Abstract
Bacille Calmette-Guérin vaccine (BCG) vaccination is used routinely in most of countries, especially developing one. The efficacy of the BCG vaccination generally decreases with time. The tuberculin skin test (TST) is a most popular diagnostic test for suspicion of tuberculosis (TB) in children till now, but it has many false positives. The interferon-gamma release assay (IGRA) is more specific than TST for detection of childhood TB, as it is more specific to Mycobacterium tuberculosis.
Evaluate the interferon gamma response and TST reaction in BCG vaccinated children in east of Egypt.
150 children were included in the study aged 1 month to 12 years; the collected data from the children included, full history taking, clinical examination, examination for the presence or absence of BCG scar under direct light. All the children had performed TST, IGRA.
TST was done for all studied group reveal 51.3% with size of reaction <5 mm, 39.3% with size of reaction = 5 to 9 mm while 9.3% with size of reaction ≥10 mm. Mean size of reaction was 4.07 mm. Interferon gamma release assay was done for all studied group reveal 5 children (3.3%) with positive test. There was significant difference between the size of TST reaction and age (P < 0.01) with old children were more frequent to show positive reaction. Also, children with age range 1 month to 1 year were frequently have negative IGRA test, while children with age range 4 years to 12 years were frequently have positive test (P < 0.01). There was moderate agreement between IGRA and TST results (Kappa [κ] = 0.475). With high agreement between IGRA and TST results in children with absent BCG scar (κ = 1000).
Therefore, Interferon gamma release assays have higher specificity and lower cross-reactions with BCG vaccination and nontuberculous Mycobacteraie than TST.
INTRODUCTION
Tuberculosis (TB) is a leading cause of death in children worldwide; however, those children are not given the priority in most national health programs.1 Worldwide, the increase of TB usually influenced by low socio-economic factors that affect health care delivery. TB in children usually follows adult TB and it is a good marker of current transmission in the community. Although advances have been made in diagnostics and new drugs for treatment of TB in adults, childhood TB has lagged behind.2 Bacille Calmette-Guérin (BCG) vaccine, the only vaccine available for TB, to protect against severe and disseminated forms of the disease in young children.3 BCG vaccination is done at birth in some countries including Egypt but in some others, it is administered at primary school entry or at puberty.4 The tuberculin skin test (TST) is the most common diagnostic test for suspected children who were in contact with TB patients or have symptoms of TB.5 TST is a delayed type hypersensitivity reaction in form of induration seen at the test sites of an individual sensitized to the tuberculin antigen.6 TST remained the only diagnostic tool for latent tuberculosis infection for decades. The results of TST have cross-reactions with BCG vaccine and environmental mycobacteria, which may lead to many false positives.7 The interferon-gamma release assay (IGRA): is an enzyme-linked immunosorbent assay (ELISA). This test is more specific than TST as it measures the in vitro production of interferon-gamma by sensitized lymphocytes in response to Mycobacterium tuberculosis-specific antigens. Therefore, these tests have a fewer false positives.8
The aim of this study is to evaluate the interferon gamma response and TST reaction in BCG vaccinated children in east of Egypt.
PATIENTS AND METHODS
This cross-sectional study was conducted in Al-Sharkia Governorate during the period from April 2014 to March 2015. The study included 150 children selected randomly during their attendance for routine visit of primary health care units. The children included in this study were divided into three age group: infancy: (1 month till 1 year), early childhood: (1 year till 4 years), late childhood (5 years till 12 years). The children were selected from multiple health offices in east of Egypt. The study was conducted in accordance with the ethical standards of the Helsinki Declaration of 1964 as revised in 2008 and was approved by our local ethical committee. Informed consent was obtained from all individuals participating in the study or their guardians.
Inclusion Criteria
The children were selected with certain parameter: BCG vaccination at birth confirmed from history and vaccination card, Good general health, Children selected from different age group, and different socioeconomic standards. No history of household contact to TB case.
Exclusion Criteria
Children with acute febrile illness, malnutrition, previous history of immunosuppressive, drugs intake, recent administration of live vaccine, and those who did not come back for TST evaluation on time.
All children will be subjected to the following:
Full history taking.
Clinical examination.
Examination for presence or absence of BCG scars under direct light.
Tuberculin Skin Test
Five tubercuin units (0.1 mL) of purified protein derivative Purified protein derivative (PPD) was injected intradermally in the volar aspect of the forearm, the diameter of induration resulted was measured 48 to 72 hours after the injection.9
Interferon-Gamma Release Assay
Peripheral venous blood samples of 1 mL were collected into 3 evacuated tubes on the day of TST reading, the first tube coated with TB-specific antigens (ESAT-6, CFP10, and TB7.7), the second tube coated with heparin-negative control, and the third tube coated with mitogen (phytohaemagglutinin) as positive control. Blood was incubated overnight at 37 °C. Plasma was then separated and stored for up to 4 weeks at 4 °C to measure the concentration of Interferon gamma (IFN-γ) by enzyme-linked immunosorbent assay using the QFT-Gold IT assay (Cellestis) following the manufacturer's instructions. The result was considered positive if the negative control IFN-γ level was <8.0 IU/mL and the value of the TB antigen minus negative control (Nil) were ≥0.35 IU/mL and ≥25% of the negative control IFN-γ value. A negative result was defined as either the TB antigen minus Nil level ≥0.35 IU/mL and <25% of Nil value or <0.35 IU/mL. Indeterminate results were defined as either a negative control IFN-γ level >8.0 IU/mL or a positive control IFN-γ response <0.5 IU/mL with a TB antigen minus negative control IFN-γ response of either <0.35 IU/mL or <25% of the negative control value.10
Statistical Analysis
All data were analyzed using SPSS 15.0 for windows (SPSS Inc. Chicago, IL). Continuous data are expressed as the median (range), and the categorical data are expressed as a number (percentage). Comparisons of differences in the categorical data between groups were performed using the Chi-square (χ2) test. Distributions of the continuous variables were analyzed by the Mann−Whitney U (MW) test for two groups or the Kruskal Wallis H (KW) test for three groups. Agreement between Quantiferon TB gold test and the TST results was analyzed using McNemar, and Kappa (κ) statistic, α = 0.05. Agreement was obtained if the McNemar was not significant and the Kappa statistic was significant. The degree of agreement was high if the Kappa value was >0.75, moderate if it was 0.4–0.75, low if it was <0.4.
RESULTS
The present study included 150 children (78 males and 72 females) who fulfilled the study criteria. Mean age was 1.4 years, 61.3% with age range 1 month and 1 year while 30% with age range 1 year and 4 years and 8.7% with age range 4 years and 12 years. Mean weight was 10.18 kg. Mean height was 71.84 cm, Table 1.
TABLE 1.
Demographic Data and TB Related Data of Studied Group
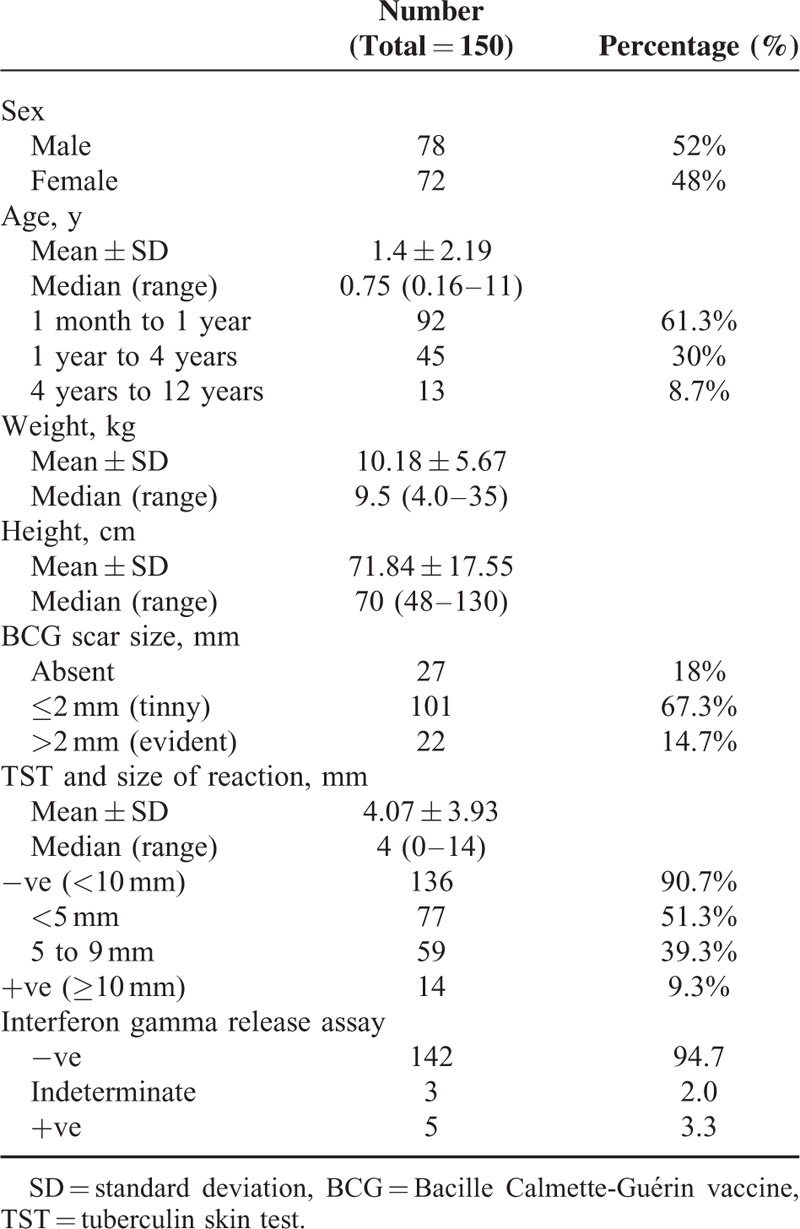
All children were examined for BCG scar size, 27 children (18%) had no scar, 101 children (67.3%) had tinny scar while 22 children (14.7%) had evident scar. TST was done for all studied group reveal 51.3% with size of reaction <5 mm, 39.3% with size of reaction = 5 to 9 mm while 9.3% with size of reaction ≥10 mm. Mean size of reaction was 4.07 mm. IGRA was done for all studied group reveal 5 children (3.3%) with positive test while 3 children (2%) with indeterminant test, and 142 children (94.7%) with negative test, Table 1.
There was a significant direct correlation between size of TST reaction and BCG scar size (r = 0.299, P < 0.01), Figure 1.
FIGURE 1.
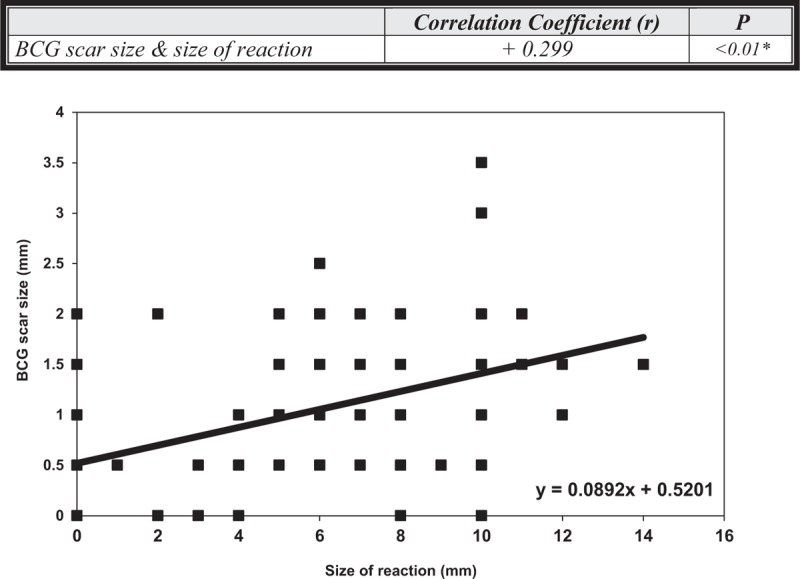
Correlation between BCG scar size (mm) and size of TST reaction (mm). There was a significant positive direct correlation between size of TST reaction and BCG scar size. BCG = Bacille Calmette-Guérin vaccine; mm = millimeter; TST = Tuberculin skin test.
There was no significant difference between sex and each of BCG scar, TST reaction, and IGRA (P = 0.683, P = 0.77, P = 76, respectively). While, there was significant difference between the BCG scar status and age of the patients as scar was more evident in old age group (P < 0.01), Table 2.
TABLE 2.
BCG Scar, TST Reaction, and IGRA Relation With Sex and Age Groups
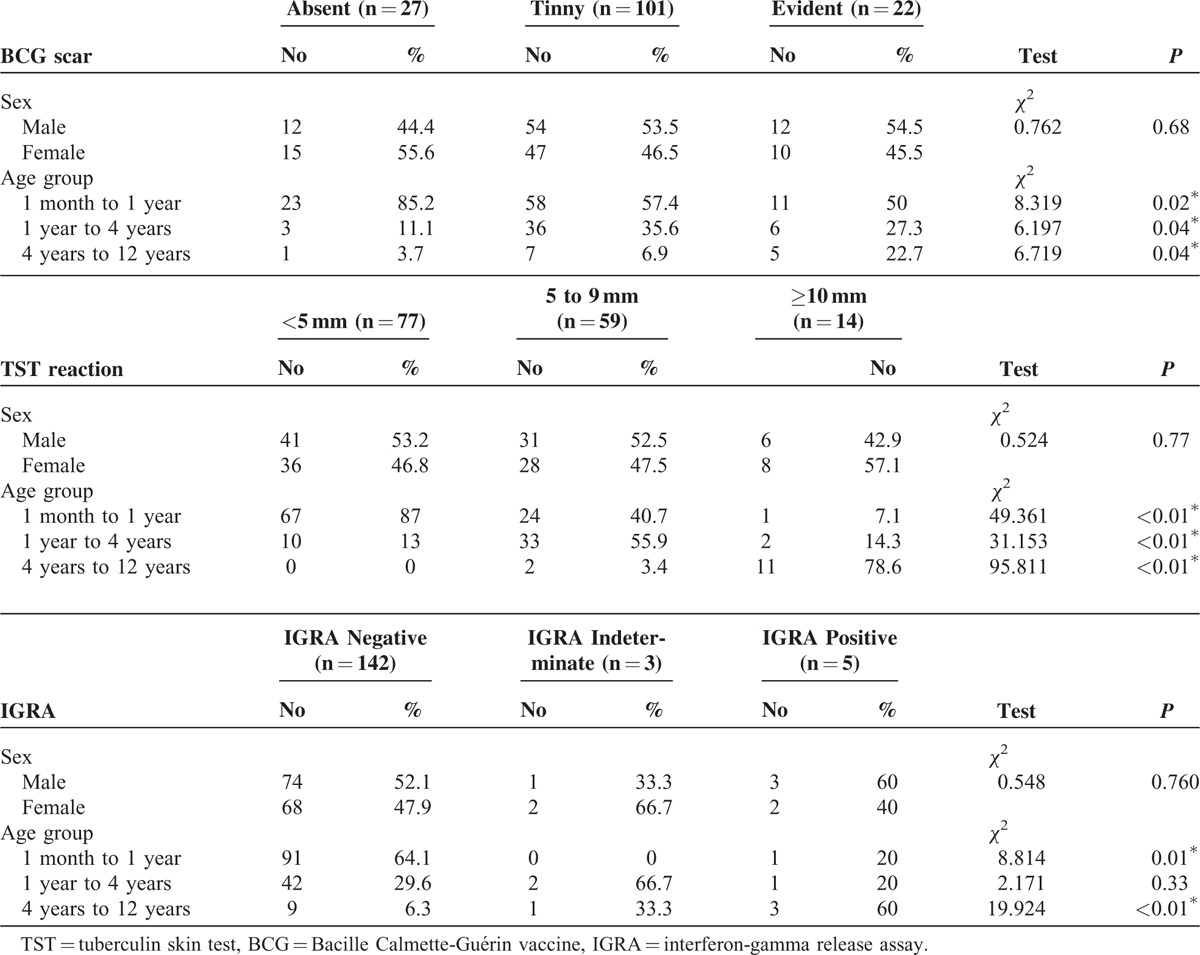
There was significant difference between the size of TST reaction and age (P < 0.01) with young children were more frequent to show reaction <5 mm than old children who more frequent to show reaction ≥10 mm. Also, children with age range 1 month to 1 year were frequently have negative IGRA test (64.1%, P = 0.01), while children with age range 4 years to 12 years were frequently have positive test (60%, P < 0.01), Table 2.
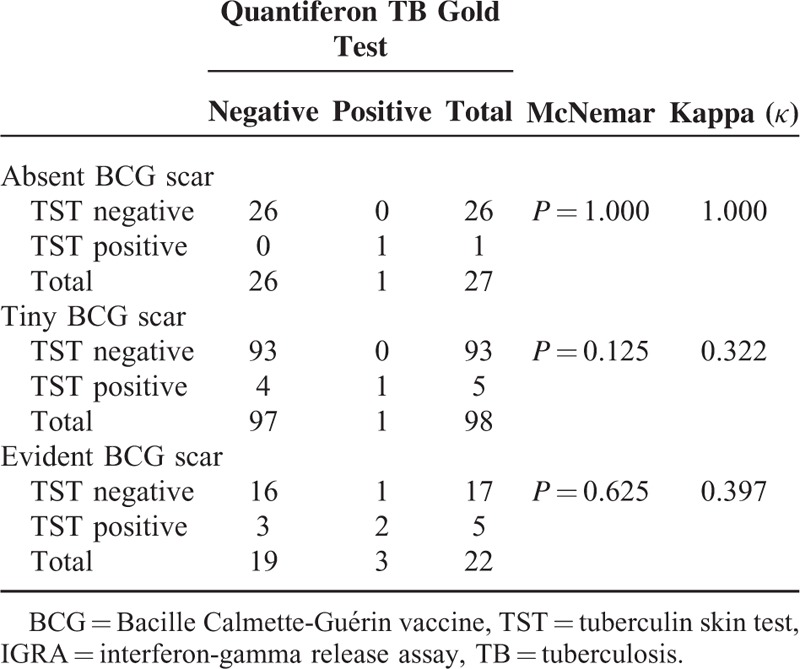
There was moderate agreement between IGRA and TST results (McNemar P = 0.070, κ = 0.475) as 8 children had discordant pairs (1 +ve IGRA/−veTST and 7 +veTST/−ve IGRA), Table 3. With stratification of TST results according to BCG scar status, we found high agreement between IGRA and TST results in children with absent BCG scar (McNemar P = 1.000, κ = 1.000,) as children with negative TST were usually negative IGRA, and low agreement in those with tinny BCG and evident scar (McNemar P = 0.125, κ = 0.322, and McNemar P = 0.625, κ = 0.397, respectively), Table 4.
TABLE 3.
Agreement Between TST and IGRA Test Results
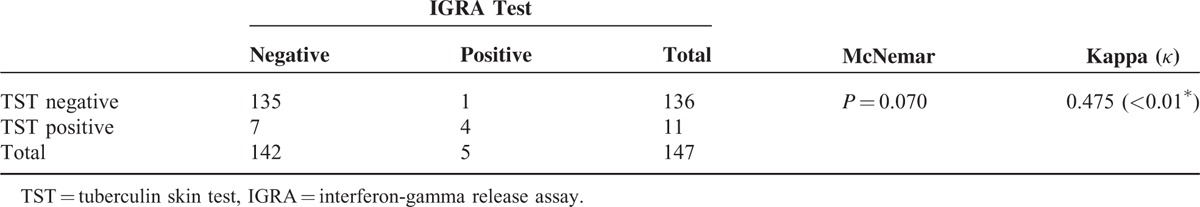
TABLE 4.
Agreement Between TST and IGRA Test Results According to BCG Scar Status
DISCUSSION
Tuberculosis in childhood (TB) is one of major public health concerns worldwide. The World Health Organization (WHO) reported that about 9 million people develop TB each year, and of whom about 1 million (11%) occur in children aged less than 15 years.4
The QuantiFERON-TB Gold in Tube method (QFN Gold IT); for detection of IGRA; has increased specificity for detection of M tuberculosis infection due to the use of 3 specific antigens. It requires a single patient visit, the interpretation is less subjective than TST and not affected by prior BCG vaccination or infection by most non TB mycobacteria.11 Positive IGRAs are more specific than TST for detection of M tuberculosis. But, it cannot distinguish active TB from LTBI.12
In our study, children with scar readings participated were more than those for whom scars were not read (82% versus 18% respectively; P < 0.001). This is comparable to Dhanawade et al13 who reported that the scar failure 8.6%. There were different reasons for scar failure such as weak vaccine, lost potency, and wrong technique.14 Wrong technique may be due to leaving prepared vaccine for long time, near a flame or in direct sunlight, also subcutaneous injection or delayed immune system response after delivery.15
Our study detect that 77 children (51.3%) had TST reaction <5 mm, 59 children (39.3%) had reaction between 5 and 9 mm, and 14 children (9.3%) had reaction ≥10 mm. 14 children was positive for TST in our vaccinated studied children, this mean that there is failure in efficacy of BCG vaccination received. In contrary, Tissot et al16 found that 48% of their subjects had a TST reaction of 10 mm diameter or more.
In studied group, 142 children (94.7%) had negative IGRA, 3 children (2%) had indeterminate IGRA, and 5 children (3.3%) had positive IGRA. There was non-significant difference between the three test results and sex (P = 0.760). This agrees with study conducted in Emirate by Al Mekaini et al,17 who reported that 660 participants had a negative IGRA (99%), 4 (0.6%) had a positive IGRA, 2 (0.3%) had an indeterminate result.
Our study detected that there was significant difference between the size of TST reaction and age (P < 0.001) with young children were more frequent to show reaction <5 mm than old children who more frequent to show reaction ≥10 mm. Hasanabadi et al,18 found that as regard to the size of TST reaction in different age groups, 27.2% of the children under the age of 12 months were negative, the PPD reaction reaches its highest level around the age of 1½ to 4 years and at this age about 28.6% of the vaccinated children showed a positive tuberculin test, between 10 mm and 14 mm.
Children with age range 1 month to 1 year were frequently have negative QNF test (64.1%, P = 0.012), while children with age range 4 years to 12 years were frequently have positive test (60%, P = 0.000). This is agree with Al Mekaini et al17 who found the overall prevalence of LTBI with positive IGRA in young children was 0.45%.
There was moderate significant agreement between QFT and TST results in our patients (κ = 0.475). In children with absent BCG scar, the agreement between QFT and TST results was high, while in those with tinny and evident scar the agreement was low (κ = 1.00, κ = 0.322, κ = 0.397, respectively), this agree with Hatice et al, and Okada et al who found that there was a statistically significant agreement between QFT-GIT and TST results (κ = 0.486; κ = 0.63, respectively).19,20 In contrary, Sayyahfar et al21 found poor agreement between TST and IGRA (κ = 0.004, P = 0.87). Sayyahfar et al and chuke et al found poor agreement between TST and IGRA kappa 0.24.21,22
The number of evident scar-IGRA positive children was more than absent scar-IGRA positive children without clinical significance (2 of 22 children and 1 of 27 children, respectively), and this is in agreement with study performed by Anuradha et al.23 In contrary, Tsiouris et al20 found the number of scar negative-IGRA positive cases was more than scar positive-IGRA positive cases. This can be explained by Zodpey24 et al who demonstrate that there was no evidence that lager BCG scar lead to increased protection against TB. Moreover, children with small scars may have more protection than those had bigger one.24
CONCLUSIONS
Therefore, IFN-γ release assays have higher specificity and lower cross-reactions with BCG vaccination and nontuberculous Mycobacteraie than TST. So, the use of IGRA may increase the efficacy for detection of children with latent TB in vaccinated children as it's more specific to M tuberculosis.
Footnotes
Abbreviations: BCG = Bacille Calmette-Guérin vaccine, IGRA = Interferone gamma release assay, LTBI = Latent tuberculosis infection, M tuberculosis = Mycobacterium tuberculosis, QNF = QuantiFERON, TB = tuberculosis, TST = Tuberculin skin test
The authors declared no potential conflict of interest with respect to this research, authership, and/or publication of this article.
REFERENCES
- 1.Marais BJ, Gie RP, Schaaf HS, et al. Childhood pulmonary tuberculosis: Old wisdom and new challenges. Am J Respir Crit Care Med 2006; 173:1078–1090. [DOI] [PubMed] [Google Scholar]
- 2.Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis 2004; 8:636–647. [PubMed] [Google Scholar]
- 3.Grindulis H, Baynham MI, Scott PH, et al. Tuberculin response 2 years after BCG vaccination at birth. Arch Dis Childhood 1984; 59:614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Mazahi MM, Sakr MA, El-Khaleegy HA, et al. Immunogenecity of neonatal BCG vaccination in children aged 6 years in Egypt, is booster dose needed? J Med Sci 2013; 13:797–802. [Google Scholar]
- 5.Mancuso JD, Tobler SK, Keep LW. Pseudoepidemics of tuberculin skin test conversions in the U.S. Army after recent deployments. Am J Respir Crit Care Med 2008; 177:1285–1289. [DOI] [PubMed] [Google Scholar]
- 6.Froeschle JE, Ruben FL, Bloh AM. Immediate hypersensitivity reactions after use of tuberculin skin testing. Clin Infect 2002; 34:e12–e13. [DOI] [PubMed] [Google Scholar]
- 7.Asl HM, Alborzi A, Pourabbas B, et al. QuantiFERON-TB Gold and tuberculin skin test for the diagnosis of latent tuberculosis infection in children. Iran J Med Sci 2015; 40:411–417. [PMC free article] [PubMed] [Google Scholar]
- 8.Detjen AK, Keil T, Roll S, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis 2007; 45:322–328. [DOI] [PubMed] [Google Scholar]
- 9.Barreto ML, Cunha SS, Pereira SM, et al. Neonatal BCG protection against tuberculosis lasts for 20 years in Brazil. Int J Tuberc Lung Dis 2005; 9:1171–1173. [PubMed] [Google Scholar]
- 10.QuantiFERON®-TB Gold (in-tube method). Package Insert. Cellestis Limited Austrailia. Available at: http://www.cellestis.com Accessed August 15, 2011. [Google Scholar]
- 11.Mohamed H, Hughes EJ, Hawkridge T, et al. Comparison of mantoux skin test with three generations of whole blood INF-γ assay for tuberculosis infection. Int J Tuberc Lung Dis 2006; 10:310–316. [PubMed] [Google Scholar]
- 12.Chiappini E, Della Bella C, Bonsignon F, et al. Potential role of M. tuberculosis specific 12- INF-γ and IL-2 ELISPOT assay in discriminating children with active or latent tuberculosis. PloS One 2012; 7:e46041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhanawade SS, Kumbhar SG, Patil VN. Scar formation and tuberculin conversion following BCG vaccination in infants: A prospective cohort study. J Family Med Prim Care 2015; 4:384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakhar BB. Neonatal BCG and scar success. Indian Pediatr 1995; 32:1323. [PubMed] [Google Scholar]
- 15.Chanabasavaiah R, Murali Mohan, Suryanarayana HV, et al. Waning of BCG scar. Indian J Tuberc 1993; 40:137–144. [Google Scholar]
- 16.Tissot F, Zanetti G, Zellweger JP, et al. Influence of Bacille Calmette-Guerin vaccination on size of tuberculin skin test reaction: to what size? Clin Infect Dis 2005; 40:211–217. [DOI] [PubMed] [Google Scholar]
- 17.Al Mekaini LA, Al Gabri ON, Narchi H, et al. The use of an interferon-gamma release assay to screen for pediatric latent tuberculosis infection in the eastern region of the Emirate of Abu Dhabi. Int J Infect Dis 2014; 23:4–7. [DOI] [PubMed] [Google Scholar]
- 18.Hasanabadi AS, HadiN, Yaghoot M. Tuberculin reaction and BCG scar in children vaccinated at birth. East Mediterr Health J 1998; 4:21–26. [Google Scholar]
- 19.Onur H, Hatipoğlu S, Arıca V, et al. Comparison of quantiferon test with tuberculin skin testforthe detection of tuberculosis infection in children. Inflammation 2012; 35:1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsiouris SJ, Austin J, Toro P, et al. Results of a tuberculosis specific interferon assay for the diagnosis of a tuberculosis infection. Int J Tuberc Lung Dis 2006; 10:939–941. [PubMed] [Google Scholar]
- 21.Sayyahfar S, Karimi A, Fahimzad A, et al. Comparison of tuberculin skin test result and Interferon gamma response to human PPD in BCG scar positive and negative children. J Epidem and Global Health 2014; 4:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuke SO, Yen NT, Laserson KF, et al. Tuberculin skin test versus interferon-gamma release assays in tuberculosis screening among immigrant visa applicants. Tuberc Res Treat 2014; 2014:217969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anuradha B, Rakh SS, Ishaq M, et al. Interferon-gamma low producer genotype +874 overrepresented in Bacillus Calmette-Guerin non responding children. Perdiatr Infec Dis J 2008; 27:325–329. [DOI] [PubMed] [Google Scholar]
- 24.Zodpey SP, Shrikhande SN, Kulkami SW, et al. Scar size and effectiveness of Bacillus Calmette Guerin (BCG) vaccination in the prevention of tuberculosis and leprosy: a case-control study. Indian J Public Health 2007; 51:184–189. [PubMed] [Google Scholar]


