Abstract
High sodium intake is a well-known risk factor for elevated blood pressure and is responsible for a higher incidence of cardiovascular events. Reports have suggested an association of sodium intake with insulin resistance (IR) and type 2 diabetes mellitus in adults. However, evidence on an association between sodium intake assessed on the basis of urinary sodium excretion and IR in adolescents is scarce. The present study aimed at investigating the association between urinary sodium excretion and IR among South Korean adolescents.
This population-based, cross-sectional study analyzed the data obtained from the Korea National Health and Nutrition Examination Survey (KNHANES) 2009 to 2010. The data of a total of 1353 adolescents (779 boys and 574 girls) were included in the final analysis. Spot urine samples were collected, and urinary sodium excretion was estimated by using the urinary sodium concentration (U[Na+]), U[Na+] to urinary creatinine ratio (U[Na+]/Cr), and U[Na+] to specific gravity unit (SGU) ratio (U[Na+]/SGU). IR was assessed by using the homeostasis model assessment of IR (HOMA-IR). Hierarchical multivariable logistic regression analysis was performed to assess the risk for a high HOMA-IR according to urinary sodium excretion.
The mean levels of U[Na+], U[Na+]/Cr, and U[Na+]/SGU were significantly higher in subjects in the highest HOMA-IR quartile (Q4) than in subjects in the lowest, second, or third quartiles (Q1–3) of HOMA-IR. The mean values of HOMA-IR and several cardiometabolic parameters tended to progressively increase with the U[Na+], U[Na+]/Cr, and U[Na+]/SGU quartiles. Q3 of U[Na+] was at a significantly higher risk than Q1 of U[Na+] of an association with Q4 of HOMA-IR, after adjustment for confounding variables. Q3 and Q4 of U[Na+]/Cr and U[Na+]/SGU, respectively, had significantly higher risks, than the respective Q1s, of an association with Q4 of HOMA-IR. The risk of an association with Q4 of HOMA-IR demonstrated significantly increasing trends with increasing quartiles of U[Na+], U[Na+]/Cr, and U[Na+]/SGU irrespective of confounding factors.
Urinary sodium excretion was positively associated with IR in South Korean adolescents. The monitoring and control of urinary sodium excretion may be recommended as an important intervention for the prevention of IR and related diseases in adolescents.
INTRODUCTION
The increased prevalence of childhood obesity worldwide is expected to increase obesity-related disorders such as insulin resistance (IR), hypertension, dyslipidemia, and type 2 diabetes mellitus (T2DM).1 IR is a condition in which the insulin-mediated disposal of glucose is impaired despite sufficient insulin production. It is related to oxidative stress and chronic low-grade inflammation resulting from adipocyte hyperplasia and hypertrophy secondary to the energy excess in obesity. IR may lead to the dysregulation of glucose metabolism and the development of T2DM.2,3 An association between IR and increased cardiovascular risk has been reported in the pediatric population. IR is also an established independent risk factor for future cardiovascular and metabolic diseases.4,5 Detection of IR, and control of the related modifiable factors during childhood may be an essential component of preventing T2DM and the associated cardiometabolic diseases that develop later in life.
Excessive sodium intake is a well-established risk factor for elevated blood pressure and is associated with a high incidence of cardiovascular events resulting in substantial medical costs.6–8 Although sodium intake has been reported to be associated with IR and T2DM in adults,9 evidence on the association between sodium intake and IR is scarce, and the study results were inconsistent.10 High dietary sodium intake during childhood seems to be related to an increased incidence of early development of cardiovascular risk factors such as high blood pressure and obesity, which are closely related to IR.11,12 However, to our knowledge, there are no studies objectively investigating the association between sodium intake and IR in adolescents.
The quantity of sodium intake can be estimated either by using a dietary recall questionnaire or through the measurement of the 24-hour urinary sodium excretion. Data based on dietary recall tends to underestimate dietary sodium intake. Whereas 24-hour urinary sodium excretion is recognized as the most objective indicator of dietary sodium intake, collection of 24-hour urine samples in children is difficult.13 Therefore, spot urine samples are preferred for the assessment of dietary sodium intake, although they are likely to be diluted or concentrated. Urinary sodium concentration (U[Na+]) corrected by using the urinary creatinine level and the specific gravity unit (SGU) are available options to compensate for the error caused by variations in the concentration of urine when using spot urine samples.14,15
Herein, the present study aimed at investigating the association between IR and urinary sodium excretion assessed with the U[Na+], urinary sodium to creatinine ratio (U[Na+]/Cr), and urinary sodium to SGU ratio (U[Na+]/SGU) among South Korean adolescents based on available nationally representative data.
METHODS
Survey Overview and Study Subjects
In this population-based, cross-sectional study, we analyzed the data of South Korean adolescents who participated in the Korea National Health and Nutrition Examination Survey (KNHANES) between 2009 and 2010. The KNHANES is a nationwide, ongoing survey of noninstitutionalized civilians, conducted since 1998 by the Division of Chronic Disease Surveillance of the Korea Centers for Disease Control and Prevention (KCDC) and the Korean Ministry of Health and Welfare. The survey was designed to assess the national health and nutritional status, and consists of health interviews, nutritional assessments, and health examination surveys. Participants were randomly selected from sampled household units by using a stratified, multistage, and probability-based sampling design, based on the component ratio of the population as well as data from the 2005 National Census Registry in South Korea.
Among the 19,491 participants sampled in the KNHANES during 2009 and 2010, data belonging to 18,138 persons were excluded. The reasons for exclusion were missing data (140) and age ≤12 or ≥19 years (17,998). Data corresponding to 1353 adolescents (779 boys and 574 girls) were finally analyzed. Parental written informed consent was obtained as the study subjects were all minors. The institutional review board of the KCDC approved the study protocol. The study data are available from the KCDC website (http://knhanes.cdc.go.kr).
Anthropometric and Biochemical Measurements
Trained staff members performed the anthropometric and biochemical measurements. Height (cm) and weight (kg) were measured with the subjects wearing light clothing without shoes, to the nearest 0.1 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). Waist circumference (WC) was measured to the nearest 0.1 cm, on a horizontal plane at the midpoint between the lower border of the rib cage and the iliac crest in standing position at the end of normal expiration. Blood pressure (BP) was measured thrice at 5-minute intervals, on the right arm in seated position, by using a mercury sphygmomanometer (Baumanometer; WA Baum Co, Copiague, NY), and the mean value of the second and third measurements was used in the analysis.
Blood and spot urine samples were obtained from the subjects after they had fasted overnight for a minimum of 8 hours. The samples were immediately processed and refrigerated before being transported in cold storage to the Central Testing Institute in Seoul, South Korea. The serum levels of fasting plasma glucose (FPG), total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) were measured by using enzymatic methods with commercially available kits (Daiichi, Tokyo, Japan) on an automatic analyzer (Hitachi 7600; Hitachi, Tokyo, Japan). The levels of low-density lipoprotein cholesterol (LDL-C) in subjects with a TG level <400 mg/dL were calculated by using Friedewald formula;16 in subjects with a TG level ≥400 mg/dL, LDL-C was measured directly by using commercially available kits (Cholestest LDL; Sekisui Medical, Tokyo, Japan). Fasting serum insulin (FSI) level was measured by using a gamma counter (1470 Wizard; PerkinElmer, Turku, Finland) with an insulin immunoradiometric kit (Biosource Europe SA, Nivelles, Belgium). IR was evaluated by using the homeostasis model assessment of IR (HOMA-IR). HOMA-IR was calculated by using the formula HOMA-IR = FSI × FPG/405.17 Subjects in the highest quartile (Q4) of HOMA-IR were defined as having a high HOMA-IR. The levels of serum creatinine and urinary creatinine and U[Na+] were measured by using kinetic colorimetry on an automatic analyzer (Hitachi 7600). Urinary specific gravity was measured by using a refractometer method on an automatic analyzer (UriSys 2400; Roche Diagnostic Co, Indianapolis, IN). SGU was calculated by using the formula (SG − 1) × 100.18 The other ratios, U[Na+]/Cr and U[Na+]/SGU, were also calculated for all subjects.
Lifestyle Variables and Nutritional Assessment
All subjects were asked about their smoking status, alcohol consumption, physical activity, monthly household income level, and sleep duration. Subjects who had smoked cigarettes on at least 1 day during the month before the survey were defined as current smokers. Similarly, subjects who had consumed at least 1 alcoholic drink on at least 1 day during the month before the survey were defined as alcohol drinkers, and those who had not, were defined as nondrinkers. On the basis of the International Physical Activity Questionnaire short form modified for Koreans, subjects were considered regular physical exercisers if they performed moderate exercise lasting ≥30 minutes per session more than 5 times per week, or performed vigorous exercise lasting ≥20 minutes per session more than 3 times per week.19 The monthly household income level was defined as lower for incomes in the lowest quartile and as higher for all others. Sleep duration was determined from the following question in the self-reported questionnaire: “How long do you sleep on average?.”
Nutritional intake, including daily total calorie, fat, and sodium intake, was assessed with a 24-hour dietary recall questionnaire administered by a trained dietician. The results were calculated by using the food composition table developed by the National Rural Living Science Institute under the Rural Development Administration.
Statistical Analyses
Subjects were categorized into quartiles of urinary sodium excretion variables or HOMA-IR level with age-specific cut-off values, which were determined separately for boys and girls, and for each the surveys conducted in 2009 and 2010. All data are presented as mean ± standard error (SE) or percentage (SE). χ2 test for categorical variables and independent t test for continuous variables were performed to analyze the differences in characteristics based on the HOMA-IR quartiles. Analysis of variance (ANOVA) was used for comparisons of cardiometabolic characteristics including HOMA-IR based on quartiles of U[Na+], U[Na+]/Cr, and U[Na+]/SGU. Hierarchical multivariable logistic regression analysis was used to evaluate the risk of an association with the highest quartile of HOMA-IR based on quartiles of U[Na+], U[Na+]/Cr, and U[Na+]/SGU. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated after adjusting for potential confounders. Model 1 did not involve any adjustments, whereas model 2 involved adjustment for age and sex. Variables adjusted in model 3 included the variables adjusted in model 2 as well as BMI, serum creatinine level, smoking status, alcohol consumption, physical activity, monthly household income level, and daily total energy and fat intake. Statistical analyses were performed by using the survey procedure of SAS (version 9.2; SAS Institute, Cary, NC) to account for the complex sampling design. Two-sided P values <0.05 were considered statistically significant.
RESULTS
Baseline Characteristics of Study Subjects
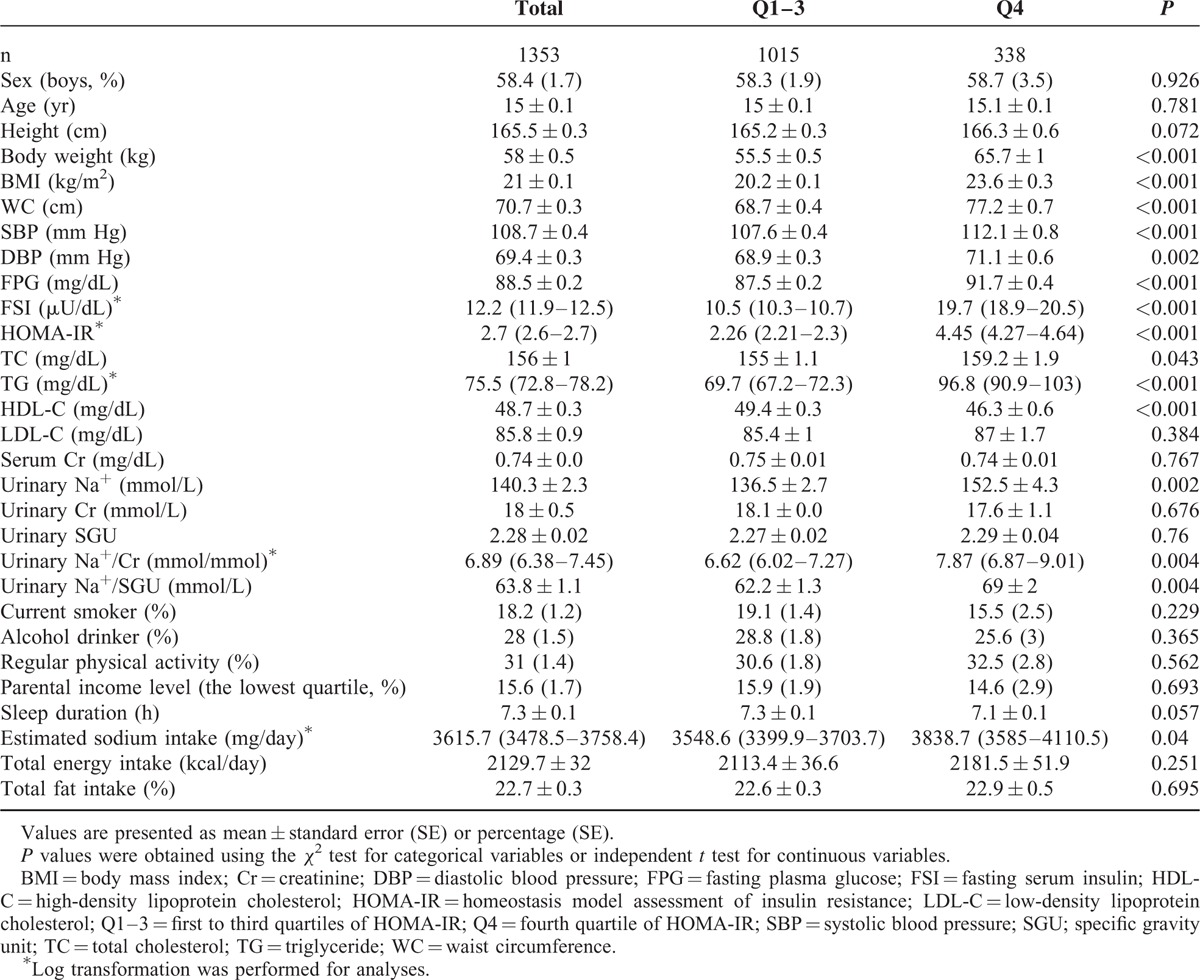
The baseline general characteristics of the study subjects according to HOMA-IR quartiles (Q1–3 vs. Q4) are shown in Table 1. There was no significant difference in sex and age distribution between the 2 HOMA-IR groups. The mean value of HOMA-IR was 2.26 in subjects in Q1–3 of HOMA-IR and 4.45 in those in Q4 of HOMA-IR (P <0.001). The mean levels of U[Na+], U[Na+]/Cr, and U[Na+]/SGU were significantly higher in subjects in Q4 than in subjects in Q1–3 of HOMA-IR. The mean values of other cardiometabolic parameters including body weight, BMI, WC, systolic BP, diastolic BP, FPG, FSI, TC, and TG were higher in Q4, whereas the mean HDL-C level was higher in Q1–3 of HOMA-IR. Serum creatinine, urinary creatinine, and urinary SGU were not significantly different between the 2 groups. The mean value of daily sodium intake from the recall questionnaire was 3838.7 mg in Q4 and 3548.6 mg in Q1–3 of HOMA-IR (P = 0.04). Sodium intake was positively associated with U[Na+] (correlation coefficient = 0.079, P = 0.035). Other lifestyle characteristics such as smoking status, alcohol consumption, physical activity, monthly household income level, sleep duration, and daily total energy and fat intake were similar between the 2 groups.
TABLE 1.
Baseline Characteristics of Study Subjects According to Quartiles of HOMA-IR
HOMA-IR and Other Cardiometabolic Parameters According to Quartiles of Urinary Sodium Excretion
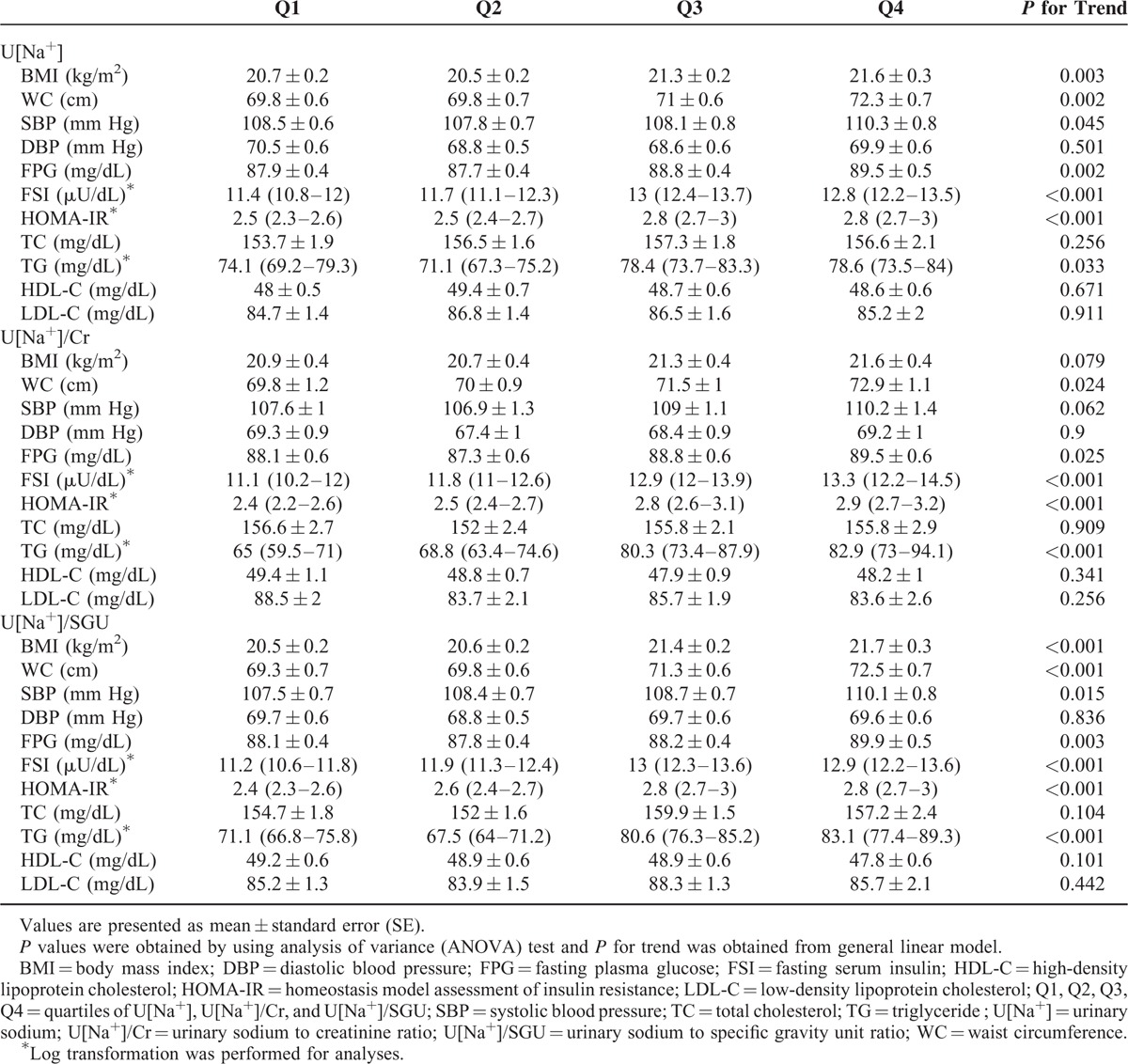
Figure 1 plots the mean values of HOMA-IR according to quartiles of U[Na+], U[Na+]/Cr, and U[Na+]/SGU. The values of HOMA-IR were progressively higher for the higher quartiles of U[Na+], U[Na+]/Cr, and U[Na+]/SGU (P for trend <0.001 for U[Na+], 0.001 for U[Na+]/Cr, and <0.001 for U[Na+]/SGU). Table 2 shows the mean levels of other cardiometabolic parameters according to quartiles of U[Na+], U[Na+]/Cr, and U[Na+]/SGU. The mean values of WC, FPG, FSI, HOMA-IR, and TG were higher for the higher quartiles of all the urinary sodium excretion parameters. The mean BMI and systolic BP were significantly higher for the higher quartiles of U[Na+] and U[Na+]/SGU.
FIGURE 1.
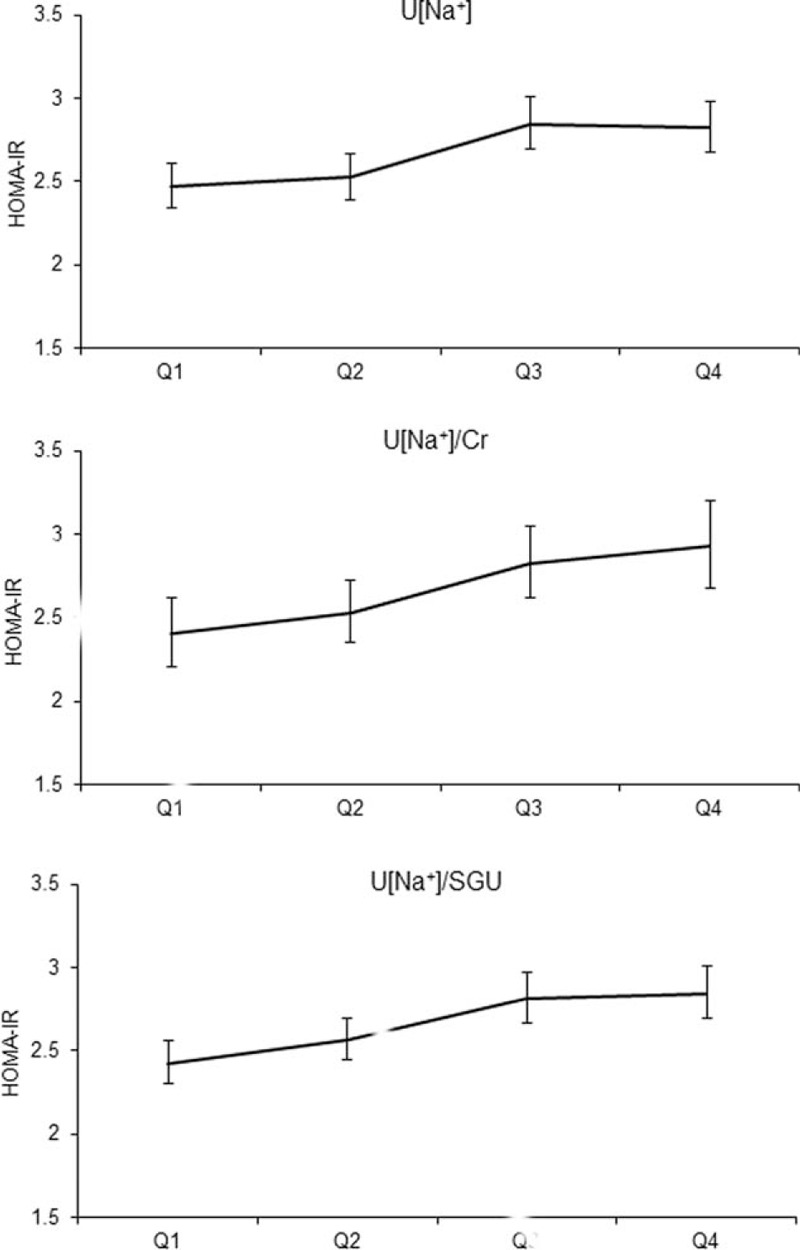
Relationship between the mean HOMA-IR and U[Na+], U[Na+]/Cr, and U[Na+]/SGU quartiles (P for trend <0.001 for U[Na+], 0.001 for U[Na+]/Cr, and <0.001 for U[Na+]/SGU).
TABLE 2.
Mean Values of HOMA-IR and Other Cardiometabolic Parameters According to Quartiles of U[Na+], U[Na+]/Cr, and U[Na+]/SGU
Risk of High HOMA-IR According to Quartiles of Urinary Sodium Excretion
Table 3 presents the ORs for the association with the highest quartile of HOMA-IR based on quartiles of urinary sodium excretion. In the unadjusted analysis (model 1), Q3 of U[Na+], compared with Q1, was significantly associated with the highest quartile of HOMA-IR (OR, 1.7; 95% CI, 1.12–2.58). The OR for Q3 of U[Na+] continued to be higher after adjusting for age and sex in model 2 (OR, 1.7; 95% CI, 1.12–2.59) and also after controlling further for BMI, serum creatinine level, smoking status, alcohol consumption, physical activity, monthly household income level, and daily total energy and fat intake in model 3 (OR, 1.74; 95% CI, 1.05–2.9). The progressive increase in the OR with the increase in the level of the quartile of U[Na+] was significant in all models (P for trend for OR = 0.007 in model 1, 0.007 in model 2, and 0.042 in model 3).
TABLE 3.
Relationship Between High HOMA-IR and Urinary Sodium Excretion
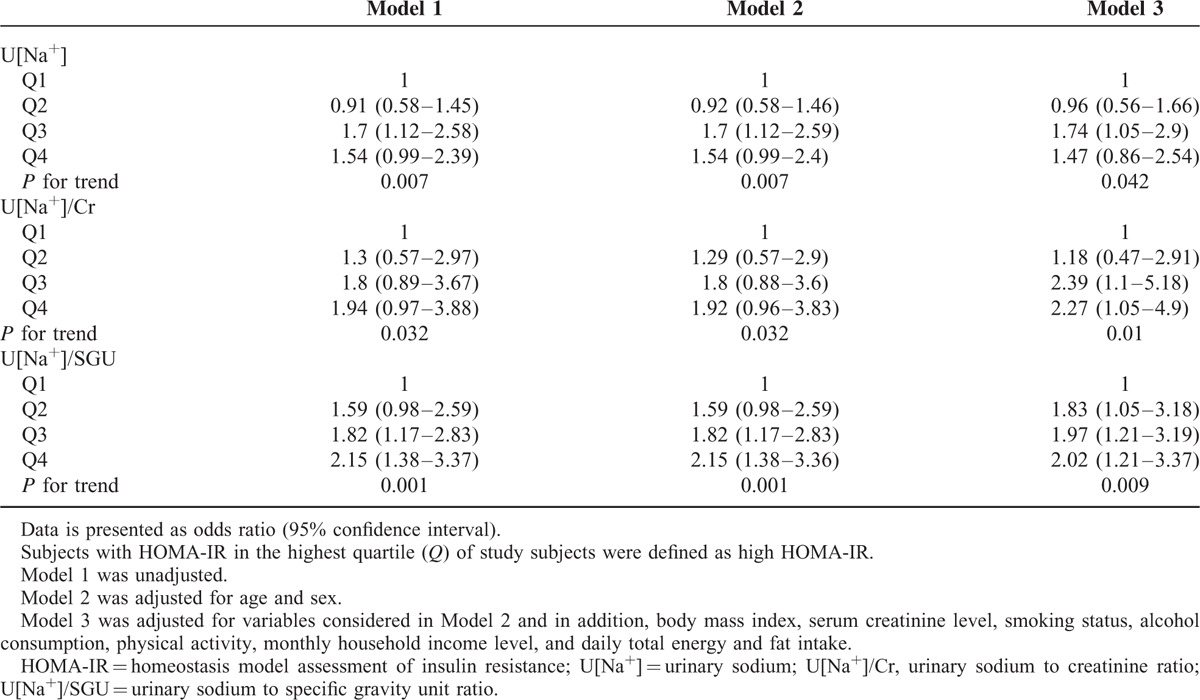
The OR for the association with the highest quartile of HOMA-IR based on quartiles for U[Na+]/Cr were not significant in model 1 and model 2 (model 1: OR [95% CI] = 1.3 [0.57–2.97] for Q2, 1.8 [0.89–3.67] for Q3, 1.94 [0.97–3.88] for Q4; model 2: OR [95% CI] = 1.29 [0.57–2.9] for Q2, 1.8 [0.88–3.6] for Q3, 1.92 [0.96–3.83] for Q4) when compared with Q1 of U[Na+]/Cr. However, in model 3, Q3 and Q4 of U[Na+]/Cr had a significantly higher OR of 2.39 (95% CI, 1.1–5.18) and 2.27 (95% CI, 1.05–4.9) compared with Q1, respectively. The ORs were higher for the higher quartiles of U[Na+]/Cr in all 3 models (P for trend for OR = 0.032 in model 1, 0.032 in model 2, and 0.01 in model 3).
When comparing the different quartiles of U[Na+]/SGU in the 3 models, in model 1, the values of OR were 1.59 (95% CI, 0.98–2.59) for Q2, 1.82 (95% CI, 1.17–2.83) for Q3, and 2.15 (95% CI, 1.38–3.36) for Q4, when compared with Q1. In model 2, Q3 and Q4 of U[Na+]/SGU also had significantly higher OR of 1.82 (95% CI, 1.17–2.83) and 2.15 (95% CI, 1.38–3.37), respectively, compared with Q1. In model 3, the values of OR were 1.83 (95% CI, 1.05–3.18) for Q2, 1.97 (95% CI, 1.21–3.19) for Q3, and 2.02 (95% CI, 1.21–3.37) for Q4. The ORs were higher for the higher quartiles of U[Na+]/SGU in all 3 models (P for trend for OR = 0.001 in model 1, 0.001 in model 2, and 0.009 in model 3).
DISCUSSION
In the present study, the levels of U[Na+], U[Na+]/Cr, and U[Na+]/SGU observed in subjects with high HOMA-IR were higher than those observed in subjects with lower HOMA-IR. The increasing trends of HOMA-IR and several cardiometabolic parameters with increasing quartiles of U[Na+], U[Na+]/Cr, and U[Na+]/SGU were statistically significant. This study also found that the risk of an association with high HOMA-IR increased progressively with higher values of U[Na+], U[Na+]/Cr, and U[Na+]/SGU, independent of confounding factors including BMI, serum creatinine level, smoking status, alcohol consumption, physical activity, monthly household income level, and daily total energy and fat intake.
To the best of our knowledge, this is the first study to investigate the association of urinary sodium excretion, with IR assessed with HOMA-IR. The findings of this study suggest that high sodium intake estimated by using objective indicators may be an independent predictor of IR and, therefore, raise the possibility that reduction of sodium intake may play a role in reducing IR in adolescents. These findings, while providing important information related to the association between sodium intake assessed by urinary sodium excretion and IR in the general adolescent population in South Korea, have public health implications.
To date, a few studies involving adults have examined the association between sodium intake and metabolic syndrome, and demonstrated a significant association between higher sodium intake and an elevated risk of metabolic syndrome.20–23 Although there are no studies investigating the association between sodium intake and IR in children and adolescents, several studies have reported an association between sodium intake and obesity-related parameters in this age group. A cohort study of 364 children and adolescents demonstrated a positive association between high dietary salt, assessed by using 24-hour urine samples, and BMI standard deviation score and percentage body fat, independent of consumption of sugar-containing beverages.11 A cross-sectional study in adolescents also demonstrated an independent association between sodium intake and body fat percentage, body fat mass, abdominal adipose tissue, and leptin levels.24 These results support the findings of the current study as IR is well known as an obesity-related complication in this age group. However, further studies are needed to confirm the association.
Although the reasons for the association between urinary sodium excretion and IR in adolescents are not clear, a few possible mechanisms have been considered. High sodium content is a common feature of highly processed foods or convenience foods, which are also adipogenic. Moreover, there is an association between the amount of salt consumed and the additional intake of sugar-sweetened soft drinks secondary to stimulation of thirst and appetite, which further results in increased energy intake and obesity.25,26 However, in the present study, it was observed that urinary sodium excretion was significantly associated with IR independent of confounding factors, including daily energy and fat intake. It suggests that there may be other factors that could explain the association between high sodium intake and IR besides body weight or BMI gain due to increased energy intake. One of the studies referred above demonstrated an independent association between sodium intake and obesity-related parameters in adolescents.24 Additionally, a high salt intake seemed to be related to an increase in fat mass, adipocyte size, and leptin level without body weight gain in an animal study. This animal study suggested the likelihood of a high-sodium diet increasing lipogenic enzymatic activity and resulting in the incorporation of glucose into lipids.27 In a rat model of metabolic syndrome, salt restriction improved insulin signaling and attenuated the inflammation of adipose tissue without reducing obesity.28 Despite discrepancies in the quantum of dietary sodium restriction, several studies have suggested that sodium intake can increase adrenal cortisol secretion as well as the local conversion of cortisone to cortisol by the 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) enzyme.29,30 Cortisol may have an important role in the pathogenesis of obesity-related metabolic conditions including IR.31 Moreover, even if aldosterone levels are suppressed in a high-sodium diet, other factors such as Rac1 or lysine-specific demethylase-1 (LSD1) could play a role in the activation of mineralocorticoid receptors leading to IR and metabolic disorders.18,32
This study has some limitations. First, owing to the cross-sectional design, a causal relationship between urinary sodium excretion and IR cannot be elucidated. Second, circadian variations may be present in the assessment of urinary sodium excretion by using random spot urine samples. Although correction with urinary creatinine or SGU has already been demonstrated as an alternative to assessing 24-h urinary albumin excretion,15,33 sodium intake can vary substantially in a single day, or during several days, and within subjects.34,35 Serial tests of urinary sodium excretion indicators are considered more informative. Also, thorough verification of these markers in children and adolescents is required to recommend their use in general clinical practice. Third, some data may be associated with recall bias as they were obtained through interviewer-administered or self-reported questionnaires.
Despite these limitations, the major strength of this study is that it is the first study on the association between urinary sodium excretion and IR in adolescents. This is a large population-based study that analyzed nationally representative data of South Korean adolescents and, therefore, provides epidemiologic evidence for the association. The analyses of this study also made use of several indicators of urinary sodium excretion.
In conclusion, urinary sodium excretion estimated by using U[Na+], U[Na+]/Cr, and U[Na+]/SGU was positively associated with IR in South Korean adolescents. The monitoring and control of urinary sodium excretion may be recommended as an important intervention to prevent IR and related diseases in adolescents.
Acknowledgment
The authors thank the Korea Centers for Disease Control and Prevention for providing the data.
Footnotes
Abbreviations: BMI = body mass index, BP = blood pressure, CI = confidence interval, FPG = fasting plasma glucose, FSI = fasting serum insulin, HDL-C = high-density lipoprotein cholesterol, HOMA-IR = homeostasis model assessment of insulin resistance, IR = insulin resistance, KCDC = Korea Centers for Disease Control and Prevention, KNHANES = Korea National Health and Nutrition Examination Survey, LDL-C = low-density lipoprotein cholesterol, OR = odds ratio, Q = quartile, SE = standard error, SGU = specific gravity unit, T2DM = type 2 diabetes mellitus, TC = total cholesterol, TG = triglyceride, U[Na+] = urinary sodium concentration, U[Na+]/Cr = urinary sodium to creatinine ratio, U[Na+]/SGU = urinary sodium to specific gravity unit ratio, WC = waist circumference
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.van der Aa MP, Fazeli Farsani S, Knibbe CA, et al. Population-based studies on the epidemiology of insulin resistance in children. J Diabetes Res 2015; 2015:362375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung KC, Jeong WS, Wild SH, et al. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care 2012; 35:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ode KL, Frohnert BI, Nathan BM. Identification and treatment of metabolic complications in pediatric obesity. Rev Endocr Metab Disord 2009; 10:167–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bocca G, Ongering EC, Stolk RP, et al. Insulin resistance and cardiovascular risk factors in 3- to 5-year-old overweight or obese children. Horm Res Paediatr 2013; 80:201–206. [DOI] [PubMed] [Google Scholar]
- 5.Saely CH, Aczel S, Marte T, et al. The metabolic syndrome, insulin resistance, and cardiovascular risk in diabetic and nondiabetic patients. J Clin Endocrinol Metab 2005; 90:5698–5703. [DOI] [PubMed] [Google Scholar]
- 6.Strazzullo P, D’Elia L, Kandala NB, et al. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ 2009; 339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 2011; 306:2229–2238. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371:612–623. [DOI] [PubMed] [Google Scholar]
- 9.Vedovato M, Lepore G, Coracina A, et al. Effect of sodium intake on blood pressure and albuminuria in Type 2 diabetic patients: the role of insulin resistance. Diabetologia 2004; 47:300–303. [DOI] [PubMed] [Google Scholar]
- 10.Sarno F, Jaime PC, Ferreira SR, et al. [Sodium intake and metabolic syndrome: a systematic review]. Arq Bras Endocrinol Metabol 2009; 53:608–616. [DOI] [PubMed] [Google Scholar]
- 11.Libuda L, Kersting M, Alexy U. Consumption of dietary salt measured by urinary sodium excretion and its association with body weight status in healthy children and adolescents. Public Health Nutr 2012; 15:433–441. [DOI] [PubMed] [Google Scholar]
- 12.He FJ, Marrero NM, Macgregor GA. Salt and blood pressure in children and adolescents. J Hum Hypertens 2008; 22:4–11. [DOI] [PubMed] [Google Scholar]
- 13.Kawano Y, Tsuchihashi T, Matsuura H, et al. Report of the Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension: (2) Assessment of salt intake in the management of hypertension. Hypertens Res 2007; 30:887–893. [DOI] [PubMed] [Google Scholar]
- 14.Newman DJ, Pugia MJ, Lott JA, et al. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin Chim Acta 2000; 294:139–155. [DOI] [PubMed] [Google Scholar]
- 15.Lee SG, Lee W, Kwon OH, et al. Association of urinary sodium/creatinine ratio and urinary sodium/specific gravity unit ratio with blood pressure and hypertension: KNHANES 2009-2010. Clin Chim Acta 2013; 424:168–173. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18:499–502. [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 18.Pojoga LH, Williams JS, Yao TM, et al. Histone demethylase LSD1 deficiency during high-salt diet is associated with enhanced vascular contraction, altered NO-cGMP relaxation pathway, and hypertension. Am J Physiol Heart Circ Physiol 2011; 301:H1862–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh JY, Yang YJ, Kim BS, et al. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J Korean Acad Fam Med 2007; 28:532–541. [Google Scholar]
- 20.Raisanen JP, Silaste ML, Kesaniemi YA, et al. Increased daily sodium intake is an independent dietary indicator of the metabolic syndrome in middle-aged subjects. Ann Med 2012; 44:627–634. [DOI] [PubMed] [Google Scholar]
- 21.Baudrand R, Campino C, Carvajal CA, et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol 2014; 80:677–684. [DOI] [PubMed] [Google Scholar]
- 22.Ge Z, Guo X, Chen X, et al. Association between 24 h urinary sodium and potassium excretion and the metabolic syndrome in Chinese adults: the Shandong and Ministry of Health Action on Salt and Hypertension (SMASH) study. Br J Nutr 2015; 113:996–1002. [DOI] [PubMed] [Google Scholar]
- 23.Oh SW, Han KH, Han SY, et al. Association of sodium excretion with metabolic syndrome, insulin resistance, and body fat. Medicine 2015; 94:e1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu H, Pollock NK, Kotak I, et al. Dietary sodium, adiposity, and inflammation in healthy adolescents. Pediatrics 2014; 133:e635–e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He FJ, Marrero NM, MacGregor GA. Salt intake is related to soft drink consumption in children and adolescents: a link to obesity? Hypertension 2008; 51:629–634. [DOI] [PubMed] [Google Scholar]
- 26.Grimes CA, Wright JD, Liu K, et al. Dietary sodium intake is associated with total fluid and sugar-sweetened beverage consumption in US children and adolescents aged 2-18 y: NHANES 2005-2008. Am J Clin Nutr 2013; 98:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonseca-Alaniz MH, Brito LC, Borges-Silva CN, et al. High dietary sodium intake increases white adipose tissue mass and plasma leptin in rats. Obesity (Silver Spring) 2007; 15:2200–2208. [DOI] [PubMed] [Google Scholar]
- 28.Hattori T, Murase T, Takatsu M, et al. Dietary salt restriction improves cardiac and adipose tissue pathology independently of obesity in a rat model of metabolic syndrome. J Am Heart Assoc 2014; 3:e001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewicka S, Nowicki M, Vecsei P. Effect of sodium restriction on urinary excretion of cortisol and its metabolites in humans. Steroids 1998; 63:401–405. [DOI] [PubMed] [Google Scholar]
- 30.Usukura M, Zhu A, Yoneda T, et al. Effects of a high-salt diet on adipocyte glucocorticoid receptor and 11-beta hydroxysteroid dehydrogenase 1 in salt-sensitive hypertensive rats. Steroids 2009; 74:978–982. [DOI] [PubMed] [Google Scholar]
- 31.Rask E, Simonyte K, Lonn L, et al. Cortisol metabolism after weight loss: associations with 11 beta-HSD type 1 and markers of obesity in women. Clin Endocrinol 2013; 78:700–705. [DOI] [PubMed] [Google Scholar]
- 32.Shibata S, Fujita T. Mineralocorticoid receptors in the pathophysiology of chronic kidney diseases and the metabolic syndrome. Mol Cell Endocrinol 2012; 350:273–280. [DOI] [PubMed] [Google Scholar]
- 33.Koo H, Lee SG, Kim JH. Evaluation of random urine sodium and potassium compensated by creatinine as possible alternative markers for 24 hours urinary sodium and potassium excretion. Ann Lab Med 2015; 35:238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann SJ, Gerber LM. Estimation of 24-hour sodium excretion from spot urine samples. J Clin Hypertens (Greenwich) 2010; 12:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee MY, Kim JH, Shin SJ, et al. Estimation of 24-hour urinary sodium excretion using spot urine samples. Nutrients 2014; 6:2360–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]


