Supplemental Digital Content is available in the text
Abstract
The aim of this study was to determine whether perinatal HIV infection (PHIV), HIV-exposed uninfected (PHEU) versus HIV-unexposed (PHU) status predicted long-term executive function (EF) deficit in school-aged Ugandan children.
Perinatal HIV status was determined by 18 months via DNA polymerase chain reaction test and confirmed at cognitive assessment between 6 and 18 years using HIV rapid-diagnostic test. Primary outcome is child EF measured using behavior-rating inventory of executive function questionnaire across 8 subscales summed to derive the global executive composite (GEC). EF was proxy-reported by caregivers and self-reported by children 11 years or older. Descriptive analyses by perinatal HIV status included derivation of mean, standard deviations (SD), number, and percent (%) of children with EF deficits warranting clinical vigilance. Raw scores were internally standardized by age and sex adjustment. EF scores warranting clinical vigilance were defined as ≥ mean + 1.5∗SD. t Tests for mean score differences by perinatal HIV status and linear-regression models were implemented in SAS version 9.4 to derive HIV status-related EF deficits (β) and 95% confidence intervals (CIs).
Proxy-reported and self-reported EF were assessed in 166 and 82 children, respectively. GEC deficit was highest for PHIV (mean = 121.9, SD = 29.9), intermediate for PHEU (mean = 107.5, SD = 26.8), and lowest for PHU (mean = 103.4, SD = 20.7; P-trend < 0.01). GEC deficit levels warranting clinical vigilance occurred in 9 (15.8%), 5 (9.3%) and 0 (0%) PHIV, PHEU, and PHU children, respectively (P-trend = 0.01). Nineteen percent (n = 32) children had deficits requiring clinical vigilance in ≥2 proxy-reported EF subscales. Of these, multisubscale deficits occurred in 35.1%, 13.0%, and 9.3% of PHIV, PHEU, and PHU respectively (P-trend = 0.001). Multivariable analyses find significantly higher GEC deficits for PHIV compared with PHU and PHEU children regardless of respondent (all P values <0.01). Proxy-reported EF performance was similar for PHEU compared with PHU; however, child self-reported GEC scores were elevated by 12.8 units (95% CI: 5.4–25.5) for PHEU compared with PHU.
PHIV had long-term EF deficits compared with other groups. Furthermore, PHEU ≥11 years may have long-term EF deficits compared with PHU, but future studies are needed to clarify this relationship. Cognitive remediation interventions with emphasis on EF may translate to improvements in long-term functional survival in HIV-affected children from sub-Saharan Africa.
INTRODUCTION
Ninety percent of children with perinatally acquired human immunodeficiency virus infection (PHIV) reside in sub-Saharan Africa. The majority of such children, given timely access to highly active antiretroviral therapy (HAART), live into adolescence and adulthood with chronic HIV infection.1,2 Yet, whether children on prolonged HAART are alive with adequate functional capacity during school-age, adolescence, and young adulthood is largely unknown.3 This understanding is essential for determining modifiable risk factors of long-term functional impairment that could be intervened upon to improve the quality of life of children expected to live for decades with chronic HIV infection.
With some exceptions,4–8 studies in the pre-HAART era found lower levels of motor, memory, and verbal functions in PHIV children compared with HIV-negative controls.3,7,9,10 Appropriate neurocognitive development is an important predictor of functional capacity. At present, it is unclear whether cognitive deficits persist despite HAART. Salient differences between pre-HAART era and contemporary PHIV cohorts limit the generalizability of previous observations to contemporary PHIV cohorts. Key differences include: current cohorts of PHIV children survive well beyond 5 years of age, HAART now commences much earlier instead of at advanced disease stages, and HIV progression-related attrition is much less common now than was typical in pre-HAART era.3 The dearth of information on neurocognitive outcomes of long-term HIV infection for PHIV children on HAART and perinatally HIV-exposed uninfected (PHEU) children in school-age and adolescence has been noted.1,11,12 In particular, how perinatal HIV infection/exposure status affects cognitive processes such as executive function (EF) that influence healthy adjustment to adult responsibilities is unknown. This information is especially needed to understand neurodevelopment trajectories of HIV-affected children from sub-Saharan Africa where HIV burden is high.
EF is a cluster of cognitive processes that enable individuals to connect past experience with present action and by doing so engage in planning, organizing, strategizing, paying of attention to details, and making necessary efforts to remember important details required for them to manage their time and space in relation to attainment of future goals.13 Healthy development of EF is crucial for functional survival in the medium and long term, yet EF deficits may be unrecognized until elementary school and beyond.14 Children with deficient EF are limited in their ability to hold things in mind, step back and reflect, have difficulty undertaking multiple tasks, making appropriate judgments and maintaining appropriate emotional control.15 To inform current knowledge gaps, we examine the hypothesis that HIV-related EF deficits are present in school-age and adolescence for PHIV despite connection to HIV care, and exist at lower but measurable degree for PHEU compared with PHU.
Key Messages.
Perinatal HIV (PHIV) infection was associated with significant global and domains-specific executive function (EF) deficit compared to perinatally HIV uninfected (PHU) children.
All child-reported EF subscales and inhibit, initiation and planning proxy-reported EF subscales were adversely affected by PHIV-infected vs. PHU- status.
Among children 11 years or older, perinatally HIV-exposed uninfected (PHEU) children have significantly greater self-reported EF deficits relative to PHU children.
We add empirical evidence that PHIV status was consistently associated with higher EF deficits compared to PHU and PHEU.
Interventions that target EF globally with emphasis on the inhibition, shift and planning subscales could be sensitive for detection of EF improvements regardless of the respondent.
METHODS
Study Setting, Study Design, and Population
Our study is implemented in Kampala, Uganda where HIV prevalence at the time of study implementation was 7.4%. An estimated 1.5 million Ugandans live with HIV/AIDS including 150,000 children 0 to 15 years’ old. HAART became partially available HIV-infected persons in Uganda from 2003.16 Treatment coverage continues to expand. As of 2014, 51% of all persons living with HIV were on HAART but HAART coverage is lower in children (39%).17
We conducted a retrospective cohort study in Kawaala Community Health Center (KCHC), Kampala, Uganda, from March 20, 2014 to July 30, 2014. We followed 168 children with or without perinatal HIV exposure or infection from birth to 6 to 18 years as part of an investigation of functional survival during school-age and adolescence. Eligibility criteria included: documented delivery of index child in a hospital setting within Kampala or its nearby rural areas between 1996 and 2008, determinable HIV status of child and birth mother during pregnancy through objective medical records, willingness to undergo testing to verify current child HIV serostatus, if negative at birth, presentation for healthcare service for any reason in outpatient ward of KCHC, and provision of caregiver consent and child assent for study participation. Children without hospital-based birth-record documentation and those with undetermined perinatal HIVinfection status were excluded. In addition, participants too ill to complete the study protocol, those for whom consent could not be obtained from adult caregivers and those unable to communicate in the local language (Luganda) or English, were excluded.
Statement of Ethical Clearance
Ethical approval was provided by the institutional review boards (IRB) of the University of Georgia (IRB Protocol # 0196), the Makerere University School of Public Health (IRB Protocol # 010) and the Uganda National Council for Science and Technology (Protocol # HS 1613). Caregivers provided written informed consent and all children provided assent for study participation.
MEASUREMENTS
Executive Cognitive Function
EF was multidimensionally assessed from child and caregiver perspective using Behavior Rating Inventory of Executive Function (BRIEF) questionnaire.18 This questionnaire is designed to assess EF behaviors in various settings for children and adolescents ages 5 to 18 years. We used the 86-item questionnaire for children and a shortened instrument version for caregiver report that included 24 questions. BRIEF was translated, culturally adapted, and validated for use in this setting. Validation included duplicate EF assessment in15 child-caregiver pairs by 2 independent raters 14 to 21 days apart. Psychometric properties (internal consistency among individual questions, inter-rater and test-retest reliabilities)19 were calculated to measure adequacy of the BRIEF for EF assessment in this setting using the %INTRACC macro.20 Caregivers provided proxy-report of EF in all children; self-reported EF measures were obtained in children ≥11 years. Before testing, respondents received snacks (fruit juice and donuts) to mitigate the distracting effect of hunger on responses. Interviews were administered by trained research assistants in the local Luganda language in well-lit ventilated research trailers away from the general patient care area.
Raw EF scores were calculated for 8 subscales and 3 EF domains. Domains included: behavioral regulatory index (BRI),composed of the shift, inhibit, and emotional control subscales; metacognition index (MCI), composed of the initiation, planning, working memory, material organization/monitoring subscales; and global executive composite (GEC), which represents sum of all subscales. In the absence of a Uganda-specific healthy population reference of executive function, raw scores for individual subscales and respective EF domains were analyzed with adjustment for age and sex instead of T-scores. Raw EF scores are analyzed primarily as linear endpoints and secondarily as a dichotomous endpoint. For the latter, EF deficits warranting clinical vigilance were empirically defined for respective domains as greater than or equal to mean score + 1.5∗standard deviation of scores within our sample. The designation of scores 1.5 standard deviations greater than the mean as warranting clinical vigilance is consistent with definition of this construct using the same instrument in a sample of children from the United States.18
Primary Determinant
Perinatal HIV-status was classified in 3 categories: PHIV, PHEU, and PHU. Perinatal HIV status was determined by 18th month of life using DNA polymerase chain reaction test and confirmed by HIV rapid diagnostic test (RDT) at cognitive testing. Child perinatal and pregnancy HIV status for birth mother were retrospectively determined by inspection of: the antenatal register, which included child and mother information in pregnancy through delivery, and the HIV-exposed infant’ (HEI) clinical chart – a database established for all children of HIV-infected women delivered at respective hospitals per standard of care. For analytic purposes, difference in outcome measure was determined for 3 contrasts: PHIV vs PHEU, PHIV vs PHU, and PHEU vs PHU.
Other Measures
For all pregnant women during index pregnancy/birth, HIV status, participation in prevention of HIV mother-to-child-transmission, antiretroviral drugs used for own care (if applicable), intrapartum prophylactic antiretroviral administration, World Health Organization HIV clinical disease stage, and linkage status to HIV-Care and treatment program were abstracted from ante-natal register. From the HEI database, we abstracted antiretroviral history (if PHIV), linkage to HIV care (if applicable), specific dates of infant HIV testing, and child HIV-status at 18th month testing. Birth weight and Apgar score of all enrolled children were abstracted from the delivery register.
Child weight and height were measured as part of current health assessment by study physicians. These values were used to calculate weight-for-age, body mass index for age, and height-for-age z scores as respective measures of short- and long-term malnutrition relative to healthy child growth relative to the world health organization 2007 reference as has been extensively described.21–23 For analytic purposes, we used only 1 of the 3 measures of malnutrition, that is most strongly associated with our outcome measure under evaluation. Laboratory tests for complete blood counts (CBC), Malaria RDT for Plasmodium falciparum, and stool assessment for helminth infections were implemented at cognitive testing. Specifically designed case-report forms were used to collect sociodemographic (age, sex, education, wealth, perceived social standing), psychosocial status (social support, anxiety, depressive symptoms), and behavioral (alcohol use, smoking and consistent bed-net use) data in child-caregiver pairs. Wealth was defined based on household assets as already extensively described elsewhere.24 Similarly, perceived social standing was measured using the MacAurthur Scale24 and caregiver-reported social support, anxiety level, and depressive symptoms were measured using adapted and standardized questionnaires as previously described.25–27 We defined anemia using WHO age- and sex-specific thresholds for hemoglobin.28 In addition to red blood cell indices measured in CBC, we defined macrocytic anemia which is linked to micronutrient deficiencies such as vitamin B-12 deficiency and microcytic anemia, which generally includes anemias owing to iron deficiency or thalassemia based on previously described age-appropriate reference ranges for mean corpuscular hemoglobin and mean corpuscular volume.29
Statistical Analyses
We analyzed preliminary data in all eligible participants collected for a pilot investigation of functional survival in HIV-affected and unaffected Ugandan children. We compared mean EF scores by perinatal HIV status and implemented bivariate analyses to identify non-HIV factors crudely associated with EF. For descriptive analyses only, a global trend test of no difference in average EF scores by HIV status was implemented using t tests for linear variables. Differences in categorical potential confounders by HIV status were tested via χ2 test. We considered factors associated with EF at a P value of ≤0.20 from descriptive analyses as potential confounders in multivariable analyses. In light of the established inverse associations between child cognition and poverty, anemia, and malnutrition,30 these variables were retained as covariates in multivariable linear regression analysis wherein differences in EF scores and associated 95% confidence intervals (CI) were calculated using a generalized estimating equations (GEE) approach. Caregivers could have multiple children in this study and this raised the potential for nonindependence of multiple children from the same home. Therefore, we addressed this analytically by implementing clustered analyses within caregiver households by specifying an exchangeable correlation matrix. Secondarily, we implemented logistic regression analysis using GEE for binomial distributions and estimated the perinatal HIV status-related odds of children having sufficiently high EF deficits to warrant clinical vigilance. For all multivariable analyses, missing confounder values were addressed using the missing indicator method so that the analytic sample included all children with data on perinatal HIV status and EF. All analyses were implemented in SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Enrolled were 166 school-aged children of 108 unique caregivers including 58 PHIV, 55 PHEU, and 53 PHU, who were on average 10.8 years’ old at cognitive testing. Of these, 82 children (further described in Table S1) were 11 years or older and self-evaluated their own EF. The average child weighed 3.4 kg at birth, 30% had Apgar score <10 at delivery. At cognitive testing, 16% were coinfected with parasites (malaria, helminthes or intestinal protozoa) and 20.4% were anemic. Across perinatal HIV status, children were comparable with respect to age, sex, birth weight, Apgar score at delivery, acute nutritional status (body mass index z score), clinical history of parasitic infection/treatment, outpatient consultation, prevalence of abnormal clinical diagnosis, and number of reported symptoms at cognitive assessment. However, PHIV children had higher levels of anemia, chronic undernutrition (height-for-age z score), whereas PHEU children were less likely than PHIV and PHEU children to currently use bed-nets. Similarly, general and organ system examination by physicians revealed greater abnormality for PHIV in the lymphatic, respiratory, and neurologic systems. (Table 1)
TABLE 1.
Health, Behavioral and Sociodemographic Description of School-aged Children From Kampala, Uganda With and Without Perinatal HIV Exposure/Infection
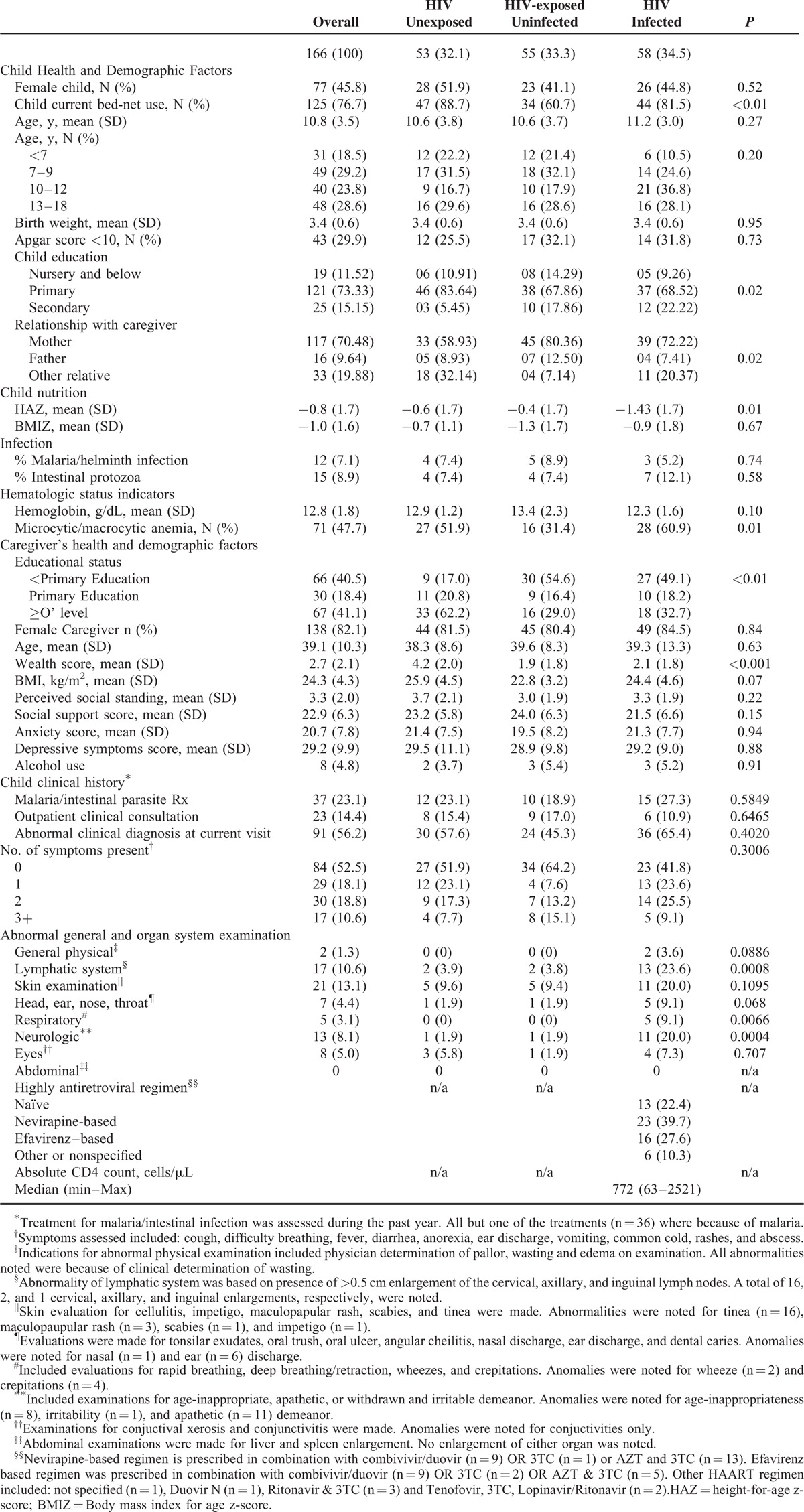
The average caregiver was 39 years’ old and the vast majority of caregivers were female (≥80%) with most attaining primary level education or lower. Caregivers of PHIV or PHEU had marginally lower BMI, and were more likely than caregivers of PHU children to have lower wealth scores and lower levels of education (Table 1). Among PHIV children, 77.6% were on HAART at cognitive testing; median CD4 cell count was 772 cells/μL (Table 1). These CD4 measures were obtained per standard of care within 3.4 (range: 0–32) months of cognitive assessment (Table S2). At time of cognitive testing, the median time in care at the health center was 5.9 (range: 0.6–16.6) years.
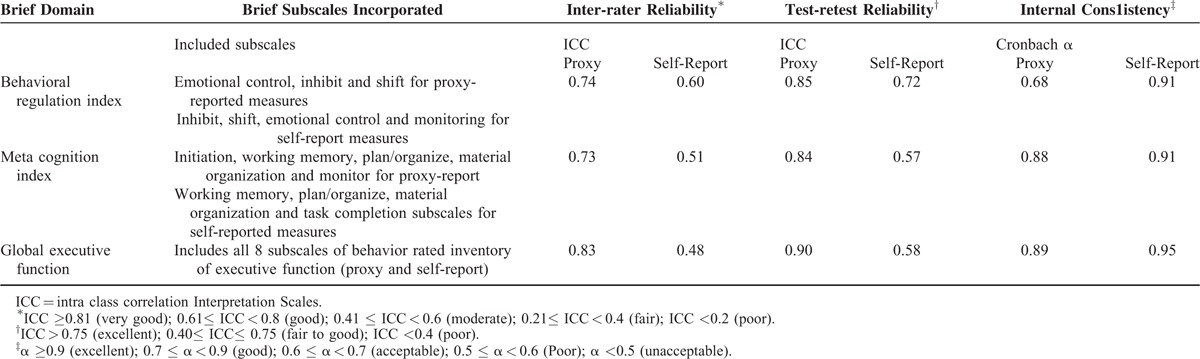
The instrument validation sample included 10 boys and 5 girls (5 PHIV, 6 PHEU, and 4 PHU) between the age of 6 and 18 years. For the proxy assessments, questions in GEC and MCI domains each demonstrated good internal consistency (Cronbach α ≥ 0.88) and questions in BRI domain exhibited acceptable internal consistency (Cronbach α = 0.68). During inter-test period of 14 to 21 days, test-retest reliability was good to excellent (intraclass correlation [ICC] ≥0.83) and inter-rater reliability was good or excellent (ICC ≥0.74) for all proxy-reported EF domains. For self-reported EF domains, questions included in respective assessments demonstrated excellent internal consistency (Cronbach α ≥ 0.91) and reliability of measures across raters was moderate (ICC = 0.48–0.60). During 14 to 21 days of repeated testing, self-reported BRI domain measures demonstrated good (ICC = 0.72) test-retest reliability, whereas test-retest reliability was fair to good for MCI (ICC = 0.57) and GEC (ICC = 0.58) domains (Table 2). Calculated scores on the inconsistency (mean = 5.1, SD = 3.2) and negativity (mean = 0.47, SD = 0.9) scales were in acceptable range, respectively, demonstrating consistency in children's responses to related questions on the BRIEF and confirming that children's responses to questions in our sample were not negatively biased.
TABLE 2.
Psychometric Property of the Behavioral Rated Inventory of Executive Function (Proxy-report) Instrument as evaluated in 15 School-aged Children Enrolled for the Pre-pilot Study
PHIV children had more deficits in all EF domains (Figure 1) and subscales (Figure 2), whether proxy or self-reported. Self-reported subscales and domains suggest a dose-dependent EF deficit with PHIV > PHEU > PHIV (Figures 1 and 2, Tables S3 & S4), whereas proxy-reported subscales and domain averages confirm most deficit for PHIV with PHEU and PHU at comparable levels (Figures 1 and 2, Tables S3 & S4). Similarly, PHIV children had more proxy-reported EF deficits warranting clinical vigilance in respective EF subscales and domains (Table S3, Table S4). PHIV were significantly less likely to have zero impaired EF subscales and correspondingly more likely to have deficits in ≥2 proxy (P = 0.001) or self-reported (P = 0.03) EF subscales (Figure 2, Table S3).
FIGURE 1.
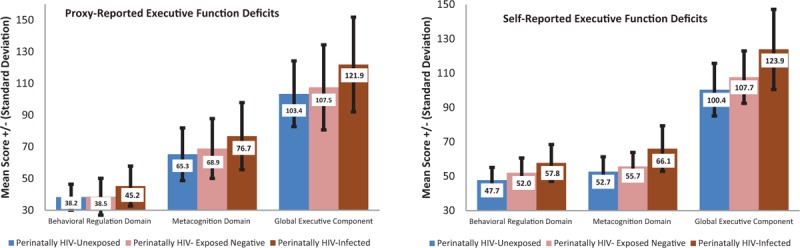
Global and domain-specific child mean executive function deficit score by perinatal HIV status ∗Self-reported scores were assessable in children 11 years or older only (n = 82).
FIGURE 2.
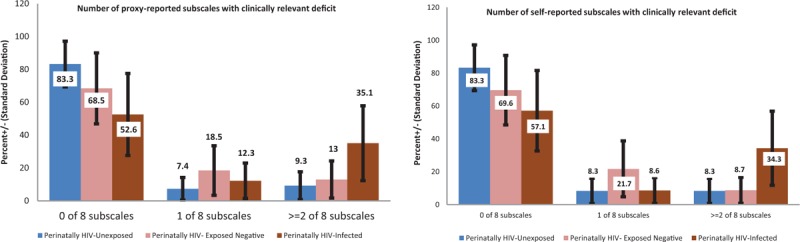
Total number of executive function subscales where scores warrant clinical vigilance in the global executive component. ∗Self-reported scores were only assessed in children 11 years or older (n = 82).
For PHIV, excess EF deficits were noted in all 3 proxy (β = 4.7–12.1; 95% CI: −1.9 to 22.3) and self-reported (β = 12.8–24.2; 95% CI: 0.02–34.7) EF domains compared with PHU or PHEU (Figure 3). For PHIV versus PHU, significantly higher proxy-reported deficits were evident in the emotional control subscale only. For PHIV versus PHEU, significantly higher EF deficits were observed in emotional control and material organization subscales only. HIV-affected status was associated with excess deficit in all domains and subscales in a dose-dependent manner such that self-reported EF deficit for PHIV versus PHU > PHEU versus PHU. Likewise, EF deficit for PHIV versus PHEU was significant in all domains and in 4 of 8 subscales (Figure 3 & Table S3). These associations were robust to adjustment for caregiver’ sociodemographic and health factors (age and education, social support, BMI) and child’ sociodemographic/behavioral health factors (age, sex, 5-minute apgar score at birth, and current versus non-current bed net use). Multivariable linear models explained as much as 27% of the variability in proxy-reported EF scores and as much as 47.3% of the variability in self-reported EF scores (Table S4).
FIGURE 3.
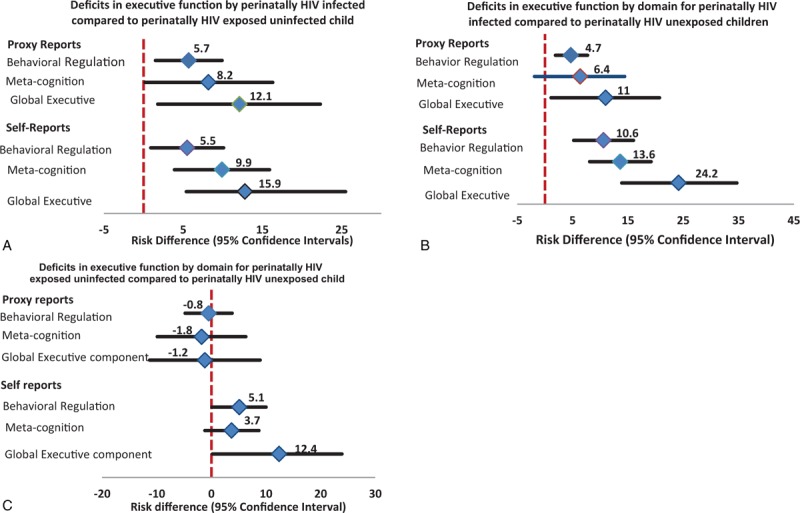
Association between perinatal HIV infection status and executive function deficits during school-age and adolescence. Estimates are derived from linear regression models. Proxy-reported measures are adjusted for: caregiver’ sociodemographic (age and education, social support, perceived social standing, and body mass index) and child’ sociodemographic and behavioral health factors (age, sex, Apgar score at birth, birth-weight, anemic vs nonanemic status, and current vs non-current bed net use). Self-reported measures are adjusted for: caregiver’ sociodemographic (education and wealth score) and child’ sociodemographic and behavioral health factors (age, sex, apgar score at birth, anemia and current vs non-current bed-net use).
There was no difference between PHEU versus PHU and PHIV versus PHEU children with respect to presence of proxy EF scores warranting clinical vigilance. However, 3.7 to 12.9 times the odds of having proxy EF scores that warrant clinical vigilance was noted in all domains for PHIV versus PHU (Table S5).
DISCUSSION
Overall our data suggest that in spite of treatment, HIV-related EF deficit persists during school-age years. In line with our hypothesis, average EF scores and the frequency of EF scores warranting clinical vigilance was highest for PHIV, intermediate for PHEU, and lowest for PHU. This finding is consistent with observations among Thai children using a different battery of neurocognitive tests.31 The 24% prevalence of EF scores warranting clinical vigilance observed in this study is intermediate between 17% observed among PHIV living in The Netherlands32 and the 39% reported among young adults from Romania infected with HIV as children.33 Our observation that PHIV were more likely to have ≥2 subscales scores warranting clinical vigilance compared with PHEU and PHU during school-age and adolescence suggests that HIV-related deficits persist in the medium to long term and corroborates similar previous observations.33 Among children 11 years or older, PHEU had significantly greater EF deficits than PHU suggesting that perinatal exposure to HIV, even without infection, detrimentally affects EF. However, we did not find significant differences in proxy-reported EF performance measures for PHEU compared with PHU per proxy-reported measures—although multisubscale deficits were more prevalent in PHEU compared with PHU.
To our knowledge, only one study has evaluated HIV-related differences in EF during school-age using the same instrument as our study.34 That study included PHIV and PHEU children of HIV-positive women from the United States (US) and found no EF differences by child HIV infection status. However, the comparability of that sample to ours may be limited as most children were exposed to illicit drugs in utero, had low birth weight, or were born preterm to birth mothers mostly classified as socioeconomically disadvantaged. Thus, the sample was de novo at extremely high risk of EF impairments. In addition, study lacked a true control group of PHU children making comparability difficult. Our finding of higher EF deficits for PHIV relative to PHU is in line with observation of moderate EF impairment among Romanians that acquired HIV parenterally,33 findings of EF deficits for PHIV versus PHU in a small study of school-aged Indian children,35 and with observations of worse performance in non-EF cognitive tests for PHIV versus PHU Ugandan school-aged children.36 Despite trends in similar direction to ours, data from school-aged children living in the Netherlands did not find significant differences in EF for PHIV versus PHU.32
For PHIV-infected children, the virus enters the central nervous system within weeks of primary infection causing neuronal damage and cell death6,37,38 that manifests in childhood as a progressive encephalopathy leading to a range of cognitive deficits and neurodevelopmental disability.37 Early HAART onset may moderate the adverse cognitive impact of HIVinfection,39 but is unlikely to reverse existing neurological insults.40 Of note, PHIV children in this have been in care at the recruitment clinic for median of 5.9 years and most were immune-competent at cognitive testing with absolute CD4 values comparable with PHIV from different settings.32,33,41,42 The mechanism of PHIV-related deficits observed is likely multifactorial including neuronal damage attributable to ongoing HIV viral replication, the presence of HAART-related side-effects that lower adherence and in turn reduces quality of life, and the ongoing impact of structural determinants of impaired child cognitive development such as poverty, malnutrition, quality of care giving, and the environment of care. These HIV-specific and non-HIV-specific factors further impair the capacity of children with PHIV infection to age-appropriately adjust to the cognitive demands of transitioning to adulthood. We observed significant differences in EF function by perinatal HIV status in spite of controlling for multiple caregiver factors (ie, age, sex, educational status, wealth, and psychosocial status) and child characteristics (apgar score, age, sex, malnutrition, anemia, and preventive health behavior such as current bednet use) known to confound this relationship. Of note, 7 of the 166 children in this study were not in school. All non-school attendees were either PHEU (n = 3) or PHIV (n = 4). Thus, relatively limited access to formal education and/or intellectually stimulating environments for HIV-affected children could have contributed to observed EF deficits.
Our study is subject to limitations that should lead to cautious interpretation of our findings. First, we estimated HIV-related mean differences in EF scores, which may not necessarily be clinically relevant. Second, we were underpowered to evaluate associations between perinatal HIV status and presence of EF scores warranting clinical vigilance. We were particularly underpowered to detect differences in EF scores for PHEU versus PHU. Third, to the extent that our enrollment strategy was conditioned on child clinic attendance for any reason, we have reason to believe that our community controls (ie PHU) may not be as healthy as children recruited from nonclinic settings such as schools. Thus, the contrasts presented could theoretically underestimate the disadvantage of perinatal HIV infection or exposure relative to healthy community controls. In addition, our instrument validation exercise despite the emphasis on psychometric reliability was limited in scope. We included a sample of 15 children across a wide age range, and did not specifically engage in evaluating other important dimensions of instrument validation such as criterion validation, content validation, construct validation, face validation, and utility. Therefore, future more robust EF validation efforts are needed and will enhance the ability to evaluate this outcome relative to an appropriate healthy reference of Ugandan children.
These limitations notwithstanding, our study has several strengths that enhance the quality of evidence presented. We are engaged in the most in-depth longitudinal analysis of perinatal HIV infection status as a determinant of mid- to long-term EF function to date in HIV-affected children. We present data on global executive component, affected EF domains and subscales to gain insight regarding most affected elements of EF and inform future interventions. Specifically, our data suggest that all EF subscales were adversely affected in PHIV compared with other groups based on child self-reported measurements and thus should be equally targeted in future interventions. Of note, interventions that target the inhibition, shift, and planning subscales could be sensitive for detection of EF improvements regardless of the respondent. Our longitudinal design, which clarifies temporal sequence, multidimensional EF of self-regulation, and analytic control for competing risk factors of child cognitive impairment are additional strengths. A further strength is the simultaneous inclusion of 3 distinct groups of children based on perinatal HIV infection and exposure status. Unlike our study, most relevant investigations to date used either PHU or PHEU as comparison groups.2 To the extent that the majority of PHEU and PHIV are exposed to similar sociodemographic, environmental, nutritional, and other caregiver factors with known impact on child neurodevelopment, the breadth of information obtained in studies including just these groups is limited.2 Last, the use of translated, culturally adapted, and internally valid cognitive assessment instrument with known reliability for EF assessment in school-aged children in the study setting is a strength of the present investigation.
We conclude that PHIV and PHEU status is associated with sustained EF impairment in school-age and adolescence among Ugandan children. Our data highlight the need to evaluate cognitive remediation interventions in HIV-affected children from sub-Saharan Africa. Larger prospective investigations are needed to confirm our findings, determine associations between perinatal HIV infection/exposure status and development of EF deficits requiring clinical vigilance, and identify potential resilience factors associated with optimal EF to inform future interventions to enhance functional survival in HIV-affected children.
Supplementary Material
Acknowledgement
We acknowledge with thanks the kind indulgence of study participants and the diligence of our field research staff.
Footnotes
Abbreviations: AIDS = Acquired Immune Deficiency Syndrome, BRI = Behavioral regulatory index, BRIEF = Behavior Rated Inventory of Executive Function, CBC = Complete blood count, CI = Confidence Interval, EF = Executive Function, GEC = Global Executive Composite, GEE = Generalized estimating equations, HAART = Highly active anti-retroviral therapy, HEI = HIV Exposed Infant, HIV = Human Immunodeficiency Virus, ICC = Intra Class Correlation, IRB = Institutional Review Board, KCHC = Kawaala Community Health Center, MCI = meta-cognition index, PHEU = Perinatally HIV exposed uninfected, PHIV = perinatally HIV infected, PHU = Perinatally HIV unexposed, RDT = Rapid Diagnostic Test, SAS = Statistical Analysis Software, SD = Standard Deviation, WHO = World Health Organization
This work was supported by a faculty research grant from the University of Georgia Research Foundation (grant number 2523) and additional seed funding support from The University of Georgia College of Public Health.
Authors’ contributions: AEE designed the study, contributed to data collection, led data analysis, interpretation, manuscript development, and refinement. FNK contributed to data analysis, data interpretation and manuscript development, critique, and revision for important intellectual content. SKZ contributed to study design, led data collection, and contributed to manuscript development. AKN and JNS contributed to data collection, data interpretation and manuscript critique, and revision for intellectually important content. MLR contributed to data collection, analysis, and data interpretation. RK contributed to data collection. MZ, NK, and CCW contributed to data interpretation, critique, and revision of the manuscript for important intellectual content. All authors were involved in the final approval of the version for peer-review.
The authors report no conflicts of interest.
REFERENCES
- 1.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, et al. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis 2014; 14:627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med 2010; 61:169–185. [DOI] [PubMed] [Google Scholar]
- 3.Le Doare K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics 2012; 130:e1326–1344. [DOI] [PubMed] [Google Scholar]
- 4.Drotar D, Olness K, Wiznitzer M, et al. Neurodevelopmental outcomes of Ugandan infants with human immunodeficiency virus type 1 infection. Pediatrics 1997; 100:E5. [DOI] [PubMed] [Google Scholar]
- 5.McGrath N, Fawzi WW, Bellinger D, et al. The timing of mother-to-child transmission of human immunodeficiency virus infection and the neurodevelopment of children in Tanzania. Pediatr Infect Dis J 2006; 25:47–52. [DOI] [PubMed] [Google Scholar]
- 6.Msellati P, Lepage P, Hitimana DG, et al. Neurodevelopmental testing of children born to human immunodeficiency virus type 1 seropositive and seronegative mothers: a prospective cohort study in Kigali, Rwanda. Pediatrics 1993; 92:843–848. [PubMed] [Google Scholar]
- 7.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics 2008; 122:e123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagenda D, Nassali A, Kalyesubula I, et al. Health, neurologic, and cognitive status of HIV-infected, long-surviving, and antiretroviral-naive Ugandan children. Pediatrics 2006; 117:729–740. [DOI] [PubMed] [Google Scholar]
- 9.Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS 2012; 26:1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potterton J, Stewart A, Cooper P, et al. The effect of a basic home stimulation programme on the development of young children infected with HIV. Dev Med Child Neurol 2010; 52:547–551. [DOI] [PubMed] [Google Scholar]
- 11.Laughton B, Cornell M, Boivin M, et al. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc 2013; 16:18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow-Mosha L, Eckard AR, McComsey GA, et al. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc 2013; 16:18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair C. School readiness. Integrating cognition and emotion in a neurobiological conceptualization of children's functioning at school entry. Am Psychol 2002; 57:111–127. [DOI] [PubMed] [Google Scholar]
- 14.Best JR, Miller PH, Naglieri JA. Relations between Executive Function and Academic Achievement from Ages 5 to 17 in a Large, Representative National Sample. Learn Individ Differ 2011; 21:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meiran N, Diamond GM, Toder D, et al. Cognitive rigidity in unipolar depression and obsessive compulsive disorder: examination of task switching, Stroop, working memory updating and post-conflict adaptation. Psychiatry Res 2011; 185:149–156. [DOI] [PubMed] [Google Scholar]
- 16.Muyanja SZ, Larke N, Rutebarika D, et al. Decreasing trends of bacteraemia among HIV-infected Ugandan adults: incidence, aetiology, clinical outcomes and effect of antiretroviral therapy in a semi-urban setting (2000-2008). Trop Med Int Health 2011; 16:756–765. [DOI] [PubMed] [Google Scholar]
- 17.The HIV and AIDS Uganda Country Progress Report 2014. In; 2014. [Google Scholar]
- 18.Gioia GA, Isquith PK, Guy SC, et al. Test review: Behavior rating inventory of executive function. Child Neuropsychol 2000; 6:235–238. [DOI] [PubMed] [Google Scholar]
- 19.Hamer R.M. Compute six intraclass correlation measures. 2000 Last Updated 11 Jan 2000 [cited 2015 August 3]; Available from: http://support.sas.com/kb/25/031.html. [Google Scholar]
- 20.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979; 86:420–428. [DOI] [PubMed] [Google Scholar]
- 21.de Onis M. The WHO Child Growth Standards. Pediatr Nutr Pract 2nd Ed 2015; 113:278–294. [DOI] [PubMed] [Google Scholar]
- 22.de Onis M, Onyango A, Borghi E, et al. Worldwide implementation of the WHO Child Growth Standards. Public Health Nutr 2012; 15:1603–1610. [DOI] [PubMed] [Google Scholar]
- 23.de Onis M, Onyango AW. WHO child growth standards. Lancet 2008; 371:204–1204. [DOI] [PubMed] [Google Scholar]
- 24.Ezeamama AE, Guwatudde D, Wang M, et al. High perceived social standing is associated with better health in HIV-infected Ugandan adults on highly active antiretroviral therapy. J Behav Med 2016; DOI 10.1007/s10865-015-9710-x. [DOI] [PubMed] [Google Scholar]
- 25.Smith Fawzi MC, Kaaya SF, Mbwambo J, et al. Multivitamin supplementation in HIV-positive pregnant women: impact on depression and quality of life in a resource-poor setting. HIV Med 2007; 8:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antelman G, Kaaya S, Wei R, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr 2007; 44:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezeamama AE, Woolfork MN, Guwatudde D, et al. Depressive and anxiety symptoms predict sustained quality of life deficits in HIV-positive Ugandan adults despite antiretroviral therapy: a prospective cohort study. Medicine (Baltimore) 2016; 95:e2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. In. Edited by Department of Nutrition for Health and Development VaMNIS. Geneva: World Health Organization; 2011. [Google Scholar]
- 29.Agarwal B. Anemia. Available at: In http://www.pediatriconcall.com/fordoctor/Diseases_a_z/article.aspx?artid=85; 2015. [Google Scholar]
- 30.Ezeamama AE, Friedman JF, Acosta LP, et al. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hyg 2005; 72:540–548. [PMC free article] [PubMed] [Google Scholar]
- 31.Puthanakit T, Aurpibul L, Louthrenoo O, et al. Poor cognitive functioning of school-aged children in thailand with perinatally acquired HIV infection taking antiretroviral therapy. AIDS Patient Care STDS 2010; 24:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen S, Ter Stege JA, Geurtsen GJ, et al. Poorer cognitive performance in perinatally HIV-infected children versus healthy socioeconomically matched controls. Clin Infect Dis 2015; 60:1111–1119. [DOI] [PubMed] [Google Scholar]
- 33.Ene L, Franklin DR, Burlacu R, et al. Neurocognitive functioning in a Romanian cohort of young adults with parenterally-acquired HIV-infection during childhood. J Neurovirol 2014; 20:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llorente AM, Brouwers P, Leighty R, et al. An analysis of select emerging executive skills in perinatally HIV-1-infected children. Appl Neuropsychol Child 2014; 3:10–25. [DOI] [PubMed] [Google Scholar]
- 35.Ravindran OS, Rani MP, Priya G. Cognitive Deficits in HIV Infected Children. Indian J Psychol Med 2014; 36:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruel TD, Boivin MJ, Boal HE, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis 2012; 54:1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong FD. Neurodevelopment and chronic illness: mechanisms of disease and treatment. Ment Retard Dev Disabil Res Rev 2006; 12:168–173. [DOI] [PubMed] [Google Scholar]
- 38.Sharer LR. Pathology of HIV-1 infection of the central nervous system. A review. J Neuropathol Exp Neurol 1992; 51:3–11. [DOI] [PubMed] [Google Scholar]
- 39.Lowick S, Sawry S, Meyers T. Neurodevelopmental delay among HIV-infected preschool children receiving antiretroviral therapy and healthy preschool children in Soweto, South Africa. Psychol Health Med 2012; 17:599–610. [DOI] [PubMed] [Google Scholar]
- 40.Whitehead N, Potterton J, Coovadia A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care 2014; 26:497–504. [DOI] [PubMed] [Google Scholar]
- 41.Garvie PA, Zeldow B, Malee K, et al. Discordance of cognitive and academic achievement outcomes in youth with perinatal HIV exposure. Pediatr Infect Dis J 2014; 33:e232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected Children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatr Infect Dis J 2013; 32:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


