Abstract
Vitamin D deficiency and vitamin D receptor (VDR) gene abnormalities confer susceptibility to tuberculosis. Toll-like receptors (TLRs), such asTLR-2, are also important mediators of inflammatory response against Mycobacterium tuberculosis. We evaluated serum vitamin D, and VDR and TLR-2 gene polymorphisms in patients with spinal tuberculosis.
This study comprised of 3 groups: spinal tuberculosis, pulmonary tuberculosis, and controls (each with 106 subjects). Enzyme-linked immunosorbent assay was used to measure vitamin D levels, and polymerase chain reaction-sequencing method was used to analyze VDR and TLR-2 gene polymorphisms. Patients were followed up for 6 months.
Vitamin D deficiency was significantly more prevalent in patients with spinal tuberculosis (P < 0.001) and pulmonary tuberculosis (P = 0.011), versus controls. The heterozygous and mutant genotypes of VDR TaqI gene were significantly associated with spinal tuberculosis (P < 0.001; odds ratio [OR] 4.74 [2.45–9.18]) and pulmonary tuberculosis (P < 0.001; OR 3.52 [1.80–6.88]) when compared with controls. The heterozygous and mutant variants of VDR ApaI gene were significantly more common in patients with spinal tuberculosis in comparison with patients with pulmonary tuberculosis (P < 0.001; OR 2.90 [1.65–5.10]) and controls (P < 0.001; OR 6.56 [3.41–12.61]). We did not observe any significantly different results for TLR-2 gene polymorphisms. Vitamin D deficiency, VDR, and TLR-2 polymorphisms did not affect the 6-month disability.
Vitamin D deficiency and VDR gene polymorphisms are significantly more prevalent in people with pulmonary and spinal tuberculosis. They may, in isolation or collectively, confer susceptibility to pulmonary and spinal tuberculosis.
INTRODUCTION
Spinal tuberculosis is a common form of extrapulmonary tuberculosis, being prevalent in more than 50% of patients with bone or joint tuberculosis.1
The vitamin D receptor (VDR) gene is located on chromosome 12q13.11. The VDR gene is responsible for making the VDR protein which is essential for the proper functioning of vitamin D. The VDR gene polymorphisms are known to confer susceptibility to tuberculosis.2 VDR gene plays a critical role in modulating monocyte and macrophage activity, and determines innate immunity against Mycobacterium tuberculosis. In addition, VDR supports the cell-cycle control system. The destruction of M tuberculosis by macrophages is hampered by inherent defects in bacterial defense mechanisms. Vitamin D (1, 25-dihydroxyvitamin D3) decreases the viability of M tuberculosis by increasing the fusion of the phagosome and lysosome in infected macrophages.3 Toll-like receptors (TLRs) and vitamin D responses may interact to orchestrate the antimicrobial reaction against M tuberculosis. TLR activation of human macrophages leads to up-regulated expression of the VDR and the vitamin D-1-hydroxylase genes, leading to production of the peptide cathelicidin and killing of intracellular M tuberculosis.4
Among common VDR gene polymorphisms, the FokI, BsmI, ApaI, and TaqI polymorphisms are 4 classical polymorphisms that have been studied in cancer and many other conditions including tuberculosis.5,6 A recent meta-analysis suggested that VDR FokI polymorphism contributes to increasing the risk of pulmonary tuberculosis in human immunodeficiency virus-negative individuals, especially in the Asian region.7FokI polymorphism in VDR gene has also been shown to be associated with the susceptibility to spinal tuberculosis in Chinese Han population.8 We selected VDR ApaI and TaqI genes, for our study, because both these genes are relatively less well studied in spinal tuberculosis.
Toll-like receptor-2 gene polymorphisms also modulate the susceptibility of patients to pulmonary tuberculosis. TLRs are mediators of innate inflammatory response and also help in developing antigen-specific adaptive immune reaction. TLR-2 recognizes lipoproteins derived from M tuberculosis, subsequently activating MyD88 adaptor like protein (Mal) and triggering a signaling pathway, and thus inducing the immune reaction. Polymorphisms in TLR-2 gene can alter this pathway, leading to increased susceptibility to tuberculosis. A recent meta-analysis revealed that TLR-2 G2258A polymorphism contributes to susceptibility to pulmonary tuberculosis, in Asian population.9 A few studies have also documented the association of TLR-2 gene polymorphisms with susceptibility to tuberculous meningitis.10,11
We aimed to assess the vitamin D status, and VDR and TLR-2 gene polymorphisms in spinal tuberculosis, pulmonary tuberculosis, and healthy controls. We also assessed the prognostic impact of VDR and TLR-2 gene polymorphisms in spinal tuberculosis.
METHODS
This was a case-control study and with a prospective follow-up. The study was performed in the Department of Neurology in collaboration with Department of Microbiology, Biochemistry, and Pulmonary Medicine at King George's Medical University, Lucknow, India. Our institution is a large tertiary care health center and a referral hospital in northern India. The study subjects were enrolled from August 2013 to July 2015. The study was approved by Institutional Ethics Committee of King George's Medical University, Lucknow, India. Written informed consent was obtained from all cases and controls that were enrolled in the study.
Inclusion Criteria
In this study, we included consecutive patients with spinal tuberculosis, pulmonary tuberculosis, and healthy controls. The diagnosis of spinal tuberculosis was entertained if a patient presenting with myelopathy had characteristic clinical features like chronic back or neck pain, fever, spinal localized tenderness, deformity, and cold abscess, and fulfilled the essential neuroimaging criteria (destruction of 2 or more contiguous vertebrae and opposed end plates, disk infection, along with a paraspinal mass or abscess). Diagnosis was histopathologically and/or bacteriologically confirmed.12
Pulmonary tuberculosis was confirmed by demonstration of acid-fast bacilli on sputum smears, at least on 2 separate occasions, in a patient with imaging abnormalities consistent with active tuberculosis.
Age and sex-matched healthy controls, from the same geographical area, were also included.
Exclusion Criteria
Patients with chronic kidney and liver diseases, malabsorption syndrome, chronic alcoholism, and patients on drugs affecting vitamin D levels (like antiepileptic drugs) were excluded from the study. Patients who were receiving vitamin D and calcium supplementation were excluded; those who were discovered to have a systemic malignancy were also excluded.
Clinical Assessment
Detailed history and physical examination was performed in all patients of spinal tuberculosis.
Laboratory Investigations
Routine blood investigations including complete hemogram, including erythrocyte sedimentation rate, blood sugar, liver and kidney function tests, serum electrolytes, and human immunodeficiency virus testing, were done in all patients with spinal and pulmonary tuberculosis.
Imaging
Chest X-ray was done in all patients with spinal and pulmonary tuberculosis. Spinal tuberculosis patients were made to undergo spinal X-ray and magnetic resonance imaging of spine with gadolinium enhancement. Magnetic resonance imaging was done using a Signa Excite 1.5 tesla instrument (General Electric Medical Systems, Milwaukee, WI). Imaging features of spinal tuberculosis such as spinal deformity, destruction of contiguous vertebrae with opposed end plates, disk involvement, and paraspinal collection were noted.
Assessment of Severity of Disease
Baseline severity assessment was done using modified Barthel index. Modified Barthel index is a 20-point scoring system, with higher score indicating a better functional status. Modified Barthel index ≤12 was considered as an indicator of poor functional status, and likewise, modified Barthel index >12 was considered as an indicator of good functional status.
Vitamin D Estimation
Blood samples for serum 25-(OH)vitamin D estimation were drawn after overnight fasting. Serum separation was done and it was stored at −80oC, till the time it was used for analysis. Serum 25-(OH)vitamin D was estimated using the enzyme immunoassay kit (MicroVue 25-[OH]vitamin D the enzyme immunoassay kit; Quidel Corporation, San Diego). The procedure was performed according to the instructions provided by the manufacturer. Vitamin D sufficiency, insufficiency, and deficiency were defined as 25-(OH)vitamin D levels ≥30 ng/mL, 10.1 to 30 ng/mL, and ≤10 ng/mL, respectively.
Polymorphism Detection
Two polymorphisms each of VDR and TLR-2 gene were analyzed. Polymorphisms which were studied for VDR were TaqI (rs731236) and ApaI (rs7975232) gene polymorphism and those for TLR-2 were Arg753Gln (rs5743708 A/G) and Pro631His (rs5743704 A/C).
We chose to analyze 2 of the 3 most common nonsynchronous polymorphisms known to be associated with TLR-2, for example, 1. 2258G>A (rs5743708; arginine replaced with glutamine at position 753; represented as Arg753Gln), 1892C>A (rs5743704; proline replaced with histidine at position 631; represented as Pro631His), and 2029C>T (rs121917864; arginine replaced with tryptophan at position 677; represented as Arg677Trp).13 We selected Arg753Gln because this has been shown to be associated significantly with risk towards tuberculosis8,9; the selection of Pro631His was done because never before has this polymorphism been detected in an Asian subset of patients with spinal tuberculosis, and is thought to be limited to European populations.13
Genomic DNA extraction was done from peripheral blood. Venous blood was collected in ethylene diamine tetraacetic acid (EDTA) vials. Genomic deoxyribonucleic acid (DNA) extraction was done using salting-out method. Phenol-chloroform method was used for DNA purification. DNA samples were stored at −20oC till they were used. Polymerase chain reaction (PCR) sequencing method was used to determine VDR and TLR-2 genotypes, using a chain termination-based method. A single set of primer sequences was designed for both the VDR polymorphisms (ApaI and TaqI) and for both the TLR-2 polymorphisms (Arg753Gln and Pro631His) (Integrated DNA Technology). The primer sequences used for VDR were: forward 5’-AGCAGAGCAGAGTTCCAAGC-3’ and reverse 5’-CACTCAGGCTGGAAGGAGAG-3’; and for TLR-2 were: forward 5’-ATGTCACAGGACAGCACTGG-3’ and reverse 5’-CGCAGCTCTCAGATTTACCC-3’ (Integrated DNA Technology). PCR amplifications were done in a 25-μL volume containing 10× assay buffer, 200 μM each of dATP, dCTP, dGTP, dTTP, 1 pm of each primer, 1.0 U of Taq DNA polymerase (Finzymes, Thermo Scientific). PCR was performed with repeated cycling of the following steps: an initial denaturation done at 94°C for 10 minutes, followed by 35 cycles of denaturing at 94°C for 45 seconds; annealing done at 62.3°C for 45 seconds for VDR and at 59.6°C for 45 seconds for TLR-2; and then extension done at 72°C for 1 minute, and a final extension at 72°C for 7 minutes followed up by cooling to 4°C. Template-free water acted as a negative control. After amplification, the products were made to go through exo-sap purification and the purified 1 to 2 μL PCR products were subjected to PCR-sequencing using BigDye Terminator Cycle Sequencing Kit v3.1 and single primer. The amplified sequencing PCR products were further purified by ethanol, EDTA, and sodium acetate precipitation. All the experiments were repeated with the second primer, as well.
Treatment
All patients of spinal tuberculosis were treated with antituberculosis treatment regimen as per World Health Organization guidelines, for duration of 9 months. In the initial 2-month intensive phase, patients were kept on isoniazid (5 mg/kg/day; maximum dose 300 mg/day), rifampicin (10 mg/kg/day; maximum dose 600 mg/day), pyrazinamide (25 mg/kg; maximum dose 2 g/day), and ethambutol (15 mg/kg/day; maximum dose 1 g/day). The intensive phase was followed by a 7-month continuation phase in which patients were maintained on isoniazid and rifampicin.14
Follow-up
Each patient of spinal tuberculosis was followed for 6 months for outcome assessment. Poor outcome and good outcome were defined as modified Barthel index ≤12 and modified Barthel index >12 at 6 months, respectively.
Statistical Analysis
Statistical analysis was done using SPSS version 16.0 (Chicago, IL). Shapiro–Wilk test was employed for testing whether the data belonged to a normally distributed population or not. Categorical and continuous variables were expressed as percentages and mean ± standard deviation, respectively. Chi-square test was used to compare the categorical variables. Wild (W), heterozygous (H), and mutant (M) genotypes of polymorphisms of VDR and TLR-2 genes were compared using age and sex-adjusted odds ratio (OR), which was calculated using binary logistic regression. Allele frequencies were estimated using SNPStats (free web-based genetic epidemiology software).15 Binary logistic regression was used to study the factors independently associated with poor outcome. All P values <0.05 were considered as significant.
RESULTS
We enrolled 106 subjects each in 3 groups: spinal tuberculosis, pulmonary tuberculosis, and age and-sex-matched healthy controls. None of the patients was human immunodeficiency virus-positive. Baseline demographic, clinical, and radiological and laboratory characteristics of 106 patients with spinal tuberculosis were compared on the basis of modified Barthel index (≤12 as poor and >12 as good functional status); the results of the same are shown in Table 1 (P values in the bold font suggest that a statistically significant difference existed in the 2 groups, depending on the baseline characteristic). The algorithm of the study is depicted in Figure 1.
TABLE 1.
Baseline Demographic, Clinical, and Radiological Characteristics of 106 Patients of Spinal Tuberculosis

FIGURE 1.
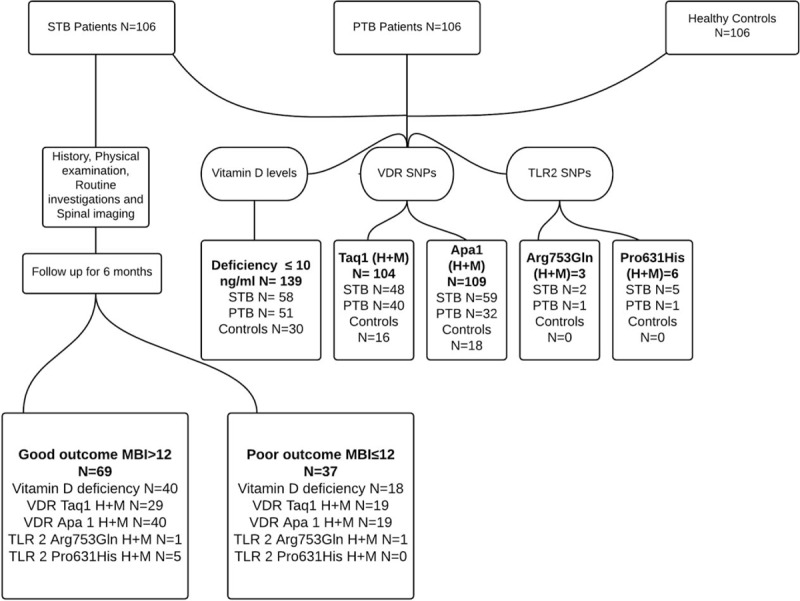
Flow diagram showing the study design. MBI = modified Barthel index, PTB = pulmonary tuberculosis, SNP = single-nucleotide polymorphism, STB = spinal tuberculosis, TLR = Toll-like receptor, VDR = vitamin D receptor.
Vitamin D Levels
Mean values of vitamin D (in ng/mL) were 11.118 ± 7.322, 12.964 ± 8.380, and 16.359 ± 7.991 in spinal tuberculosis, pulmonary tuberculosis, and healthy controls, respectively. Vitamin D deficiency frequency was significantly high in patients of spinal tuberculosis (P < 0.001) and pulmonary tuberculosis (P = 0.011) as compared with healthy controls (Table 2).
TABLE 2.
Vitamin D Status Among Spinal and Pulmonary Tuberculosis Patients, and Healthy Controls

VDR Gene Polymorphisms
The combined frequency of heterozygous (TC) and mutant (CC) genotypes (TC + CC) of VDR TaqI polymorphisms were significantly more in spinal tuberculosis (P < 0.001; OR = 4.74 [2.45–9.18]) and pulmonary tuberculosis (P < 0.001; OR 3.52 [1.80–6.88]) cases as compared with healthy controls. Likewise, combined frequency of heterozygous (TG) and mutant (GG) genotypes of VDR ApaI gene polymorphisms (TG + GG) were significantly more frequent in spinal tuberculosis (P < 0.001; OR 6.56 [3.41–12.61]) and pulmonary tuberculosis (P = 0.022; OR 2.13 [1.10–4.11]) cases as compared with controls. On comparison of data of spinal and pulmonary tuberculosis groups, heterozygous and mutant genotypes of VDR ApaI polymorphisms (TG + GG) were significantly more frequent in spinal tuberculosis (P < 0.001; OR 2.903 [1.651–5.105]), whereas no significant difference between the 2 arms was observed in heterozygous and mutant genotypes of VDR TaqI polymorphisms (TC + CC) (Table 3).
TABLE 3.
Distribution of Genotypes of VDR and TLR-2 Genes Among Spinal and Pulmonary Tuberculosis, and Healthy Controls
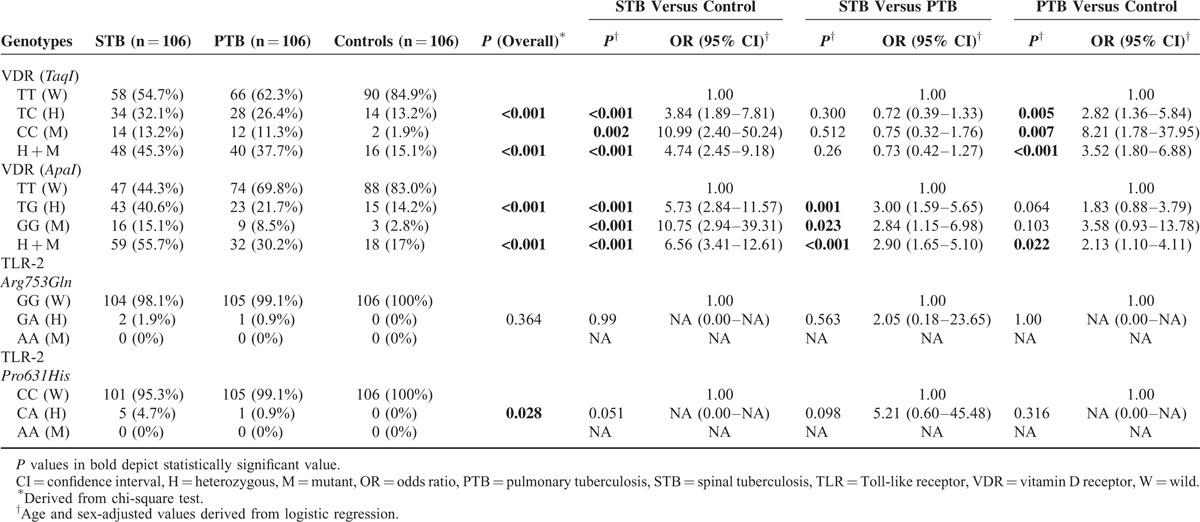
On genotypes analysis, heterozygous and mutant genotypes of VDR TaqI polymorphisms were significantly more common in spinal tuberculosis (P < 0.001; OR 3.84 [1.89–7.81] and P = 0.002; OR 10.99 [2.40–50.24], respectively) and pulmonary tuberculosis (P = 0.005; OR 2.82 [1.36–5.84] and P = 0.007; OR 8.21 [1.78–37.95], respectively) versus healthy controls. No significant difference was observed in the occurrence of heterozygous and mutant genotypes of VDR TaqI polymorphisms in 2 tuberculosis groups. VDR ApaI polymorphisms, heterozygous, and mutant genotypes were significantly more common in spinal tuberculosis (P < 0.001; OR 5.73 [2.84–11.57] and P < 0.001; OR 10.75 [2.94–39.31], respectively) as compared with healthy controls, whereas no significant difference was observed in pulmonary tuberculosis cases in comparison with controls. Heterozygous and mutant genotypes of VDR ApaI polymorphisms were significantly more common in spinal tuberculosis (P = 0.001; OR 3.00 [1.59–5.65] and P = 0.023; OR 2.84 [1.15–6.98], respectively) group in comparison with pulmonary tuberculosis group.
Comparison of allele frequencies revealed that C allele of TaqI polymorphisms was significantly more common in spinal tuberculosis and pulmonary tuberculosis groups (P < 0.001; OR 4.45 [2.52–7.84] and P < 0.001; OR 3.50 [1.97–6.22], respectively) as compared to healthy controls. Likewise, G allele of ApaI polymorphism was significantly common in spinal tuberculosis and pulmonary tuberculosis cases (P < 0.001; OR 4.97 [2.92–8.47] and P = 0.003; OR 2.18 [1.24–3.83], respectively) as compared with healthy controls. Further, on comparing spinal tuberculosis and pulmonary tuberculosis, G allele of Apa1 polymorphism was observed to be significantly associated with spinal tuberculosis cases (P < 0.001; OR 2.28 [1.46–3.55]).
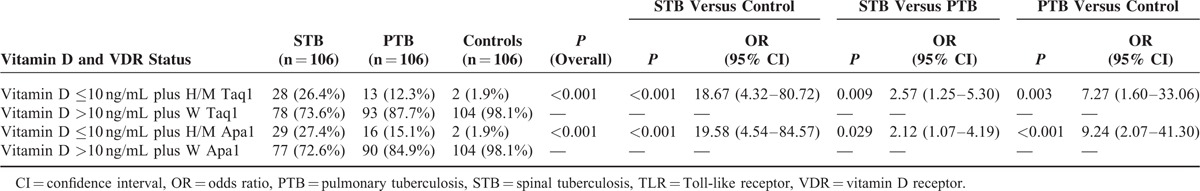
Number of individuals having both vitamin D deficiency and heterozygous and mutant genotypes of Taq1 polymorphism of VDR gene were significantly more prevalent in spinal tuberculosis in comparison with controls (P < 0.001) and pulmonary tuberculosis (P = 0.009). Number of individuals having both vitamin D deficiency and heterozygous and mutant genotypes of Taq1 polymorphism of VDR gene were significantly more prevalent in pulmonary tuberculosis in comparison with controls (P = 0.003). Similarly, number of individuals having both vitamin D deficiency and heterozygous and mutant genotypes of Apa1 polymorphism of VDR gene were significantly more prevalent in spinal tuberculosis in comparison with controls (P < 0.001) and pulmonary tuberculosis (P = 0.029); and number of individuals having both vitamin D deficiency and heterozygous and mutant genotypes of Apa1 polymorphism of VDR gene were significantly more prevalent in pulmonary tuberculosis in comparison with healthy controls (P < 0.001) (Table 4).
TABLE 4.
Combined Effect of Vitamin D Deficiency and VDR Polymorphisms in Patients With Spinal and Pulmonary Tuberculosis, and Healthy Controls
TLR-2 Gene Polymorphisms
We did not observe any mutant genotype of the polymorphisms of TLR-2, Arg753Gln and Pro631His, in any of the 3 arms. Heterozygous forms of Arg753Gln (GA) were observed in 2 cases of spinal tuberculosis group and 1 case of pulmonary tuberculosis group. On similar lines, heterozygous forms of Pro631His (CA) were observed in 5 cases of spinal tuberculosis group and 1 case of pulmonary tuberculosis group. All the differences were insignificant.
Vitamin D Levels, VDR and TLR-2 Gene Polymorphisms, and Baseline Disease Severity
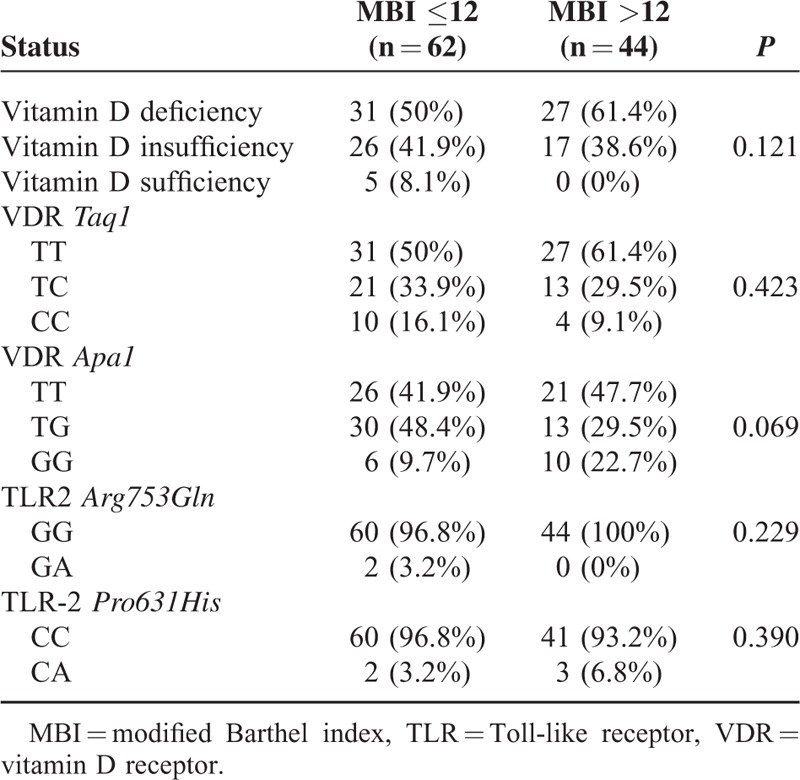
Vitamin D levels did not show any significant difference among the patients with good functional status (modified Barthel index >12) and those with poor functional status (modified Barthel index ≤12). Likewise, no significant difference was observed between the 2 subgroups in the frequencies of genotypes of VDR and TLR-2 polymorphisms (Table 5).
TABLE 5.
Distribution of Vitamin D Status and Polymorphisms According to Severity of Spinal Tuberculosis
Vitamin D Levels, VDR and TLR-2 Gene Polymorphisms, and 6 Months Outcome
On univariate analysis, the factors which were associated with poor outcome were fever (P < 0.001), quadriparesis (P = 0.002), spinal deformity (P < 0.001), constitutional symptoms (P = 0.003), bladder involvement (P = 0.016), disk involvement (P = 0.001), paraspinal collection (P = 0.023), raised ESR (P = 0.003), and involvement of cervical spine (P = 0.011). Multivariate analysis revealed that quadriparesis and spinal deformity were independent predictors of poor outcome. Vitamin D status and polymorphisms of VDR and TLR-2 genes did not affect the outcome.
DISCUSSION
We observed that amongst clinical parameters at baseline, a shorter duration of illness, paraparesis, quadriparesis, spinal deformity, and bladder involvement were significantly associated the poor functional status (modified Barthel index ≤12). Similarly, in terms of spinal imaging, involvement of cervical spine, involvement of disc, presence of paraspinal collection, narrowing of joint space, anterior subligamentous extension, and involvement of pedicles were significantly more prevalent in those with poor functional status.
We noted that the mean vitamin D level was lower and the frequency of vitamin D deficiency (≤10 ng/mL) was higher in patients with spinal tuberculosis and pulmonary tuberculosis as compared with controls. The association of vitamin D deficiency with increased risk of pulmonary tuberculosis is well documented; we observed similar association for spinal tuberculosis as well. A level of 10 ng/mL of 25-OHvitamin D in children is considered crucial to promote bone mineralization and calcium homeostasis. A concentration 20 to 50 ng/mL is considered sufficient for effective immune function.16 Results of a meta-analysis suggest that significantly increased risk of tuberculosis exists if the estimated serum vitamin D level is ≤12.5 nmol/L (pooled OR 4.6) or falls between 13 and 25 nmol/L (pooled OR 3.8).17 The immunomodulatory effects of 25-OHvitamin D against Mycobacterium are mediated by several mechanisms. Vitamin D enhances the innate immunity of the host by enhancing the production of many antimicrobial peptides, like cathelicidin (a family of proteins found in lysosomes of macrophages and polymorphonuclear leukocytes).18,19 The protective role of vitamin D against tuberculosis results from enhancement of innate immunity by several immune mechanisms like monocyte activation, T-cell suppression, and cytokine synthesis.3,20–23 In addition, vitamin D enhances the autophagy of the infected cells, thus restricting the intracellular replication of M tuberculosis in macrophages. Endoplasmic reticulum calcium induces autophagy when it is stimulated by vitamin D3.24
FokI polymorphism in VDR gene may be associated with the susceptibility to spinal tuberculosis. Zhang et al,8 in a Chinese population, demonstrated the association of spinal tuberculosis with ff genotype of FokI polymorphisms of VDR gene. Selvaraj et al,25 on an Indian population, have documented a significantly higher occurrence of ff genotype of FokI polymorphisms in patients with spinal tuberculosis. Our findings suggest that TaqI and ApaI polymorphisms of the VDR gene were also associated with spinal tuberculosis. Similar observations are also available for pulmonary tuberculosis. In our study, however, ApaI polymorphisms seemed to correlate more with spinal tuberculosis when compared with pulmonary tuberculosis. In an Indian study, TaqI polymorphism was found associated with high risk of smear-positive tuberculosis. In the same study, aa genotype of ApaI polymorphism was strongly associated with smear-positive tuberculosis in Muslim population. They have also reported an increased frequency of mutant tt genotype of TaqI VDR polymorphism in female Indian patients.26 The exact role of VDR-TaqI and VDR-ApaI genes, which are located near the 3 prime untranslated region of the VDR gene, is not precisely clear. Abnormalities in these genetic regions are likely to affect transcriptional regulation, mRNA stability, or protein translational efficiency, and thus, affect structure and functioning of VDR protein.27
The TLR-2 gene polymorphism is said to contribute to the risk of tuberculosis disease. We, however, did not find any significant association of TLR-2 polymorphisms with either pulmonary tuberculosis or spinal tuberculosis cases. Although studies demonstrate that there is a strong association between TLR-2 polymorphisms and the development of tuberculous meningitis and miliary tuberculosis, and indicate that TLR-2 responses influence the dissemination of M tuberculosis, no such analysis is present in reference to spinal tuberculosis.11 Our analysis of TLR-2 gene polymorphisms is novel in this regard, even though without a positive or a negative correlation.
An association between the VDR and TLR-2 receptor has been demonstrated. TLR-2 stimulation of macrophages induce the expression of VDR and the enzyme which catalyzes the conversion of 25-(OH)vitamin D3 to an active 1,25(OH)2 vitamin D3. The VDR and 1,25(OH)2 vitamin D3 complex then activates the gene encoding peptide cathelicidin (LL-37), leading to destruction of intracellular M tuberculosis.28
The control population in our study demonstrated a high percentage of people with insufficient levels (64.2%), and also those with deficiency (28.3%) of vitamin D. A hospital study catering to population from a similar geographic distribution also indicates that vitamin D insufficiency (38%) and deficiency (31%) exists in very high numbers.29 It shows that lower levels of vitamin D are highly prevalent in patients with pulmonary tuberculosis when compared to patients with other illnesses and healthy controls. Given the data that such low levels are detected in the apparently healthy population, a larger population seems to be at risk to develop one or the other form of tuberculosis. One need not, therefore, wonder why tuberculosis is a public health problem in India that is home to almost 25% of all new cases of tuberculosis occurring globally per year. In terms of absolute numbers, India has the highest prevalence, and also incidence in the world.30
Statistically, existence of a control arm takes care of changes in terms of errors in estimation or overall low (or high) levels of a variable of interest by computing the results in terms of relative levels rather than absolute levels. Results of a meta-analysis show that the odds of detecting vitamin D deficiency in patients with tuberculosis are very high if the level of vitamin D is ≤12.5 nmol/L.17 Thus, our results hold relevance even with lower levels of vitamin D in the control population, but may not predict the susceptibility of the control population which needs to be studied in the form of a cohort. For better understanding of the effect, a multicenter study sampling different geographic regions may be planned.
We conclude that vitamin D deficiency and VDR gene polymorphisms are more prevalent in patients with pulmonary tuberculosis and spinal tuberculosis. Their effect in isolation or in unison may confer susceptibility towards pulmonary and spinal tuberculosis.
Footnotes
Abbreviations: DNA = deoxyribonucleic acid, EDTA = ethylene diamine tetraacetic acid, mRNA = messenger RNA, PCR = polymerase chain reaction, TLR = Toll-like receptor, VDR = vitamin D receptor
AP, RKG, and HSM contributed equally in this work.
Conflict of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
- 1.Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med 2011; 34:440–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao L, Tao Y, Zhang L, et al. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis 2010; 14:15–23. [PubMed] [Google Scholar]
- 3.Selvaraj P, Harishankar M, Afsal K. Vitamin D: immuno-modulation and tuberculosis treatment. Can J Physiol Pharmacol 2015; 93:377–384. [DOI] [PubMed] [Google Scholar]
- 4.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006; 311:1770–1773. [DOI] [PubMed] [Google Scholar]
- 5.Gandini S, Gnagnarella P, Serrano D, et al. Vitamin D receptor polymorphisms and cancer. Adv Exp Med Biol 2014; 810:69–105. [DOI] [PubMed] [Google Scholar]
- 6.McClung JP, Karl JP. Vitamin D and stress fracture: the contribution of vitamin D receptor gene polymorphisms. Nutr Rev 2010; 68:365–369. [DOI] [PubMed] [Google Scholar]
- 7.Xu C, Tang P, Ding C, et al. Vitamin D receptor gene FOKI polymorphism contributes to increasing the risk of HIV-negative tuberculosis: evidence from a meta-analysis. PLoS One 2015; 10:e0140634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HQ, Deng A, Guo CF, et al. Association between FokI polymorphism in vitamin D receptor gene and susceptibility to spinal tuberculosis in Chinese Han population. Arch Med Res 2010; 41:46–49. [DOI] [PubMed] [Google Scholar]
- 9.Wang J-J, Xia X, Tang S-D, et al. Meta-analysis on the associations of TLR2 gene polymorphisms with pulmonary tuberculosis susceptibility among Asian populations. PloS One 2013; 8:e75090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caws M, Thwaites G, Dunstan S, et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog 2008; 4:e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thuong NT, Hawn TR, Thwaites GE, et al. A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun 2007; 8:422–428. [DOI] [PubMed] [Google Scholar]
- 12.Bhandari A, Garg RK, Malhotra HS, et al. Outcome assessment in conservatively managed patients with cervical spine tuberculosis. Spinal Cord 2014; 52:489–493. [DOI] [PubMed] [Google Scholar]
- 13.Ioana M, Ferwerda B, Plantinga TS, et al. Different patterns of Toll-like receptor 2 polymorphisms in populations of various ethnic and geographic origins. Infect Immun 2012; 80:1917–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Treatment of Tuberculosis: Guidelines. WHO/HTM/TB/2009.420. 4th ed. World Health Organization; 2012. Available at: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf; 2010 Accessed on January 9, 2016. [Google Scholar]
- 15.Sole X, Guino E, Valls J, et al. SNPStats: a web tool for the analysis of association studies. Bioinformatics 2006; 22:1928–1929. [DOI] [PubMed] [Google Scholar]
- 16.Esposito S, Lelii M. Vitamin D and respiratory tract infections in childhood. BMC Infect Dis 2015; 15:487.doi: 10.1186/s12879-015-1196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng J, Wu G, Yang W, et al. A serum vitamin D level < 25nmol/l pose high tuberculosis risk: a meta-analysis. PLoS One 2015; 10:e0126014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhan I, Camargo CA, Jr, Wenger J, et al. Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol 2011; 127:1302–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoo AL, Chai LY, Koenen HJ, et al. Vitamin D(3) down-regulates proinflammatory cytokine response to Mycobacterium tuberculosis through pattern recognition receptors while inducing protective cathelicidin production. Cytokine 2011; 55:294–300. [DOI] [PubMed] [Google Scholar]
- 20.Chandra G, Selvaraj P, Jawahar MS, et al. Effect of vitamin D3 on phagocytic potential of macrophages with live Mycobacterium tuberculosis and lymphoproliferative response in pulmonary tuberculosis. J Clin Immunol 2004; 24:249–257. [DOI] [PubMed] [Google Scholar]
- 21.Estrella JL, Kan-Sutton C, Gong X, et al. A novel in vitro human macrophage model to study the persistence of Mycobacterium tuberculosis using vitamin D(3) and retinoic acid activated THP-1 macrophages. Front Microbiol 2011; 2:67.doi: 10.3389/fmicb.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhalla AK, Amento EP, Serog B, et al. 1,25- Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol 1984; 133:1748–1754. [PubMed] [Google Scholar]
- 23.Vidyarani M, Selvaraj P, Jawahar MS, et al. 1, 25 Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine 2001; 40:128–134. [DOI] [PubMed] [Google Scholar]
- 24.Wu S, Sun J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov Med 2011; 11:325–335. [PMC free article] [PubMed] [Google Scholar]
- 25.Selvaraj P, Kurian SM, Chandra G, et al. Vitamin D receptor gene variants of BsmI, ApaI, TaqI, and FokI polymorphisms in spinal tuberculosis. Clin Genet 2004; 65:73–76. [DOI] [PubMed] [Google Scholar]
- 26.Sharma PR, Singh S, Jena M, et al. Coding and non-coding polymorphisms in VDR gene and susceptibility to pulmonary tuberculosis in tribes, castes and Muslims of Central India. Infect Genet Evol 2011; 11:1456–1461. [DOI] [PubMed] [Google Scholar]
- 27.Uitterlinden AG, Fang Y, van Meurs JB, et al. Vitamin D receptor gene polymorphisms in relation to vitamin D related disease states. J Steroid Biochem Mol Biol 2004; 89-90:187–193. [DOI] [PubMed] [Google Scholar]
- 28.Larcombe L, Orr P, Turner-Brannen E, et al. Effect of vitamin D supplementation on Mycobacterium tuberculosis-induced innate immune responses in a Canadian Dené First Nations Cohort. PLoS One 2012; 7:e40692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karoli R, Fatima J, Gupta SS, et al. Vitamin D deficiency in medical patients at a teaching hospital in North India. J Assoc Physicians India 2015; 63:35–39. [PubMed] [Google Scholar]
- 30.World Health Organization. Global tuberculosis report 2015. Geneva: World Health Organization; 2015. Available at: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf Accessed March 21, 2016. [Google Scholar]


