Abstract
This study investigated the oncologic impact of loss of SMAD4 expression in resected left-sided pancreatic cancer and its correlation with tumor metabolism.
From 2005 to 2011, the medical records of patients who underwent radical distal pancreatectomy for resectable pancreatic cancer were retrospectively reviewed. Formalin-fixed, paraffin embedded tissue from 32 patients was investigated. Clinicopathological characteristics, immunostaining of SMAD4, and positron emission tomography-based parameters were analyzed in relation to oncologic outcomes.
Thirteen patients were women and 19 were men, with a mean age of 63 ± 9.4 years. Mean resected tumor size was 3.3 ± 1.5 cm. Ten patients (31.3%) showed loss of SMAD4 expression. No significant clinicopathological differences were noted according to SMAD4 expression (P > 0.05); however, patients with loss of SMAD4 showed significantly poorer disease-free survival (mean 57.4 months vs mean 17.6 months, P = 0.006). As a cut-off value, a SUVmax of 4.5 was found to be predictive of loss of SMAD4 with a sensitivity of 75% and a specificity of 84.6%. In logistic regression analysis, SUVmax>4.5 was found to infer a 16-fold higher risk for loss of SMAD4 in resected left-sided pancreatic cancers (Exp[β] = 16.5, P = 0.012, 95% confidence interval: 1.832–148.606).
Loss of SMAD4 is associated with poor oncologic outcomes. SUVmax can predict loss of SMAD4 in resected left-sided pancreatic cancer. SUVmax may be a clinical biomarker for detecting loss of SMAD4 expression and predicting early systemic metastasis.
INTRODUCTION
Pancreatic cancer results from an accumulation of somatic mutations in multiple genes, including K-ras, p16, p53, and SMAD4.1SMAD4 is a major tumor-suppressing gene involved in the development of pancreatic cancer. Inactivation of SMAD4 occurs more frequently in pancreatic cancer than in other cancers harboring K-ras, p16, and p53 mutations.2
SMAD4 is suggested to be a useful prognostic marker in pancreatic cancer. SMAD4 deficiency rapidly promotes K-ras-initiated neoplasms3 and enhances metastasis more frequently than that which is seen in Smad4-intact tumors.4 Iacobuzio-Donahue et al5 performed autopsies on 76 patients with pancreatic cancer and found that SMAD4-negative status was highly correlated with widespread metastasis. Thus, preoperative determination of SMAD4 status could be of use in stratifying patients for appropriate treatment.
Several studies support the potential role of SMAD4 in the clinical oncology of pancreatic cancer.6–8 Others, however, report contradictory results, showing loss of SMAD4 expression to be significantly associated with better survival after resection: Biankin et al9 evaluated deleted in pancreatic cancer 4 (DPC4)/SMAD4 expression in relation to pancreatic cancer outcomes. They found that loss of DPC4/Smad4 expression was correlated with resectability and improved survival after resection. Winter et al10 found regional lymph node metastasis to be the only factor predictive of distant metastasis; SMAD4 was not associated with recurrence or early cancer-related death. These results highlight the continuing controversy regarding the clinical application of SMAD4 in pancreatic cancer treatment.
In this study, we evaluated the oncologic impact of SMAD4 expression on resected pancreatic cancer. To exclude potential bias from other periampullary cancers, we focused only on left-sided pancreatic cancer. Additionally, we assessed SMAD4 expression in relation to 18-fluoro-deoxyglucose (18FDG) positron emission tomography/positron emission tomography-computed tomography (PET/PET-CT) to determine the clinical implications of SMAD4 expression in the clinical oncology of resectable pancreatic cancer.
METHODS
Review of Medical Records
From January 2005 to December 2011, 50 patients at our institute underwent radical distal pancreatosplenectomy for left-sided pancreatic cancer. Ductal adenocarcinoma was confirmed in all patients. Fifteen patients with preoperative neoadjuvant treatment and 9 without available paraffin-embedded tissue blocks were excluded (Figure 1). We reviewed the medical records of 32 patients who underwent resection for national comprehensive cancer network guideline-based resectable left-sided pancreatic cancer.11 Clinicopathological characteristics, such as age, gender, clinical presentation, tumor size, histopathological features, and follow-up data, were reviewed and recorded. The Institutional Review Board of Yonsei University College of Medicine approved this study protocol.
FIGURE 1.
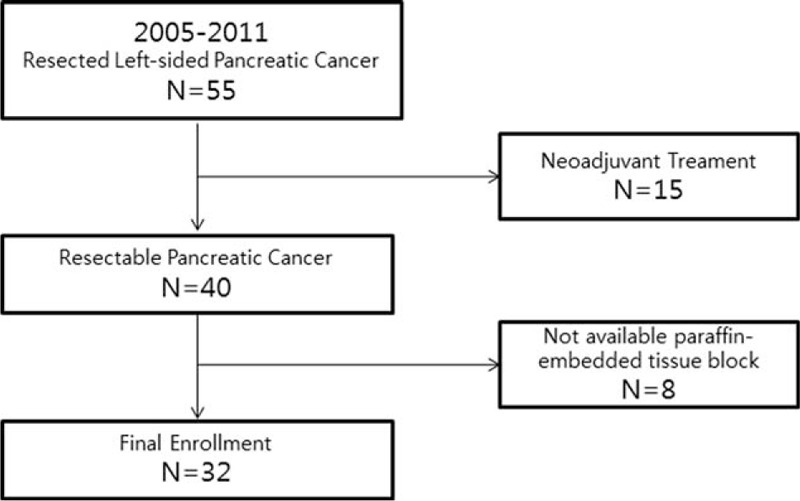
Patient enrolment, schematic illustration showing patient enrollment in this study. Among 55 patients who underwent resection of left-sided pancreatic cancer, 32 patients were selected.
PET-CT Analysis
In the present study, PET-CT imaging was conducted as follows: 18F-FDG PET scans were obtained on a dedicated PET-CT scanner (Discovery STe, GE Healthcare). Patients were instructed to fast for at least 6 hours before the scans, and a glucose level of ≤140.0 mg/dL was administered to all patients. Scans were started at 60 minutes after intravenous injection of 5.5 MBq/kg of 18F-FDG. CT scans were initially performed at 30 mA and 130 kVp without contrast-enhancement for attenuation correction. Then, PET scans were performed with a 3 min/bed position in a 3-dimensional mode. PET/CT images were reconstructed using an iterative algorithm, ordered subset expectation maximization with attenuation correction. 18F-FDG PET/CT images were reviewed and analyzed by 2 nuclear medicine physicians. Maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV2.5), and total lesion glycolysis (TLG) on PET images were measured using commercially available imaging software (MIM-6.4, MIM software Inc., Cleveland, OH, USA)14. Each tumor was examined with a spherical volume of interest (VOI) that included the entire lesion in the axial, sagittal, and coronal planes. Using CT images, 18F-FDG uptake of normal organs, such as the bowel, stomach, and liver, was not included in the VOI. The SUVmax of the VOI was calculated as (decay-corrected activity/tissue volume)/(injected dose/body weight). MTV2.5 was defined as a total tumor volume with SUV ≥2.5. MTV and mean SUV of the VOI were automatically calculated. TLG was calculated as (mean SUV) × (MTV). In patients with SUVmax <2.5, MTV2.5 and TLG were not measured. Metabolic volume was calculated according to the steepest gradient of FDG uptake, the so-called PET-Edge calculation (MTVEdge).15 For edge detection, the cursor was dragged out over the lesion until 3 orthogonal guiding lines covered the boundaries of the lesion. Then, the edge detection software automatically calculated metabolic tumor volume in 3 dimensions.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections were deparaffinized and rehydrated for antigen retrieval. Immunohistochemical analysis used prediluted anti-Smad4 (Abcam, #ab13786) according to the manufacturer's instructions. All slides were reviewed by 2 pathologists from the Korean CFC, a domestic pathology laboratory company specializing in immunohistochemistry (http://www.koreacfc.com/intro/intro01.asp), who were blinded to the oncologic outcomes and values of the PET-based parameters. SMAD4 staining intensity was scored as 0 for loss of expression and 1 for intact expression (Figure 2).
FIGURE 2.
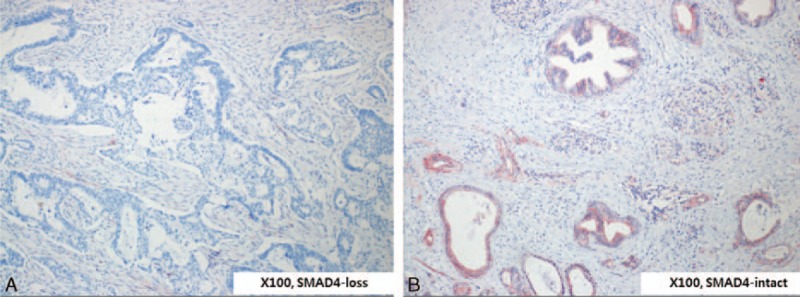
Expression of SMAD4 in resected left-sided pancreatic cancer. According to SMAD4 expression on pathological examination of immunostaining results, patients were divided into 2 groups: SMAD4-loss (A) and SMAD4-intact (B).
Statistical Analysis
Statistical analysis was performed using SPSS II software (IBM Corporation, Chicago, IL). Continuous variables were expressed as a mean ± standard deviation, and categorical variables were expressed as a frequency (percent). Receiver operating characteristic (ROC) curves were used to determine optimal cutoff values for PET-based parameters in order to predict SMAD4 expression. Univariate analysis was performed using Chi-square with Fisher's exact test, and Mann–Whitney's U test was applied for statistical assessment of the associations between clinicopathological characteristics and PET-based parameters. Odds ratios and their 95% confidence intervals were estimated using logistic regression analysis. Disease-specific survival was calculated from the time of diagnosis to death or last follow-up day, and disease-free survival was calculated from the time of surgery to disease recurrence (local, systemic, both). In particular, the period of time from surgery to systemic recurrence, as initial recurrence, was defined as systemic metastasis-free survival. Survival was analyzed by the Kaplan–Meier method. Survival outcomes were compared by the log-rank test, and Cox's proportional hazards model was applied for multivariate analysis. All P-values <0.05 were considered statistically significant.
RESULTS
Patient Demographics
All patients underwent distal pancreatosplenectomy; 13 were women and 19 were men, with a mean age of 63 ± 9 years. Sixteen patients had clinical symptoms. Abdominal pain and discomfort was the most common symptom, which was reported in 16 patients. Indigestion was noted in 5 patients, weight loss in 2 patients, and anorexia/nausea in 1 patient. Of the tumors, 25 were located in the pancreatic body, 5 in the tail, and 2 in the body and tail. Mean tumor size was 3.3 ± 1.5 cm in the maximal diameter. Five patients required a combined resection, such as that of the adrenal gland, a left nephrectomy, or segmental resection of the transverse colon. Stage I was noted in 1 patient, Stage IIA in 13, Stage IIB in 16, and Stage III in 2. Margin-negative resection (R0) was reported in 27 patients (84.4%). Post-adjuvant chemotherapy was administered to 23 patients (71.9%). During the follow-up period (mean 37.2 months [range, 1.5–96.97]), the 5-year disease-specific survival was 38.9% (17 cancer-related mortalities among 32 patients), and disease-free survival was 43.4% (21 postoperative cancer recurrences among 32 patients at 5 years after surgery).
SMAD4 Expression in Resected Left-Sided Pancreatic Cancer
Among 32 patients who underwent radical pancreatectomy for left-sided pancreatic cancer, 22 (68.8%) showed intact SMAD4 expression, and the other 10 (31.3%) had no expression of SMAD4 (Figure 2A and B).
Oncologic Impact of SMAD4 Expression in Resected Left-Sided Pancreatic Cancer
No significant differences were observed in clinicopathological characteristics according to SMAD4 expression in patients with resected left-side pancreatic cancer (Table 1). However, pancreatic cancer patients with intact SMAD4 expression showed significantly longer disease-free survival, compared to patients without SMAD4 expression (mean 57.4 months [95% CI: 41.4–73.3] vs mean 17.6 months [95% CI: 6.3–28.9], P = 0.006, Figure 3).
TABLE 1.
Clinicopathological Characteristics According to SMAD4 Expression in Resected Left-Sided Pancreatic Cancer
FIGURE 3.
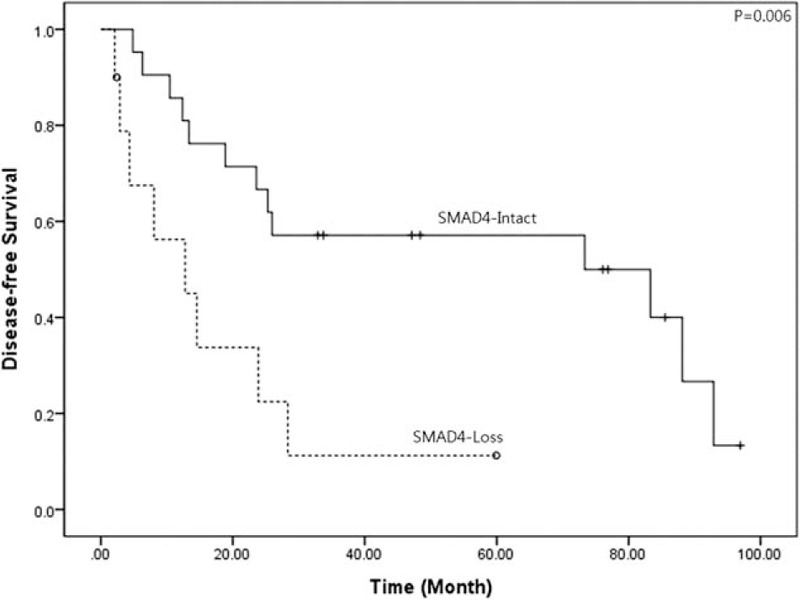
Disease-free survival according to SMAD4 expression in resected left-sided pancreatic cancer. According to SMAD4 expression, a significant difference in disease-free survival was noted in resected left-sided pancreatic cancer (mean 57.4 months [95% CI: 41.4–73.3] vs mean 17.6 months [95% CI: 6.3–28.9], P = 0.006). CI = confidence interval.
Values of PET-Based Parameters in Predicting Loss of SMAD4 Expression in Resected Left-Sided Pancreatic Cancer
We noted that resected left-sided pancreatic cancers with intact SMAD4 expression had relatively lower values for PET-based parameters (Figure 4). Among PET-based parameters, only SUVmax was statistically different according to SMAD4 expression in resected left-sided pancreatic cancer (5.4 ± 2.2 vs 3.6 ± 1.7, P = 0.025, Table 2).
FIGURE 4.
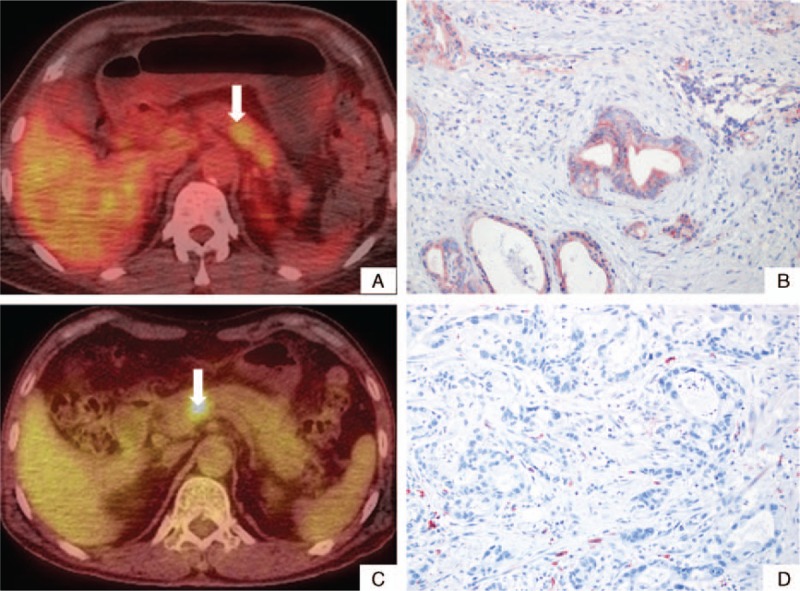
Signal intensities of FDG-uptake according to SMAD4 expression. The signal intensity of FDG-uptake in preoperative PET-CT scan is related to SMAD4 expression in resected left-sided pancreatic cancer. Low-signal intensity in FDG-PET-CT (A) was found to represent intact SMAD4 expression (B), whereas high-signal intensity of FDG uptake (C) reflected no SMAD4 expression (D). FDG = fluoro-deoxyglucose, PET-CT = positron emission tomography-computed tomography.
TABLE 2.
Differences in PET-Based Parameters According to SMAD4 Expression in Resected Left-Sided Pancreatic Cancer
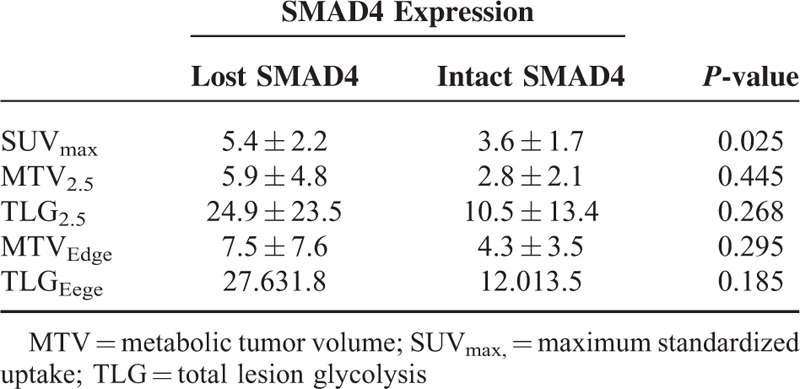
According to ROC analysis, a SUVmax of 4.5 was suggested as a cutoff value for predicting loss of SMAD4 in resected left-sided pancreatic cancer, with a sensitivity of 75% and a specificity value of 84.6% (AUC=0.798, P = 0.025, 95% confidence interval: 0.594–1.000, Figure 5A). In subsequent logistic regression analysis, SUVmax>4.5 was found to infer a 16-fold greater risk for loss of SMAD4 in resected left-sided pancreatic cancer (Exp[β]=16.5, P = 0.012, 95% confidence interval: 1.832–148.606).
FIGURE 5.
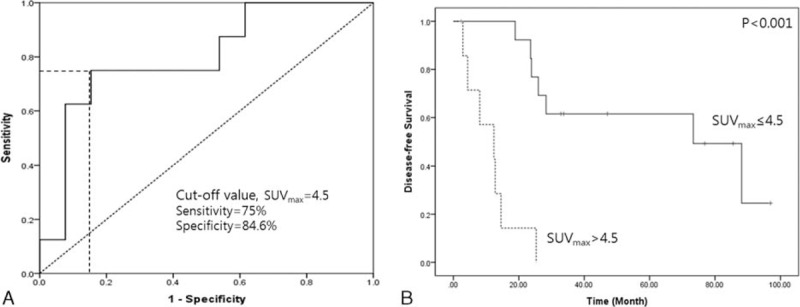
Optimal cut-off value for SUVmax to predict loss of SMAD4 and recurrence. In ROC analysis, a SUVmax of 4.5 was found to be predictive of tumor recurrence with a sensitivity of 75% and specificity of 84.6% (A). Subsequent survival analysis demonstrated that SUVmax =4.5 discriminated disease-free survival (mean 63.9 months [95% CI: 46.0–81.7] vs 11.5 months [95% CI: 5.9–17.1], P < 0.001) (B). CI = confidence interval, ROC = receiver operating characteristic, SUVmax = maximum standardized uptake.
Prediction of Initial Recurrence as Systemic Metastasis
SUVmax >4.5 predicted early cancer recurrence in resected left-side pancreatic cancer (mean 63.9 months [95% CI: 46.0–81.7] vs 11.5 months [95% CI: 5.9–17.1], P < 0.001, Figure 5B). In particular, resected pancreatic cancers without SMAD4 expression showed earlier systemic metastasis, compared to those with intact SMAD4 expression (22.3 months [95% CI: 8.9–35.7] vs 62.5 months [95% CI: 46.7–78.3], P = 0.022) (Figure 6A). In addition, resected pancreatic cancers with a SUVmax >4.5 were also associated with early systemic metastasis (14.3 months [95% CI: 8.0–20.5] vs 66.1 months [95% CI: 47.3–84.8], P < 0.001) (Figure 6B). In Cox's proportional hazards model, both loss of SMAD4 expression (Exp [β] = 4.620, P = 0.039) and SUVmax >4.5 (Exp [β] = 11.650, P = 0.003) were independent prognostic factors for predicting early systemic metastasis.
FIGURE 6.
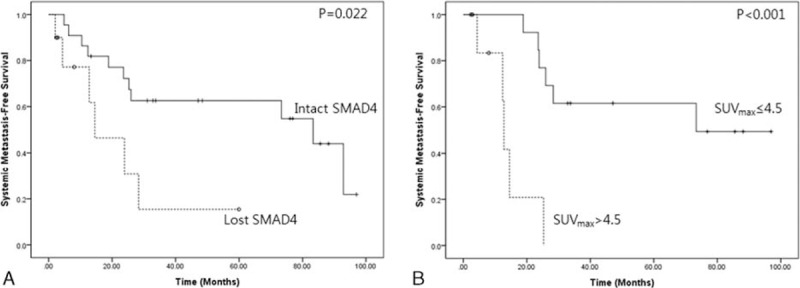
Systemic metastasis-free survival according to SMAD4 expression and SUVmax value, 4.5. Loss of SMAD4 was associated with earlier systemic metastasis in resected left-sided pancreatic cancer (22.3 months [95% CI: 8.9–35.7] vs 62.5 months [95% CI: 46.7–78.3], P = 0.022) (A), and SUVmax >4.5 was also related to early systemic metastasis (14.3 months [95% CI: 8.0–20.5] vs 66.1 months [95% CI: 47.3–84.8], P < 0.001) (B). CI = confidence interval, SUVmax = maximum standardized uptake.
DISCUSSION
Although SMAD4 expression is potentially involved in pancreatic cancer, a relationship between loss of or reduced SMAD4 expression and poor oncologic outcomes has not been consistently observed. Smith et al16 performed a meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Therein, loss of SMAD4 expression was noted in a median of 25% of patients (range, 24–85% of 540 patients), with no significant overall association between SMAD4 expression and survival from resected pancreatic cancer. Studies reporting no oncologic significance for SMAD4 in pancreatic cancer mostly involved patients with pancreatic head cancer and only a small proportion of patients with body/tail cancer: Winter et al10 studied 110 patients with pancreatic head cancer and 15 patients with left-sided pancreatic cancer. This suggests the possibility of contamination by other periampullary cancers, such as cancers of the bile duct, ampulla of Vater, or duodenum. In periampullary cancers, the exact origin of a cancer is often unclear. Therefore, our study focused on resected left-sided pancreatic cancer in order to minimize issues related to pathological contamination and to provide information about clinical applications for resectable pancreatic cancer.
The present results clearly demonstrated that loss of SMAD4 expression has a significant adverse oncologic impact on resected left-sided pancreatic cancer (Figure 3). In addition, a potential correlation between SMAD4 expression and SUVmax was an important observation (Figure 4 and Table 2). Multivariate analysis showed that mean SUVmax > 4.3 is an independent predictive factor for determining loss of SMAD expression (Exp[β] = 10.0, P = 0.028).
Several studies have suggested potential clinical applications for SMAD4 expression in treatment of pancreatic cancer. Oshima et al17 reported that immunohistochemically detected SMAD4 expression strongly predicts oncologic outcomes for resected pancreatic cancer. They showed that loss of SMAD4 immunolabeling is an independent and significant prognostic factor for poor overall and disease-free survival. Crane et al18 demonstrated chemotherapeutic agent effectiveness according to SMAD4 immunostaining of locally advanced pancreatic cancer, showing that SMAD4 expression is correlated with local, rather than distant, dominant patterns of disease progression.
In clinical situations, preoperative SMAD4 expression in resectable pancreatic cancer can be determined by endoscopic ultrasound (EUS)-guided fine needle aspiration biopsy (FNAB). Although extensive gastroenterologist experience increases the safety and high diagnostic performance of EUS-guided FNAB,19 the following disadvantages of EUS-guided FNAB should be considered: (1) the procedure is invasive and operator-dependent. (2) Immunostaining for targeted protein, such as SMAD4, takes time. (3) Rebiopsy is often recommended because of insufficient tissue material.
Meanwhile, PET/PET-CT is noninvasive and provides useful information on diagnosis, staging, and biological behavior for treating pancreatic cancer in preoperative settings.20 Several studies have shown the oncological significance of preoperative PET/PET-CT in resected pancreatic cancer.12,13,21,22 According to our results, loss of SMAD4 expression is associated with increases in PET-based parameters (Table 2). In particular, SUVmax > 4.5 predicted loss of SMAD4 expression with 75% sensitivity and a 15.4% false-positive rate (Figure 5A). More importantly, oncologic outcomes were clearly differentiated by a SUVmax cut-off of 4.5 (Figure 5B), as shown in survival differences according to SMAD4 expression (Figure 3). In addition, both loss of SMAD4 expression (Exp [β] = 4.620, P = 0.039) and SUVmax > 4.5 (Exp [β] = 11.650, P = 0.003) were independent predictive factors of early systemic metastasis.
This study was retrospective in design and included a small study population. Therefore, selection bias could not be avoided. However, to the best of our knowledge, this study is the first to correlate SMAD4 expression and tumor metabolism (PET-based parameters) in resected pancreatic cancer. The potential correlations between SMAD4 expression and PET-based parameters suggest a patient-oriented surgical approach to treating resectable pancreatic cancer. Despite some debate, the literature shows that loss of SMAD4 is associated with dissemination of pancreatic cancer cells. If PET-based parameters, such as SUVmax, are indeed found to be of use as representative clinical biomarkers for SMAD4 expression and early systemic recurrence, then alternative surgical strategies (e.g., neoadjuvant treatment followed by pancreatectomy) could be recommended according to SUVmax values, even for resectable pancreatic cancers. The validity of such approaches should first be explored in a larger number of patients.
Footnotes
Abbreviations: 18FDG = 18-fluoro-deoxyglucose, DPC4 = deleted in pancreatic cancer 4, EUS = endoscopic ultrasound, FNAB = fine needle aspiration biopsy, MTV2.5 = metabolic tumor volume, PET/PET-CT = positron emission tomography/positron emission tomography-computed tomography, ROC = receiver operating characteristic, SUVmax = maximum standardized uptake value, TLG = total lesion glycolysis, VOI = volume of interest
Woo Jung Lee and Mijin Yun equally contributed as the corresponding author.
Funding: this study was supported by a faculty research grant of Yonsei University College of Medicine (6-2013-0163).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Goggins M, Kern SE, Offerhaus JA, et al. Progress in cancer genetics: lessons from pancreatic cancer. Ann Oncol 1999; 10 Suppl 4:4–8. [PubMed] [Google Scholar]
- 2.Iacobuzio-Donahue CA, Song J, Parmiagiani G, et al. Missense mutations of MADH4: characterization of the mutational hot spot and functional consequences in human tumors. Clin Cancer Res 2004; 10:1597–1604. [DOI] [PubMed] [Google Scholar]
- 3.Bardeesy N, Cheng KH, Berger JH, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev 2006; 20:3130–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izeradjene K, Combs C, Best M, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell 2007; 11:229–243. [DOI] [PubMed] [Google Scholar]
- 5.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009; 27:1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voorneveld PW, Stache V, Jacobs RJ, et al. Reduced expression of bone morphogenetic protein receptor IA in pancreatic cancer is associated with a poor prognosis. Br J Cancer 2013; 109:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toga T, Nio Y, Hashimoto K, et al. The dissociated expression of protein and messenger RNA of DPC4 in human invasive ductal carcinoma of the pancreas and their implication for patient outcome. Anticancer Res 2004; 24 (2C):1173–1178. [PubMed] [Google Scholar]
- 8.Singh P, Srinivasan R, Wig JD. SMAD4 genetic alterations predict a worse prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas 2012; 41:541–546. [DOI] [PubMed] [Google Scholar]
- 9.Biankin AV, Morey AL, Lee CS, et al. DPC4/Smad4 expression and outcome in pancreatic ductal adenocarcinoma. J Clin Oncol 2002; 20:4531–4542. [DOI] [PubMed] [Google Scholar]
- 10.Winter JM, Tang LH, Klimstra DS, et al. Failure patterns in resected pancreas adenocarcinoma: lack of predicted benefit to SMAD4 expression. Ann Surg 2013; 258:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006; 13:1035–1046. [DOI] [PubMed] [Google Scholar]
- 12.Lee JW, Kang CM, Choi HJ, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med 2014; 55:898–904. [DOI] [PubMed] [Google Scholar]
- 13.Choi HJ, Kang CM, Lee WJ, et al. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography in patients with resectable pancreatic cancer. Yonsei Med J 2013; 54:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang CM, Cho A, Kim H, et al. Clinical correlations with (18)FDG PET scan patterns in solid pseudopapillary tumors of the pancreas: still a surgical enigma? Pancreatology 2014; 14:515–523. [DOI] [PubMed] [Google Scholar]
- 15.Wanet M, Lee JA, Weynand B, et al. Gradient-based delineation of the primary GTV on FDG-PET in non-small cell lung cancer: a comparison with threshold-based approaches, CT and surgical specimens. Radiother Oncol 2011; 98:117–125. [DOI] [PubMed] [Google Scholar]
- 16.Smith RA, Tang J, Tudur-Smith C, et al. Meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Br J Cancer 2011; 104:1440–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshima M, Okano K, Muraki S, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg 2013; 258:336–346. [DOI] [PubMed] [Google Scholar]
- 18.Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol 2011; 29:3037–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamao K, Sawaki A, Mizuno N, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol 2005; 40:1013–1023. [DOI] [PubMed] [Google Scholar]
- 20.Dibble EH, Karantanis D, Mercier G, et al. PET/CT of cancer patients: part 1, pancreatic neoplasms. AJR Am J Roentgenol 2012; 199:952–967. [DOI] [PubMed] [Google Scholar]
- 21.Xu HX, Chen T, Wang WQ, et al. Metabolic tumour burden assessed by (1)(8)F-FDG PET/CT associated with serum CA19-9 predicts pancreatic cancer outcome after resection. Eur J Nucl Med Mol Imaging 2014; 41:1093–1102. [DOI] [PubMed] [Google Scholar]
- 22.Epelbaum R, Frenkel A, Haddad R, et al. Tumor aggressiveness and patient outcome in cancer of the pancreas assessed by dynamic 18F-FDG PET/CT. J Nucl Med 2013; 54:12–18. [DOI] [PubMed] [Google Scholar]


