP after adjustment for sex and age in the pooling (Table 4), with the biggest coefficients in G2n (b = 0.0824, P = 1.43E-07). Similarly, although only 8 and 2 of 19 coefficients remained significant in Korčula and Orkney population but not in other 2 populations, the trends of all correlation coefficients in pooling analysis were the same as those in the analysis of each individual population.
DISCUSSION
Given the prevalence of hypertension in Chinese Han, Croatian's Vis and Korčula, and Scottish Orkney cohorts (23.9%, 47.6%, 46.2%, and 29.6 %, respectively), hypertension continues to be a major health problem in the investigated populations, and identification of biomarkers that might translate new knowledge into prevention and treatment of hypertension is a pressing need. The differences in prevalence of hypertension among different populations might be caused by the differences in age distribution (the median age [interquartile range] were 46 [41–50], 57 [47–67], 57 [45–69], and 54 [41–65] in Chinese Han, Croatian Korčula and Vis, and Scottish Orkney, respectively). In addition, the genetic isolation of the Croatian Korčula and Vis could be the genetic factor contributing to the observed difference of incidence of BP among these different populations.
Glycans have been reported to be associated with several diseases, such as systemic lupus erythematosus, glomerulonephritis, inflammatory bowel disease, Alzheimer disease, and progressive mild cognitive impairment.18,23–27 Based on a combination of approaches of a cross-sectional survey at population level and an association study at glycomic level, this multiple centers’ study aimed to examine a possible association between hypertension and IgG glycosylation. The present study is the 1st such example based on a large-scale population-based study that demonstrates a possible association between blood pressures and IgG glycosylation in both Asian and Europeans.
The results showed that 5 IgG glycosylation traits (G2n, GP12, GP14, GP12n, and GP14n) are strongly and statistically significantly associated with both prehypertension and hypertension. Other 17 glycan traits (GP4, GP6, GP13, GP15, GP18, GP4n, GP6n, GP13n, GP15n, FGS/[F + FG + FGS], FG2S2/[FG2 + FG2S1 + FG2S2], FBG2S2/[FBG2 + FBG2S1 + FBG2S2], FtotalS1/FtotalS2, FS1/FS2, FBStotal/FStotal, G0n, and Bn/[Fn + FBn]) are significantly associated only with hypertension. It should be noted that association of these traits with prehypertension, while insignificant after stringent multiple testing correction we used, is in many cases marginally significant, and exhibited consistent sign of association, albeit the correlations were weaker (Table 2). Furthermore, 25 glycan traits are correlated with SBP (Table 3), while 19 glycan traits are also correlated with DBP (Table 4). Although analysis of individual populations does not reveal statistical significance, the pooled analysis does, and the effect estimates are very consistent – in terms of sign and the magnitude – across populations (Tables 3 and 4). The main issue here is that hypertension strongly associates with age, and since IgG glycans are also highly associated with both chronological and biological ages, the correction for chronological age is removing a significant part of associations between glycans and biological age.47
The same concerns other glycan traits: although not statistically significant, we see strong enrichment of low P-values in comparison of normal versus prehypertension group (e.g., 19 of 77 P-values are <0.05, with null expectation of P < 1E-4, exact binomial P < 1E-8). The results from our previous study suggested that N-glycosylation of plasma proteins is affected by both age and sex, and that changes in the levels of glycan features can be associated with metabolic syndrome components including BMI, SBP, DBP, and fasting plasma glucose simultaneously.31 The present data suggested that blood pressures are associated with individual variation in the extent of fucosylation, the degree of branching, levels of sialylation, and levels of glycosylation. Individual variation in IgG glycome composition is very high.29 Despite the absence of a direct genetic template for individual glycans, their levels are up to 80% heritable48 and associate with different genetic44 and epigenetic loci.48
Ebringer and Doyle (1970)49 reported that serum IgG levels are significantly higher in 118 severely hypertensive patients compared with a group of 163 normotensive blood donors, suggesting that the raised levels of serum IgG may be an index of vascular damage induced by hypertension. Ohara et al (2014)50 reported that urinary excretion of IgG is significantly elevated in nondiabetic hypertension patients compared to the healthy controls and in type 2 diabetic patients with hypertension compared to those with type II diabetes only. Also, serum IgG antibodies to Chlamydia pneumoniae were reported to be associated with essential hypertension in a highly homogeneous population from Majorca, Balearic Islands, Spain.51 The present study showed that the quantitative changes of IgG glycosylation are associated with hypertension, besides the level of IgG.
Alterations of the immune response have been implicated in the pathogenesis of hypertension for more than 5 decades. White and Grollman (1964)52 reported that immunosuppression attenuates hypertension in rats with partial renal infarction. Guzik et al (2007)53 found that the degree of hypertension caused by chronic angiotensin II infusion is markedly blunted in RAG-1−/− mice, which lack both T and B cells. Marvar et al (2010)54 further found that T cells are essential for the development of deoxycorticosterone acetate-salt and norepinephrine-induced hypertension, suggesting that hypertension has been involved in both central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Through examination on hypertensive response of mice that have severe combined immunodeficiency, Crowley et al (2008)55 showed that T cells are essential for the development of the angiotension II induced hypertension and immunoefficiency can lead to decreased left ventricular hypertrophy, cardiac fibrosis, and albuminuria following angiotension II administration. Several studies showed that cytokines produced by T cells and other inflammatory cells contribute to hypertension.56–59
Recently, Harrison et al (2011)60 reviewed the relation between inflammation, immunity, and hypertension, and proposed that hypertensive stimuli (such as angiotension II and salt) cause a modest elevation in blood pressure (prehypertension), then lead to neoantigen formation, promoting cell activation and entering kidney and vasculature, and then T cells-derived signals such as IL-17 promote entry of other inflammatory cells such as macrophages releasing cytokines that cause vasoconstriction and promote sodium and water absorption, and ultimately start severe hypertension. Furthermore, based on the evidences from both basic and clinical studies, Montecucco et al (2011)61 proposed the possible direct role of inflammation in the pathophysiology of essential hypertension. Our findings that the level of IgG galactosylation (G2n, the percentage of digalactosylated structures in total neutral IgG glycans; GP12, GP14, GP12n, and GP14n, all with digalactosylation structure) are significantly lower in the participants with prehypertension, suggesting that IgG galactosylation might occur at the early stage of pathogenesis of hypertension, and play role in the development of severe hypertension from prehypertension.
Glycosylation of the IgG, Fc-, or Fab-fragments has a role in enhancing or blocking the pro- and anti-inflammatory effector functions.62 IgG mediates pro- and anti-inflammatory activities mainly through the engagement of its constant region (Fc part) with distinct Fcg receptors. The differential compositions of the N-glycans determine the effector functions by altering the affinity of IgG to activating or inhibitory FcγRs.63 A recent study showed that highly galactosylated IgG1 mediates anti-inflammatory activity by facilitating the association of the inhibitory receptor FcγRIIB and a C-type lectin-like receptor dectin-1.64 In addition, the lack of core fucosylation of anti-HPA-1a–specific IgG1 also dramatically increases the binding affinity to FcγRIIIa and FcγRIIIb but not FcγRIIa.65 The findings of the association between hypertension and the number of terminal galactose in IgG (e.g., glycan without galactose [GP4, GP4n, GP4, GP6n, and G0n] increases, while glycans with galactose [GP12, GP13, GP14, GP15, GP18, GP12n, GP13n, GP14n,GP15n, and G2n] decreases from normal to hypertension, and also the similar pattern of glycans correlation with SBP or DBP, or the core fucosylation or bisecting GlcNAc of IgG, e.g., GP14, GP14n, and Bn/(Fn + FBn) decreases successively from normal to hypertension, and negatively correlates with SBP or DBP) corresponds to the alteration of the affinity of IgG to activating or inhibitory FcγRs, and suggests that galactosylation or core fucosylation of IgG contribute to the increase of blood pressure via inflammatory signaling.
IgG also acquires anti-inflammatory properties depending on Fc sialylation. This differential sialylation may provide a switch from innate anti-inflammatory activity in the steady state to generate adaptive pro-inflammatory effects upon antigenic challenge.66 Sialylation of IgG exerts anti-inflammatory effects,67 through interactions with the lectin receptor specific intercellular adhesion molecule-3-grabbing nonintegrin-related-1 or dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin.68 IgG with disialylation (FG2S2/[FG2 + FG2S1 + FG2S2] and FBG2S2/[FBG2 + FBG2S1 + FBG2S2]) increases with hypertension, while other IgG sialylation (GP18, FGS/[F + FG + FGS], FtotalS1/FtotalS2, and FS1/FS2) decreases with hypertension, suggesting that sialylation is associated with hypertension via decreasing adaptive pro-inflammatory effects of IgG. This is further supported by the observation of the opposite associations: positive correlation of SBP with FG2S2/(FG2 + FG2S1 + FG2S2) and FBG2S1/(FBG2 + FBG2S1 + FBG2S2), but negative with GP18, FtotalS1/FtotalS2, and FS1/FS2.
The strength of this study is that it is the 1st population-based study on the association of IgG N-glycosylation and hypertension, and we found that the N-glycome profile is differed between the normal, hypertension, and hypertension. However, several limitations must be considered when interpreting these findings. First, the present study is a cross-sectional study, thus it cannot judge whether IgG N-glycosylation changes are causal for hypertension or just parallel with hypertension. Second, hypertension is diagnosed only after at least 2 separate visits to a clinic, whereas we used a single measurement in this multiple ethnic cross-sectional study. Thirdly, no other risk factors (such as BMI, smoking, diabetes, etc.) were controlled in this study. The absence of controlling for potential risk factors may confound the association, but at certain extend the consistent results among populations investigated with different ethnic background might help this study to profile objectively the association observed between IgG glycosylation and hypertension.
In conclusion, the present study showed that galactosylation of IgG is moderately associated with prehypertension and hypertension, while certain types of IgG N-glycan traits (sialylation, core fucosylation, and bisecting N-GlcNAc) are associated with hypertension, but not prehypertension. Sialylation, bisecting N-GlcNAc, galactosylation, and core fucosylation of IgG are also associated with SBP or DBP. Considering that galactosylation, sialylation, and core fucosylation of IgG involve directly in inflammation pathways, and inflammation plays a direct role in the pathophysiology of essential hypertension, our findings suggested that individual variation of N-glycosylation of IgG might contribute to hypertension via its effects on pro- and anti-inflammation pathways.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank the National Natural Science Foundation of China (81370083, 81273170, 81573215, 81373099), the National “12th Five-Year” Plan for Science and Technology Support, China (2012BAI37B03), the Joint Project of the Australian National Health and Medical Research Council and the National Natural Science Foundation of China (NHMRC-APP1112767-NSFC 81561128020), Connecting Australian-European Science and Innovation Excellence (CAESIE-Priming Grant 1747853), Australian National Health and Medical Research Council (NHMRC-APP1046711), and ECU Industry Collaboration Scheme 2013 (G1001368) and EU-fp-7 Pain-Omics (602736) for the support. MS was supported by the Importation and Development of High-Calibre Talents Project of Beijing Municipal Institutions (CIT&TCD201404185); and YW was supported by Beijing Higher Education Young Elite Teacher Project (YETP1671) and Beijing Nova Program (Z141107001814058); and work of YA was supported by Russian Science Foundation (14-14-00313), and OP was supported by The Medical Research Council UK and the Croatian Science Foundation (8875).
Footnotes
Abbreviations: BMI = body mass index, DBP = diastolic blood pressure, Fc = fragment crystallizable region, Ig = immunoglobulin, IgG = immunoglobulin G, SBP = systolic blood pressure
Conceived and designed the experiments: WW, GL, IR, and QZ; performed the experiments: YW, LK, XY, KT, JD, MN, JW, OP, YL, JK, SG, MP, LW, and JZ; analyzed the data: YW, LK, XG, and YA; wrote the paper: YW, WW, GL, LK, IR, YZ, IU, MS, IR, and HC; and all authors have read, and confirm that they meet ICMJE criteria for authorship.
This work was supported by grants from the National Natural Science Foundation of China (81370083, 81273170, 81573215, 81373099), the National “12th Five-Year” Plan for Science and Technology Support, China (2012BAI37B03), the Joint Project of the Australian National Health and Medical Research Council and the National Natural Science Foundation of China (NHMRC-APP1112767-NSFC 81561128020), Connecting Australian-European Science and Innovation Excellence (CAESIE-Priming Grant 1747853), Australian National Health and Medical Research Council (NHMRC-APP1046711), and ECU Industry Collaboration Scheme 2013 (G1001368) and EU-fp-7 Pain-Omics (602736). MS was supported by the Importation and Development of High-Calibre Talents Project of Beijing Municipal Institutions (CIT&TCD201404185), and YW was supported by Beijing Higher Education Young Elite Teacher Project (YETP1671) and Beijing Nova Program (Z141107001814058). Work of YA was supported by Russian Science Foundation (14-14-00313), and OP was supported by The Medical Research Council UK and the Croatian Science Foundation (8875)
The funders have no roles in study design, experiments manipulation, data collection and analysis, and manuscript preparation and results interpretation.
The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, et al. Writing Group Members. Heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation 2010; 121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA 2002; 287:1003–1010. [DOI] [PubMed] [Google Scholar]
- 4.Ehret GB, Munroe PB, Rice KM, et al. International Consortium for Blood Pressure Genome-Wide Association Studies. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011; 478:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 2001; 104:545–556. [DOI] [PubMed] [Google Scholar]
- 6.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypert 2009; 23:363–384. [DOI] [PubMed] [Google Scholar]
- 7.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013; 346:f1325. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens 2006; 24:215–233. [DOI] [PubMed] [Google Scholar]
- 9.Haslam DW, James WP. Obesity. Lancet 2005; 366:1197–1209. [DOI] [PubMed] [Google Scholar]
- 10.Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 2002; 288:1882–1888. [DOI] [PubMed] [Google Scholar]
- 11.Mesas AE, Leon-Muñoz LM, Rodriguez-Artalejo F, et al. The effect of coffee on blood pressure and cardiovascular disease in hypertensive individuals: a systematic review and meta-analysis. Am J Clin Nutr 2011; 94:1113–1126. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya A, Forman JP. Vitamin D and hypertension: current evidence and future directions. Hypertension 2010; 56:774–779. [DOI] [PubMed] [Google Scholar]
- 13.Lawlor DA, Smith GD. Early life determinants of adult blood pressure. Curr Opin Nephrol Hypertens 2005; 14:259–264. [DOI] [PubMed] [Google Scholar]
- 14.Marshall IJ, Wolfe CD, McKevitt C. Lay perspectives on hypertension and drug adherence: systematic review of qualitative research. BMJ 2012; 345:e3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauc G. Sweet secret of multicellular life. Biochim Biophys Acta 2006; 1760:525–526. [DOI] [PubMed] [Google Scholar]
- 16.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta 1999; 1473:4–8. [DOI] [PubMed] [Google Scholar]
- 17.Marek KW, Vijay IK, Marth JD. A recessive deletion in the GlcNAc-1-phosphotransferase gene results in peri-implantation embryonic lethality. Glycobiology 1999; 9:1263–1271. [DOI] [PubMed] [Google Scholar]
- 18.Parekh RB, Dwek RA, Sutton BJ, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 1985; 316:452–457. [DOI] [PubMed] [Google Scholar]
- 19.Varki A, Baum L, Bellis S, et al. Working group report: the roles of glycans in hemostasis, inflammation and vascular biology. Glycobiology 2008; 18:747–749. [DOI] [PubMed] [Google Scholar]
- 20.McGraw-Hill, Junqueira LC, Carneiro J. Basic Histology. 2003; ISBN 0838505902. [Google Scholar]
- 21.Wright A, Morrison SL. Effect of glycosylation on antibody function: implications for genetic engineering. Trends Biotechnol 1997; 15:26–32. [DOI] [PubMed] [Google Scholar]
- 22.Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther 2007; 7:1401–1413. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi E, Shinzaki S, Fujii H, et al. Role of aberrant IgG glycosylation in the pathogenesis of inflammatory bowel disease. Proteomics Clin Appl 2016; 10:384–390. [DOI] [PubMed] [Google Scholar]
- 24.van Timmeren MM, van der Veen BS, Stegeman CA, et al. IgG glycan hydrolysis attenuates ANCA-mediated glomerulonephritis. J Am Soc Nephrol 2010; 21:1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trbojević Akmačić I, Ventham NT, Theodoratou E, et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm Bowel Dis 2015; 21:1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vučković F, Krištić J, Gudelj I, et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol 2015; 67:2978–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundström SL, Yang H, Lyutvinskiy Y, et al. Blood plasma IgG Fc glycans are significantly altered in Alzheimer's disease and progressive mild cognitive impairment. J Alzheimers Dis 2014; 38:567–579. [DOI] [PubMed] [Google Scholar]
- 28.Kaneshige H. Nonenzymatic glycosylation of serum IgG and its effect on antibody activity in patients with diabetes mellitus. Diabetes 1987; 36:822–828. [DOI] [PubMed] [Google Scholar]
- 29.Pucić M, Knezević A, Vidic J, et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics 2011; 10:M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikolac Perkovic M, Pucic Bakovic M, Kristic J, et al. The association between galactosylation of immunoglobulin G and body mass index. Prog Neuropsychopharmacol Biol Psychiatry 2014; 48:20–25. [DOI] [PubMed] [Google Scholar]
- 31.Lu JP, Knezevic A, Wang YX, et al. Screening novel biomarkers for metabolic syndrome by profiling human plasma N-glycans in Chinese Han and Croatian populations. J Proteome Res 2011; 10:4959–4969. [DOI] [PubMed] [Google Scholar]
- 32.Rudan I, Campbell H, Rudan P. Genetic epidemiological studies of eastern Adriatic Island isolates, Croatia: objective and strategies. Coll Antropol 1999; 23:531–546. [PubMed] [Google Scholar]
- 33.Rudan I, Biloglav Z, Vorko-Jovic A, et al. Effects of inbreeding, endogamy, genetic admixture, and outbreeding on human health: a (1001 Dalmatians) study. Croat Med J 2006; 47:601–610. [PMC free article] [PubMed] [Google Scholar]
- 34.Rudan I, Marusic A, Jankovic S, et al. “10001 Dalmatians:” Croatia launches its national biobank. Croat Med J 2009; 50:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McQuillan R, Leutenegger AL, Abdel-Rahman R, et al. Runs of homozygosity in European populations. Am J Hum Genet 2008; 83:359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003; 42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 37.Ruhaak LR, Steenvoorden E, Koeleman CA, et al. 2-Picoline-borane: a non-toxic reducing agent for oligosaccharide labeling by reductive amination. Proteomics 2010; 10:2330–2336. [DOI] [PubMed] [Google Scholar]
- 38.Ruhaak LR, Hennig R, Huhn C, et al. Optimized workflow for preparation of APTS-labeled N-glycans allowing high-throughput analysis of human plasma glycomes using 48-channelmultiplexed CGE-LIF. J Proteome Res 2010; 9:6655–6664. [DOI] [PubMed] [Google Scholar]
- 39.Royle L, Campbell MP, Radcliffe CM, et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal Biochem 2008; 376:1–12. [DOI] [PubMed] [Google Scholar]
- 40.Pucic M, Knezevic A, Vidic J, et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics 2011; 10:010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007; 8:118–127. [DOI] [PubMed] [Google Scholar]
- 42.Leek JT, Johnson WE, Parker HS, et al. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012; 28:882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aulchenko YS, de Koning DJ, Haley C. Genome wide rapid association using mixed model and regression: a fast and simple method for genome wide pedigree-based quantitative trait loci association analysis. Genetics 2007; 177:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauc G, Huffman JE, Pucic M, et al. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet 2013; 9:e1003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aulchenko YS, Ripke S, Isaacs A, et al. GenABEL: an R library for genome-wide association analysis. Bioinformatics 2007; 23:1294–1296. [DOI] [PubMed] [Google Scholar]
- 46.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ Accessed at April 11, 2014. [Google Scholar]
- 47.Krištić J, Vučković F, Menni C, et al. Glycans are a novel biomarker of chronological and biological ages. J Gerontol A Biol Sci Med Sci 2014; 69:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menni C, Keser T, Mangino M, et al. Glycosylation of Immunoglobulin G: role of genetic and epigenetic influences. PLoS One 2013; 8:e82558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebringer A, Doyle AE. Raised serum IgG levels in hypertension. Br Med J 1970; 2:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohara N, Hanyu O, Hirayama S, et al. Hypertension increases urinary excretion of immunoglobulin G, ceruloplasmin and transferrin in normoalbuminuric patients with type 2 diabetes mellitus. J Hypertens 2014; 32:432–438. [DOI] [PubMed] [Google Scholar]
- 51.Zabay JM, Marco J, Soler J, et al. Association of HLA-DRB3∗0202 and serum IgG antibodies to Chlamydia pneumoniae with essential hypertension in a highly homogeneous population from Majorca (Balearic Islands, Spain). J Hum Hypertens 2005; 19:615–622. [DOI] [PubMed] [Google Scholar]
- 52.White FN, Grollman A. Autoimmune factors associated with infarction of the kidney. Nephron 1964; 204:93–102. [DOI] [PubMed] [Google Scholar]
- 53.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marvar PJ, Thabet SR, Guzik TJ, et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 2010; 107:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crowley SD, Song YS, Lin EE, et al. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 2010; 298:R1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tran LT, MacLeod KM, McNeill JH. Chronic etanercept treatment prevents the development of hypertension in fructose-fed rats. Mol Cell Biochem 2009; 330:219–228. [DOI] [PubMed] [Google Scholar]
- 57.Venegas-Pont M, Manigrasso MB, Grifoni SC, et al. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 2010; 56:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schrader LI, Kinzenbaw DA, Johnson AW, et al. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol 2007; 27:2576–2581. [DOI] [PubMed] [Google Scholar]
- 59.Brands MW, Banes-Berceli AK, Inscho EW, et al. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension 2010; 56:879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrison DG, Guzik TJ, Lob HE, et al. Inflammation, immunity, and hypertension. Hypertension 2011; 57:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montecucco F, Pende A, Quercioli A, et al. Inflammation in the pathophysiology of essential hypertension. J Nephrol 2011; 24:23–34. [DOI] [PubMed] [Google Scholar]
- 62.Basta M, Branch DR. 7(th) International Immunoglobulin Conference: Mechanisms of Action. Clin Exp Immunol 2014; 178 Suppl 1:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Ann NY Acad Sci 2012; 1253:170–180. [DOI] [PubMed] [Google Scholar]
- 64.Karsten CM, Pandey MK, Figge J, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med 2012; 18:1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kapur R, Kustiawan I, Vestrheim A, et al. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood 2014; 123:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006; 313:670–673. [DOI] [PubMed] [Google Scholar]
- 67.Anthony RM, Nimmerjahn F, Ashline DJ, et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 2008; 320:373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol 2010; 30 Suppl 1:S9–S14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


