Abstract
Previous studies suggested that diabetes mellitus (DM) was associated with risk and mortality of cancer, but studies investigating the correlation between DM and lung cancer prognosis remain controversial. Herein, a meta-analysis was performed to derive a more precise estimate of the prognostic role of DM in lung cancer.
Medline and Embase were searched for eligible articles from inception to October 25, 2015. The pooled hazard ratio (HR) with its 95% confidence interval (95% CI) was calculated to evaluate the correlation between DM and lung cancer prognosis. Subgroup meta-analysis was performed based on the histology and the treatment methods.
A total of 20 cohort studies from 12 articles were included in the meta-analysis. Also, 16 studies investigated the overall survival (OS) and 4 studies investigated the progression-free survival (PFS). DM was significantly associated with the inferior OS of lung cancer with the pooled HR 1.28 (95% CI: 1.10–1.49, P = 0.001). The association was prominent in the nonsmall cell lung cancer (NSCLC) subgroup (HR 1.35, 95%CI: 1.14–1.60, P = 0.002), whereas the association was not significant in the small cell lung cancer (SCLC) subgroup (HR 1.33, 95% CI: 0.87–2.03, P = 0.18). When NSCLC patients were further stratified by treatment methods, DM had more influence on the surgically treated subgroup than the nonsurgically treated subgroup. There was no obvious evidence for publication bias by Begg's and Egger's test.
The results of this meta-analysis exhibit an association of DM with inferior prognosis amongst lung cancer patients, especially the surgically treated NSCLC patients. Given the small number of studies included in this meta-analysis, the present conclusion should be consolidated with more high-quality prospective cohort studies or randomized controlled trials.
INTRODUCTION
Lung cancer is the most common cancer and the leading cause of cancer-related deaths worldwide.1 Despite diagnosis and therapeutic advances, the prognosis of lung cancer patients is still unsatisfactory.2 To guide decision-making for therapeutic strategies for lung cancer patients and improve their prognosis, a better understanding of the relevant factors affecting lung cancer prognosis is urgently needed. In addition to some established indicator for survival, such as age, smoking status, histology, and stage, diabetes mellitus (DM) maybe another effective prognostic factors for lung cancer patients. Epidemiologic evidence suggests that people with DM are at a significantly higher risk of cancer incidence or mortality, such as breast, bladder, gastric, prostate, and kidney.3–7 A recent meta-analysis demonstrated that pre-existing DM might increase the risk of lung cancer, especially among female diabetic patients.8 However, evidence on the correlation between DM and outcome of lung cancer is conflicting and indefinite.9–11 To derive a more precise estimate of the prognostic value of DM in lung cancer patients, we performed this meta-analysis of eligible published articles according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).12
METHODS
Search Strategy
We searched the electronic databases Medline and Embase from inception to October 25, 2015 by Internet Explorer (version8.0, Microsoft Corp), for articles investigating the correlation of DM with any prognostic outcome in lung cancer patients. Our search strategy included terms for diabetes (diabetes, diabetes mellitus, and glucose) and lung cancer (lung cancer, lung neoplasm, and lung carcinoma). All references in retrieved articles were also reviewed manually for possible inclusions. Our search strategy was limited to English language and human studies. No additional unpublished study was identified.
Study Selection
We included studies investigating the effect of DM on overall survival (OS) or progression-free survival (PFS) in lung cancer patients, and the DM diagnosis were determined by the criterions described by the American Diabetes Association or the World Health Organization. We excluded studies for which no hazard ratio (HR) with its 95% confidence interval (95% CI) could be elicited from any form of outcomes. Provided that multiple articles based on the same population, only the most informative and/or the recently published article was enrolled.
Data Extraction and Quality Assessment
Relevant data were extracted from all the eligible studies by 2 investigators independently using a purpose-designed form. The results were compared and discrepancies were resolved by mutual discussions. The following items, if available, were extracted from the articles included: first author's name, publication year, data source, study recruitment years, follow-up period, tumor histology, treatment method, age, sample size, HR with its 95% CI for the association of DM and lung cancer, and statistical adjustments for confounding factors. HR that was not directly reported was calculated according to the data presented in the graphs or tables. On condition that there were no enough data in the origin article, we contacted the corresponding author by e-mail. Quality assessment for studies included in this meta-analysis was conducted according to the Newcastle Ottawa scale (NOS) criteria.13 Because all analyses were based on previous published studies, no ethical approval and patient consent were necessary.
Statistical Analysis
We calculated the pooled HRs with their corresponding 95% CIs to assess the prognostic significance of DM status in lung cancer patients, and the HR >1 implied an inferior prognosis for patients with DM. A random-effect model was adopted in this meta-analysis since considerable heterogeneity presented.14 Statistical heterogeneity between studies was evaluated using Cochrane Q test and the chi-squared test. The I2 values ≥50% indicated significant heterogeneity.15 For additional analyses, subgroup meta-analysis was performed on the basis of the histology (SCLC or NSCLC) and the treatment methods (undergoing resection or nonsurgical treatment). Sensitivity analysis was performed by sequential omission of individual studies to examine the stability of the outcomes in this meta-analysis. Furthermore, Begg's and Egger's tests were applied to evaluate the potential publication bias.16,17 All analyses were performed using Stata software (version 12.0, Stata Corp, College Station, TX). A 2-tailed P value <0.05 was considered significant in statistical tests.
RESULTS
Literature Search
Figure 1 represented the steps of retrieving articles for inclusion in the meta-analysis. Our original search identified 4103 potentially relevant articles, of which we scanned the titles and abstracts. After further evaluating the articles identified, 12 articles with a total of 15,180 lung cancer patients stratifying by DM status were included in the meta-analysis.9–11,18–26
FIGURE 1.
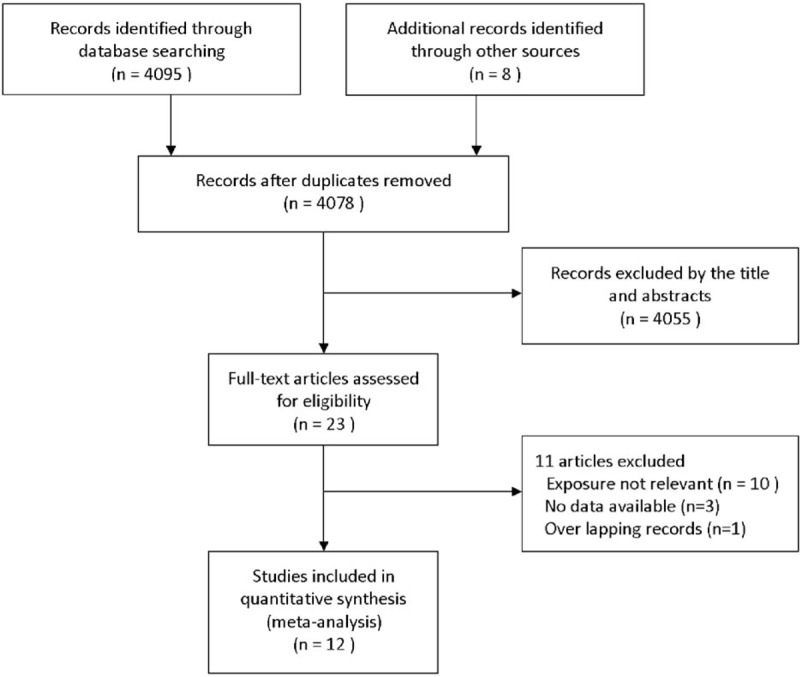
Flowchart representing the articles selection process.
Study Characteristics
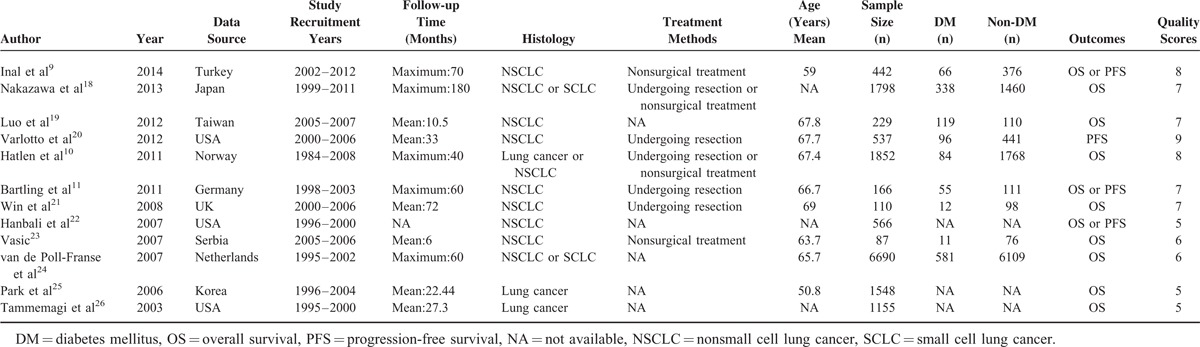
The main characteristics of 12 studies included in the meta-analysis were summarized in Table 1. A total of 20 cohorts from 12 studies were included, which were published between 2003 and 2014. Of the 20 cohorts, 16 cohorts9–11,18,19,21–26 with a total of 14,643 lung cancer cases investigated the OS and 4 studies9,11,20,22 with a total of 1711 lung cancer patients investigated the PFS. The outcomes of all the 20 cohorts were adjusted for several potential confounders, including age, gender, smoking status, performance status, body mass index, and so on. Study quality was assessed according to the NOS, and the mean score was 6.7.
TABLE 1.
Main Characteristic of 12 Eligible Studies in this Meta-Analysis
Meta-analysis
To evaluate the prognostic significance of DM status in lung cancer patients, a meta-analysis was conducted on HRs of OS and PFS. As shown in Figure 2, the pooled HR with its corresponding 95% CI of OS in 16 cohorts was 1.28 (95% CI: 1.10–1.49, P = 0.001). Sensitivity analysis through sequential omission of single study did not change the original outcomes, which supported the credibility and stability of the results. Furthermore, subgroup meta-analyses were conducted by stratifying based on histology and treatment methods. Subgroup analysis by histology suggested DM is associated with worse OS in NSCLC (HR 1.36, 95% CI: 1.12–1.67, P = 0.002), but not the SCLC (HR 1.33, 95% CI: 0.87–2.03, P = 0.18) (Figure 3). However, the association between DM and OS in SCLC is inconclusive due to limited data. After that, we conducted subgroup analysis stratified by treatment methods in NSCLC patients. DM seemed have more effect on surgically treated NSCLC (HR 1.71, 95% CI: 0.94–3.08, P = 0.08) than NSCLC treated by the nonsurgical method (HR 1.53, 95% CI: 0.52–4.52, P = 0.44) (Figure 4), though there was no significant correlation between DM and OS in the 2 subgroups. The insignificant association between DM and OS in NSCLC when stratified by treatment methods might be due to the exclusion of some data that could not be stratified. In addition, 4 studies were eligible for examining the relationship between DM and PFS in NSCLC patients. As shown in Figure 5, the pooled HR of the PFS was 1.12 (95% CI: 0.59–2.10, P = 0.73), which should be regarded with caution due to inadequate amount of studies included. Considering the pooled data about DM and lung cancer survival, DM status is predicted to have a significant poor prognostic effect on OS in lung cancer patients, especially in NSCLC.
FIGURE 2.
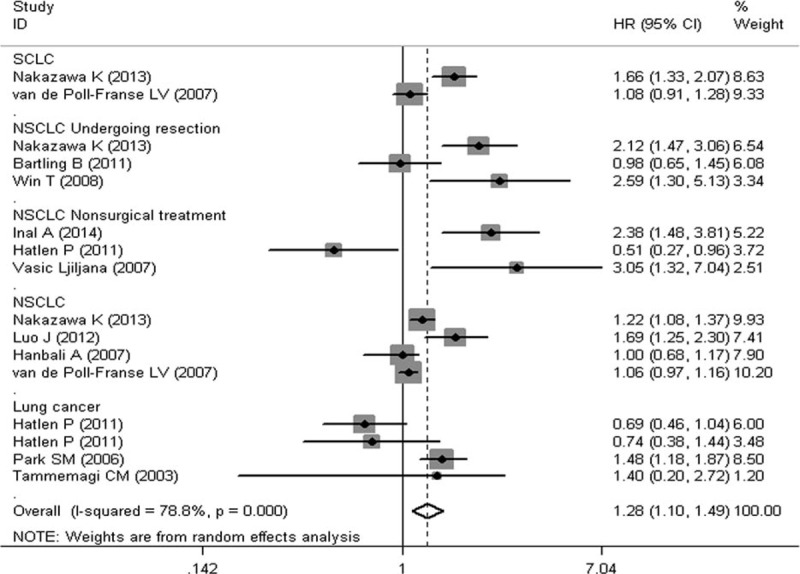
Meta-analysis of the association between diabetes mellitus and overall survival in lung cancer.
FIGURE 3.
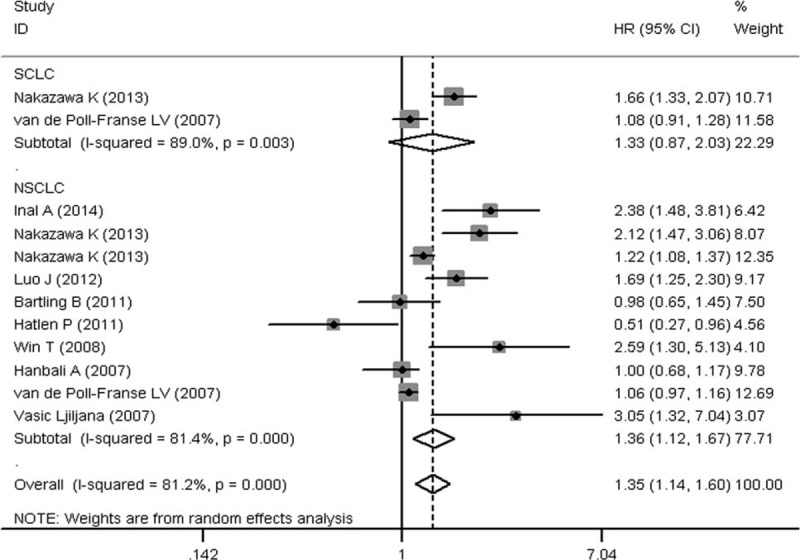
Subgroup meta-analysis of the association between diabetes mellitus and overall survival in lung cancer according to histology (SCLC or NSCLC). NSCLC = non small cell lung cancer, SCLC = small cell lung cancer.
FIGURE 4.
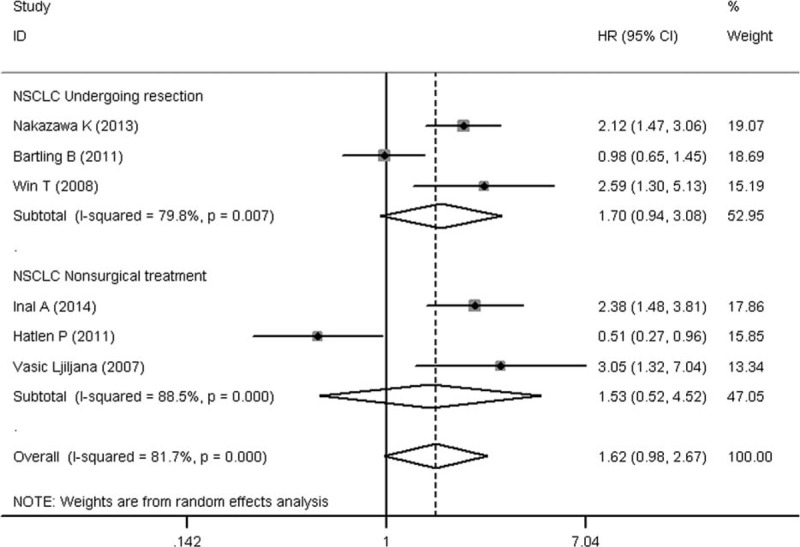
Subgroup meta-analysis of the association between diabetes mellitus and overall survival in NSCLC according to the treatment methods (undergoing resection or nonsurgical treatment). NSCLC = non small cell lung cancer.
FIGURE 5.
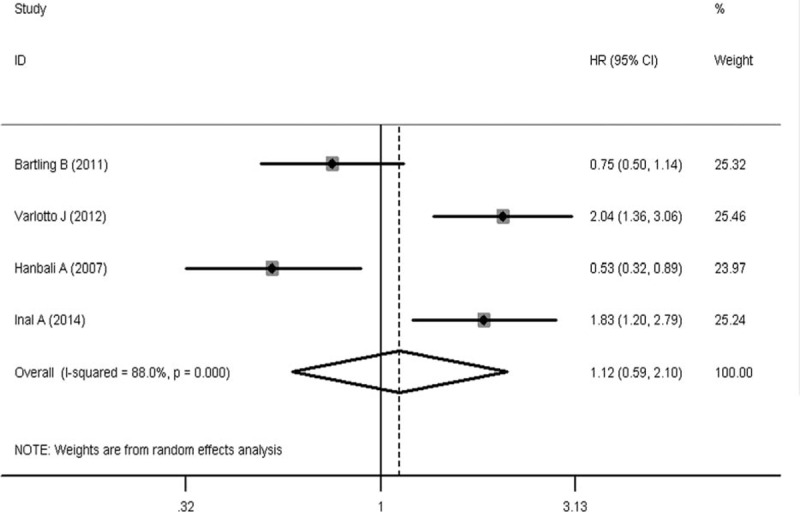
Meta-analysis of the association between diabetes mellitus and progression-free survival in NSCLC. NSCLC = non small cell lung cancer.
Publication Bias Analyses
The funnel plot, Begg's test, and Egger's test were performed to detect publication bias in the meta-analysis. No obvious publication bias was revealed after assessing the funnel plot for the eligible studies (Figure 6). In addition, the results from Begger's and Egger's test for the studies evaluating OS in lung cancer did not reveal obvious publication bias (Pbegg's = 0.82 and Pegger's = 0.28).
FIGURE 6.
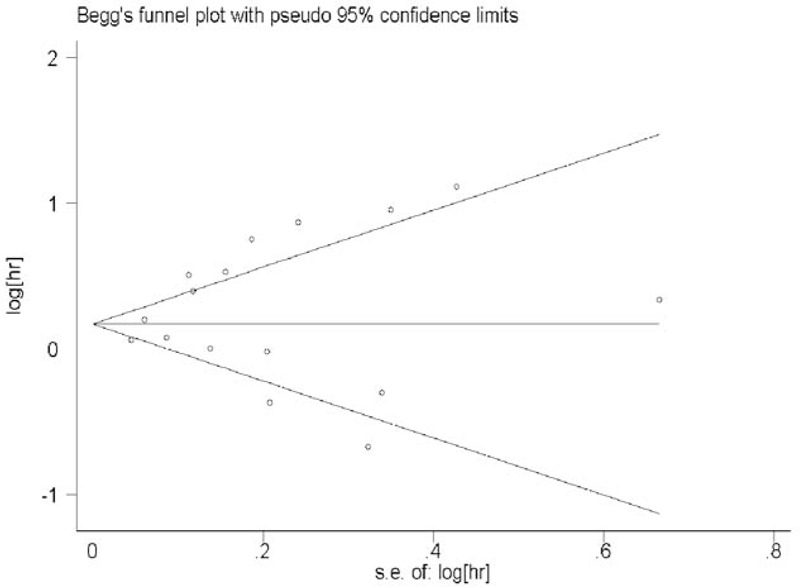
Funnel plot of hazard ratios for the overall survival in lung cancer patients.
DISCUSSION
This meta-analysis summarized the previous studies to evaluate the effect of DM on the prognosis of lung cancer patients. In our meta-analysis, the included articles were limited to the English language because the non-English language articles were generally in poor quality and tended to bring about more bias. The results of this meta-analysis indicated that DM was associated significantly with a worse OS in lung cancer patients. When subgroup analysis was performed stratifying by histology, worse survival was presented in the NSCLC subgroup other than the SCLC subgroup. However, the association between DM and OS in SCLC should be regarded with caution because of only 2 eligible studies included. Furthermore, when stratifying by treatment methods among the NSCLC patients, the association of DM with surgically treated NSCLC subgroup was more prominent than the nonsurgically treated NSCLC subgroup narrowly. However, there was no significant association between DM and the PFS in NSCLC patients with only 4 eligible studies included.
Although epidemiologic evidence support a role for DM in lung cancer progression, the genuine biological linkage between DM and lung cancer is still uncertain. Several mechanisms have been proposed to explain the negative effect of DM on the survival of lung cancer. Hyperinsulinemia, hyperglycaemia, and metabolic disorder of cancer cells may be the potential factors contributing to the development of lung cancer.27,28 Elevated insulin levels, which respond to insulin resistance, may have impact on cancer-promoting through the insulin-like growth factor-1 (IGF-1) pathway.29 The IGF-1 pathway is regarded as an important promoter of tumor progression in certain studies30,31 and IGF-1 receptor inhibitor may contribute to the cancer therapy.32,33 In addition, hyperglycemia and the metabolic disorder of cancer cells may accelerate the proliferation of lung cancer cells.28 Han et al have made an important discovery that high glucose can promote cancer proliferation via the induction of epidermal growth factor (EGF) expression and transactivation of EGF receptor.34 Further research by De Rosa et al indicate that EGF pathways are associated with cancer metabolism and inhibition of EGF pathways may have the synergistic antitumor effect with the therapeutic strategies targeting glucose metabolism through reversal of Warburg effect and reactivation of oxidative phosphorylation in NSCLC.35 However, except for the cancer-promoting effects of DM, there may be some other feasible effects of DM on the poor survival in lung cancer patients. For example, patients with DM generally present with more advanced stages of lung cancer, which may contribute to the inferior OS of lung cancer patients with DM.36 Furthermore, lung cancer patients with pre-existing DM may receive less aggressive treatment because of a greater risk of chemotherapy-related toxicity.36 As for the histology of lung cancer, different results between NSCLC and SCLC subgroups indicate that the influence of DM might differ on the NSCLC and SCLC patients. However, the difference may also be due to only 2 studies focusing on the SCLC subgroup. Further research is needed to clarify the pathophysiologic mechanisms by which DM may have different effects on NSCLC and SCLC patients.
Compared with the previous study by Barone et al,37 our meta-analysis has several strengths. Compared with Barone's study, which included only 4 studies focusing on lung cancer mortality and could inevitably include more risk of bias, Our meta-analysis included 20 eligible studies with a total of 15,180 lung cancer patients stratified by DM status, which should provide a stronger statistical power and have less risk of bias. Furthermore, subgroup analyses by the histology and treatment methods were also performed in our meta-analysis, and indicated that DM might be an independent prognostic factor for NSCLC, especially for the surgically treated NSCLC subgroup. These strengths above all provide a more persuasive evidence for the prognosis role of DM in lung cancer patients.
However, several limitations should be pointed out. First, studies were much inconsistent in their confirmation of DM, study population, duration of follow-up, and adjustment for confounding variables, which might produce a high level of heterogeneity across the analysis. Second, the meta-analysis did not think over the methods of DM therapy used or their impact on lung cancer outcomes. Different therapies may have different effects on cancer progression.38,39 Third, DM durations varied in different studies and we could not investigate whether DM duration have effect on lung cancer outcomes. Finally, our search was restricted to published articles in electronic databases and no additional unpublished studies or original data were taken into account, which might bring about publication bias.
In conclusion, our meta-analysis showed a significant correlation between DM state with worse survival in lung cancer patients, especially in the surgically treated NSCLC subgroup. On the basis of outcomes of this meta-analysis, more attention should be paid to lung cancer patients with pre-existing DM. Given the small number of studies included in this meta-analysis, the present conclusion should be consolidated with more high-quality prospective cohort studies or randomized controlled trials.
Acknowledgment
The authors thank Dr. Xia Li from Shandong University for her kind help in the statistics.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, DM = diabetes mellitus, EGF = epidermal growth factor, HR = hazard ratio, IGF = insulin-like growth factor, NA = not available, NSCLC = nonsmall cell lung cancer, OS = overall survival, PFS = progression-free survival, SCLC = small cell lung cancer
Funding: this research was supported by the Natural Science Foundations of Shandong Province (No. ZR2014HM100) and Technology Development Projects of Shandong Province (No. 2015GSF118109 and No. 2015GSF118129).
LZ and HC equally contributed to this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Dillman RO, McClure SE. Steadily improving survival in lung cancer. Clin Lung Cancer 2014; 15:331–337. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Zhang X, Gu C, et al. Diabetes mellitus is associated with breast cancer: systematic review, meta-analysis, and in silico reproduction. Panminerva Med 2015; 57:101–108. [PubMed] [Google Scholar]
- 4.Fang H, Yao B, Yan Y, et al. Diabetes mellitus increases the risk of bladder cancer: an updated meta-analysis of observational studies. Diabetes Technol Ther 2013; 15:914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimoyama S. Diabetes mellitus carries a risk of gastric cancer: a meta-analysis. World J Gastroenterol 2013; 19:6902–6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai H, Xu Z, Xu T, et al. Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: a meta-analysis of 11 cohort studies. Diabetes Metab Res Rev 2015; 31:336–343. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Li H, Gu L, et al. The impact of diabetes mellitus on renal cell carcinoma prognosis: a meta-analysis of cohort studies. Medicine 2015; 94:e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JY, Jeon I, Lee JM, et al. Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer 2013; 49:2411–2423. [DOI] [PubMed] [Google Scholar]
- 9.Inal A, Kaplan MA, Kucukoner M, et al. Is diabetes mellitus a negative prognostic factor for the treatment of advanced non-small-cell lung cancer? Revista Portuguesa Pneumologia 2014; 20:62–68. [DOI] [PubMed] [Google Scholar]
- 10.Hatlen P, Gronberg BH, Langhammer A, et al. Prolonged survival in patients with lung cancer with diabetes mellitus. J Thorac Oncol 2011; 6:1810–1817. [DOI] [PubMed] [Google Scholar]
- 11.Bartling B, Simm A, Sohst A, et al. Effect of diabetes mellitus on the outcome of patients with resected non-small cell lung carcinoma. Gerontology 2011; 57:497–501. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8:336–341. [DOI] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 18.Nakazawa K, Kurishima K, Tamura T, et al. Survival difference in NSCLC and SCLC patients with diabetes mellitus according to the first-line therapy. Med Oncol 2013; 30:367. [DOI] [PubMed] [Google Scholar]
- 19.Luo J, Chen YJ, Chang LJ. Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients. Lung Cancer 2012; 76:242–247. [DOI] [PubMed] [Google Scholar]
- 20.Varlotto J, Medford-Davis LN, Recht A, et al. Confirmation of the role of diabetes in the local recurrence of surgically resected non-small cell lung cancer. Lung Cancer 2012; 75:381–390. [DOI] [PubMed] [Google Scholar]
- 21.Win T, Sharples L, Groves AM, et al. Predicting survival in potentially curable lung cancer patients. Lung 2008; 186:97–102. [DOI] [PubMed] [Google Scholar]
- 22.Hanbali A, Al-Khasawneh K, Cole-Johnson C, et al. Protective effect of diabetes against metastasis in patients with non-small cell lung cancer. Arch Int Med 2007; 167:513. [DOI] [PubMed] [Google Scholar]
- 23.Vasić L. Locally advanced non-small cell lung cancer, pretreatment prognostic factors: disease stage, tumor histopathological characteristics, the patient-related factors. Arch Oncol 2007; 15:19–23. [Google Scholar]
- 24.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, et al. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. International journal of cancer. J Int Cancer 2007; 120:1986–1992. [DOI] [PubMed] [Google Scholar]
- 25.Park SM, Lim MK, Shin SA, et al. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol 2006; 24:5017–5024. [DOI] [PubMed] [Google Scholar]
- 26.Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Impact of comorbidity on lung cancer survival. International journal of cancer. J Int Cancer 2003; 103:792–802. [DOI] [PubMed] [Google Scholar]
- 27.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin 2010; 60:207–221. [DOI] [PubMed] [Google Scholar]
- 28.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh P, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol 2014; 31:805. [DOI] [PubMed] [Google Scholar]
- 30.Cardillo TM, Trisal P, Arrojo R, et al. Targeting both IGF-1R and mTOR synergistically inhibits growth of renal cell carcinoma in vitro. BMC Cancer 2013; 13:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodt P, Samani A, Navab R. Inhibition of the type I insulin-like growth factor receptor expression and signaling: novel strategies for antimetastatic therapy. Biochem Pharmacol 2000; 60:1101–1107. [DOI] [PubMed] [Google Scholar]
- 32.Singh RK, Gaikwad SM, Jinager A, et al. IGF-1R inhibition potentiates cytotoxic effects of chemotherapeutic agents in early stages of chemoresistant ovarian cancer cells. Cancer Let 2014; 354:254–262. [DOI] [PubMed] [Google Scholar]
- 33.Wilky BA, Rudek MA, Ahmed S, et al. A phase I trial of vertical inhibition of IGF signalling using cixutumumab, an anti-IGF-1R antibody, and selumetinib, an MEK 1/2 inhibitor, in advanced solid tumours. Br J Cancer 2015; 112:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han L, Ma Q, Li J, et al. High glucose promotes pancreatic cancer cell proliferation via the induction of EGF expression and transactivation of EGFR. PloS One 2011; 6:e27074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Rosa V, Iommelli F, Monti M, et al. Reversal of Warburg effect and reactivation of oxidative phosphorylation by differential inhibition of EGFR signaling pathways in non-small cell lung cancer. Clin Cancer Res 2015; 21:5110–5120. [DOI] [PubMed] [Google Scholar]
- 36.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, et al. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer 2007; 120:1986–1992. [DOI] [PubMed] [Google Scholar]
- 37.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 2008; 300:2754–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JH, Kim TI, Jeon SM, et al. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer 2012; 131:752–759. [DOI] [PubMed] [Google Scholar]
- 39.Chen KH, Shao YY, Lin ZZ, et al. Type 2 diabetes mellitus is associated with increased mortality in Chinese patients receiving curative surgery for colon cancer. Oncologist 2014; 19:951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]


