Abstract
The objective of this study was to examine the association of estimated glomerular filtration rate (eGFR) and urine albumin to creatinine ratio (ACR) with hearing impairment among diabetic adults in Korea. The study was based on data from Korea National Health and Nutrition Examination Survey 2011 to 2012. Participants were 1206 diabetic adults, aged over 19 years, who completed audiometric testing supervised by nationally certified clinicians. Hearing impairment was defined in three grades: no hearing impairment (pure-tone average 0–25 dB), slight hearing impairment (26–40 dB), and disabling hearing impairment (>40 dB) in the better ear at frequencies 0.5, 1, 2, 3, 4 and 6 kHz. Using logistic regression, risk of hearing impairment was assessed after having controlled for confounding factors. Higher levels of ACR and lower levels of eGFR correlated with an increase in percentage of disabling hearing impairment both unilaterally and bilaterally (P < 0.001). Controlling for possible confounding covariates, odds ratios for hearing impairment showed tendency to increase in higher ACR groups (P for trend = 0.029). Similar pattern was examined between eGFR and hearing impairment (P for trend = 0.006). Odds ratios were 1.981 (1.146, 3.424) for ACR Q4 and 2.773 (1.286, 5.983) for eGFR < 60 mL/min. Fall in eGFR and rise in ACR correlated with severity of hearing impairment. The association existed independently of age, sex, body mass index (BMI), smoking, drinking, exercise, new onset of diabetes, education, income, mental stress, noise exposure, and metabolic syndrome.
INTRODUCTION
Diabetes mellitus inflicts systemic damage in various parts of the human body, affecting eyes, kidneys, nerves, and vasculature. Diabetes-related hearing impairment is thought to involve sensorineural and microvascular damages. Transmission by the cochlear nerve and the eighth cranial nerve are impeded by neuropathic and microangiopathic changes common in diabetes, and hyperglycemic condition leads to microvascular damages and oxidative stress to the cochlear vasculature.1 Hypertension and metabolic syndrome that frequently accompany diabetes exacerbate such damages.
Kidney function has long been known as a prognostic factor of diabetes, associated with changes in glomerular filtration rate (GFR) and albumin to creatinine ratio (ACR).2,3 Microvascular damage occurs to an extent in both nephropathy and hearing impairment, especially in a condition of significant metabolic imbalance, as in the case of patients with diabetes. There have been several studies that have separately associated decreasing estimated glomerular filtration rate (eGFR) or increasing ACR with hearing impairment.4–7 There has not yet been a study that has sought to associate the stages of both eGFR and ACR with varying degrees of hearing impairment severity among patients with diabetes. The present paper will seek to confirm this association, and go on to assess whether it holds independently of demographic, socioeconomic, and clinical factors.
RESEARCH DESIGN AND METHODS
Study Population and Data Collection
The study was based on the Korea National Health and Nutrition Examination Survey (KNHANES), a cross-sectional health examination and survey supervised and conducted by Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention, and Division of Health and Nutrition Survey. It is a nationwide surveillance system that aims to monitor and ultimately improve health and nutritional status of the Korean population. KNHANES was initiated in 1998, and from then on was performed in the following sequence: KNHANES I (1998), KNHANES II (2001), KNHANES III (2005), KNHANES IV (2007–2009), KNHANES V (2010–2012), and KNHANES VI (2013–2015). The target population of KNHANES is noninstitutionalized Korean citizens residing in Korea, selected by stratified multi-stage clustered probability design. While KNHANES I to III had been conducted every 3 years and their data published in bulk, from KNHANES IV onwards data have been collected and assessed annually on an all-year-round basis by adopting the Rolling Sampling Survey method. Every year, 20 households are selected for each primary sampling unit, and 192 units are drawn from approximately 200,000 geographically defined primary sampling units. Health examination, health interview, and nutrition survey are proceeded after participants sign informed consent forms.
Data used in the present study were retrieved from KNHANES V, specifically from years 2011 to 2012. A total number of 16,576 individuals participated in the health interview and examination. From this number, 3717 individuals of below 19 years of age were excluded. Eleven thousand four hundred seventy eight non-diabetic individuals were excluded from the remaining 12,859 participants. Then, 175 cases of missing data were eliminated. The final study subjects were 1206 in number.
Measurements
eGFR was calculated using the modification of diet in renal disease study equation.8 ACR was obtained from random urine, most preferably first-voided spot urine. Pure-tone audiometric (PTA) testing was conducted using a SA203 audiometer inside a soundproof audiobooth positioned in the KNHANES mobile examination center. Trained otolaryngologists certified by the Korean Society of Otorhinolaryngology performed and supervised each test. Standard supra-auricular headphones and buttons were handed out to participants aged over 12 years, and instructions were given by clinicians on the testing method. Subjects were to push the button when they thought they heard a tone, and result transmission program would automatically send and record their responses. Air conduction thresholds were measured bilaterally, and the threshold was set at a point, which the subject responded to 50% of given tones. Subjects were tested on frequencies 0.5, 1, 2, 3, 4, and 6 kHz.
Definitions
Diabetes was defined as fasting blood glucose level ≥126 mg/dL (≥7.0 mmol/L), based on the diagnostic criteria published by World Health Organization. The definition also included respondents who were on diabetes medication and those who replied “yes” to having been diagnosed of diabetes by a doctor or health professional.
In terms of eGFR, subjects were allocated into three groups: eGFR ≥90 mL/min, 60 ≤ eGFR < 90, and eGFR < 60 mL/min. As for ACR, they were categorized into quartiles: Q1, Q2, Q3, and Q4. To note, the National Kidney Foundation defined normal and mildly increased range of ACR as <30 mg/g, moderately increased albuminuria as 30 to 300 mg/g, and severely increased albuminuria as >300 mg/g.
Hearing impairment was categorized in correspondence to severity, based on WHO's guideline on grades of hearing impairment. In total, subjects were identified into three grades: no hearing impairment (pure-tone average 0–25 dB), slight hearing impairment (26–40 dB), and disabling hearing impairment (>40 dB) in the better ear at frequencies 0.5, 1, 2, 3, 4 , and 6 kHz. KNHANES used PTA >40 dB as a defining criteria of hearing impairment.
Current smoker was defined as an individual in the habit of smoking, who has smoked ≥5 packs in a lifetime. Heavy drinking was defined as an average of 30 g/day of alcohol consumption per day. The lowest quartile of income was used as a representative index of the overall income. Exercise included moderate exercise of 5 days a week (≥30 minutes per day) and intensive exercise of 3 days a week (≥20 minutes per day). Definitions of mental health were based on participants’ answers to a questionnaire. Mental stress accounted for replies that reported significant stress in daily lives, melancholia for those that acknowledged to have undergone depression for over 2 consecutive weeks in the recent year, and suicidal ideation for those that admitted to having thought of committing suicide at least once in the recent year.
Noise exposure was analyzed in three categories, occupational, leisure-time, and momentary, and defined based on responses to a questionnaire. Occupational noise exposure was corresponded to those who replied that they have been in a working environment heavily exposed to loud sounds of machines or generators for more than 3 years. Leisure-time noise exposure was defined as any daytime noise exposure outside working environment. It included listening to high-volume music with earphones in a noisy environment, such as a bus or a subway. It also included noise exposure outside work of more than 5 hours a week, such as sounds of traffic, machines, karaoke, or concert music. Loud sounds of gunshots or explosions were identified as momentary noise exposure.
Hypertension was defined as systolic pressure ≥140 mm Hg and diastolic pressure ≥90 mm Hg, or as use of antihypertensive medication. Criteria by American Heart Association and the National Heart, Lung and Blood Institute & International Diabetes Federation was used in definition of metabolic syndrome.
Statistical Analyses
Statistical analyses were performed using the SAS 9.2 software (SAS institute, Inc., Cary, NC), combining two separate data from KNHANES 2011 and 2012. Clinical characteristics of study subjects were presented as mean ± standard error (SE) or as %(SE). P values of less than 0.05 were considered as statistically significant. Correlation analyses were utilized to assess and visualize relation between hearing impairment severity and fall in eGFR or rise in ACR. Logarithmic transformation was performed in analyzing ACR to normally distribute its variables. Age-, sex-adjusted regression analysis was then used to examine correlation that eGFR, log albumin to creatinine ratio (logACR), and glycated hemoglobin (HbA1C) hold for different degrees of frequencies. Multivariate logistic regression analysis was used after controlling for demographic, socioeconomic, and clinical factors. Odds ratios (OR) were calculated for every eGFR stage and ACR quartile, and individual ORs of all factors controlled for in the multivariate model were included for further information.
RESULTS
Baseline Characteristics
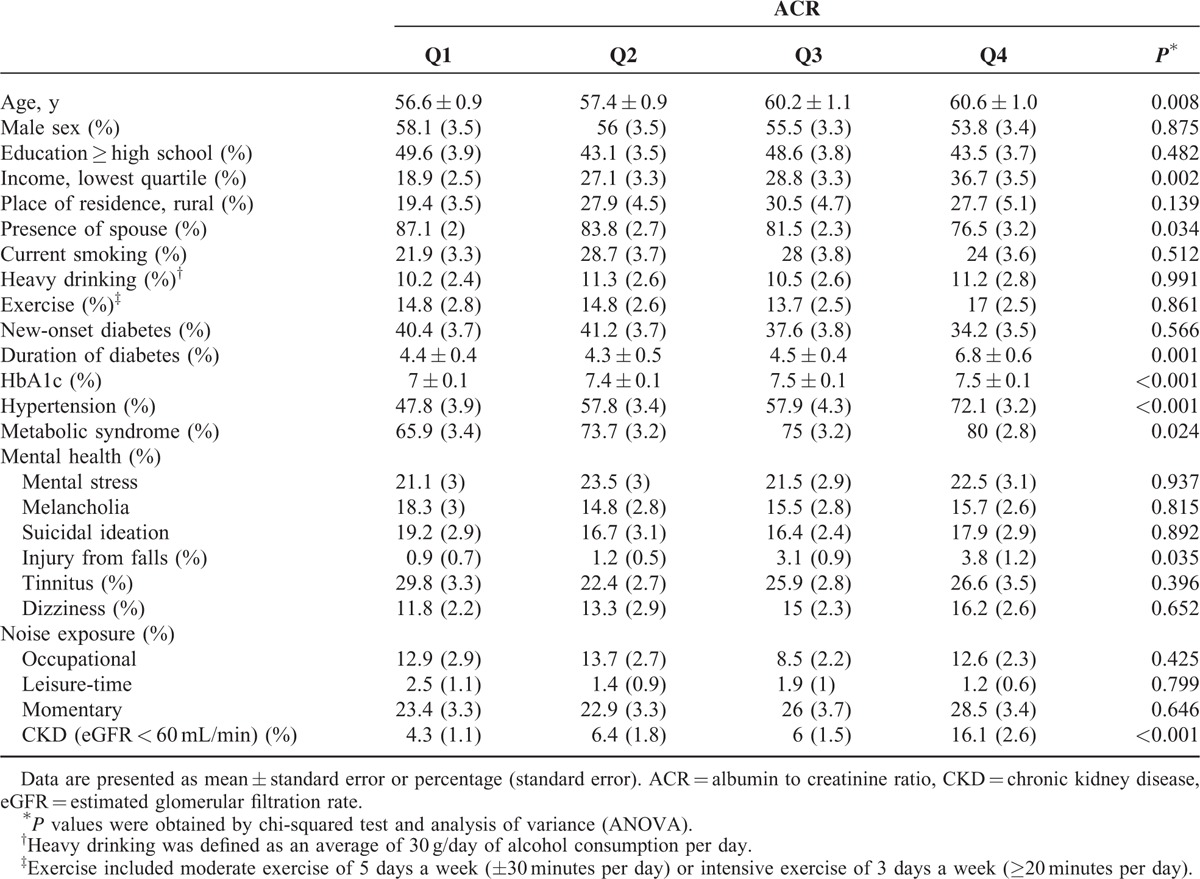
Mean age of the subjects was 58.6 ± 0.5. Mean duration of diabetes was 4.9 ± 0.2 years and mean HbA1C was 7.3 ± 0.1%. Clinical parameters of study participants are compared among ACR quartiles (Q1−Q4) in Table 1. Age, the lowest quartile of income, presence of spouse, duration of diabetes, HbA1C, hypertension, metabolic syndrome, injury from falls, and diagnosis of Chronic Kidney Disease (eGFR <60 mL/min) displayed statistical significance (P < 0.05) in relation to levels of ACR.
TABLE 1.
Baseline Characteristics of the Korean Adults with Diabetes Aged over 19 Years by ACR Quartiles From Q1 to Q4, KNHANES 2011, 2012 (n = 1206)
Severity of Hearing Impairment
In Figure 1a and b, percentage of subjects with PTA ≤40 dB in both ears decreased from GFR ≥90 mL/min to GFR <60 mL/min and also from Q1 to Q4. As for eGFR, both unilateral and bilateral PTA >40 dB displayed increases from the lower stage to the next. ACR Q3 to Q4 showed higher percentages of bilateral PTA >40 dB than Q1, and the percentages increased from Q3 to Q4. ACR Q2 to Q4 displayed higher percentages of unilateral PTA >40 dB than Q1. In Figure 1c and d, percentage of subjects without hearing impairment (0–25 dB) in both ears steadily decreased from a lower eGFR stage to the next. On the contrary, percentage of those with slight hearing impairment (26–40 dB) and disabling hearing impairment (>40 dB) showed a gradual increase. Similarly, from ACR Q1 onwards, percentage of subjects without hearing impairment showed a consecutive decrease, while those with disabling hearing impairment increased.
FIGURE 1.
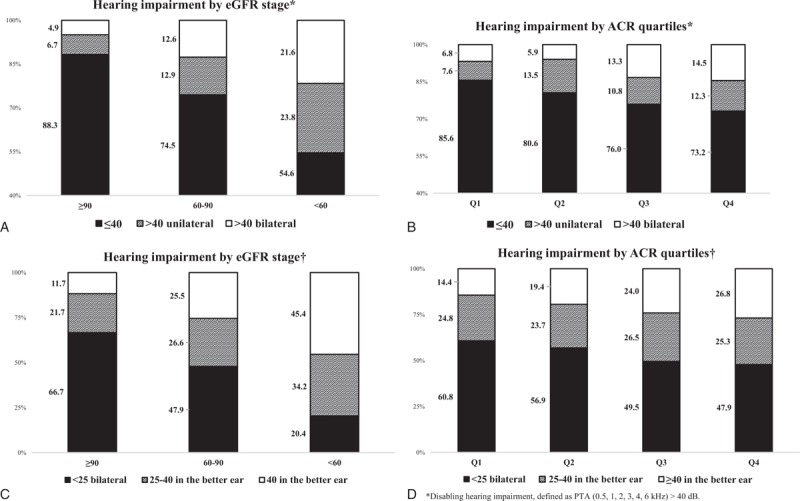
Prevalence of hearing impairment in accordance with eGFR stage and ACR quartiles. eGFR = estimated glomerular filtration rate, ACR = albumin to creatinine ratio.
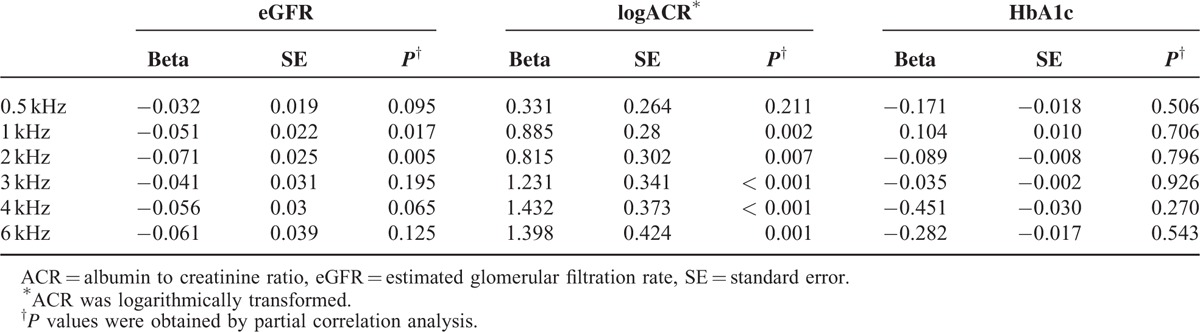
Frequencies
In Table 2, eGFR showed negative regression coefficient for all frequencies after having been adjusted for age and sex, while logACR showed positive regression coefficient. eGFR showed statistical significance at frequencies 1 and 2 kHz and logACR at frequencies 1, 2, 3, 4 and 6 kHz (P < 0.05). logACR generally showed stronger correlation in higher frequencies, but clear-cut patterns were not observed. Hearing impairment existed across all frequencies in relation to eGFR and ACR. HbA1c was not associated with results of pure tone audiometric test in all hearing frequencies.
TABLE 2.
The Age- and Sex-Adjusted Partial Correlation of eGFR, logACR, and HbA1c With Results of Pure Tone Audiometric Test in all Hearing Frequencies
Confounder Adjustment
Calculations on Table 3 had been based on the definition of disabling hearing impairment, as being PTA >40 dB hearing level threshold (KNHANES criteria). For independent variables ACR and eGFR, ORs (95% CI) were calculated after having controlled for various confounding factors. The controlled factors were: age, sex, BMI, current smoking, heavy drinking, exercise, new-onset diabetes, education, income, mental stress, noise exposure, and metabolic syndrome.
TABLE 3.
Multivariable-Adjusted Odds Ratios (95% CI) for the Association of Hearing Impairment Holds with ACR Quartiles and eGFR Stages in Korean Adults with Diabetes Aged Over 19 years, KNHANES 2011, 2012 (n = 1206)
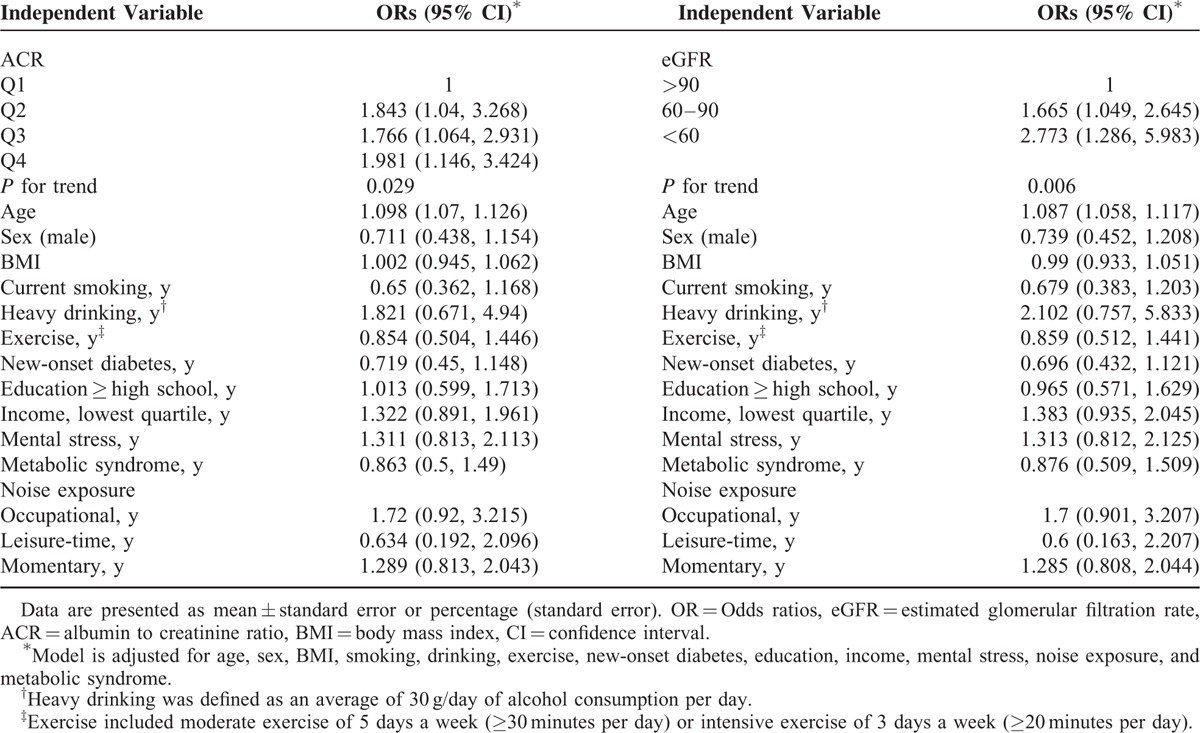
As for ACR, trends in values of OR were analyzed in quartiles, from Q1 to Q4, the reference group being Q1. Higher quartiles of ACR (Q2–4) had higher ORs than Q1. Although there had been a slight decrease in OR from Q2 to Q3, Q4 notably held the highest OR among all quartiles (P = 0.029).
For eGFR, the reference group was eGFR >90 mL/min. ORs showed a continuous increase going from eGFR >90 to eGFR <60 mL/min, the values reaching notably high for eGFR <60 mL/min (OR = 2.773) (P = 0.006).
ORs (95% CI) of the controlled factors have been displayed in Table 3 as well. With the exception of age, ORs of all controlled factors were found to be statistically insignificant. Meanwhile, age showed only a slight increase in OR.
CONCLUSIONS
In this KNHANES-based study focused on diabetic patients, correlation was found between kidney function deterioration and hearing impairment severity. It was indicated from the results that eGFR and ACR maintain their relevance to hearing impairment even after controlling for age, sex, BMI, current smoking, heavy drinking, exercise, new-onset diabetes, education, income, mental stress, noise exposure, and metabolic syndrome.
A number of studies are in line with our results. Vilayur et al4,9 demonstrated that moderate CKD was independently associated with hearing loss. Presence of metabolic syndrome or CKD was also found to increase hearing level thresholds in a study conducted on Koreans.6 Lin et al10 found that comorbidity of diabetes hinders improvement from idiopathic sudden sensorineural hearing loss. Urine albumin excretion rate and serum creatinine levels were also associated with severity of hearing impairment.7,11
Exact mechanism underlying association between nephropathy and hearing impairment still remains uncertain. Glomeruli and the inner ear share similar physiologic structures in transporting fluids and electrolytes, and both have microvasculature sensitive to changes in blood flow and pressure.12,13 Effects of uremia and electrolyte imbalances have also been used to explain both nephropathy and hearing impairment.14,15 Cosgrove et al16 had suggested that prevalence of otopathology in patients with Alport Syndrome may be due to similar antigenic structure between basement membranes of glomeruli and cochlea.
Diabetes-related hearing loss involves sensorineural and microvascular damages that disrupt transmission of auditory signals from the cochlea to the brain. Examination of the autopsied diabetic patients revealed atrophy of the spiral ganglion and demyelination of the eighth cranial nerve.17 Microvascular endothelial damage that leads to nephropathy and retinopathy in diabetic patients may also inflict damages to the cochlear vasculature. Autopsies on diabetic patients presented thickened basilar membranes, microangiopathic changes to stria vascularis, and luminal narrowing of the internal auditory artery.17,18
Diabetic patients suffer from many other comorbid diseases, such as hypertension, peripheral arterial disease, and atherosclerosis.19,20 Diabetes and hypertension can have synergistic effects on hearing loss, exacerbating microvascular damage, and cochlear ischemia.21 Diabetes-related hearing loss co-exists with an atherosclerotic mechanism. C-reactive protein was found to mediate relationship between diabetes and hearing impairment, indicating an inflammatory process to sensorineural hearing loss.1 Such conditions impair the kidney as well, injuring its filtration, and clearance functions.
Hypertension is both the risk factor and the consequence of renal insufficiency.22 Cardiovascular mortality was doubled in patients with Stage 3 CKD, and tripled in patients with Stage 4 CKD; lower eGFR and higher albuminuria were associated with risk of cardiovascular disease.23,24 Kidney also is the major clearance site of uremic toxin and advanced glycation end products (AGEs), and failure of renal function leads to AGE accumulation and to unimpeded oxidative stress and damage.25 Yuan et al26 reported that CKD, hypertension, and dyslipidemia had cumulative effects on carotid atherosclerosis in patients with type 2 diabetes. Diabetes and its comorbidities work as risk factors of renal insufficiency, but are in turn heightened in consequence. Intensification of comorbidities and accumulation of toxic materials further damages the auditory function. Results from the present study show that hearing impairment increases in severity as renal function deteriorates. As Dalton et al27 found, patients with diabetic nephropathy were more likely to develop hearing impairment than those with diabetes alone.
A number of studies have reported that bilateral hearing loss seems to be associated more with diffuse or systemic exposure, while unilateral hearing loss with more focal processes.28,29 Bilateral hearing loss is a rarer and more serious form of the two, and the differences may be examined in relation to severity of diabetes or CKD.29 In this study, both unilateral and bilateral PTA >40 dB hearing impairment correlated with higher degrees of renal insufficiency.
Diabetes-related hearing impairment was found to be negatively correlated with eGFR and positively with ACR across all frequencies, according to the present study's results. There were inconsistencies in prior studies regarding hearing frequencies in diabetic patients. Different studies attributed high- or low-frequency hearing loss to diabetes-related hearing impairment, and some studies have reported hearing loss across all frequencies.30 Age-related hearing loss starts in high frequencies and progresses to low frequencies, and differences in mean age of target population may have led to such wide discrepancies among previous studies. After adjusting for age in Table 2, our results were consistent with Bainbridge et al31 that reported diabetes-related hearing impairment across all frequencies.
Even after adjustment of various confounding factors in Table 3, eGFR and ACR remained to be significantly associated with severity of hearing impairment. Some of these factors were reported to be risk factors of hearing impairment as a whole, including history of diabetes, heavy smoking, noise exposure, cardiovascular risks, and unhealthy eating.28,32–35 Although there were conflicting findings among studies, they seemed to agree on the fact that metabolic imbalances affect auditory functions. Risk factors of diabetes-related hearing impairment included low high density lipoprotein cholesterol, coronary heart disease, peripheral neuropathy, and poor health.1 Hearing impairment in diabetic patients was also said to be mediated by neuropathy, microangiopathy, inflammation, and hyperglycemia.36 Age was also an important confounding factor. Hearing impairment generally increases with age, and presbycusis is ranked as the most common cause of hearing loss.37 Therefore, it was essential to distinguish between impairment caused naturally by age and by diabetic nephropathy. These previously confirmed confounding factors did not affect the correlation between renal dysfunction and auditory impairment in this study.
The present study is not without limitations. Due to cross-sectional design of this study, a causal relationship cannot be established between renal dysfunction and hearing impairment. ACR is subject to day-to-day intra-individual variability, and inaccuracies are likely to exist. The study did not control for medications adversely affecting hearing loss, and the definition of diabetes included those who self-reported to having been diagnosed with diabetes. There is room for more research in the subject of this study. As the study was based on a target population of over 19 years of age, it is yet to be examined whether the correlation of kidney function and hearing impairment holds for young diabetic patients as well. Duration and severity of diabetes may also be tied into this study. Advantages of the present study are in its large sample size and credible data source.
In conclusion, an increase in severity of hearing impairment is associated with a decline in renal function for diabetic patients. Clinicians caring for patients with diabetes need to note the possibility that a fall in eGFR and a rise in ACR may correlate with worsening auditory function. The correlation stands independently of confounding factors such as age, sex, BMI, current smoking, heavy drinking, exercise, new-onset diabetes, education, income, mental stress, noise exposure, and metabolic syndrome.
Footnotes
Abbreviations: ACR = urine albumin to creatinine ratio, BMI = body mass index, CI = confidence interval, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, KNHANES = Korean National Health and Nutrition Examination Survey, OR = odd ratio, PTA test = pure tone audiometric test
DHK and JC contributed equally to this study and are both corresponding authors.This research was not supported by any funding.
The authors declare no conflicts of interest.
REFERENCES
- 1.Bainbridge KE, Cheng YJ, Cowie CC. Potential mediators of diabetes-related hearing impairment in the U.S. population: National Health and Nutrition Examination Survey 1999-2004. Diabetes Care 2010; 33:811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James MT, Grams ME, Woodward M, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis 2015; 66:602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berhane AM, Weil EJ, Knowler WC, et al. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol 2011; 6:2444–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilayur E, Gopinath B, Harris DC, et al. The association between reduced GFR and hearing loss: a cross-sectional population-based study. Am J Kidney Dis 2010; 56:661–669. [DOI] [PubMed] [Google Scholar]
- 5.Jamaldeen J, Basheer A, Sarma AC, et al. Prevalence and patterns of hearing loss among chronic kidney disease patients undergoing haemodialysis. Australas Med J 2015; 8:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang SH, Jung da J, Cho KH, et al. The association between metabolic syndrome or chronic kidney disease and hearing thresholds in Koreans: the Korean National Health and Nutrition Examination Survey 2009-2012. PLoS One 2015; 10:e0120372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen FC, Hsieh CJ. Severity of hearing impairment is positively associated with urine albumin excretion rate in patients with type 2 diabetes. J Diabetes Investig 2014; 5:743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145:247–254. [DOI] [PubMed] [Google Scholar]
- 9.Jakic M, Mihaljevic D, Zibar L, et al. Sensorineural hearing loss in hemodialysis patients. Coll Antropol 2010; 34 Suppl 1:165–171. [PubMed] [Google Scholar]
- 10.Lin CF, Lee KJ, Yu SS, et al. Effect of comorbid diabetes and hypercholesterolemia on the prognosis of idiopathic sudden sensorineural hearing loss. Laryngoscope 2016; 126:142–149. [DOI] [PubMed] [Google Scholar]
- 11.Kakarlapudi V, Sawyer R, Staecker H. The effect of diabetes on sensorineural hearing loss. Otol Neurotol 2003; 24:382–386. [DOI] [PubMed] [Google Scholar]
- 12.Arnold W. Inner ear and renal diseases. Ann Otol Rhinol Laryngol Suppl 1984; 112:119–124. [DOI] [PubMed] [Google Scholar]
- 13.Thodi C, Thodis E, Danielides V, et al. Hearing in renal failure. Nephrol Dial Transplant 2006; 21:3023–3030. [DOI] [PubMed] [Google Scholar]
- 14.Adler D, Fiehn W, Ritz E. Inhibition of Na+,K+-stimulated ATPase in the cochlea of the guinea pig. A potential cause of disturbed inner ear function in terminal renal failure. Acta Otolaryngol 1980; 90:55–60. [DOI] [PubMed] [Google Scholar]
- 15.Albertazzi A, Cappelli P, Di Marco T, et al. The natural history of uremic neuropathy. Contrib Nephrol 1988; 65:130–137. [DOI] [PubMed] [Google Scholar]
- 16.Cosgrove D, Samuelson G, Meehan DT, et al. Ultrastructural, physiological, and molecular defects in the inner ear of a gene-knockout mouse model for autosomal Alport syndrome. Hear Res 1998; 121:84–98. [DOI] [PubMed] [Google Scholar]
- 17.Makishima K, Tanaka K. Pathological changes of the inner ear and central auditory pathway in diabetics. Ann Otol Rhinol Laryngol 1971; 80:218–228. [DOI] [PubMed] [Google Scholar]
- 18.Wackym PA, Linthicum FH., Jr Diabetes mellitus and hearing loss: clinical and histopathologic relationships. Am J Otol 1986; 7:176–182. [PubMed] [Google Scholar]
- 19.American Diabetes Association Peripheral arterial disease in people with diabetes. Diabetes Care 2003; 26:3333–3341. [DOI] [PubMed] [Google Scholar]
- 20.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002; 287:2570–2581. [DOI] [PubMed] [Google Scholar]
- 21.Duck SW, Prazma J, Bennett PS, et al. Interaction between hypertension and diabetes mellitus in the pathogenesis of sensorineural hearing loss. Laryngoscope 1997; 107:1596–1605. [DOI] [PubMed] [Google Scholar]
- 22.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382:339–352. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352. [DOI] [PubMed] [Google Scholar]
- 25.Modaresi A, Nafar M, Sahraei Z. Oxidative stress in chronic kidney disease. Iran J Kidney Dis 2015; 9:165–179. [PubMed] [Google Scholar]
- 26.Yuan C, Lai CW, Chan LW, et al. Cumulative effects of hypertension, dyslipidemia, and chronic kidney disease on carotid atherosclerosis in Chinese patients with type 2 diabetes mellitus. J Diabetes Res 2014; 2014:179686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalton DS, Cruickshanks KJ, Klein R, et al. Association of NIDDM and hearing loss. Diabetes Care 1998; 21:1540–1544. [DOI] [PubMed] [Google Scholar]
- 28.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999-2004. Arch Intern Med 2008; 168:1522–1530. [DOI] [PubMed] [Google Scholar]
- 29.Sara SA, Teh BM, Friedland P. Bilateral sudden sensorineural hearing loss: review. J Laryngol Otol 2014; 128 Suppl 1:S8–S15. [DOI] [PubMed] [Google Scholar]
- 30.Hong O, Buss J, Thomas E. Type 2 diabetes and hearing loss. Dis Mon 2013; 59:139–146. [DOI] [PubMed] [Google Scholar]
- 31.Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med 2008; 149:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol 2009; 30:139–145. [DOI] [PubMed] [Google Scholar]
- 33.Ciletti L, Flamme GA. Prevalence of hearing impairment by gender and audiometric configuration: results from the National Health and Nutrition Examination Survey (1999-2004) and the Keokuk County Rural Health Study (1994-1998). J Am Acad Audiol 2008; 19:672–685. [DOI] [PubMed] [Google Scholar]
- 34.Lohi V, Hannula S, Ohtonen P, et al. Hearing impairment among adults: the impact of cardiovascular diseases and cardiovascular risk factors. Int J Audiol 2015; 54:265–273. [DOI] [PubMed] [Google Scholar]
- 35.Shargorodsky J, Curhan SG, Eavey R, et al. A prospective study of cardiovascular risk factors and incident hearing loss in men. Laryngoscope 2010; 120:1887–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bainbridge KE, Hoffman HJ, Cowie CC. Risk factors for hearing impairment among U.S. adults with diabetes: National Health and Nutrition Examination Survey 1999-2004. Diabetes Care 2011; 34:1540–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horikawa C, Kodama S, Tanaka S, et al. Diabetes and risk of hearing impairment in adults: a meta-analysis. J Clin Endocrinol Metab 2013; 98:51–58. [DOI] [PubMed] [Google Scholar]


