Supplemental Digital Content is available in the text
Abstract
Angina pectoris is a treatable symptom that is associated with mortality and decreased quality of life. Angina eradication is a primary care goal of care after an acute myocardial infarction (AMI). Our aim was to evaluate factors influencing angina pectoris 1 year after an AMI.
From January 2005 to December 2013, 1547 patient received primary percutaneous intervention in our hospital for an acute ST-segment elevation myocardial infarction (MI). Of these patients, 1336 patients did not experience post-MI angina during a 1-year follow-up, and 211 patients did. Univariate and multivariate logistic regression analyses were performed to identify the factors influencing angina pectoris 1 year after an AMI. Propensity score matched analyses were performed for subgroups analyses.
The average age of the patients was 61.08 ± 12.77 years, with a range of 25 to 97 years, and 82.9% of the patients were male. During 1-year follow-up, 13.6% of the patients experienced post-MI angina. There was a longer chest pain-to-reperfusion time in the post-MI angina group (P = 0.01), as well as a higher fasting sugar level, glycohemoglobin (HbA1C), serum creatinine, troponin-I and creatine kinase MB (CK-MB). The post-MI angina group also had a higher prevalence of multiple-vessel disease. Manual thrombectomy, and distal protective device and intracoronary glycoprotein IIb/IIIa inhibitor injection were used frequently in the no post-MI angina group. Antiplatelet agents and post-MI medication usage were similar between the 2 groups. Multivariate logistic regression analyses demonstrated that prior MI was a positive independent predictor of occurrence of post-MI angina. Manual thrombectomy use and drug-eluting stent implantation were negative independent predictors of post-MI angina. Higher troponin-I and longer chest pain-to-reperfusion time exhibited a trend toward predicting post-MI angina.
Prior MIs were strong, independent predictors of post-MI angina. Manual thrombectomy and drug-eluting stent implantation could decrease the occurrence of angina pectoris 1 year after an AMI, decrease long-term healthy costs, and increase post-MI quality of life.
INTRODUCTION
Treating angina symptoms is a critical goal for myocardial infarction (MI) patients, during the acute stage of an MI and for post-MI medical care. Post-MI angina has been associated with adverse cardiac outcomes, including poor performance function, decreased health-related quality of life, recurrent MI, and mortality.1,2 According to a previous study, the prevalence of post-MI angina was 19.9% during a 1-year of follow-up period.3 Identifying the population with post-MI angina is an important first step in treating residual angina, gaining a more complete understanding of those patients who are at the greatest risk for post-MI angina, and developing a management plan that could decrease the occurrence of post-MI angina after AMI.
To address this gap in the published knowledge, we examined the prevalence and factors associated with angina 1 year after AMI, and identified important predictive factors.
METHODS
Patient Collection and Groups
From January 2005 to December 2013, 1547 patients received primary percutaneous intervention (PCI) in our hospital for an acute ST-segment elevation myocardial infarction. During a 1-year follow-up, 1336 patients did not experience post-MI angina. This group was considered the no post-MI angina group. The remaining 211 patients experienced post-MI angina and were as the post-MI angina group. General demographics, risk factors, the severity of MI, timing of primary PCI, laboratory examination, infarcted territory, characteristics of coronary artery disease, primary PCI angiography, and pre- and post-MI medication were compared between the 2 groups. Univariate and multivariate logistic regression analyses were performed to identify factors influencing angina pectoris 1 year after an AMI. Propensity score matched analyses were performed for subgroup analyses of influenced factors.
Different Strategies for Acute ST-Segment Elevation Myocardial Infarction With a Thrombus
In our insurance system, the aspiration catheter used for acute ST-segment elevation MI (STEMI) and thrombus suction was provided for usage since July, 2009. After that, the rate of the aspiration catheter used for acute STEMI increased from 42.2% to 61.1% at our hospital. The ESC and AHA/ACC Guidelines on myocardial revascularization list aspiration thrombectomy during primary PCI in STEMI, as a Class IIa, level of evidence-B, indication.4,5 Therefore, aspiration thrombuctomy was performed for primary PCI in STEMI according to a visible thrombus, coronary flow below TIMI 2 flow, and the operator's decision. In addition, aspiration thrombectomy was not performed if the infarcted artery was relatively small or if the aspiration catheter could not pass through the target lesion.
According to ESC and AHA/ACC guidelines for STEMI, glycoprotein (GP) IIb/IIIa inhibitor usage is listed as a Class IIa, level of evidence-C, indication when there is angiographic evidence of a massive thrombus, slow or no-reflow, or a thrombotic complication.4,5 At our hospital, additional intracoronary GP IIb/IIIa inhibitor injection was used for the patients with subtotal or total occlusion of infarct-related arteries with heavy thrombus-burden plaques. At our hospital, intracoronary tirofiban injection was done at a high bolus dosage of 25 ug/kg after thrombus aspiration and was then given intravenously at a maintenance dosage of 0.15 ug/kg/min for 24 hours.
According to ESC and AHA/ACC guidelines for STEMI, the routine use of distal protection devices is listed as a Class III, level of evidence-C, indication.4,5 Therefore, the distal protection device PercuSurge GuardWire (Medtronic AVE, Santa Rosa, CA) was used according to the operator's experience and in patients with a vessel size greater than 3.5 mm and with high thrombus burden at our hospital.
Comprehensive inpatient and outpatient data were collected from medical record abstractions and patient interviews were collected. The Institutional Review Committee on Human Research at our institution approved the study protocol.
Definitions
Typical angina was characterized by at least 1 of the following: chest pain occurring at rest or during minimal exertion and usually lasting less than 20 minutes (without nitroglycerin administration), new onset of severe flank pain (i.e., within 1 month), or a crescendo pattern of chest pain (more severe, prolonged, or at an increased frequency than previously experienced).
Study End-Points
The primary end-point of this study was angina pectoris during 1 year of follow-up. This was defined as visits to emergency departments or re-admissions for angina 1 year after a primary PCI.
Statistical Analysis
Data are expressed as percentages and means ± standard deviations. Categorical variables were compared using a χ2 test. Continuous variables were compared using an analysis of t test. Continuous variables among the 2 groups were analyzed using a 2-way analysis of variance.
Univariate and multivariate logistic regression analyses were performed to identify the significant factors influencing angina pectoris 1 year after an AMI. A univariate logistic regression analysis was used to examine the candidate factors that were associated with 1-year angina. If the candidate factors had a significant difference by univariate logistic regression or a linear regression test, we entered the factors of prior MI, peak troponin-I level, chest pain-to-reperfusion time, multiple-vessel disease, manual thrombectomy, and drug-eluting stents (DES) implantation into the multivariate logistic regression analysis to identify factors associated with angina pectoris 1 year after an AMI. All tests for statistical significance were 2-tailed with a P value of less than 0.05.
In order to advance the evaluation of the efficacy of manual thrombectomy and the utility of adjunctive administration of GP IIb/IIIa inhibitors, subgroups analyses were performed. Because the patients were not randomly assigned, there was some bias in baseline characteristics. In order to figure out the clinical effect of manual thrombectomy, the patients on whom distal protection and intracoronary GP IIb/IIIa inhibitor were injected were initially excluded and a propensity score matched analysis was performed as a 2-to-1 matched analysis using a logistic regression model for nonthrombectomy versus thrombectomy to adjust for differences in baseline characteristics (Supplemental Table 1). Using the estimated logits, the nonthrombectomy group and the thrombectomy group had the closest estimated logit value. The baseline covariates were compared between these 2 groups and were similar. In order to explore the clinical effect of intracoronary GP IIb/IIIa injection, we included the patients who had a total or subtotal occlusion with a high thrombus burden and excluded the patients with whom distal protection was used. A propensity score matched analysis was performed as a 1-to-1 matched analysis using a logistic regression model for the thrombectomy alone group versus the thrombectomy plus intracoronary GP IIb/IIIa injection group to adjust for differences in baseline characteristics (Supplemental Table 2). Using the estimated logits, the no intracoronary GP IIb/IIIa injection group versus the intracoronary GP IIb/IIIa injection group had the closest estimated logit value. The baseline covariates were compared between these 2 groups and were similar.
All statistical analyses were performed using SPSS 22.0 software (SPSS Inc, Chicago, IL).
RESULTS
Baseline Characteristics of Study Patients
Table 1 shows the baseline characteristics of the study patients. The average age of the patients was 61.08 ± 12.77 years, with a range of 25 to 97 years, and 82.9% of the patients were male. During a 1-year follow-up, 13.6% of patients experienced post-MI angina. The age and the percentage of males were similar between the 2 groups. The percentage of patients with hypertension, diabetes, prior MI, end stage renal disease (ESRD), and who were smokers was higher in the post-MI angina group. The percentage of patients with a prior MI was significantly different between the groups (6.2% vs 15.2%, no post-MI angina vs post-MI angina groups, P < 0.001). Systolic blood pressure (SBP) was similar between the 2 groups. The percentage of patients who had a Killip class ≧3 was higher in the post-MI angina group, but the difference did not reach significance (P = 0.147). The timing of a primary PCI as being a holiday PCI, reperfusion time and door-to-needle time were similar between the 2 groups. There was a longer chest pain-to-reperfusion time in the post-MI angina group (233.28 ± 230.65 vs 283.76 ± 408.63 minutes, no post-MI angina vs post-MI angina groups, P = 0.01).
TABLE 1.
Patient Characteristics by Presence or Absence of 1-Yr Post-MI Angina
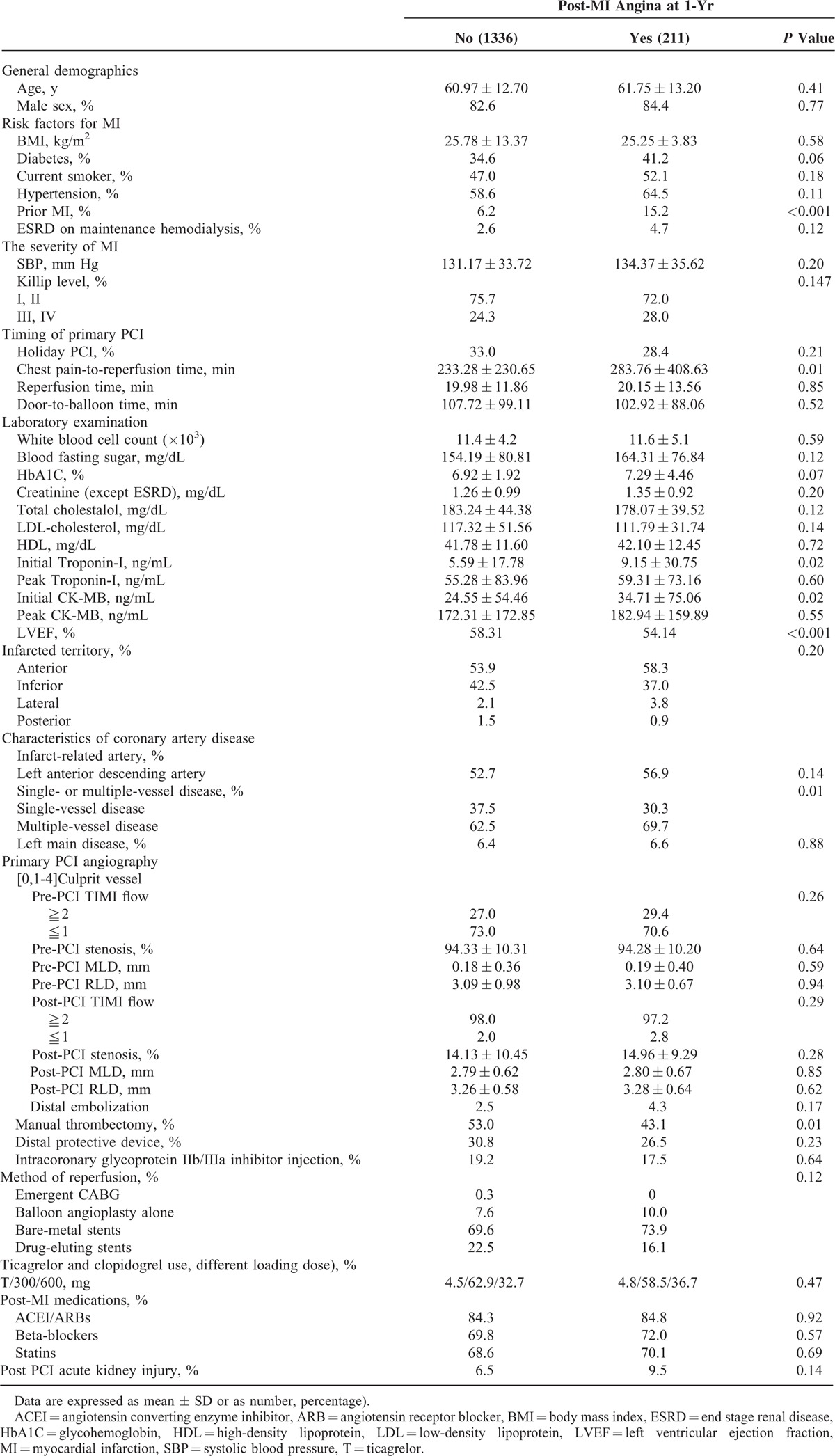
A higher fasting blood fasting sugar level (154.19 ± 80.81 vs 164.31 ± 76.84 mg/dL, P = 0.12), HbA1C (6.92 ± 1.92 vs 7.29 ± 4.46%, P = 0.07), serum creatinine (1.46 ± 1.65 vs 1.73 ± 1.95 mg/dL, P = 0.04), troponin-I (5.59 ± 17.78 vs 9.15 ± 30.75, P = 0.02) and CK-MB (24.55 ± 54.46 vs 34.71 ± 75.06, P = 0.02) were noted in the post-MI angina group (no post-MI angina vs post-MI angina groups, respectively). Peak troponin-I and CK-MB were not significantly different between the 2 groups. The white blood cell counts and lipid profiles were also not significantly different. A lower left ventricular ejection fraction (LVEF) was presented in the post-MI angina group.
The infarcted territory was similar between the 2 groups (P = 0.20). The no post-MI angina group had a greater percentage of inferior wall MIs (42.5% vs 37.5%, no post-MI angina vs post-MI angina groups). The post-MI angina group had a greater percentage of anterior wall MIs (53.9% vs 58.3%, no post-MI angina vs post-MI angina groups). The post-MI angina group had a significantly greater percentage of patients with multiple-vessel disease (62.5% vs 69.7%, no post-MI angina vs post-MI angina groups, P = 0.001). The percentage of left main disease was similar between the 2 groups (6.4% vs 6.6%, no post-MI angina vs post-MI angina groups, P = 0.88).
Pre- and postprimary PCI angiographies showed similar results for TIMI flow, stenosis, minimal luminal diameter (MLD), and reference luminal diameter (RLD) between the 2 groups. Manual thrombectomy, and distal protective device and intracoronary GP IIb/IIIa inhibitor injections were used more frequent in the no post-MI group versus the post-MI angina group (53.0% vs 43.1%, P = 0.01; 30.8% vs 26.5%, P = 0.23; 19.2% vs 17.5%, P = 0.64, respectively). DES implantation after a primary PCI was higher in the no post-MI group, but was not significantly different compared with the post-MI angina group (22.5% vs 16.1%, P = 0.12). The ticagrelor and different clopidogrel loading doses were similar between the 2 groups (P = 0.47). Post-MI medications including angiotensin converting enzyme inhibitors/angiotensin receptor blockers, and B-blockers, and statins were also similar between the 2 groups. However, there was a higher incidence of post-PCI acute kidney injury (AKI) in the post-MI angina group (6.5% vs 9.5%, no post-MI angina vs post-MI angina groups, P = 0.14).
Predictors of Post-MI Angina
Table 2 shows the predictors of post-MI angina. The univariate logistic regression analyses identified a prior MI (odd ratio (OR): 2.699, 95% confidence interval (CI): 1.744–4.177, P < 0.001), chest pain-to-reperfusion time (OR: 1.000, 95% CI: 1.000–1.001, P = 0.048), multiple-vessel disease (OR: 1.378, 95% CI: 1.007–1.886, P = 0.045), manual thrombectomy use (OR: 0.673, 95% CI: 0.502–0.902, P = 0.008), and DES implantation (OR: 0.663, 95% CI: 0.450–0.979, P = 0.039) as factors with a significant predictive value. The percentage of diabetes (OR: 1.327, 95% CI: 0.987–1.185, P = 0.061) and ESRD (OR: 0.663, 95% CI: 0.902–3.793, P = 0.093) also displayed a trend toward having a positive predictive value.
TABLE 2.
Univariate Analysis and Multivariate Logstic Regression Analysis for Presence or Absence of 1-Yr Post-MI Angina
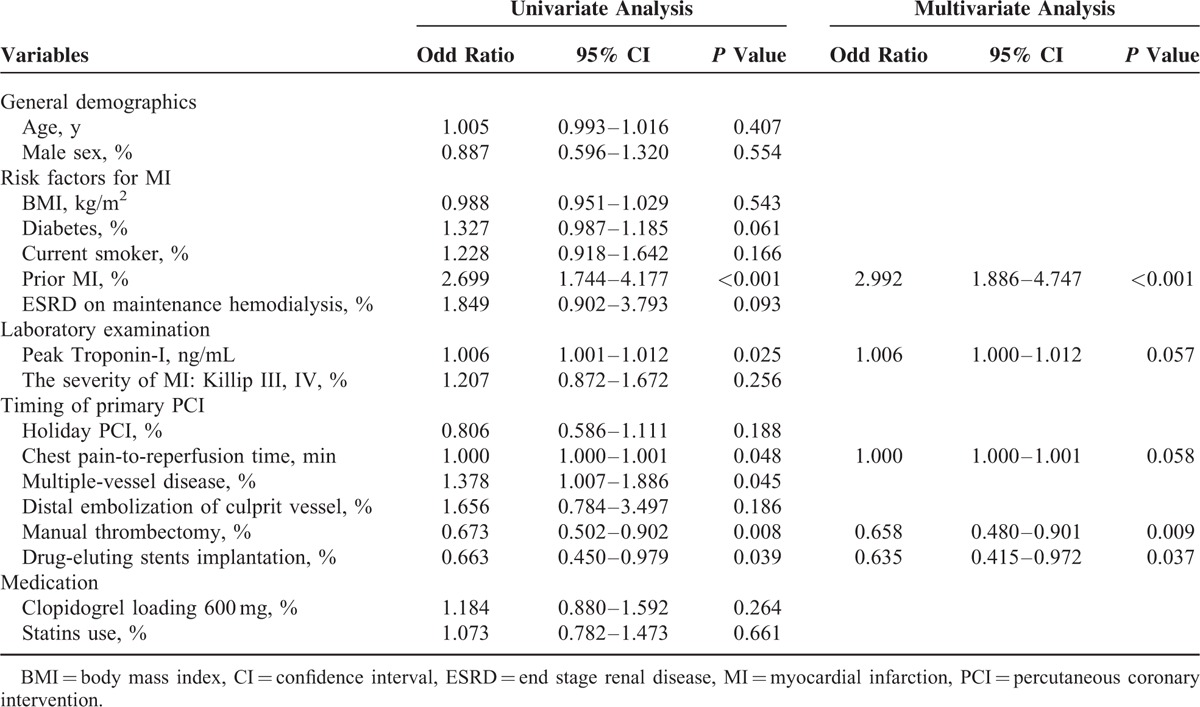
Multivariate logistic regression analysis identified that a prior MI (OR: 2.992, 95% CI: 1.886–4.747, P < 0.001) was a positive independent predictors of occurrence of post-MI angina. Manual thrombectomy use (OR: 0.658, 95% CI: 0.480–0.901, P = 0.009) and DES implantation (OR: 0.635, 95% CI: 0.415–0.972, P = 0.037) could decrease the occurrence of post-MI angina. Higher troponin-I (OR: 1.006, 95% CI: 1.000–1.012, P = 0.057) and longer chest pain-to-reperfusion time (OR: 1.000, 95% CI: 1.000–1.001, P = 0.058) displayed a trend toward being predictors that could increase occurrence of post-MI angina.
Comparison Between the Nonthrombectomy and Thrombectomy Groups
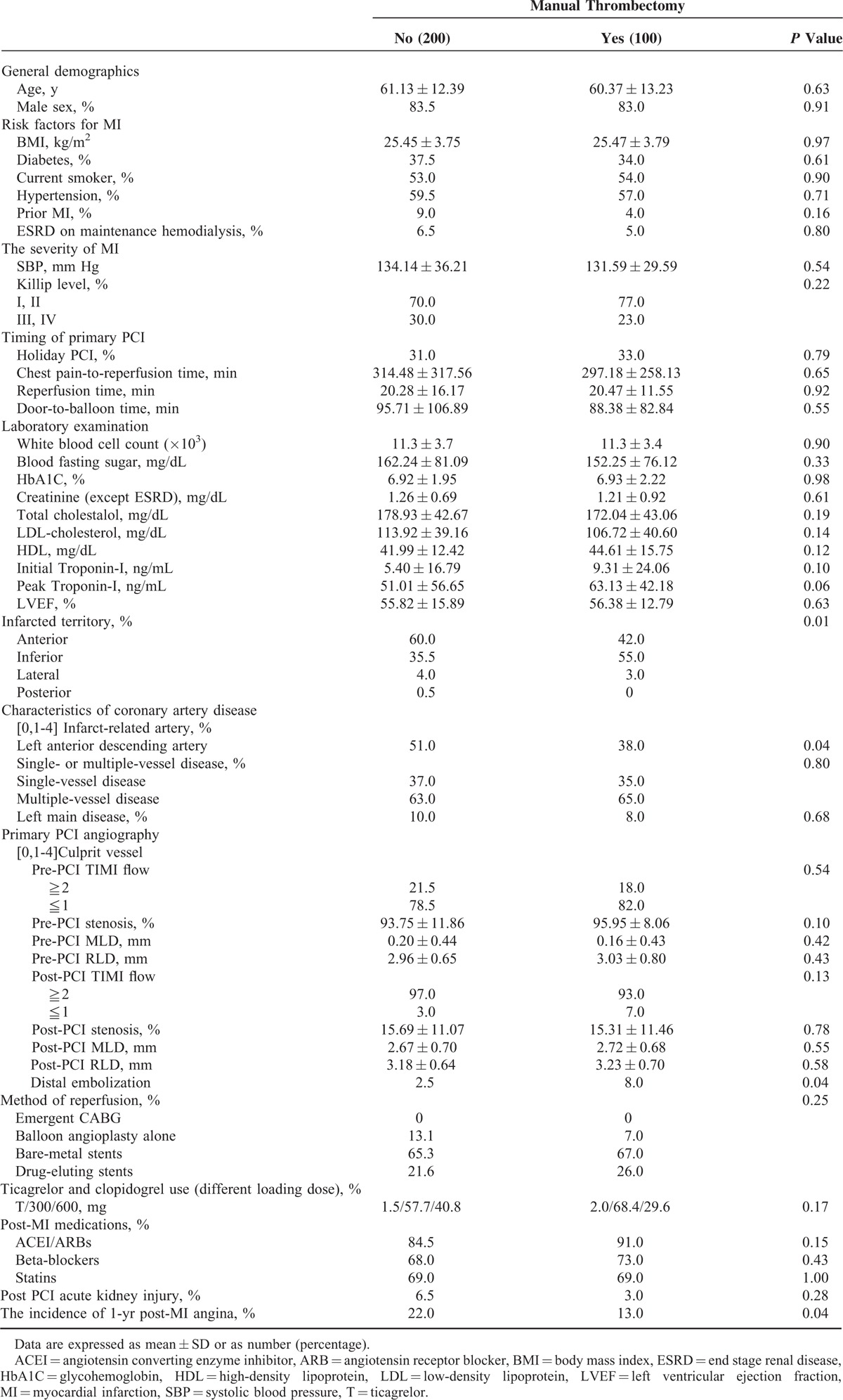
Table 3 shows a comparison between the nonthrombectomy and thrombectomy groups. Baseline characteristics are listed in Supplemental Table 1 and many differences were noted. The patients with distal protective device and intracoronary Glycoprotein (GP) IIb/IIIa injections were excluded first, and only patients with a manual thrombectomy or not were compared. After propensity score matched analyses and a 2-to-1 ratio match, the baseline characteristics of the study patients became similar (Table 3). The general demographics, risk factors for MI, the severity of MI, timing of primary PCI, primary PCI angiography, method of reperfusion, ticagrelor and clopidogrel usage, post-MI medication usage, and post PCI acute kidney injury were similar between the nonthrombectomy and thrombectomy groups. Only the presence of infarcted territory in the anterior wall (P = 0.01) and left anterior artery (P = 0.04) dominated in the nonthrombectomy group. The thrombectomy group had a higher level of initial (5.40 ± 16.79 ng/mL vs 9.31 ± 24.06 ng/mL; P = 0.10) and peak troponin-I (51.01 ± 56.65 ng/mL vs 63.13 ± 42.18 ng/mL; P = 0.06) than the nonthrombectomy group. The incidence of distal embolization was higher in the thrombectomy group. However, the thrombectomy group still had better results regarding recurrent post-MI angina (22.0% vs 13.0%; P = 0.04).
TABLE 3.
Patient Characteristics by Manual Thrombectomy After Propensity Score Match (Exclude the Patients With Distal Protective Device and Intracoronary Glycoprotein IIb/IIIa Inhibitor Injection)
Comparison Between Thrombectomy Alone and Thrombectomy Plus Intracoronary GP IIb/IIIa Injection
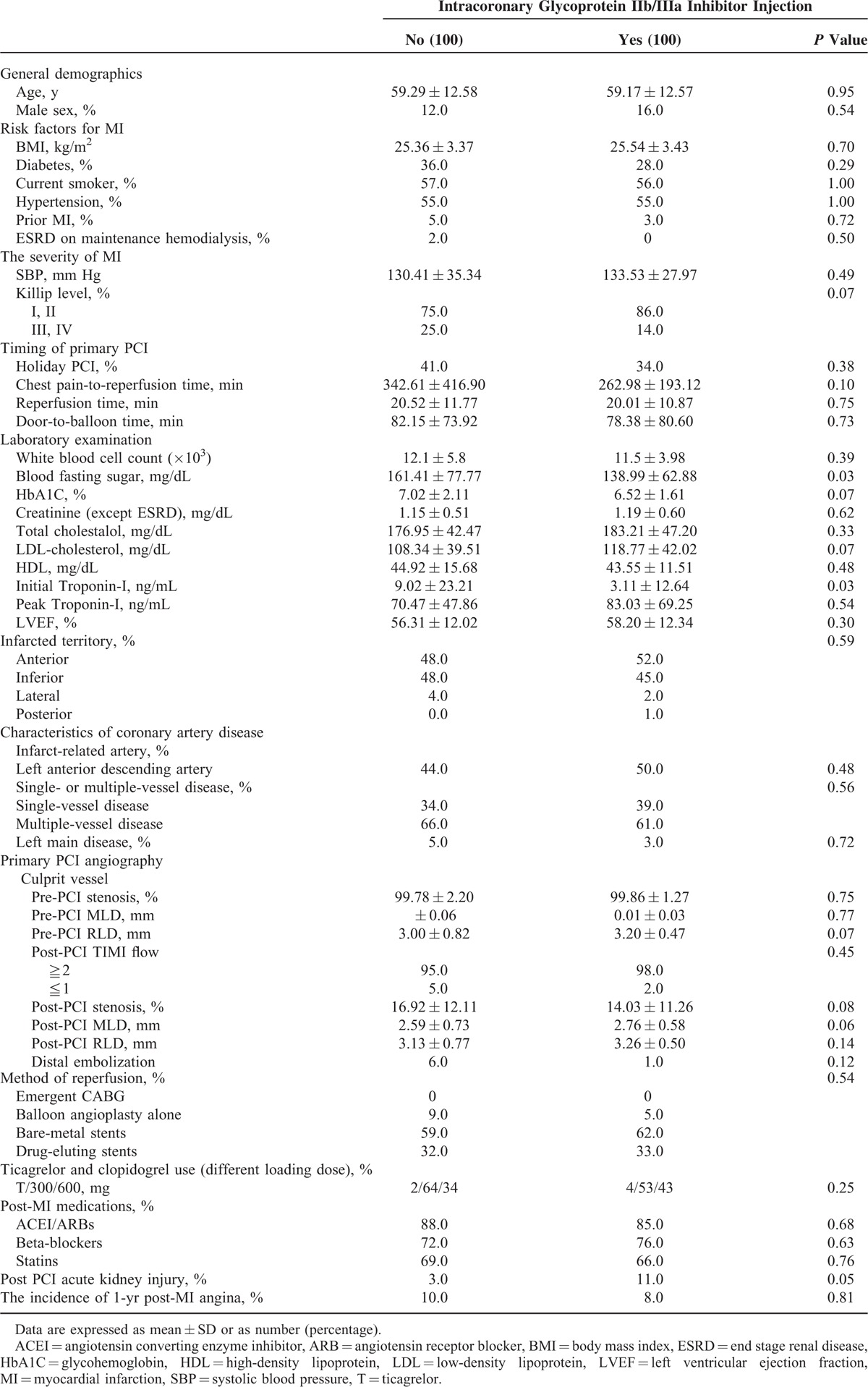
Table 4 shows a comparison between thrombectomy alone and thrombectomy plus intracoronary GP IIb/IIIa injection. The patients with subtotal occlusion or total occlusion with a high thrombus burden were included first. Baseline characteristics of these patients are listed in Supplemental Table 2; some differences were noted. Manual thrombectomy use plus intracoronary GP IIb/IIIa injection and manual thrombectomy alone (no GP IIb/IIIa injection) were compared. After propensity score matched analyses and a 1-to-1 ratio match, the baseline characteristics of the study patients were similar (Table 4). The general demographics, risk factors for MI, the severity of MI, timing of primary PCI, primary PCI angiography, method of reperfusion, ticagrelor and clopidogrel usage, and post-MI medication usage were all similar between the thrombectomy alone and thrombectomy plus intracoronary GP IIb/IIIa injection groups. Only the blood fasting sugar level (161.41 ± 77.77 mg/dL vs 138.99 ± 62.88 mg/dL, P = 0.03), and initial level of troponin-I (9.02 ± 23.21 ng/mL vs 3.11 ± 12.64 ng/mL, P = 0.03), and the incidence of post PCI AKI were higher in the thrombectomy alone group versus the thrombectomy plus intracoronary GP IIb/IIIa injection group, respectively. There was no significant difference in recurrent post-MI angina between the 2 groups (10.0% vs 8.0%, thrombectomy alone group vs the thrombectomy plus intracoronary GP IIb/IIIa injection group, P = 0.81).
TABLE 4.
Patient Characteristics by Intracoronary Glycoprotein IIb/IIIa Inhibitor Injection (After Propensity Score Match)
DISCUSSION
Although mortality associated with an AMI has been steadily declining,6 this trend has been accompanied by a growing need to manage the postdischarge and chronic care of patients after an AMI more effectively. In particular, rehospitalization for acute coronary syndromes (ACS) and coronary revascularization is still common after an AMI, which impact patient's quality of life and increase healthcare costs. According to previous reports, approximately 1 in 5 patients experienced angina 1 year after hospitalization for MI.3 A recent study estimated that rates of rehospitalization due to ACS and revascularization were 6.8% and 4.1%, respectively.7 In our study, 13.6% of patients experienced post-MI angina during a 1-year follow-up. Because a primary goal of cardiac care after MI is to identify and treat angina symptoms, understanding the prevalence of post-MI angina and its associated factors is critical to understanding post-MI angina. Several risk models have identified clinical factors associated with higher risk of mortality8 and all-cause rehospitalization.9 Thus, identifying factors associated with persistent angina can enable clinicians to remain vigilant for the condition and the adverse outcomes that accompany it.
The pathogenesis of post-MI angina remains unclear. The potential reasons are sudden vessel closure,10 coronary spasm,11 multivessel disease and incomplete revascularization,12 and vessel overexpansion.13 Endothelial dysfunction also plays an important role in post-MI angina and leads to a variety of pathophysiologic processes, including vasospasm, excessive thrombosis, and abnormal vascular proliferation.14 Some important polymorphisms linked to endothelial dysfunction and included calcium/calmodulin-dependent kinase IV,15 phospholipase A2,16 and G-Protein-Coupled Receptor Kinase 2.17 In addition, circulating microRNAs have specific patterns in the patients with cardiovascular problems, and therefore offer therapeutic targets in cardiovascular disease,18 and play an important role in the re-endothelialization process postangioplasty.19
Many prior studies have investigated the frequency and predictors of death after AMI8,20 or composites of major adverse cardiac events (which include death).21 However, few prior studies have focused on preventing post-MI angina, which could decrease mortality rates. Therefore, additional focus is needed on other nonfatal outcomes, such as rehospitalizations, procedures, health status, quality of life and cost. Post-MI angina not only consumes healthcare resources, but also is stressful to patients and may adversely affect their quality of life. Understanding the factors that influence post-MI angina is important to creating strategies to try to treat these patients and prevent post-MI angina. According to a previous study, patients with 1-year angina were more likely to be younger, nonwhite males, have had prior angina, have undergone prior coronary artery bypass graft (CABG) surgery, experienced recurrent angina at rest during their hospitalization, have a history of continued smoking, and have significant depressive symptoms.3 In our study, a prior MI was a positive, independent predictor of post-MI angina. Importantly, manual thrombectomy use and DES implantation could decrease the occurrence of post-MI angina. In addition, higher troponin-I and longer chest pain-to-reperfusion time displayed a trend toward influencing the occurrence of post-MI angina.
A manual thrombus aspiration is often performed during a PCI to achieve patent coronary blood flow. Meta-analyses studies of randomized trials revealed that the effectiveness of manual thrombus aspiration is unclear, especially in regards to mortality.22 A recent large prospective randomized controlled trial, the Thrombus Aspiration during ST-Segment Elevation (TASTE) study, showed that a routine thrombus aspiration before PCI did not reduce the 30-day and 1-year mortality in patients with STEMI.23,24 The major limitation of the aspiration catheter is its relatively small inner diameters, which causes difficulty when retrieving diffuse, organized, and distal thrombi.25 Furthermore, data from optical coherence tomography used to evaluate an intracoronary thrombus demonstrated that substantial amounts of residual thrombi are present in most patients after a catheter aspiration, even in patients with a patent coronary angiography.26 However, routine manual thrombectomy is still performed for AMI patients because the procedure is not difficult to perform and is not related to an adverse outcome. In addition, some studies stated that thrombectomy in STEMI may decrease the long-term risk for death or cardiac re-hospitalization,27 and improve myocardial perfusion, when compared to conventional PCI.28 In our study, manual thrombectomy use could have decreased the occurrence of post-MI angina. We hypothesize that manual thrombectomy may remove some thrombus, inflammatory cytokines, and small emboli in small vessels, which may be related to the occurrence of post-MI angina. In the subgroup analyses, the thrombectomy group had a higher level of initial and peak troponin-I than the nonthrombectomy group. The incidence of distal embolization was also higher in the thrombectomy group; the problem may be related to the aspiration catheter incidentally pushing emboli to distal vessels during manual thrombectomy. However, the thrombectomy group still had better results in regard to recurrent post-MI angina versus the nonthrombectomy group (22.0% vs 13.0%; P = 0.04).
In our study, a distal protective device was used in 30.3% of patients. According to a previous study, Wu et al suggest that PCI with a distal protective device appears to have favorable late angiographic results at the target site for the patients with an AMI.29 In our study, treatment with a distal protection device to prevent coronary microvascular obstruction was demonstrated to increase the occurrences of short-term and long-term symptom-free outcomes.30 In our institution, distal protective devices still were used for AMI with selective patients with high thrombus burden and a vessel diameter over 3.5 mm, even though this is not recommended by the current guidelines. Current ESC and AHA guidelines recommend that intracoronary GP IIb/IIIa inhibitor injection should be considered for patients with angiographic evidence of a massive thrombus, slow or no-reflow, or a thrombotic complication.31 In addition, primary PCI with thrombectomy and intracoronary GP IIb/IIIa inhibitor injection still prevented coronary microvascular obstruction and decreased the incidence of post-MI angina when compared with conventional PCI.30 In our study, intracoronary GP IIb/IIIa inhibitor injection was used for 19% of patients. In our subgroup analyses, blood fasting sugar level, initial level of troponin-I, and the incidence of post PCI AKI were higher in the thrombectomy alone group. In addition, when compared with thrombectomy alone group, the incidence of recurrent post-MI angina did not decrease after primary PCI in conjunction with thrombectomy and intracoronary GP IIb/IIIa inhibitor injection.
Balloon angioplasty was used for AMI if the patient had small vessels with reference luminal diameter smaller than 2.5 mm, or only emboli without luminal irregularities, or stent thrombosis. In our study, 2.3% of patients had small vessel disease with a reference luminal diameter smaller than 2.5 mm. Acute, subacute, or late stent thrombosis happened in 2.9% of patients. Balloon angioplasty alone for AMI was performed in 8% of patients for the reasons stated above.
Prior studies and meta-analyses studies suggest that using DESs in STEMI patients is safe, efficacious, and associated with decreased mortality rates and a reduction in the need for repeat revascularization procedures as compared with treatment with bare-metal stents.32–34 In our study, DES implantation was a negative independent predictor of occurrence of post-MI angina and could also decrease the occurrence of post-MI angina.
There are several potential limitations of this study. First, this was a retrospective study in a single center. Second, the presence of angina symptoms is partially dependent on a patient's level of activity; sedentary patients may have significant cardiac disease, but may not report angina symptoms because of lack of activity. Therefore, the prevalence of post-MI angina may be underestimated. Third, the enrolled data covered a long period of time. Technical improvements during that period may have influenced our results. Fourth, even though propensity score matched analyses were performed, some selective bias still existed. Fifth, we did not explore the mechanism of post-MI angina and further investigation about the changes in molecular biology about post-MI angina should be performed.
Despite the limitations and being a retrospective study, we identified that the patients who have a high risk of developing post-MI angina are ones who had a prior MI, higher troponin-I, and longer chest pain-to-reperfussion time. Manual thrombectomy and DES implantation could decrease angina pectoris 1 year after AMI, decrease long-term healthy costs, and increase post-MI quality of life. In the future, it will be essential to perform, large, randomized, prospective investigations for advanced evaluations of the effect of these factors in reducing post-MI angina and concurrent changes in molecular biology.
CONCLUSIONS
A prior MI was a strong, independent predictor of occurrence of post-MI angina. Manual thrombectomy and drug-eluting stent implantation could decrease angina pectoris 1 year after an acute MI, decrease long-term healthy costs, and increase post-MI quality of life.
Human Rights Statements and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions. Informed consent was obtained from all patients before being included in the study.
Supplementary Material
Footnotes
Abbreviations: ACS = acute coronary syndrome, AKI = acute kidney injury, AMI = acute myocardial infarction, CABG = coronary artery bypass graft, CI = confidence interval, CK-MB = creatine kinase MB, DES = drug-eluting stent, ESRD = end stage renal disease, GP = Glycoprotein, HbA1C = glycohemoglobin, LVEF = left ventricular ejection fraction, MI = myocardial infarction, MLD = minimal luminal diameter, OR = odd ratio, PCI = primary percutaneous intervention, RLD = reference luminal diameter, STEMI = ST-segment elevation myocardial infarction, TASTE = Thrombus Aspiration during ST-Segment Elevation
C-YF has equal contribution as a first author. W-CL and C-YF have equal contribution as correspondence author.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Brand FN, Larson M, Friedman LM, et al. Epidemiologic assessment of angina before and after myocardial infarction: the Framingham study. Am Heart J 1996; 132:174–178. [DOI] [PubMed] [Google Scholar]
- 2.Pocock SJ, Henderson RA, Clayton T, et al. Quality of life after coronary angioplasty or continued medical treatment for angina: three-year follow-up in the RITA-2 trial. J Am Coll Cardiol 2000; 35:907–914. [DOI] [PubMed] [Google Scholar]
- 3.Maddox TM, Reid KJ, Spertus JA, et al. Angina at 1 year after myocardial infarction. Arch Intern Med 2008; 168:1310–1316. [DOI] [PubMed] [Google Scholar]
- 4.Gabriel SP, James Stefan K, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST segment elevation. Eur Heart J 2012; 33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 5.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 6.Krumholz HM, Wang Y, Chen J, et al. Reduction in acute myocardial infarction mortality in the United States: risk-standardized mortality rates from 1995-2006. JAMA 2009; 302:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold SV, Smolderen KG, Kennedy KF, et al. Risk factors for rehospitalization for acute coronary syndromes and unplanned revascularization following acute myocardial infarction. J Am Heart Assoc 2015; 4:e001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT investigators. Circulation 2000; 101:2557–2567. [DOI] [PubMed] [Google Scholar]
- 9.Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure. Update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes 2010; 3:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansour M, Carrozza JP, Kuntz RE, et al. Frequency and outcome of chest pain after two new coronary interventions (atherectomy and stenting). Am J Cardiol 1992; 69:1379–1382. [DOI] [PubMed] [Google Scholar]
- 11.Jeremias A, Kutscher S, Haude M, et al. Nonischemic chest pain induced by coronary interventions. A prospective study comparing coronary angioplasty and stent implantation. Circulation 1998; 98:2656–2658. [DOI] [PubMed] [Google Scholar]
- 12.Hollman J, Grüntzig AR, Douglas JS, et al. Acute occlusion after percutaneous transluminal coronary angioplasty: a new approach. Circulation 1983; 68:725–732. [DOI] [PubMed] [Google Scholar]
- 13.Fischell TA, Derby G, Tse TM, et al. Coronary artery vasoconstriction routinely occurs after percutaneous transluminal coronary angioplasty: a quantitative arteriographic analysis. Circulation 1988; 78:1323–1334. [DOI] [PubMed] [Google Scholar]
- 14.Hamasaki S, Tei C. Effect of coronary endothelial function on outcomes in patients undergoing percutaneous coronary intervention. J Cardiol 2011; 57:231–238. [DOI] [PubMed] [Google Scholar]
- 15.Santulli G, Cipolletta E, Sorriento D, et al. CaMK4 gene deletion induces hypertension. J Am Heart Assoc 2012; 1:e001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galasso G, Santulli G, Piscione F, et al. The GPIIIA PlA2 polymorphism is associated with an increased risk of cardiovascular adverse events. BMC Cardiovasc Disord 2010; 10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santulli G, Trimarco B, Iaccarino G. G-protein-coupled receptor kinase 2 and hypertension. High Blood Press Cardiovasc Prev 2013; 20:5–12. [DOI] [PubMed] [Google Scholar]
- 18.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol (Oxf) 2015; 213:60–83. [DOI] [PubMed] [Google Scholar]
- 19.Santulli G, Wronska A, Uryu K, et al. A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J Clin Invest 2014; 124:4102–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004; 291:2727–2733. [DOI] [PubMed] [Google Scholar]
- 21.Park HW, Yoon CH, Kang SH, et al. Early- and late-term clinical outcome and their predictors in patients with ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction. Int J Cardiol 2013; 169:254–261. [DOI] [PubMed] [Google Scholar]
- 22.De Luca G, Navarese EP, Suryapranata H. A meta-analytic overview of thrombectomy during primary angioplasty. Int J Cardiol 2013; 166:606–612. [DOI] [PubMed] [Google Scholar]
- 23.Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013; 369:1587–1597. [DOI] [PubMed] [Google Scholar]
- 24.Lagerqvist B, Fröbert O, Olivecrona GK, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med 2014; 371:1111–1120. [DOI] [PubMed] [Google Scholar]
- 25.Stone GW. Simple aspiration in acute myocardial infarction: too simple to be true? Eur Heart J 2012; 33:3005–3007. [DOI] [PubMed] [Google Scholar]
- 26.Onuma Y, Thuesen L, van Geuns RJ, et al. Randomized study to assess the effect of thrombus aspiration on flow area in patients with ST-elevation myocardial infarction: an optical frequency domain imaging study—TROFI trial. Eur Heart J 2013; 34:1050–1060. [DOI] [PubMed] [Google Scholar]
- 27.Adlbrecht C, Distelmaier K, Bonderman D, et al. Long-term outcome after thrombectomy in acute myocardial infarction. Eur J Clin Invest 2010; 40:233–241. [DOI] [PubMed] [Google Scholar]
- 28.Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med 2008; 358:557–567. [DOI] [PubMed] [Google Scholar]
- 29.Wu CJ, Yang CH, Fang CY, et al. Six-month angiographic results of primary angioplasty with adjunctive PercuSurge GuardWire device support. Catheter Cardiovasc Interv 2005; 64:35–42. [DOI] [PubMed] [Google Scholar]
- 30.Lee WC, Chen HC, Fang HY, et al. Comparison of different strategies for acute ST-segment elevation myocardial infarction with high thrombus burden in clinical practice: symptom-free outcome at one year. Heart Lung 2015; 44:487–493. [DOI] [PubMed] [Google Scholar]
- 31.Yip HK, Wu CJ, Chang HW, et al. Impact of tirofiban on angiographic morphologic features of high-burden thrombus formation during direct percutaneous coronary intervention and short-term outcomes. Chest 2003; 124:962–968. [DOI] [PubMed] [Google Scholar]
- 32.Mauri L, Silbaugh TS, Garg P, et al. Drug-eluting or bare-metal stents for acute myocardial infarction. N Engl J Med 2008; 359:1330–1342. [DOI] [PubMed] [Google Scholar]
- 33.Piscione F, Piccolo R, Cassese S, et al. Effect of drug-eluting stents in patients with acute ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: a meta-analysis of randomised trials and an adjusted indirect comparison. Eurointervention 2010; 5:853–860. [DOI] [PubMed] [Google Scholar]
- 34.Suh HS, Song HJ, Choi JE, et al. Drug-eluting stents versus bare-metal stents in acute myocardial infarction: a systematic review and meta-analysis. Int J Technol Assess Health Care 2011; 27:11–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


