Abstract
Kawasaki disease (KD) is a systemic vasculitis of unknown etiology. IFNG gene encoding interferon (IFN)-γ, produced by natural killer cells and T cells, has been suggested to play an important role in the immunopathogenesis of Kawasaki disease. The aim of this study was to examin the correlation of gene polymorphisms of the IFNG gene and plasma levels of IFN-γ in KD patients and their outcomes.
A total of 950 subjects (381 KD and 569 controls) were recruited. Three tagging single-nucleotide polymorphisms (rs2069718, rs1861493, rs2069705) were selected for TaqMan allelic discrimination assay. Clinical phenotypes, coronary artery lesions (CAL), coronary artery aneurysms (CAA) and intravenous immunoglobulin (IVIG) treatment outcomes were collected for analysis. Plasma IFN-γ levels were also measured with an enzyme-linked immunosorbent assay.
Polymorphisms of the IFNG gene were significantly different between the normal controls and KD patients. The G allele of rs1861493 conferred a better response to IVIG treatment in KD patients. AA allele frequencies of rs1861493 were also associated with a significantly higher risk of CAA in KD patients. Furthermore, the plasma IFN-γ level was lower in the AA allele than in the GG allele of rs1861493 both before and after IVIG treatment in KD patients.
This study provides the first evidence supporting an association between IFNG gene polymorphisms, susceptibility of KD, IVIG responsiveness, and plasma IFN-γ levels in KD patients.
INTRODUCTION
Kawasaki disease (KD) is an acute febrile systemic vasculitis of unknown etiology, and it has a predilection for the involvement of coronary arteries in childhood.1 The clinical manifestations of KD are fever for >5 days, strawberry tongue, conjunctival inflammation, cervical lymphadenopathy, polymorphous rashes, and brawny edema of the hands and feet.2 In addition, KD is also the most common cause of acquired heart diseases in children.2,3 More than 20% of patients with KD may develop coronary artery lesions (CALs) if not given adequate treatment with intravenous immunoglobulin (IVIG), which in turn increase risk of coronary artery fistula formation,4 myocardial infarction, or coronary artery aneurysm (CAA).5 Clinical and epidemiology evidence indicates higher incidence rates of KD in Japan, followed by Korea and Taiwan, and lowest in Europe.5 Thus, susceptibility genetic polymorphisms might play an important role in the immunopathogenesis of KD.
The mounting evidence of the clinical and epidemiologic characteristics of KD strongly support the hypothesis that an infectious agent may be the inducing factor.2 Till now, most researchers believe that imbalance of the immune system and abnormal Th1/Th2/Treg profiles are the key immunopathogenesis in KD.6–9 IFN-γ, a Th1 cytokine secreted mostly by natural killer cells and CD8+ T cells, can recruit macrophages and T cells into coronary arteries, thereby contributing to the production of reactive oxygen species, stimulating matrix metalloproteinases, and inducing tissue factor expressions.10,11 Recently, it was shown that IFN-γ was significantly increased in KD patients before IVIG treatment and the higher level of IFN-γ was associated with patients with CAL than those without CAL.12 The IFNG, encoded IFN-γ, gene polymorphisms have been reported by various genetic association studies to also be implicated in asthma susceptibility,13,14 infectious disease,15 coronary artery disease16 and autoimmune diseases.17,18 Therefore, the aim of this study was to investigate in KD patients the correlation of IFNG gene polymorphisms and the plasma levels of IFN-γ and their outcomes.
PATIENTS AND METHODS
Patients
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB No. 98–0037B), and written informed consent was obtained from parents or guardians of the participants. Three hundred and eighty-one KD patients and 569 controls were studied. Blood samples from age-matched control subjects, who were admitted because of respiratory tract infections (such as acute pharyngitis, acute tonsillitis, croup, acute bronchitis, and acute bronchiolitis), were used for genotyping. All patients of KD were treated with a single high dose of IVIG (2 g/kg) in 12 hours, as previously described.7 Patients with symptoms that did not fully match the KD criteria of American Heart Association were excluded. We used SONOS 5500 or 7500 cardiac scanner (Philips, Andover, MA) with a 5- to 8-MHz sector phased array transducers in this study. All patients of KD underwent a series of pulse Doppler, two-dimensional, and color flow echocardiogram at least 3 times within 8 weeks from the onset of the illness as previously described.7 Echocardiographic follow-up was performed every 3 to 6 months in the first year for KD patients with abnormal coronary artery, and then once annually until the affected coronary arteries become normal, as in our previous studies.7,19,20 We used 2-dimensional echocardiography to visualize the diameter of the left and right coronary arteries on the parasternal short-axis view of the aorta.4 In an echocardiogram, a CAL is defined as the internal diameter of the coronary artery being >3 mm (4 mm, if the subject was older than 5 years) or the internal diameter of a segment being at least 1.5 times that of an adjacent segment.21,22 Transient coronary artery ectasia or dilatation, which disappeared within the initial 8 weeks after the onset of illness, was not identified as CAL.21,22 The CAA was defined as internal diameter of coronary artery being >4 mm or a segment being at least 1.5 times that of an adjacent segment in children older than 5 years according to JCS Joint Working Group.21,22 IVIG responsiveness (or resistance) was defined as defervescence 48 hours after the completion of IVIG administration and no fever (defined as a ear temperature >38°C) recurrence for at least 7 days, with marked normalization or improvement of inflammatory signs.20,23 Blood samples were immediately stored in heparin tubes, and were stored at −80°C until performing assay, as in our previous studies.7
DNA Extraction
Blood cells were treated with 0.5% SDS lysis buffer and then protease K (1 mg/mL) for 4 hours at 60°C to digest the nuclear proteins. Total DNA was extracted using a Gentra extraction kit followed by 70% alcohol precipitation.
Genotyping
In total, 3 tagging single-nucleotide polymorphisms (SNPs) of IFNR (rs2069718, rs1861493, rs2069705) with a minimum allelic frequency of >10% in the Han Chinese population were selected from the HapMap database (http://hapmap.ncbi.nlm.nih.gov/). The rs2069718 was located on the 5’UTR of IFNG, and rs1861493 and rs2069705 of the IFNG gene were located in the introns. Genotyping was carried out using a TaqMan Allelic Discrimination Assay (Applied Biosystems, Foster City, CA), as previously described.24 Quantification of PCR was performed using a 96-well microplate with an ABI 9700 Realtime PCR system, with the following thermal cycling conditions: denaturing at 95°C for 10 minutes, followed by 40 cycles of denaturing at 92°C for 15 seconds and annealing and extension at 60°C for 1 minute, as previously described.24 Fluorescence was quantified using System SDS software version 1.2.3 (Applied Biosystems).
Measurement of Cytokines by an Enzyme-Linked Immunosorbent Assay
We used an enzyme-linked immunosorbent assay to measure human interferon (IFN)-γ according to the manufacturer's instructions.
Statistical Analysis
All data are presented as the mean ± standard deviation. Quantitative data were analyzed using 1-way analysis of variance where appropriate. The least significant difference test was used for post-hoc testing where appropriate. Changes in the data before and after IVIG treatment were tested by the paired sample t test. All statistical analyses were performed by using SPSS version 13.0 for Windows XP (SPSS software, Inc, Chicago, IL) and JMP 9.0 for Windows. The genotypes and allele frequencies associated with the susceptibility of KD and disease outcomes (CAL formation, IVIG treatment response, and aneurysm) were analyzed with the χ2 test. The χ2 test with 1 degree of freedom was used to perform the Hardy-Weinberg equilibrium. Linkage disequilibrium (LD) was assessed for haplotype blocks, which were defined using the default setting of the Haploview software 4.1. Two-sided P values <0.05 were considered statistically significant.
RESULTS
Association of IFNG Gene Polymorphisms With Susceptibility to KD
Table 1 demonstrates the clinical background in this study. There were 381 KD patients and 569 controls in this study. Male subjects accounted for 66.8% of patients of KD and 56% of controls. The mean ages of the cases and controls were 1.7 ± 1.6 years and 5.7 ± 4.9 years, respectively, with children predominantly in this study. In total, 12.9% of patients of KD were resistant to initial IVIG treatment, 9.7% development with CAL, and 4.2% with CAA. The SNP genotypes were in line with the Hardy-Weinberg equilibrium for cases and controls (Table 2). Figure 1 showed the LD plot of IFN-γ gene and the haplotype block structure of rs2069718, rs1861493, and rs2069705 in KD patients. In the comparison of the allele distribution between the KD patients and controls, rs2069718 of IFNG showed significant associations with KD under the genotype model (P = 0.0242).
TABLE 1.
Basal Characteristics of KD Patients and Normal Controls
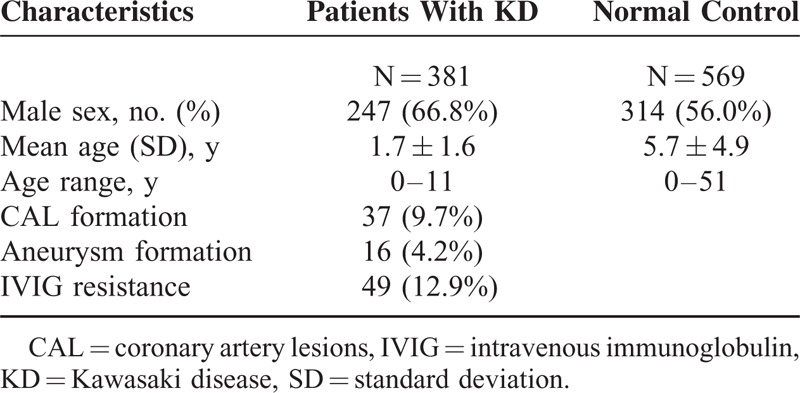
TABLE 2.
Genotype and Allele Frequencies of the IFNG Gene in Controls and Patients With Kawasaki Disease
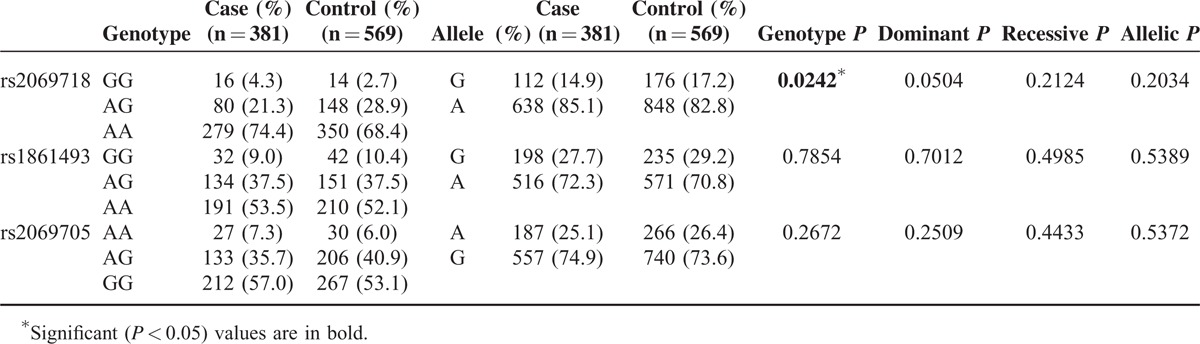
FIGURE 1.
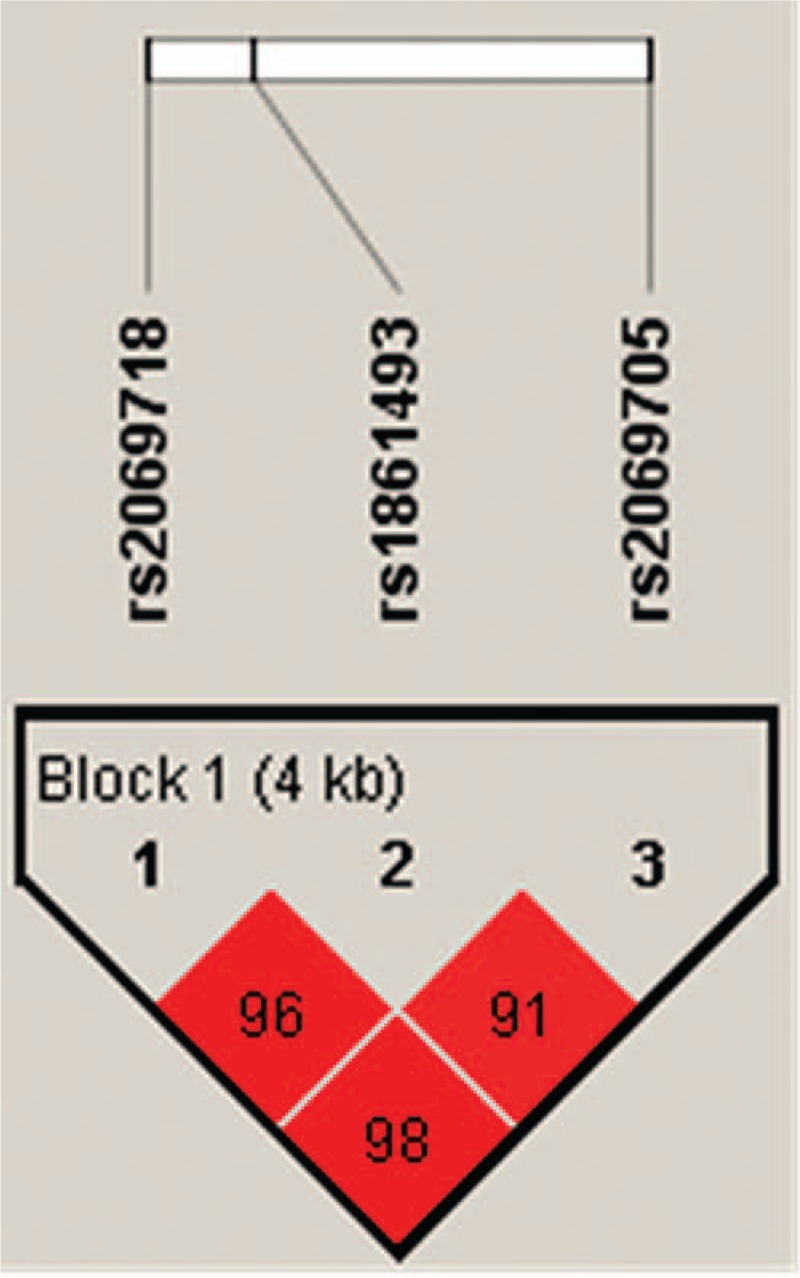
Linkage disequilibrium plot of IFNG gene and the haplotype block structure of rs2069718, rs1861493, and rs2069705 in Kawasaki disease patients. The number on the cell is the logarithm of the odds score of D’.
Association Between IFNG Gene Polymorphisms and Aneurysm Formation and IVIG Responsiveness
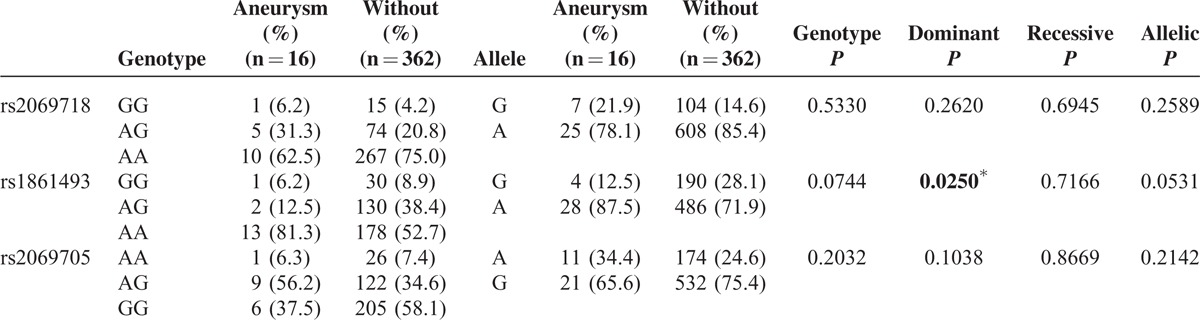
As shown in Table 3, the G allele of rs1861493 conferred a better response to IVIG treatment in KD patients (P = 0.0407). In addition, the dominant model of G allele frequency of rs1861493 conferred a significantly lower risk for CAA in KD patients (P = 0.0250) (Table 4). However, in the comparison of the allele distribution and the risk of CAL formation, there were no differences between the genotypes of IFNG and CAL formation (P = 0.2924 in rs2069718, P = 0.6057 in rs1861493, P = 0.2456 in rs2069705).
TABLE 3.
Genotyping and Allele Frequency of IFNG Single-Nucleotide Polymorphisms in Patients With Kawasaki Disease Responding or Not Responding to Intravenous Immunoglobulin Treatment
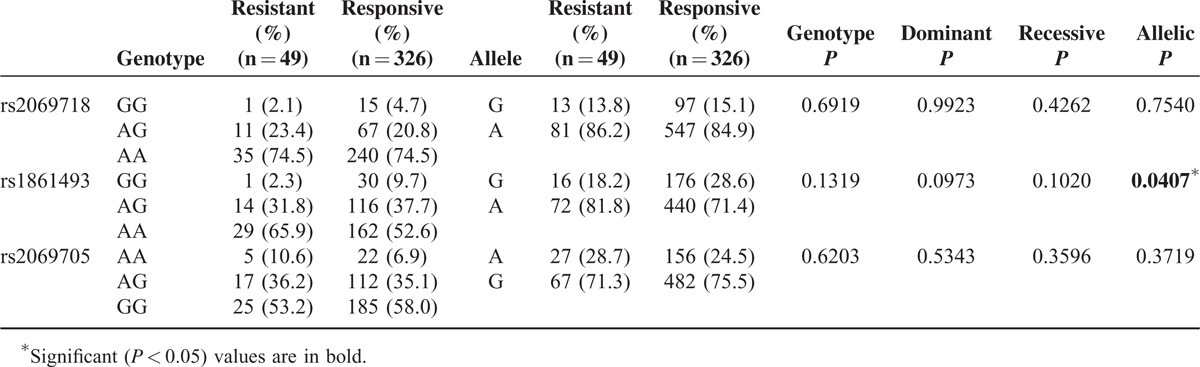
TABLE 4.
Genotyping and Allele Frequency of IFNG Single-Nucleotide Polymorphism in Patients With Kawasaki Disease With Aneurysm or Without Aneurysm
Plasma IFN-γ Levels in KD Patients With different IFNG Genotypes
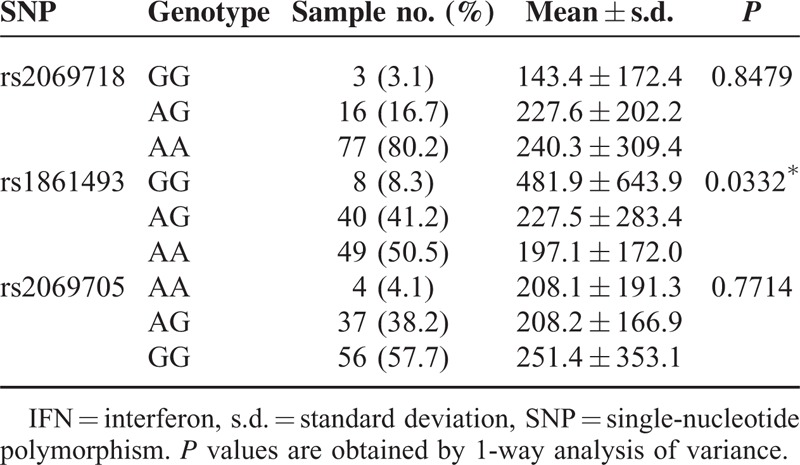
Then, we investigated the association between IFNG genetic variation and the plasma IFN-γ levels in KD patients. As shown in Tables 5 and 6, there was a significant genetic difference between rs1861493 of IFNG and the plasma IFN-γ levels in KD patients. The plasma IFN-γ levels were lower in the AA allele than in the GG allele of rs1861493 both before and after IVIG treatment (P = 0.008 and 0.009, respectively). However, there was no genetic variation association between rs2069718 and rs2069705 of IFNG and the plasma IFN-γ levels in KD patients.
TABLE 5.
Associations of Plasma IFN-γ With Genotype in Kawasaki Disease Patients Before Intravenous Immunoglobulin
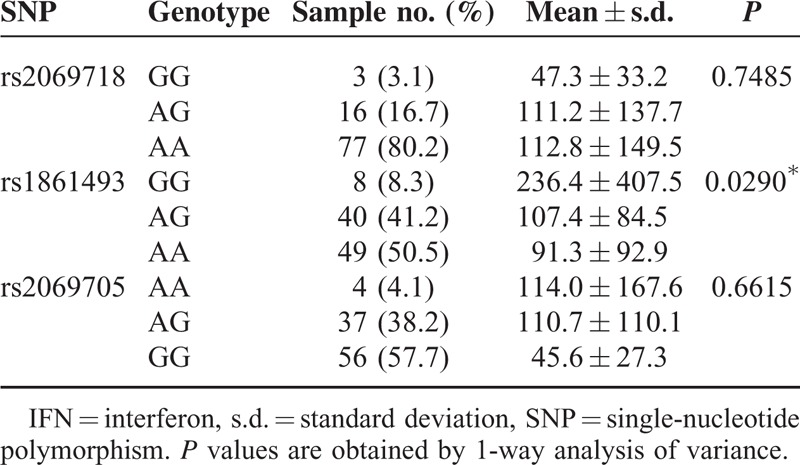
TABLE 6.
Associations of Plasma IFN-γ With Genotype in Kawasaki Disease Patients After Intravenous Immunoglobulin Treatment
DISCUSSION
The pathogenesis of KD remains unknown and KD results from an undefined infectious process in a genetically predisposed individual with a “double hit model.”25 Several studies have indicated an imbalanced immunity between Th1/Th2 response in KD patients.26 In this study, we found that there was an IFNG genotype difference between the normal controls and KD patients. The G allele of rs1861493 conferred a better response to IVIG treatment in KD patients. In addition, the AA allele frequencies of rs1861493 were associated with a significantly higher risk of CAA in KD patients. Furthermore, the plasma IFN-γ levels were lower in the AA allele than in the GG allele of rs1861493 both before and after IVIG treatment in KD patients.
Although growing evidence indicates that the cytokine profiles are associated with the pathogenesis of KD, the precise immunopathogenesis of KD remains unclear. Recently, we have shown that Th1/Th26 and Th17/Treg27 cytokine profiles are associated with the pathogenesis of KD, and this significantly predicted the treatment and prognosis of KD.7 Recently, Wang et al12 have demonstrated that the IFN-γ levels in the main KD patients appeared to stay within the normal range, and that was consistent with Matsubara et al's28 observation that only 30% of KD patients show a slightly increased IFN-γ level. They also found a decrease in IFN-γ-producing CD3+ T cells during the acute stage of KD.29 Although no previous report has linked IFN-γ levels with risk of CAA in the KD patients, lower IFN-γ levels are associated with abdominal aortic aneurysm30,31 and popliteal artery aneurysms formation.32 Moreover, by using expression quantitative trait locus meta-analysis, the genotype of rs1861493 showed a significant association with IFNG gene expression in blood,33 which provides a reliable explanation of the association of rs1861493 and the IFN-γ level. Supporting this observation, our study has found that IFN-γ levels were lower in the AA allele of rs1861493, which was associated with a significantly higher risk of CAA in KD patients.
IFNG gene polymorphisms have also been involved in asthma susceptibility,13,14,34 infectious disease,15 coronary artery disease,16 autoimmune diseases,17,18,35 and type I diabetes mellitus36 in some genetic association studies. Kumar et al13 first described a significant association of an intronic SNP of the IFNG gene with asthma and identified the association of rs1861494 A/G polymorphism with asthma, which may control the IFN-γ levels and thus modulate asthma pathogenesis. In accordance with these findings, we also observed an expression difference between the AA allele and GG allele in KD patients. Moreover, the rs1861494 has been associated with white adults with polymyositis,37 risk of development of pulmonary tuberculosis,38 and the symptomatic score of acute Q fever.39
Growing evidence suggests that genetics play a major role in the immunopathogenesis of KD26 and findings in the past decade have contributed to a major breakthrough in the genetic study of KD. In Asia, Onouchi et al40 discovered a susceptibility loci on chromosome 19q13.2, and further identified the functional SNP of inositol 1,4,5-trisphosphate 3-kinase C in the disease activity of KD. Furthermore, the identification of several genomic regions has been linked to the pathogenesis of KD, including CD40,41 BLK,41 TARC/CCL17,7 and FCGR2A.42 In addition, we also observed an association between genomic hypomethylation of FCGR2A and susceptibility to KD and IVIG resistance.43 In this study, we revealed that IFNG gene polymorphisms were not only associated with the susceptibility of KD, but were also significantly associated with disease outcomes of KD patients of Taiwanese population.43 Therefore, future studies of IFNG gene polymorphisms in KD patients of other ethnic groups could be interesting and important.
In conclusion, our results gain insights into the association of IFNG gene polymorphisms with the susceptibility of KD as well as IFNG gene polymorphisms and the plasma IFN-γ levels with the development of CAA in KD patients.
Footnotes
Abbreviations: CAA = coronary artery aneurysms, CAL = coronary artery lesions, IVIG = intravenous immunoglobulin, KD = Kawasaki disease, LD = Linkage disequilibrium, SNP = single nucleotide polymorphism
The authors report no conflicts of interest.
This study was supported by grants from the Ministry of Science and Technology of Taiwan (102–2314-B-182A-022 and 102–2314-B-182–053-MY3) and grants from Chang Gung Memorial Hospital (CMRPG8D0521, CMRPG8D1561, CMRPG8E0021, CMRPG8E0031, CMRPF6E0041, CMRPG8E0051, CMRPG8E0061, OMRPG8D0011, and CMRPG8C1082).
The authors have indicated that they have no financial interests relevant to this article to disclose.
REFERENCES
- 1.Kawasaki T, Kosaki F, Okawa S, et al. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 1974; 54:271–276. [PubMed] [Google Scholar]
- 2.Wang CL, Wu YT, Liu CA, et al. Kawasaki disease: infection, immunity and genetics. Pediatr Infect Dis J 2005; 24:998–1004. [DOI] [PubMed] [Google Scholar]
- 3.Burns JC, Glode MP. Kawasaki syndrome. Lancet 2004; 364:533–544. [DOI] [PubMed] [Google Scholar]
- 4.Liang CD, Kuo HC, Yang KD, et al. Coronary artery fistula associated with Kawasaki disease. Am Heart J 2009; 157:584–588. [DOI] [PubMed] [Google Scholar]
- 5.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 2004; 114:1708–1733. [DOI] [PubMed] [Google Scholar]
- 6.Kuo HC, Wang CL, Liang CD, et al. Association of lower eosinophil-related T helper 2 (Th2) cytokines with coronary artery lesions in Kawasaki disease. Pediatr Allerg Immunol 2009; 20:266–272. [DOI] [PubMed] [Google Scholar]
- 7.Lee CP, Huang YH, Hsu YW, et al. TARC/CCL17 gene polymorphisms and expression associated with susceptibility and coronary artery aneurysm formation in Kawasaki disease. Pediatr Res 2013; 74:545–551. [DOI] [PubMed] [Google Scholar]
- 8.Ko TM, Kuo HC, Chang JS, et al. CXCL10/IP-10 is a biomarker and mediator for Kawasaki disease. Circ Res 2015; 116:876–883. [DOI] [PubMed] [Google Scholar]
- 9.Guo MM, Tseng WN, Ko CH, et al. Th17- and Treg-related cytokine and mRNA expression are associated with acute and resolving Kawasaki disease. Allergy 2015; 70:310–318. [DOI] [PubMed] [Google Scholar]
- 10.Springall R, Amezcua-Guerra LM, Gonzalez-Pacheco H, et al. Interferon-gamma increases the ratio of matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 in peripheral monocytes from patients with coronary artery disease. PloS One 2013; 8:e72291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulte DJ, Yilmaz A, Shimada K, et al. Involvement of innate and adaptive immunity in a murine model of coronary arteritis mimicking Kawasaki disease. J Immunol 2009; 183:5311–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Wang W, Gong F, et al. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum 2013; 65:805–814. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Ghosh B. A single nucleotide polymorphism (A –> G) in intron 3 of IFNgamma gene is associated with asthma. Genes Immun 2008; 9:294–301. [DOI] [PubMed] [Google Scholar]
- 14.Loisel DA, Tan Z, Tisler CJ, et al. IFNG genotype and sex interact to influence the risk of childhood asthma. The Journal of allergy and clinical immunology 2011; 128:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanchan K, Jha P, Pati SS, et al. Interferon-gamma (IFNG) microsatellite repeat and single nucleotide polymorphism haplotypes of IFN-alpha receptor (IFNAR1) associated with enhanced malaria susceptibility in Indian populations. Infect Genet Evol 2015; 29:6–14. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Kang SW, Chung JH, et al. Polymorphisms of the Interferon gamma gene and coronary artery disease in the Korean population. Mol Biol Rep 2012; 39:5425–5432. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Bermudez M, Lopez-Mejias R, Gonzalez-Juanatey C, et al. Analysis of the interferon gamma (rs2430561, +874T/A) functional gene variant in relation to the presence of cardiovascular events in rheumatoid arthritis. PloS One 2012; 7:e47166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K, Cho SK, Sestak A, et al. Interferon-gamma gene polymorphisms associated with susceptibility to systemic lupus erythematosus. Ann Rheum Dis 2010; 69:1247–1250. [DOI] [PubMed] [Google Scholar]
- 19.Kuo HC, Onouchi Y, Hsu YW, et al. Polymorphisms of transforming growth factor-beta signaling pathway and Kawasaki disease in the Taiwanese population. J Hum Genet 2011; 56:840–845. [DOI] [PubMed] [Google Scholar]
- 20.Kuo HC, Liang CD, Wang CL, et al. Serum albumin level predicts initial intravenous immunoglobulin treatment failure in Kawasaki disease. Acta Paediatr 2010; 99:1578–1583. [DOI] [PubMed] [Google Scholar]
- 21.Shulman ST, De Inocencio J, Hirsch R. Kawasaki disease. Pediatr Clin North Am 1995; 42:1205–1222. [DOI] [PubMed] [Google Scholar]
- 22.Kuo HC, Yu HR, Juo SH, et al. CASP3 gene single-nucleotide polymorphism (rs72689236) and Kawasaki disease in Taiwanese children. J Hum Genet 2011; 56:161–165. [DOI] [PubMed] [Google Scholar]
- 23.Kuo HC, Yang KD, Liang CD, et al. The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatr Allergy Immunol 2007; 18:354–359. [DOI] [PubMed] [Google Scholar]
- 24.Kuo HC, Lin YJ, Juo SH, et al. Lack of association between ORAI1/CRACM1 gene polymorphisms and Kawasaki disease in the Taiwanese children. J Clin Immunol 2011; 31:650–655. [DOI] [PubMed] [Google Scholar]
- 25.Lidar M, Lipschitz N, Langevitz P, et al. The infectious etiology of vasculitis. Autoimmunity 2009; 42:432–438. [DOI] [PubMed] [Google Scholar]
- 26.Kuo HC, Hsu YW, Wu MS, et al. Intravenous immunoglobulin, pharmacogenomics, and Kawasaki disease. J Microbiol Immunol Infect 2016 F; 49:1–7. [DOI] [PubMed] [Google Scholar]
- 27.Kuo HC, Lo MH, Hsieh KS, et al. High-dose aspirin is associated with anemia and does not confer benefit to disease outcomes in Kawasaki disease. PloS One 2015; 10:e0144603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol 1990; 56:29–36. [DOI] [PubMed] [Google Scholar]
- 29.Matsubara T, Katayama K, Matsuoka T, et al. Decreased interferon-gamma (IFN-gamma)-producing T cells in patients with acute Kawasaki disease. Clin Exp Immunol 1999; 116:554–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao M, Liu CL, Lv BJ, et al. Plasma cytokine levels and risks of abdominal aortic aneurysms: A population-based prospective cohort study. Ann Med 2015; 47:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King VL, Lin AY, Kristo F, et al. Interferon-gamma and the interferon-inducible chemokine CXCL10 protect against aneurysm formation and rupture. Circulation 2009; 119:426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdul-Hussien H, Hanemaaijer R, Kleemann R, et al. The pathophysiology of abdominal aortic aneurysm growth: corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J Vasc Surg 2010; 51:1479–1487. [DOI] [PubMed] [Google Scholar]
- 33.Westra HJ, Peters MJ, Esko T, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nature genetics 2013; 45:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.24995662Loisel DA, Tan Z, Tisler CJ, et al. IFNG genotype and sex interact to influence the risk of childhood asthma. J Allerg Clin Immunol 2011; 128:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tangwattanachuleeporn M, Sodsai P, Avihingsanon Y, et al. Association of interferon-gamma gene polymorphism (+874A) with arthritis manifestation in SLE. Clin Rheumatol 2007; 26:1921–1924. [DOI] [PubMed] [Google Scholar]
- 36.Morris GA, Lowe CE, Cooper JD, et al. Polymorphism discovery and association analyses of the interferon genes in type 1 diabetes. BMC Genet 2006; 7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chinoy H, Salway F, John S, et al. Interferon-gamma and interleukin-4 gene polymorphisms in Caucasian idiopathic inflammatory myopathy patients in UK. Ann Rheum Dis 2007; 66:970–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abhimanyu, Bose M, Jha P. Indian Genome Variation Consortium. Footprints of genetic susceptibility to pulmonary tuberculosis: cytokine gene variants in north Indians. Indian J Med Res 2012; 135:763–770. [PMC free article] [PubMed] [Google Scholar]
- 39.Wielders CC, Hackert VH, Schimmer B, et al. Single nucleotide polymorphisms in immune response genes in acute Q fever cases with differences in self-reported symptoms. Eur J Clin Microbiol Infect Dis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onouchi Y, Gunji T, Burns JC, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet 2008; 40:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YC, Kuo HC, Chang JS, et al. Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat Genet 2012; 44:522–525. [DOI] [PubMed] [Google Scholar]
- 42.Onouchi Y, Ozaki K, Burns JC, et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet 2012; 44:517–521. [DOI] [PubMed] [Google Scholar]
- 43.Kuo HC, Chang JC, Kuo HC, et al. Identification of an association between genomic hypomethylation of FCGR2A and susceptibility to Kawasaki disease and intravenous immunoglobulin resistance by DNA methylation array. Arthritis Rheum 2015; 67:828–836. [DOI] [PubMed] [Google Scholar]


