Supplemental Digital Content is available in the text
Abstract
The aim of the study is to compare Roux-en-Y gastric bypass (RYGB) surgery versus medical treatment for type 2 diabetes mellitus (T2DM) in obese patients.
Bariatric surgery can achieve remission of T2DM in obese patients. RYGB surgery has been performed as one of the most common surgical treatment options for obese patients with T2DM, but the efficacy of RYGB surgery comparing with medical treatment alone has not been conclusively determined.
A systematic literature search identified randomized controlled trials (RCTs) evaluating RYGB surgery versus medical treatment for T2DM in obese patients was conducted in PubMed, Embase, Cochrane Database, and Cochrane Clinical Trials Registry. This systematic review and meta-analysis were performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. The primary outcome was T2DM remission. Additional analyses comprised hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), body mass index (BMI), waist circumference, serum lipid level, blood pressure, medication use, and adverse events. Random-effects meta-analyses were calculated and presented as weighted odds ratio (OR) or mean difference (MD) with 95% confidence intervals (CI).
Six RCTs concerning 410 total obese T2DM patients were included. Follow-up ranged from 12 to 60 months. RYGB surgery was associated with a higher T2DM remission rate (OR: 76.37, 95% CI: 20.70–281.73, P < 0.001) and serum level of high-density lipoprotein cholesterol (MD: 0.24 mmol/L, 95% CI 0.18–0.30 mmol/L, P < 0.001) than medical treatment alone. HbA1c (MD: –1.25%, 95% CI: –1.88% to –0.63%, P < 0.001), BMI (MD: –6.54 kg/m2, 95% CI: –9.28 to –3.80 kg/m2, P < 0.001), waist circumference (MD: –15.60 cm, 95% CI: –18.21 to –13.00 cm, P < 0.001), triglyceride (MD: –0.87 mmol/L, 95% CI: –1.17 to –0.57 mmol/L, P < 0.001), low-density lipoprotein cholesterol (MD: –0.32 mmol/L, 95% CI: –0.62 to –0.02 mmol/L, P = 0.04), systolic blood pressure (MD: –2.83 mm Hg, 95% CI: –4.88 to –0.78 mm Hg, P < 0.01) were lower after RYGB surgery. However, FPG (MD: –1.58 mmol/L, 95% CI: –3.58 to 0.41 mmol/L, P = 0.12), total cholesterol (MD: –0.40 mmol/L, 95% CI: –0.92 to 0.12 mmol/L, P = 0.13), and diastolic blood pressure (MD: 0.28 mm Hg, 95% CI: –1.89 to 2.45 mm Hg, P = 0.80) were not significantly different between the 2 treatment groups. The medicine use and quality of life were solely improved in the surgical group. Nutritional deficiencies and anemia were noted more frequently in the RYGB group.
RYGB surgery is superior to medical treatment for short- to medium-term remission of T2DM, improvement of metabolic condition, and cardiovascular risk factors. Further RCTs should address the safety and long-term benefits of RYGB surgery on obese patients with T2DM.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) and obesity are 2 of the common chronic diseases that occur frequently among people in the whole world and remain an unsolved problem for global health care.1–4 The association between obesity and diabetes is well established by that obese individuals are accompanied with an increased risk for T2DM, and 90% of patients with T2DM have an excess body weight.5,6 Furthermore, effective weight loss in obese subjects with or without T2DM is associated with improvements of metabolic condition.7–9 Conventional medical treatment of T2DM is usually a multimodal approach consisting of an appropriate diet, exercise, and various pharmacotherapeutics. However, few patients manage to satisfy the targets of T2DM treatment concepts.10
Although originally developed solely as a weight loss therapy, it has been shown that bariatric surgery not only leads to substantial weight loss but also to T2DM remission for patients with severe obesity.11 Bariatric surgery has been recommended for severely obese patients with a body mass index (BMI) of >40 kg/m2 or >35 kg/m2 with obesity-related comorbidities.12 Recently, more and more studies have indicated that bariatric surgery in nonseverely obese patients (BMI of <35 kg/m2) might be even superior to medical therapy with regard to diabetes remission, improvements of metabolic condition, and cardiovascular risk factors.13–15 So far, it even appeared that a great number of T2DM patients with mild-moderate obesity (BMI of >30 kg/m2 and <40 kg/m2) have becoming the majority of subjects undergone bariatric surgery.16–19
As a growing body of literature reported the outcomes of bariatric surgery for treatment of T2DM in obese patients, even some randomized controlled trials (RCTs) with longer-term follow-up outcomes have been completed recently.20–22 Several reviews evaluating bariatric surgery against medical treatment in obese patients have been published recently.23–25 However, a critically appraised pooled summary and meta-analysis of the available RCTs on solely Roux-en-Y gastric bypass (RYGB) surgery comparing with medical treatment alone is still missing, despite RYGB surgery is one of the most common bariatric surgery and RCTs were level I evidence for the clinical study.
The objective of this systematic review and meta-analysis was to evaluate the effectiveness of RYGB surgery versus medical treatment for T2DM in obese patients. T2DM remission served as the main outcome. Furthermore, hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), BMI, waist circumference, serum lipid level, blood pressure, medication use, and adverse events were evaluated.
METHODS
Search Strategy
We conducted a systematic review of the English-language literature published up to December 2015 by searching abstracts in PubMed, Embase, Cochrane Database, and Cochrane Clinical Trials Registry, using the search terms: [bariatric surgery OR obesity surgery OR metabolic surgery OR Roux-en-Y gastric bypass] AND [medical therapy OR nonsurgical treatment] AND [type 2 diabetes OR morbid obesity] AND [randomized controlled trial OR randomized clinical trial]. Additional cross-referencing was carried out for all the included studies. This systematic review was performed according to the recommendations of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.26 Two researchers (YY and YS) independently searched for literatures, selected studies, assessed quality, and extracted data from articles and then cross-checked. Any disagreement was resolved by consulting a third reviewer (GY). As all analyses were based on previous published studies, ethics approval was not required for this systematic review.
Inclusion Criteria
RCTs evaluated RYGB surgery in patients with T2DM and investigated medical treatment as comparator were included in the meta-analysis if each treatment group included patients with BMI of 30 kg/m2 or more and the mean BMI of each treatment group was 30 kg/m2 or more. The RYGB procedure performed in order to improve metabolic conditions in patients with T2DM and obesity regardless of laparoscopic or open way. The rate of diabetes remission, HbA1c, FPG, BMI, waist circumference, serum lipid level, blood pressure, medication use, and adverse events were reported. Case reports, prospective studies, letters, comments, reviews, and animal studies were excluded.
Data Extraction and Quality Assessment
The primary outcome was T2DM remission. Different definitions for diabetes remission were used as reported in the article. Furthermore, secondary outcomes comprised HbA1c, FPG, BMI, waist circumference, serum lipid level, blood pressure, medication use, and adverse events. Outcome parameters of all the included studies were extracted by using a standardized data form. Additionally, general characteristics of studies, baseline characteristics of patients, inclusion and exclusion criteria of patients, details of interventions, and definition of outcomes were extracted from the included studies. In the case of missing data, the study authors were contacted for completion. Previously published follow-up data were also included to complete the outcome parameters. The methodological quality of the included RCTs was assessed as described by the Cochrane Handbook for Systematic Reviews.27
Statistical Analysis
All statistical analyses were performed using Review Manager 5.2 software (the Cochrane Collaboration, http://www.cochrane.org). For dichotomous data, the odds ratio (OR) was calculated. For continuous data, the mean difference (MD) was calculated for the effect size based on the mean and standard deviation given in the retrieved studies. Missing mean and standard deviations at study end were calculated from other statistics if needed, such as values of mean change from baseline or baseline standard deviations. For all analyses, a random-effects model with 95% confidence intervals (CI) was used to adjust for possible variations in the treatment effect between the studies. Heterogeneity was assessed by the I2 statistic, with values of >50% considered to indicate significant heterogeneity. The P value for the overall effect was calculated using the z test, with significance set at P < 0.05. Sensitivity analysis and estimation of publication bias were also performed.
RESULTS
Study Selection
The flow diagram of study selection procedure is shown in Figure 1. A total of 39 articles were retrieved by literature searches. A full-text review was performed on 15 articles. After evaluating articles according to selection, 6 eligible RCTs published with 10 full-length articles which met the inclusion criteria were finally included,20–22,28–30 as the RCT by Courcoulas et al, Ikramuddin et al, Schauer et al, and Mingrone et al had additional 1-year, 1-year,1-year, and 2 years outcomes published, respectively.31–34
FIGURE 1.
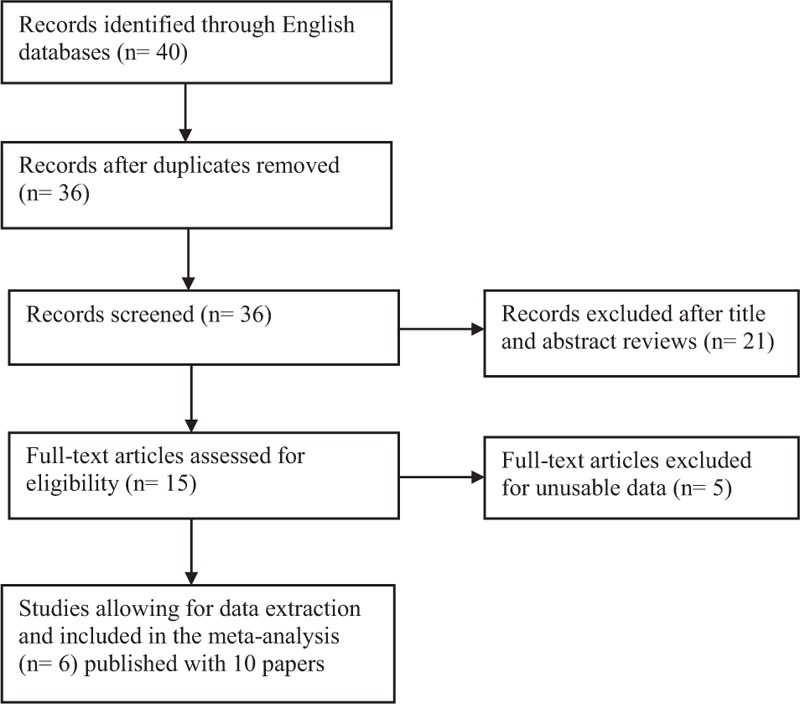
Flow diagram of included and excluded studies.
Study Characteristics
These trials included a total of 410 diabetic patients with 204 who underwent RYGB and 206 who received medical treatment alone, and 57% were women. The baseline characteristics of patients in each treatment group are summarized in Table 1. The 6 included studies were conducted in USA (3), China (1), Italy (1), and in the USA and Taiwan (1). There were no significant differences among the study groups in baseline characteristics, except for serum lipid levels in study by Mingrone et al, in which higher values of total cholesterol, low-density lipoprotein, and triglycerides in the medical treatment group were found comparing with the RYGB group.34 The mean BMI of the study population was >35 kg/m2, except for the RCT by Ikramuddin et al and Liang et al was <35 kg/m2, but the mean BMI was >40 kg/m2 in study by Mingrone et al. In those 3 studies, the mean BMIs of the study population were 34.6 kg/m2, 30.4 kg/m2, and 45.2 kg/m2, respectively. All studies included patients with T2DM, and the mean duration of T2DM in each treatment group was ranged from 5.7 to 10.6 years. The mean HbA1c level of the study population was ranged from 7.9% to 10.5%. The mean age of the treatment group was ranged from 43.5 to 52.6 years. Study follow-up ranged from 12 to 60 months.
TABLE 1.
Description of Patients at Baseline From Studies Included in the Meta-Analysis
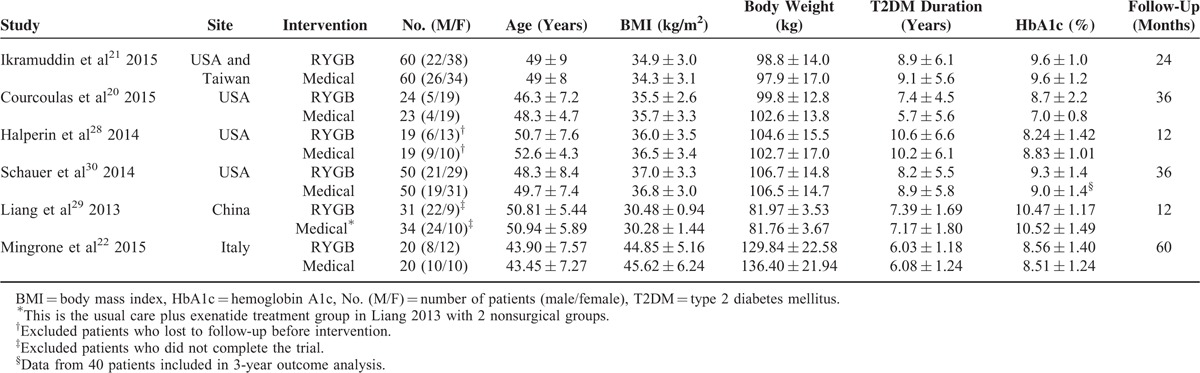
The characteristics of patient recruitment and intervention details of all included RCTs are summarized in Tables S1and S6, respectively. All 6 studies included patients with BMI of 30 kg/m2 or more, and one22 of which performed exclusively in patients with BMI of 35 kg/m2 or more. Surgical treatment was standardized in all studies, and RYGB surgery was performed laparoscopically in 4 studies.21,22,29,30 Within all included RCTs, bariatric surgery was compared with nonsurgical treatment which generally comprised reducing energy intake, increasing physical activity, weight management, and receiving medications for control of hyperglycemia, dyslipidemia, and hypertention, directed by a multidisciplinary team. Medical treatment was standardized in 4 studies, which was modeled on both the Diabetes Prevention Program35 and the Look AHEAD trial protocol36 in studies by Ikramuddin et al and Courcoulas et al, the Why WAIT program37 in study by Halperin et al, and the American Diabetes Association guidelines38 in study by Schauer et al. The details of medication use during the study follow-up were reported by all RCTs but one20 (Table S5). Furthermore, the goal of medical treatment was reported in 4 RCTs,21,22,29,30 which was modification of medications until the patient reached the target HbA1c level <7.0% in 3 studies21,22,29 and of 6.0% or less in a study by Schauer et al. The same medical treatment for the nonsurgical group was also offered to the RYGB group in 2 studies.21,30
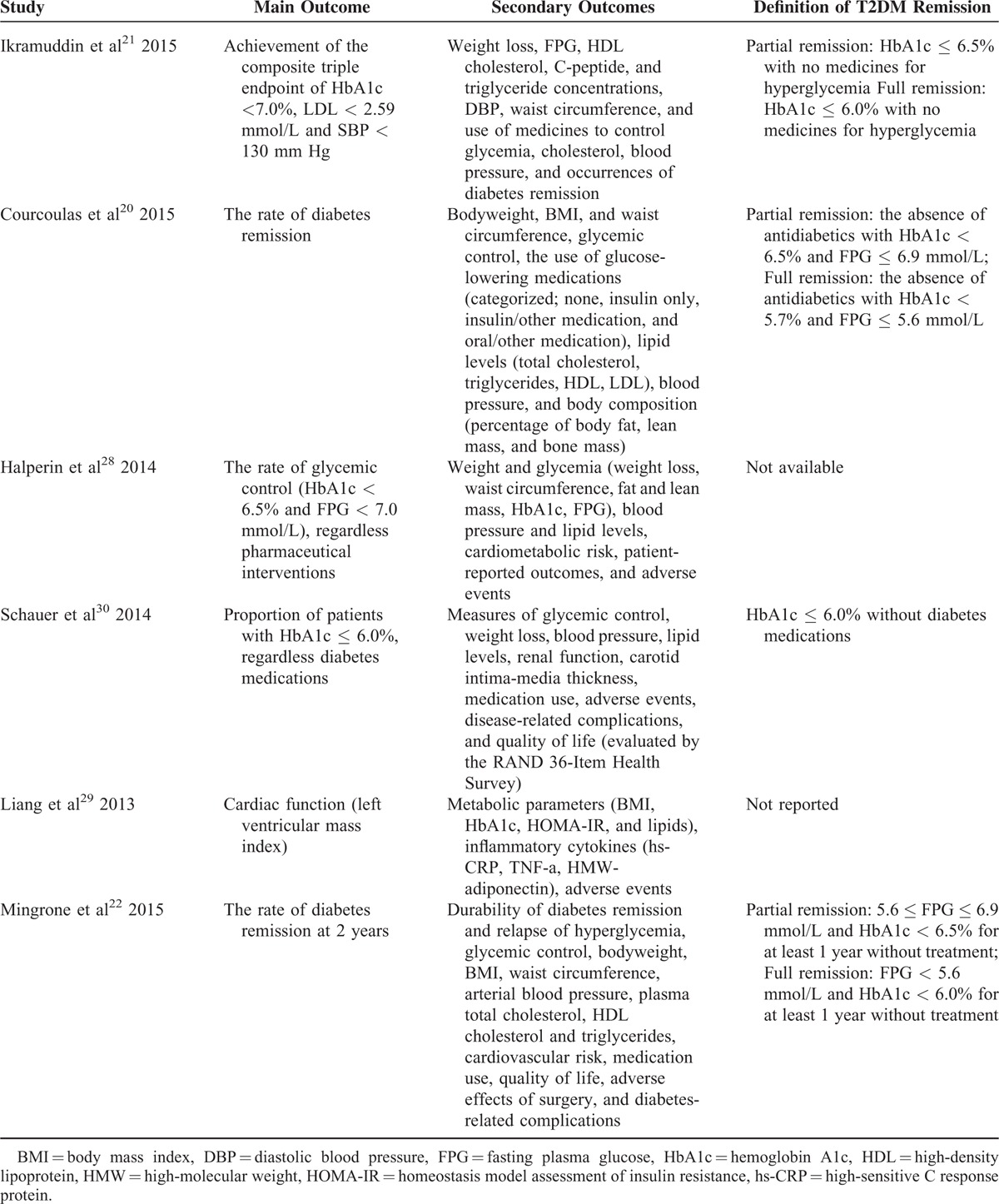
Remission of T2DM was reported by 5 RCTs with different definition for diabetes remission in 4 studies according to the study reported,20–22,30 and 1 study did not report a definition.29 The target HbA1c level for T2DM remission ranged from 5.7% to 6.5%. What is more, 2 kinds of definition, full and partial remission, were given by studies by Ikramuddin et al, Courcoulas et al, and Mingrone et al. Two studies demanded solely a target HbA1c level of 6.0% or less without diabetes medication,21,30 the other 2 studies additionally demanded an FPG level <5.6 mmol/L.20,22 The definition of outcome in each study was resumed in Table 2. We included the total (full and partial) T2DM remission data in meta-analysis. We calculated the mean values at study end from mean change variables and baseline values, and imputed baseline standard deviations in studies by Halperin et al and Courcoulas et al.
TABLE 2.
Outcome Definition
Study Quality
The results of risk bias assessment are shown in Table S2. The sequence generation for randomization was adequate in all studies. Concealment of group allocation was unclear in 1 study.29 In none of these studies, patients and healthcare providers were blind. In 5 studies not all randomized individuals were analyzed.20–22,29,30 Missing outcome data were not addressed in 1 study.29 All but one29 study were free of selective reporting.
Medication Use
All the included studies reported the changes of medication use during follow-up (Table S3). All studies reported the number of medicines for control of hyperglycemia, dyslipidemia, and hypertension in the surgical group was significantly reduced more than the medical treatment group. Furthermore, the quality of life was solely significantly improved after RYGB surgery in 2 studies (Table S4).22,30 However, medication use in patients who received medical treatment alone was frequently reported without significant improvement at study end.
Adverse Events
All studies reported adverse events in the publication, and adverse events are listed in Table S5. There was heterogeneity in the definitions of adverse events among studies. Although there were no deaths during operation, 1 postoperative complications merit particular attention in a study by Ikramuddin et al.32 That was 1 patient undergone RYGB surgery developed a leak from the jejunojejunostomy ultimately led to anoxic brain injury, lower extremity amputation, and long-term disability. In a study by Mingrone et al, 1 patient in the medical group suffered fatal myocardial infarction that led to the death during the 5-year follow-up.22 After RYGB surgery, 160 adverse events were reported in 199 patients, included 35 developed hypoglycemic episode, 11 developed anemia, 8 developed anastomotic ulcer, 4 developed intestinal obstruction, and 2 developed anastomotic leak. In the medical treatment group, 119 adverse events were reported in 190 patients, included 39 developed hypoglycemic episode, 7 developed renal calculus, and 6 developed anemia. Depression with suicide attempt developed in 1/199 of surgically treated and in 1/190 of medically treated individuals. It is worth mentioning, nutritional deficiencies were noted more frequently in the RYGB group, mainly a greater incidence of iron-deficiency anemia.
Meta-Analysis Results
T2DM Remission
Five of the 6 studies reported T2DM remission rates.20–22,29,30 The T2DM remission rate was 56.81% (100/176) in the surgical group and 0% (0/162) in the medical treatment group. The pooled analysis of T2DM remission rates revealed a significantly higher remission rate after RYGB surgery than after medical treatment alone (OR: 76.37, 95% CI: 20.70–281.73, P < 0.001) (Figure 2).
FIGURE 2.
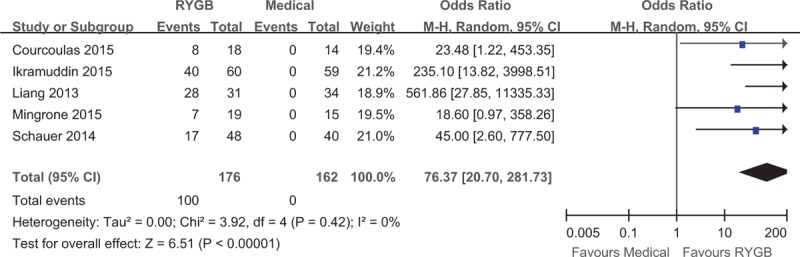
Forest plot of the type 2 diabetes mellitus remission rate after RYGB surgery compared to medical treatment. The remission rate was higher in the RYGB group than the medical group (OR: 76.37, 95% CI: 20.70–281.73, P < 0.001). CI = confidence interval, OR = odds ratio, RYGB = Roux-en-Y gastric bypass.
Serum HbA1c Level
All the included studies reported HbA1c levels in the study end. The pooled analysis revealed that the serum HbA1c level was lower by the end of postsurgical follow-up than after medical treatment alone (MD: –1.25%, 95% CI: –1.88% to –0.63%, P < 0.001) (Figure 3A).
FIGURE 3.
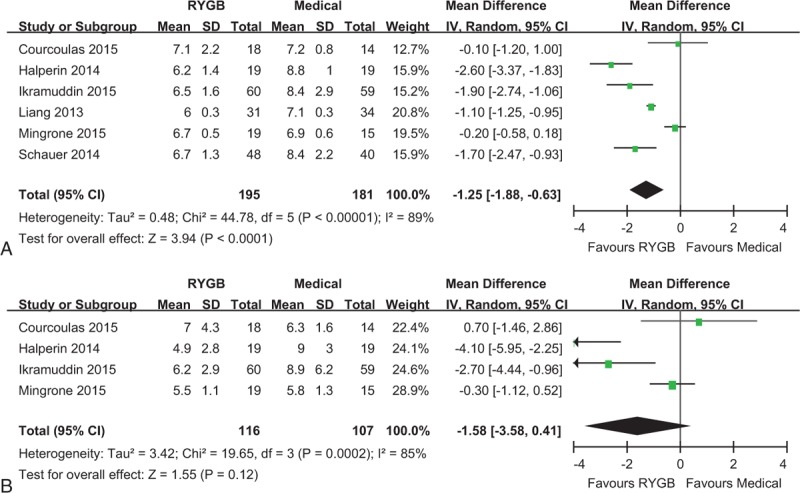
Forest plot of HbA1c (A) and FPG (B) level after RYGB surgery compared to medical treatment. The HbA1c level was lower in the RYGB group than the medical group (MD: –1.25, 95% CI: –1.88 to –0.63, P < 0.001). The FPG level was similar in RYGB and medical groups (MD: –1.58, 95% CI: –3.58 to 0.41, P = 0.12). CI = confidence interval, FPG = fasting plasma glucose, HbA1c = hemoglobin A1c, MD = mean difference, RYGB = Roux-en-Y gastric bypass.
FPG
Four of the 6 studies reported FPG levels in the study end.20–22,28 Meta-analysis revealed no statistical significance between the 2 groups in terms of FPG level (MD: –1.58 mmol/L, 95% CI: –3.58 to 0.41 mmol/L, P = 0.12) (Figure 3B).
BMI
All the included studies reported the BMI at study end as a parameter of weight loss. Meta-analysis showed a significant lower BMI in individuals undergone RYGB surgery than those received medical therapy alone (MD: –6.54 kg/m2, 95% CI: –9.28 to –3.80 kg/m2, P < 0.001) (Figure 4A). The result suggested that RYGB surgery is associated with a more powerful effect on weight loss.
FIGURE 4.
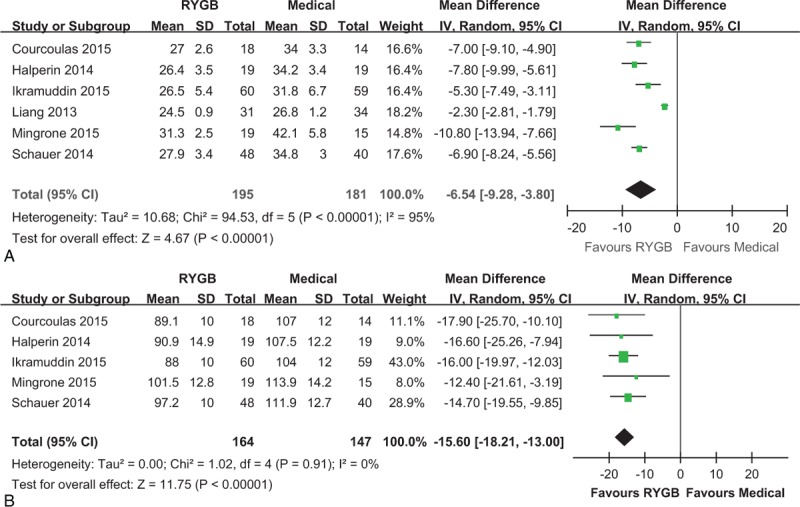
Forest plot of BMI (A) and waist circumference (B) level after RYGB surgery compared to medical treatment. Both BMI (MD: –6.54, 95% CI: –9.28 to –3.80, P < 0.001) and waist circumference (MD: –15.60, 95% CI: –18.21 to –13.00, P < 0.001) were lower in the RYGB group than the medical group. BMI = body mass index, CI = confidence interval, MD = mean difference, RYGB = Roux-en-Y gastric bypass.
Waist Circumference
Five of the 6 studies reported the waist circumference as the index of abdominal fat mass.20–22,28,30 Meta-analysis showed a significant lower waist circumference in individuals undergone RYGB surgery than those received medical therapy alone (MD: –15.60 cm, 95% CI: –18.21 to –13.00 cm, P < 0.001) (Figure 4B). The result suggested that RYGB surgery can more powerfully reduce abdominal fat mass.
Serum Lipid Profiles
Triglyceride concentrations are available for 5 studies.20–22,28,29 Triglyceride concentrations decreased more after RYGB surgery than after medical treatment alone (MD: –0.87 mmol/L, 95% CI: –1.17 to –0.57 mmol/L, P < 0.001) (Figure 5A). Furthermore, high-density lipoprotein cholesterol increased more (MD: 0.24 mmol/L, 95% CI: 0.18–0.30 mmol/L, P < 0.001) and low-density lipoprotein cholesterol decreased more (MD: –0.32 mmol/L, 95% CI: –0.62 to –0.02 mmol/L, P < 0. 05) after RYGB surgery than after medical treatment alone (Figure 5C and D). However, total cholesterol was not significantly different between surgical and medical treatment (MD: –0.40 mmol/L, 95% CI: –0.92 to 0.12 mmol/L, P = 0.13) (Figure 5B).
FIGURE 5.
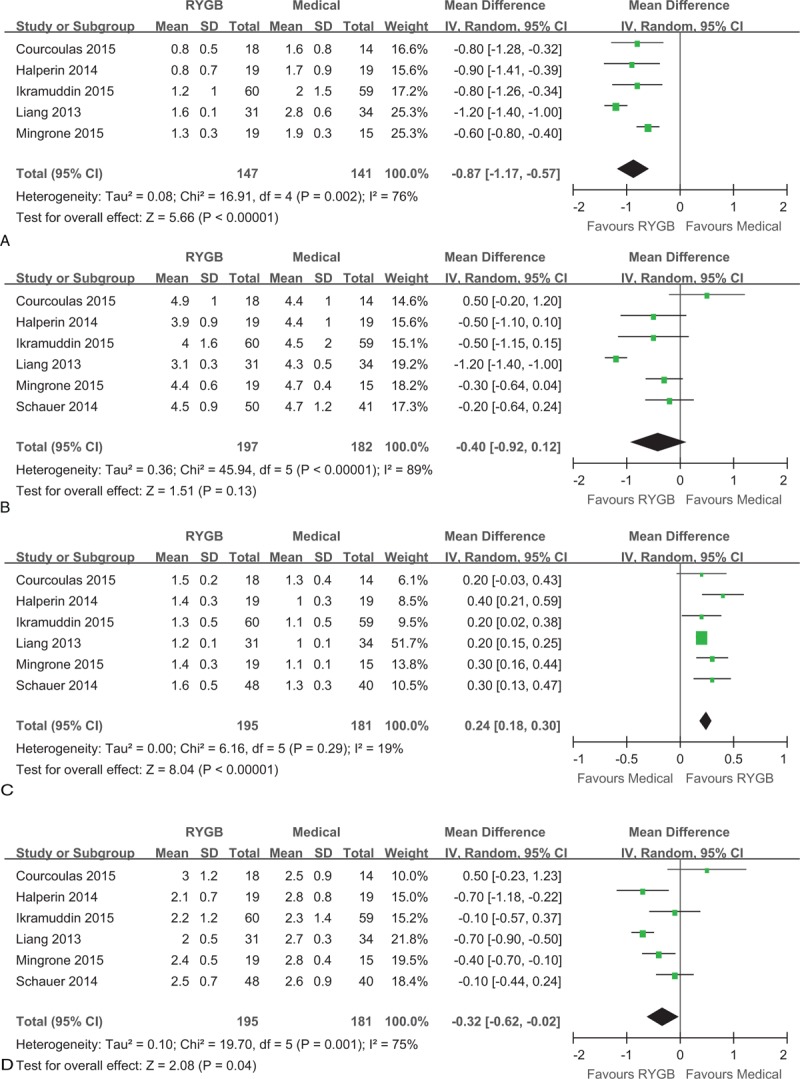
Forest plot of triglyceride (A), total cholesterol (B), high-density lipoprotein cholesterol (C), and low-density lipoprotein cholesterol (D) after RYGB surgery compared to medical treatment. The levels of triglyceride (MD: –0.87, 95% CI: –1.17 to –0.57, P < 0.001) and low-density lipoprotein cholesterol (MD: –0.32, 95% CI: –0.62 to –0.02, P = 0.04) were lower in the RYGB group than the medical group. The total cholesterol level was similar in RYGB and medical groups (MD: –0.40, 95% CI: –0.92 to 0.12, P = 0.13). The high-density lipoprotein cholesterol (MD: 0.24, 95% CI: 0.18–0.30, P < 0.001) level was higher in the RYGB group than the medical group. CI = confidence interval, MD = mean difference, RYGB = Roux-en-Y gastric bypass.
Blood Pressure
All 6 studies reported systolic blood pressure, but diastolic blood pressure was available for 5 studies. The pooled analysis revealed that systolic blood pressure was lower by the end of postsurgical follow-up than after medical treatment alone (MD: –2.83 mm Hg, 95% CI: –4.88 to –0.78 mm Hg, P < 0.01) (Figure 6A). However, diastolic blood pressure was not significantly different between the 2 treatment groups (MD: 0.28 mm Hg, 95% CI: –1.89 to 2.45 mm Hg, P = 0.80) (Figure 6B).
FIGURE 6.
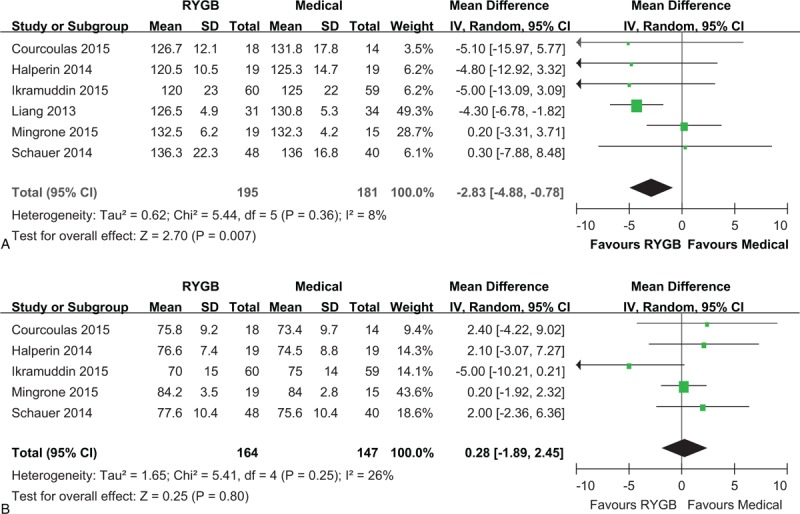
Forest plot of systolic blood pressure (A) and diastolic blood pressure (B) after RYGB surgery compared to medical treatment. The systolic blood pressure was lower in the RYGB group than the medical group (MD: –2.83, 95% CI: –4.88 to –0.78, P < 0.01). The diastolic blood pressure was similar in RYGB and medical groups (MD: 0.28, 95% CI: –1.89 to 2.45, P = 0.80). CI = confidence interval, MD = mean difference, RYGB = Roux-en-Y gastric bypass.
Sensitivity Analysis and Publication Bias
Sensitivity analysis was performed to determine the significance of results by sequentially repeating the meta-analysis excluding 1 study at a time. The results of this analysis suggested that the pooled OR and MD values were not significantly affected except for FPG result. The pooled MD value of FPG was significantly affected after excluded 2 longer-term follow-up studies (data not shown).20,22 As statistically significant data are published more frequently than nonsignificant data, our results may be influenced by publication bias (Figure 7).
FIGURE 7.
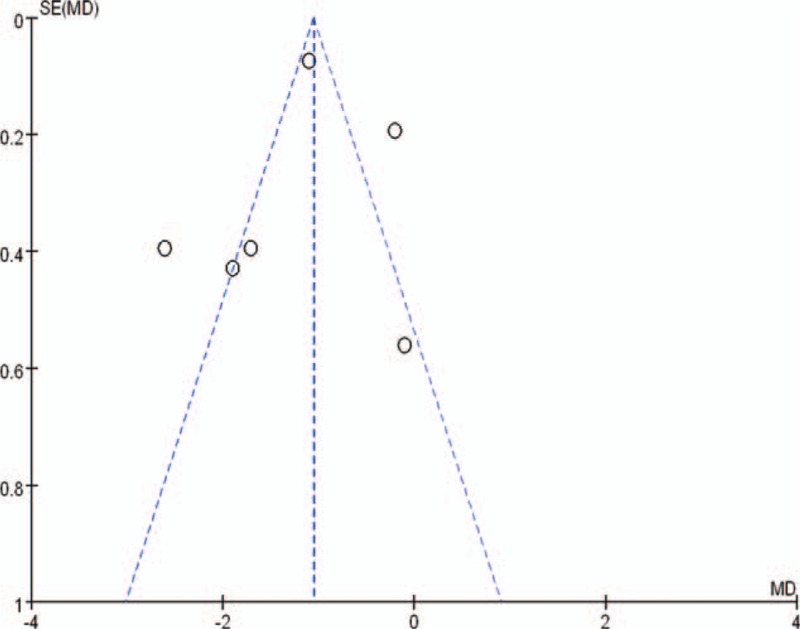
Funnel plot of HbA1c for assessing publication bias. HbA1c = hemoglobin A1c.
DISCUSSION
This systematic review and meta-analysis demonstrates that RYGB surgery is more efficient than medical treatment alone for T2DM in obese patients. According to the results of pooled analysis, RYGB surgery is superior in terms of short- to medium-term (12–60 months) T2DM remission. After RYGB surgery, HbA1c, triglycerides, and low-density lipoprotein cholesterol decreased more, and high-density lipoprotein cholesterol increased more. Furthermore, RYGB surgery led to greater weight loss and abdominal fat mass reduction, and greater reductions in use of antidiabetic, antihypertensive, and lipid-lowering drugs. Although nutritional deficiencies and anemia were noted more frequently in the RYGB group, the quality of life was improved more in the surgical group.22,30 There was no significant difference of FPG level between the 2 groups after included 2 longer-term studies, which may due to relapsed hyperglycemia during longer-term follow-up in the surgical group and more efficient antidiabetic drugs used in the medical group.20,22 As a consequence, RYGB surgery should be considered an efficient treatment option for T2DM in obese patients, but continued monitoring of glycogenic control is warranted because of potential relapse of hyperglycemia in some patients.
The effect of RYGB surgery on diabetes remission seems to be strictly different compared to the effect of medical treatment alone, as 56.81% (100/176) of patients undergone RYGB surgery and none of the 162 patients received medical treatment alone attained diabetes remission. In the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS), 67.9% of obese patients with T2DM in the RYGB group were not taking medication 1 year after surgery.39 In the recent meta-analysis from Ribaric et al, relative to diabetes and weight in comparative studies of bariatric surgery versus conventional medical therapy, the overall T2DM remission rate for surgery versus conventional group was 63.5% and 15.6%, respectively.25 Nowadays, more and more nonseverely obese patients (BMI <35 kg/m2) with T2DM received bariatric surgery have also achieved ideal goals, as 5 of the 6 included studies in our meta-analysis recruited participants with BMI <35 kg/m2.20,21,28–30 In fact, less obese patient who choose to receive bariatric surgery are more likely to have severe diabetes, as the mean duration of T2DM in each treatment group was ranged from 5.7 to 10.6 years in our meta-analysis. Our results of T2DM remission are somewhat lower than previous reported studies; such differences could be explained by the greater severity, longer duration of diabetes and longer-term follow-up in our population, as well as a stricter definition of diabetes remission based on American Diabetes Association guidelines used in studies conducted in recent years.20,21,30 With regard to the pooled analysis of T2DM remission, our results (OR: 76.37, 95% CI: 20.70–281.73) is consistent with a network meta-analysis from Muller-Stich et al compared RYGB surgery with medical treatment in nonseverely obese patients (OR: 55.1, 95% CI: 12.2–248.5).24
The results of our meta-analysis showed that RYGB surgery was significantly more efficient than medical treatment alone, with regard to improving of obesity, hyperglycemia, hyperlipidemia, and arterial hypertention, indicating the improvements of metabolic condition and cardiovascular risk factors.40,41 Unlike specific medical treatment with poor outcomes associated with massive quantities of medication used to stringently control hyperglycemia, hyperlipidemia, and arterial hypertention, RYGB surgery seems to have a multifactorial effect on T2DM and its associated comorbidities.42 Although the significant and durable caloric restriction and body weight loss might explain some of the greater improvements in hyperglycemia, hyperlipidemia, and arterial hypertention after RYGB surgery compared with medical treatment alone, numerous studies have indicated that RYGB surgery caused favorable outcomes independent of caloric restriction and weight loss.43 Currently several potential mechanisms are being discussed, the bile acid-mediated regulation of metabolism via activation of farnesoid X receptor and TGR5,44–46 the jejunal nutrient sensor that may be enhanced by bypassing the jejunum,47 the altered microbiota in small intestine,48 the reprogramming of intestinal glucose metabolism after intestinal reconstruction,49 as well as the decreased obesity-induced and inflammation-mediated insulin resistance,50 all of which has been linked to improvements of metabolic condition after RYGB surgery. Unraveling the precise mechanisms underlying the effects induced by RYGB surgery may potentially lead to promising targets of novel drugs for treatment metabolic diseases.
Despite the discussions above, it is important to notice that there are some negative metabolic consequences after RYGB surgery, the rate of overall adverse events was higher in the surgical group with 80.40% (160/199) compared to the medical group with 62.63% (119/190). As the RYGB procedure comprised of gastric restriction and intestinal bypass, malabsorption of micronutrients (including vitamins, calcium, zinc, and iron) is a problem after surgery. Despite protocol requirements for supplementation, nutritional deficiencies and iron deficiency anemia were common after RYGB surgery.51,52 There are also other complications after RYGB surgery and all we need to focus on, which included a variety of different forms, such as anastomotic leaks, stenosis, small bowel obstruction, gastrointestinal hemorrhage, internal hernias, and so on. Other negative effects such as dumping syndrome and gastroesophageal reflux disease are more likely to torment patient undergone RYGB surgery.53 The complication rate, mortality rate, and reoperation rate not mentioned here are also important determinants in recommending surgical intervention for diabetes management. Bariatric surgery is not without risks, the mortality up to 30 days was 0.16% for RYGB surgery in a meta-analysis included 361 studies of 85 048 patients.54 In the American College of Surgeons Bariatric Surgery Network database, the 30-day complication rate was 5.9% for RYGB surgery.55 It is important to notice that the merit of RYGB surgery treatment of obese patients with T2DM depends on whether potential risks make benefits acceptable.
Although this meta-analysis provided comprehensive evidence that to which extent efficacy of RYGB surgery for T2DM in obese patients compared with medical treatment alone, some limitations must be taken into account. First, we included a small number of studies and as the included participants, as available RCTs on RYGB surgery against medical treatment alone in obese patients with T2DM is rare to date. Additionally, we only searched for English literature and significant data are published more frequently than nonsignificant data, our results may be influenced by publication bias. Second, there were certain differences in respect to baseline characteristics of patients, length of follow-up, implementation of interventions, and criteria for T2DM remission among the different studies. Third but not the last, the quality assessment of the included studies revealed moderated quality within all RCTs, as patients and health providers were not blinded in all included studies. As all of above limitations may affect the accuracy of this meta-analysis, data were pooled conservatively with random-effects models.56 The quantity and quality of available evidence limit our conclusion. However, most of the treatment effect could be reproduced in a sensitivity analysis by sequentially repeating the meta-analysis excluding 1 study at a time, and the fixed-effects model did not change significantly. Despite the discussions above, the pooled data of currently available evidence showed that RYGB surgery is more efficient for treatment of obese T2DM patients than medical treatment alone.
As there is no consensus on reporting adverse events of surgical and medical interventions, and little available data, we could not perform a meta-analysis on surgical risks. This meta-analysis included studies with follow-up periods ranged from 12 to 60 months just represented short to medium-term benefits of improvements of metabolic condition and cardiovascular risk factors. Theoretically, such improvements have the potential to reduce cardiovascular morbidity and mortality in the long term, as shown in nonrandomized studies.41,57 Long-term outcomes of RYGB surgery for metabolic conditions, diabetes-related complications, and mortality, should to be evaluated among obese patients. Surgeons should focus on the potential surgical risks and long-term benefits fully of using RYGB surgery to reduce the complications and mortality associated with T2DM. Well-designed studies evaluating surgical risks and long-term outcomes should be conducted in the future.
CONCLUSION
This meta-analysis showed the short to medium-term superiority of RYGB surgery to medical treatment, with regard to T2DM remission, improvement of metabolic condition, and cardiovascular risk factors. Additionally, well-designed studies with consistent definition of adverse events, as well as a larger number of RCTs with long-term follow-up (>60 months) are needed to evaluate the safety and long-term benefits of RYGB surgery on obese patients with T2DM.
Supplementary Material
Acknowledgments
The authors thank Shanghai Diabetes Institute, Shanghai Jiao Tong University Affiliated Sixth People's Hospital for their generous assistance.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, FPG = fasting plasma glucose, HbA1c = hemoglobin A1c, MD = mean difference, OR = odds ratio, PRISMA = preferred reporting items for systematic reviews and meta-analyses, RCTs = randomized controlled trials, RYGB = Roux-en-Y gastric bypass, T2DM = type 2 diabetes mellitus
YY and YS have contributed equally to this study.
This study was supported by the Key Project of Shanghai Health and Family Planning Commission (grant No. 201440026).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011; 377:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatrics 2014; 168:561–566. [DOI] [PubMed] [Google Scholar]
- 3.Bhutani J, Bhutani S. Worldwide burden of diabetes. Indian J Endocrinol Metab 2014; 18:868–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skrha J. Diabetes mellitus–a global pandemic. Keynote lecture presented at the Wonca conference in Prague in June 2013. Eur J Gen Pract 2014; 20:65–68. [DOI] [PubMed] [Google Scholar]
- 5.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev 2014; 15:504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009; 9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care 2015; 38:1161–1172. [DOI] [PubMed] [Google Scholar]
- 8.Lebherz C, Lehrke M. [Prevention of cardiovascular disease in patients with type 2 diabetes. Deutsche Medizinische Wochenschrift (1946) 2015; 140:645–649. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz SS, Kohl BA. Glycemic control and weight reduction without causing hypoglycemia: the case for continued safe aggressive care of patients with type 2 diabetes mellitus and avoidance of therapeutic inertia. Mayo Clinic Proc 2010; 85 12 Suppl:S15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali MK, Bullard KM, Saaddine JB, et al. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med 2013; 368:1613–1624. [DOI] [PubMed] [Google Scholar]
- 11.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351:2683–2693. [DOI] [PubMed] [Google Scholar]
- 12.SAGES Guidelines Committee. SAGES guideline for clinical application of laparoscopic bariatric surgery. Surg Obes Related Dis 2009; 5:387–405. [DOI] [PubMed] [Google Scholar]
- 13.Abbatini F, Capoccia D, Casella G, et al. Type 2 diabetes in obese patients with body mass index of 30–35 kg/m2: sleeve gastrectomy versus medical treatment. Surg Obes Relat Dis 2012; 8:20–24. [DOI] [PubMed] [Google Scholar]
- 14.Scopinaro N, Adami GF, Papadia FS, et al. Effects of gastric bypass on type 2 diabetes in patients with BMI 30 to 35. Obes Surg 2014; 24:1036–1043. [DOI] [PubMed] [Google Scholar]
- 15.Serrot FJ, Dorman RB, Miller CJ, et al. Comparative effectiveness of bariatric surgery and nonsurgical therapy in adults with type 2 diabetes mellitus and body mass index <35 kg/m2. Surgery 2011; 150:684–691. [DOI] [PubMed] [Google Scholar]
- 16.Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA 2013; 309:2250–2261. [DOI] [PubMed] [Google Scholar]
- 17.Maglione MA, Gibbons MM, Livhits M, et al. AHRQ Comparative Effectiveness Reviews Bariatric Surgery and Nonsurgical Therapy in Adults With Metabolic Conditions and a Body Mass Index of 30. 0 to 34. 9 kg/m2. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. [PubMed] [Google Scholar]
- 18.Li Q, Chen L, Yang Z, et al. Metabolic effects of bariatric surgery in type 2 diabetic patients with body mass index < 35 kg/m2. Diabetes Obes Metab 2012; 14:262–270. [DOI] [PubMed] [Google Scholar]
- 19.Reis CE, Alvarez-Leite JI, Bressan J, et al. Role of bariatric-metabolic surgery in the treatment of obese type 2 diabetes with body mass index <35 kg/m2: a literature review. Diabetes Technol Ther 2012; 14:365–372. [DOI] [PubMed] [Google Scholar]
- 20.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015; 150:931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol 2015; 3:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015; 386:964–973. [DOI] [PubMed] [Google Scholar]
- 23.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013; 347:f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller-Stich BP, Senft JD, Warschkow R, et al. Surgical versus medical treatment of type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis. Ann Surg 2015; 261:421–429. [DOI] [PubMed] [Google Scholar]
- 25.Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg 2014; 24:437–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8:336–341. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J, Green S. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 510. 2009. [Google Scholar]
- 28.Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014; 149:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang Z, Wu Q, Chen B, et al. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013; 101:50–56. [DOI] [PubMed] [Google Scholar]
- 30.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014; 370:2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014; 149:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013; 309:2240–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366:1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012; 366:1577–1585. [DOI] [PubMed] [Google Scholar]
- 35.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002; 25:2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007; 30:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamdy O, Carver C. The Why WAIT program: improving clinical outcomes through weight management in type 2 diabetes. Curr Diabetes Reports 2008; 8:413–420. [DOI] [PubMed] [Google Scholar]
- 38.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterli R, Borbely Y, Kern B, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg 2013; 258:690–694.discussion 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batsis JA, Miranda WR, Prasad C, et al. Effect of bariatric surgery on cardiometabolic risk in elderly patients: a population-based study. Geriatr Gerontol Int 2015; doi: 10.1111/ggi.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boido A, Ceriani V, Cetta F, et al. Bariatric surgery and prevention of cardiovascular events and mortality in morbid obesity: mechanisms of action and choice of surgery. Nutr Metab Cardiovasc Dis 2015; 25:437–443. [DOI] [PubMed] [Google Scholar]
- 42.Albeladi B, Bourbao-Tournois C, Huten N. Short- and midterm results between laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy for the treatment of morbid obesity. J Obes 2013; 2013:934653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol 2014; 2:152–164. [DOI] [PubMed] [Google Scholar]
- 44.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009; 10:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penney NC, Kinross J, Newton RC, et al. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes 2015; 39:1565–1574. [DOI] [PubMed] [Google Scholar]
- 47.Duca FA, Bauer PV, Hamr SC, et al. Glucoregulatory relevance of small intestinal nutrient sensing in physiology, bariatric surgery, and pharmacology. Cell Metab 2015; 22:367–380. [DOI] [PubMed] [Google Scholar]
- 48.Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab 2015; 22:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 2013; 341:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell 2013; 152:673–684. [DOI] [PubMed] [Google Scholar]
- 51.Verger EO, Aron-Wisnewsky J, Dao MC, et al. Micronutrient and protein deficiencies after gastric bypass and sleeve gastrectomy: a 1-year follow-up. Obes Surg 2016; 26:785–796. [DOI] [PubMed] [Google Scholar]
- 52.Kotkiewicz A, Donaldson K, Dye C, et al. Anemia and the need for intravenous iron infusion after Roux-en-Y gastric bypass. Clinical medicine insights. Blood Disord 2015; 8:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best practice & research. Clin Gastroenterol 2014; 28:741–749. [DOI] [PubMed] [Google Scholar]
- 54.Buchwald H, Estok R, Fahrbach K, et al. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery 2007; 142:621–632.discussion 632-625. [DOI] [PubMed] [Google Scholar]
- 55.Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 2011; 254:410–420.discussion 420–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 57.Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015; 313:62–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


