Abstract
Migraine has been associated with sleep disturbances. Relationship between sleep quality and migraine frequency is yet to be determined. The present study aimed to investigate sleep disturbances among low-frequency, moderate-frequency, high-frequency, and chronic migraineurs, with and without auras, with well-controlled confounding variables.
This cross-sectional controlled study included 357 subjects from an outpatient headache clinic in Taiwan. Standardized questionnaires were utilized to collect demographic, migraine, sleep, depression, anxiety, and restless leg syndrome characteristics in all participants. According to frequency of migraine attacks, patients were divided into 4 groups: with 1 to 4 migraine days per month, 5 to 8 migraine days in a month, 9 to 14 migraine days in a month, and >14 migraine days per month. The Pittsburgh Sleep Quality Index (PSQI) and subgroup items were used to evaluate sleep quality. The association between migraine frequency and sleep quality was investigated using multivariable linear regression and logistic regression.
The PSQI total score was highest in patients with high frequent migraine (10.0 ± 3.4) and lowest in controls (7.0 ± 3.4) with a significant trend analysis (P for trend = 0.006). Migraine frequency had an independent effect on the items “Cannot get to sleep within 30 minutes” (P < 0.001), “Wake up in the middle of the night or early morning” (P < 0.001), “Bad dreams” (P = 0.001), “Pain” (P = 0.004), and “Quality of sleep” (P < 0.001). The result showed the effect of migraine frequency in both the aura-present (P for trend = 0.008) and the aura-absent subgroups (P for trend = 0.011).
High migraine frequency correlates with poor sleep quality and a higher prevalence of poor sleepers. These associations occur in migraine with aura and without aura.
INTRODUCTION
Migraine is one of the most common headache disorders, characterized by lateralized, intense, throbbing, or pulsatile sensations in the head. According to the Global Burden of Disease report, migraine is the sixth most burdensome disease worldwide, and the first among neurological diseases.1 Migraine attacks are often associated with nausea, vomiting, sound sensitivity, and light sensitivity. Migraine can lead to poor quality of life and considerable disability in migraineurs. In addition, migraine is shown to be comorbid with several psychiatric conditions such as anxiety and depression.2 It has been suggested that there is a relationship between migraine and sleep disorders. However, the relationship between migraine frequency and sleep remains uncertain.
Quality of sleep is associated with life satisfaction. In the general population, one-third of adults are affected by poor sleep.3 Considering the high prevalence of sleep disturbances and the close relationship between sleep and quality of life, one can regard sleep quality as an important indicator of quality of life.3 Disturbed sleep is a particularly common problem among migraineurs (children or adult), affecting 30% to 50% of migraine patients. Moreover, sleep disruption can be a trigger of migraine attacks, which are improved with sufficient restful sleep. Typically, people with chronic migraine are prone to morning headaches due to sleep insufficiency.4–6
Recent studies have shown a higher prevalence of poor sleep quality in patients with migraine compared to people without migraine.4,7–13 Two large longitudinal studies from Norway revealed a bidirectional association between insomnia and primary headache.14,15 Additionally, a prior systematic review suggested that migraine and tension-type headaches were significantly related to insomnia.16 Another population-based cross-sectional study linking headaches and sleep in Denmark revealed a high prevalence of concurrent headache and sleep problems.17 Seidel et al have reported that sleep quality was particularly poor in patients with 8 or more migraine days per month and markedly better in control subjects than in migraine patients.7 Collectively, these studies suggest that there is an association between sleep and migraine.
Nevertheless, migraine with or without aura, detailed differentiated migraine frequency and other confounding factors affecting sleep quality, such as anxiety, depression, and restless legs syndrome (RLS), need to be taken into account. It is suggested that the information is useful for the development of preventive and therapeutic strategies for alleviating the burden of both migraine and poor sleep quality in these and other understudied populations.
We hypothesized that migraine frequency was correlated with poor sleep quality, and that migraine impact on sleep quality was independent of whether 1 experiences auras. We evaluated the relationship between sleep quality and migraine frequency while controlling for the comorbidities of anxiety, depression, and restless legs syndrome (RLS). The pathophysiological interrelation between migraine and sleep was discussed.
METHODS
Patients
This cross-sectional controlled study enrolled 357 subjects attending an outpatient headache clinic in the Department of Neurology at TSGH between June 2013 and May 2015. Of those, 34 subjects were with chronic migraine (≥15/month), 60 were with high-frequency migraine (9–14/month), 44 were with medium frequency migraine (5–8/month), and 185 were with low-frequency migraine (1–4/month). In addition, 134 sex- and age-matched healthy volunteers were selected using several criteria, including no family history of migraine and no previous diagnosis of other primary or secondary headache disorders, except for episodic tension-type headaches (<6 days/year). In addition, subjects who completed the screening questionnaire were subsequently interviewed by the corresponding author (F-CY), a board-certified neurologist and headache specialist, to confirm the diagnosis according to the International Classification of Headache Disorders, 3rd edition (beta version) (ICHD-III beta).18 Consecutive migraine patients with and without aura were enrolled according to the criteria of the International Headache Society.18 To diagnose migraine more accurately and exclude other sleep-related headaches (such as hypnic headache) in this study, all subjects who completed the screening questionnaire were subsequently interviewed by the corresponding author (F-CY), a board-certified neurologist and headache specialist to evaluate their eligibility for migraine, based on the ICHD-III beta.18
After giving informed consent, the patients were interviewed with a structured questionnaire packet containing an “RLS symptoms screening questionnaire” from the Restless Legs Syndrome Foundation (http://giic.rgps.on.ca/files/24c%20Living%20with%20Restless%20Legs%20Syndrome%20Booklet%20RLS%20Foundation.pdf), the Pittsburgh Sleep Quality Index (PSQI),19 the Beck's Depression Inventory (BDI),20 and the hospital anxiety and depression subscales (HADS).21 The “RLS symptoms screening questionnaire” consists of 11 questions. If a patient answers “yes” to a majority of these questions, there is a high probability of RLS. The BDI contains 21 items with a maximum score of 63. Patients who had a score ≥18 on the BDI were classified as depressed. The PSQI estimates one's sleep quality over the prior month. It consists of 7 component scores, which are added together to produce a final score. A PSQI score of ≥6 was considered indicative of a poor sleeper.19 The HADS is a 14-item scale, with 7 items related to anxiety and 7 items related to depression. Each item is rated on a 4-point scale (0, not at all; 1, sometimes; 2, often; and 3, all the time), giving a maximum subscale score of 21 for anxiety and for depression.22 The Migraine Disability Assessment questionnaire (MIDAS), a 5-item questionnaire that was designed to evaluate disability during the previous 3 months, was used in this study.23 This study protocol was approved by the Institutional Review Broad of Tri-Service General Hospital (TSGH), Taiwan and written informed consent was obtained for all participants prior to enrollment.
Data Analysis
Values of continuous variable were presented as mean with standard deviation and that of categorical variable were presented as number and percentage. We tested the linear trend of the distribution of the subjects’ characteristics across the study groups (control, low, medium, high, and chronic) using the Cochran–Armitage chi-square test for categorical variables and using linear contrasts of univariate linear regression for continuous variables. To assess the effect of migraine frequency on sleep quality, we tested the linear trend of PSQI items and total score across the study groups using linear contrasts in multivariable linear regressions for continuous variables and linear contrasts in multivariable logistic regressions for categorical variables. Finally, both univariate and multivariable logistic regression analyses were used to identify the independent factors of PSQI total score ≥6 (poor sleep quality). All data analyses were carried out with SPSS 22 (IBM SPSS, Armonk, NY: IBM Corp).
RESULTS
Characteristics of the Study Cohort
The demographic and clinical characteristics of each group are reported in Table 1. There were no significant differences among the 5 study groups in age, gender ratio, body mass index (BMI), and attained education level (Table 1). Less than one-third of the enrolled subjects with migraine experienced auras (61/225; 27.1%).
TABLE 1.
Characteristics of the Study Population (N = 357)
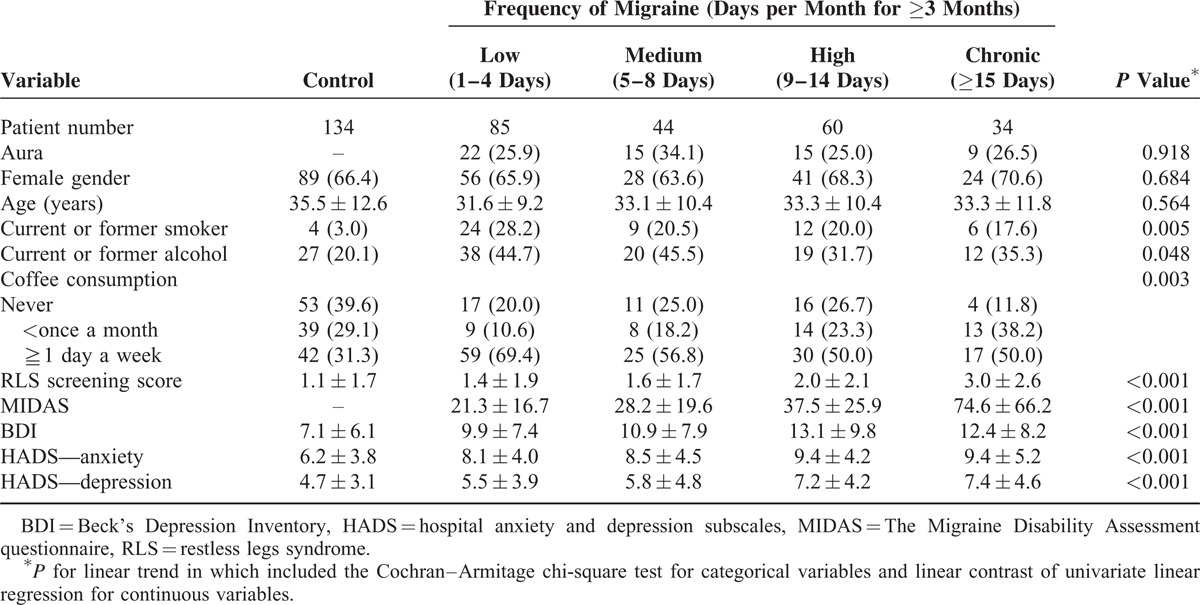
Linear trend analyses showed that greater migraine frequency correlated positively with MIDAS as well as higher levels of RLS screening scores, BDI indication of depression, HADS indication of anxiety, and HADS indication of depression (Table 1). Additionally, we found that subjects in the control group were less likely to be smokers, less likely to drink alcohol, and less likely to consume coffee than subjects in the migraine groups (Table 2). Gender ratio, age, BMI, and education level did not differ across the migraine frequency groups overall, in the with-aura subgroup, or in the without-aura subgroup (data regarding BMI and education level are not shown).
TABLE 2.
Sleep Quality in Migraine-Free Controls and in Migraine Frequency Groups (N = 357)
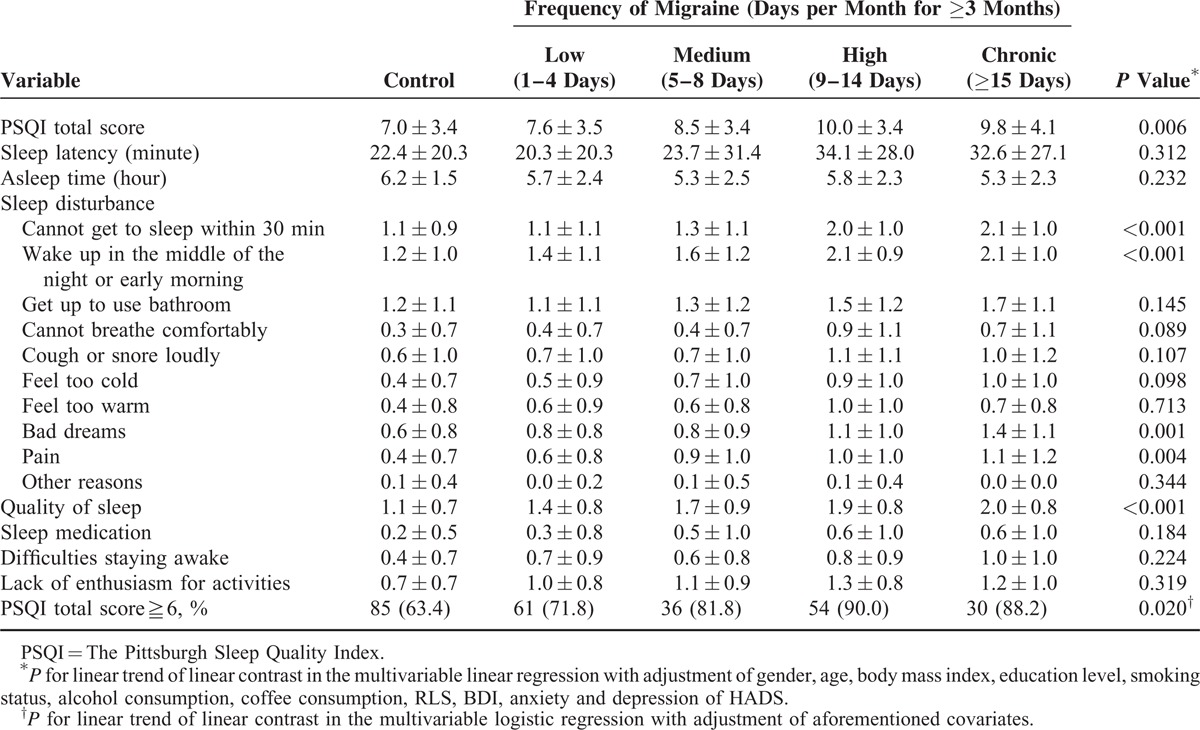
Effect of Migraine Frequency on Sleep Quality
We next examined the influence of migraine frequency on PSQI scores. As shown in Table 2, greater migraine frequency was associated generally with greater PSQI total score (P = 0.006) with adjustment of baseline characteristics. The high and chronic frequency groups had similar PSQI scores (Figure 1A). Regarding particular PSQI items, migraine frequency had an independent effect on the items “Cannot get to sleep within 30 minutes,” “Wake up in the middle of the night or early morning,” “Bad dreams,” “Pain,” and “Quality of sleep,” with the trend leveling off between the high and chronic frequency groups (Table 2). Our data showed that greater migraine frequency was associated with a higher prevalence of poor sleep quality (P = 0.020). Like PSQI scores, the prevalence of poor sleep quality was similar between the high and chronic migraine frequency groups.
FIGURE 1.
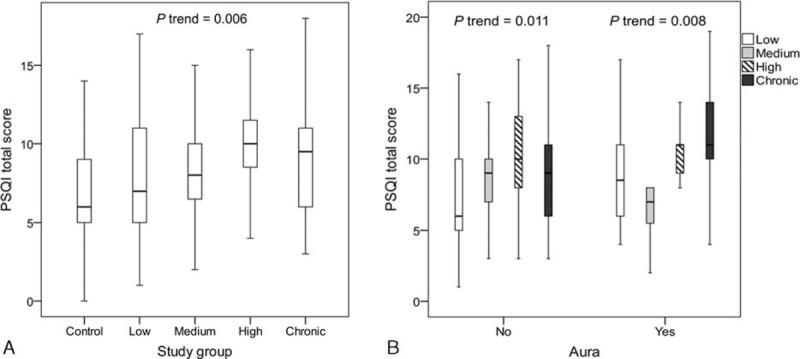
(A) Median and interquartiles of PSQI total score across the study groups. (B) Median and interquartiles of PSQI total score stratified by aura in the migraine frequency groups. PSQI = The Pittsburgh Sleep Quality Index.
Independent Factor of Poor Sleep Quality
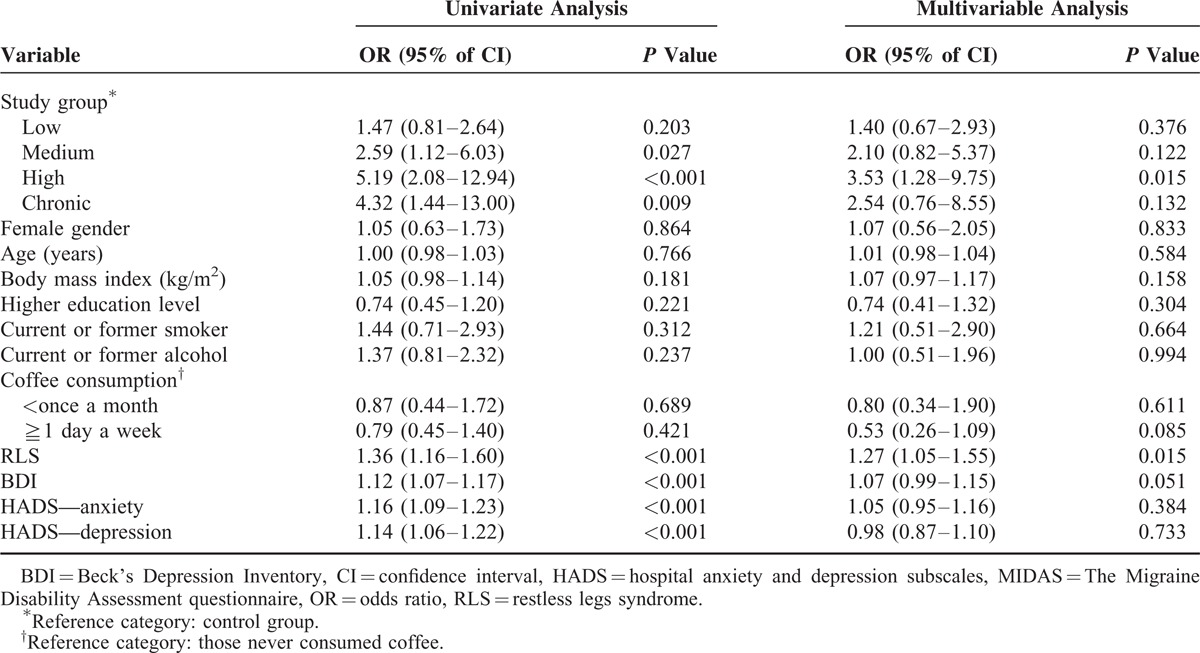
The univariate and multivariable analysis results regarding our investigation of migraine frequency as an independent factor of poor sleep quality (PSQI total score ≥ 6) are reported in Table 3. The univariate analyses revealed that subjects in the medium, high, and chronic migraine frequency groups were more likely to have poor sleep quality than control subjects. RLS, BDI indicated depression, HADS indicated anxiety, and HADS indicated depression correlated with a higher prevalence of poor sleep quality (Table 3). However, only high migraine frequency and RLS screening scores were identified as independent factors of poor sleep quality after adjusting for baseline characteristics (Table 3).
TABLE 3.
Factors Associating With Poor Sleep Quality (PSQI total score≧6)
We performed a subgroup analysis to evaluate the effect of migraine frequency on the PSQI total score stratified by the presence of aura in the migraine groups. Linear trend analyses demonstrated that the effect of migraine frequency was observed in both the aura-present subgroup (P = 0.008) and the aura-absent subgroup (P = 0.011) (Figure 1B).
DISCUSSION
In this study, we found that quality of sleep was associated with migraine frequency, comparing 4 migraine groups differentiated based on the number of days with migraine per month and an age- and gender-matched healthy control group. The PSQI total score was strongly associated with migraine frequency after adjustment for well-known confounding factors, such as gender, age, BMI, education level, smoking status, alcohol consumption, coffee consumption, RLS screening scores, BDI score, HADS anxiety score, and HADS depression score. Moreover, sleep quality (as indicated by the PSQI) correlated strongly with migraine frequency regardless of whether patients had migraine with aura or without aura. Overall, PSQI total scores were highest for the high and chronic frequency groups (i.e., ≥8 days/month of migraine headaches), and sleep quality was poorest in subjects with chronic migraine. In addition, these patients most often reported sleep disturbances related to the PSQI items “cannot get to sleep within 30 minutes,” “wake up in the middle of the night or early morning,” “bad dreams,” and “pain.”
According to the IHS classification and ICHD-III beta criteria, migraine can be classified as chronic migraine (attacks on ≥15 days/month and for >3 months) or episodic migraine (others that do not fulfill the criteria for chronic migraine).18 A previous study divided migraineurs into 3 subgroups based on the number of days with migraine attacks per month (1–4 days/month, 5–7 days/month, ≥8 days/month). The authors found that sleep quality was particularly poor in patients with 8 or more migraine days per month.7 However, the possible relationship between sleep quality and more detailed differentiation of migraine frequency (such as 9–14 days/month, ≥15 days/month) remained uncertain. Therefore, we designed this study comparing 4 migraine groups with more detailed differentiation based on the number of days with migraine per month and an age- and gender-matched healthy control group to investigate the relationship between migraine frequency and sleep quality. Indeed, our study demonstrates subgroup differences and correlations in migraine frequency and sleep quality.
Unlike previous studies in the literature, our study had a control group and 4 migraine subgroups differentiated according to migraine frequency. Our study was designed in consideration of anxiety, depression, and RLS as potential confounding factors. Similar to our results, Seidel and colleagues 7 found that PSQI scores and poor subjective quality of sleep were higher in migraineurs than in controls. Additionally, our study revealed that migraineurs’ sleep disturbances are attributed to “go to sleep within 30 minutes,” “wake up in the middle of the night or early morning,” and “bad dreams.” Consistent with our findings, Karthik et al found a high prevalence of poor sleepers among patients with “migraine without aura,” who reported difficulty in sleep initiation, sleep maintenance, and early morning waking.9 Furthermore, our subgroup of migraine with aura also had similar findings.
Kelman and Reins4 found that migraineurs have difficulty staying sleep and are prone to waking up with headaches (71% of migraineurs) early in the morning. Similarly, the migraine group subjects in our study reported “wake up in the middle of the night or early morning.”
Migraine has many comorbid disorders, the most common of which are depression and anxiety, which have been reported to occur in 63.8% and 60.4% of migraine patients, respectively.24 Here, we found that high BDI, HADS anxiety, and HADS depression scores were associated with higher migraine frequency (linear trends). Schürks et al showed that RLS prevalence in migraine ranged from 8.7% to 39.0% and identified RLS as an important comorbidity of migraine.25 Additionally, results from a prior systematic review and a recent longitudinal cohort study suggest that migraine is associated with an increased probability of RLS.25,26 Similarly, we observed a strong positive association between RLS screening score and greater migraine frequency. Hence, our findings support the notion that migraine frequency correlates with anxiety, depression, and the possibility of RLS.
In our study, smokers, alcohol drinkers, and coffee drinkers tended to have higher migraine frequency, consistent with a previous study.27 These findings are consistent with the possibility that smoking, alcohol consumption, and coffee consumption may be important triggers of migraine attacks. Additionally, in this study, high percentages of nonsmokers and nondrinkers were found; this may be due to cultural differences.28 Our univariate factor analysis indicated that subjects with medium, high, and chronic migraine frequency were more likely to have poor sleep quality than control subjects. Meanwhile, high RLS screening scores, BDI, HADS anxiety, and HADS depression scores also correlated with a higher prevalence of poor sleep quality. Subsequent multivariate factor analysis indicated that high migraine frequency and RLS are independent predictors of poor sleep quality after adjusting for baseline characteristics.
Sleep and migraine may link in a bidirectional way and share some pathophysiological mechanisms. First, regarding the mechanism of impact of sleep disturbances on headache, previous experimental studies have shown that sleep deprivation increases self-reported pain.29 Additionally, sleep deprivation may lead to a disturbance of the descending pain inhibitory control system.30,31 In addition, deficiency of serotonin descending pain inhibitory was suggested to be associated with migraine pathophysiology.32 Second, regarding the mechanism of impact of headaches on sleep, chronic pain may lead to alterations of neuron activity in the raphe magnus, which can regulate the sleep cycle.33 Therefore, this type of alteration may have an influence on sleep. Third, migraine and sleep disorders may share some pathophysiological mechanisms. The pathophysiology of migraine includes cortical spreading depression, activation and sensitization of the trigeminovascular system, and excitatory-inhibitory imbalance of the dura, brainstem, cortex, and subcortical regions.34–36 Furthermore, a prior review suggested that the hypothalamic orexinergic system plays a role in the association between sleep and the development of a migraine headache.37 Orexin-containing neurons in the hypothalamus fire in wakeful states, and disruption of orexinergic signaling results in excessive sleepiness. Orexinergic cells affect not only monoaminergic activity across the sleep cycle, but also pain modulation. Meanwhile, orexin may affect trigeminovascular tone. Furthermore, migraine attacks can be triggered by stress, fatigue, sleep deprivation, or poor sleep habits, which activate the hypothalamus and orexin system simultaneously. The pineal gland synthesizes and secretes melatonin, which is stimulated by darkness and inhibited by light in a 24-hour circadian pattern.38 Low urinary melatonin and 6-sulfatoxymelatonin levels have been associated with migraine.39,40 The melatonin level may not only play a role in the pathophysiology of migraine, but may also predispose people to awakening from rapid eye movement (REM) sleep with a headache.41 Collectively, these evidences suggest a bidirectional relationship between sleep and migraine and sharing of some pathophysiological mechanisms.
Interestingly, the high subscores for the PSQI items “cannot get to sleep within 30 minutes,” “wake up in the middle of the night or early morning,” and “bad dreams” in our higher migraine frequency groups may be related to the fact that dreams, including nightmares, occur during REM sleep. Hsu et al found that waking up with a migraine attack occurred most often during REM sleep.42 Indeed, REM sleep disruption is suggested to be an underlying mechanism of bad dreams, untimely waking, and nocturnal migraine attacks.13,43,44 Our findings are in agreement with Vgontzas et al45 who reported 48.8% of migraineurs complain of sleeping difficulties. In this study, many of the patients would “wake up in the middle of the night or early morning.” A previous study found that most patients with hypnic headaches have a history of migraine or coexisting migraine.46 Additionally, it has been reported that migraine is a sleep-related headache and that migraine-related symptoms may disturb sleep quality. In our study, although we carefully ruled out the presence of hypnic headache, “Waking up in the middle of the night or early morning” may be one of the sleep-related symptoms of migraine. Further studies are needed to verify this hypothesis.
In the present study, we found that sleep quality was associated with migraine frequency in adults. It has been also reported that migraine may lead to nonrapid eye movement (NREM) parasomnias and sleep-related movement disorders in children.47 Furthermore, previous studies showed that migraine may disturb sleep architecture, with excessive daytime sleepiness, higher prevalence of cosleeping with family, and disorders in initiating and maintaining sleep in developmental age.48–52 Additionally, migraines may have some impacts on perceptual organizational ability,53 mood,54 and motor coordination55 in school-aged children. Collectively, the interrelationship and influences between migraine and sleep may not only occur in adults but also in adolescents and children.
The strengths of this study are the controlled study design, the large number of subjects, the differentiated subgroups, the use of validated questionnaires, our consideration of comorbid anxiety, depression and restless leg syndrome, our analysis of migraine subgroups (with or without aura), the similarity of the study groups regarding demographics, and our robust statistical analysis.
Notwithstanding, several limitations of the study must be taken into consideration. First, we used a cross-sectional design, which restricts the causal inference between migraine and sleep quality. Second, our study population consists of patients who visited the Tri-service General Hospital outpatient department, thereby limiting the broad generalizability of the findings. Third, subjective sleep quality and habits were evaluated with the self-rated PSQI scoring system; we did not measure sleep with an objective assessment. Regarding, PSQI scores, it should also be mentioned that the mean PSQI score for the control group was 7.0 ± 3.4 (poor sleepers, 63.4%), which is higher than that from a previous cross-sectional study in Austria7, but similar to data obtained for premenopausal women in Taiwan (6.1 ± 2.2; poor sleepers, 60.8%).56 It could be that PSQI scores differ between Asian and European subjects. Fourth, the RLS screening score in Table 1 can only suggest the probability of an RLS diagnosis rather than RLS severity. Further studies are warranted with use of the IRLS to clarify the relationship between RLS severity and sleep quality among migraineurs.57 Fifth, migraines often present with prodromal symptoms including sleepiness and postdromal symptoms including fatigue.58,59 This would potentially confound the outcome in this study. However, these detailed prodrome or postdrome symptoms were unavailable in this study. Further studies are warranted to establish the potential relationship between premonitory/ postdromal symptoms and sleep quality. Lastly, the chronic frequency group was relatively small. A future study for recruitment of more chronic migraine patients is warranted.
In conclusion, poor sleep quality and a higher prevalence of poor sleepers are associated with greater migraine frequency regardless of auras. High migraine frequency and RLS emerged as independent factors of poor sleep quality. These findings may have clinical implications. In particular, pharmacological episodic migraine prevention may reduce the tendency for migraine sufferers to become poor sleepers.
Footnotes
Abbreviations: BDI = Beck's Depression Inventory, BMI = body mass index, HADS = hospital anxiety and depression subscales, ICHD-III beta = international classification of headache disorders, 3rd Edition (beta version), MIDAS = The Migraine Disability Assessment questionnaire, NREM = nonrapid eye movement, PSQI = The Pittsburgh Sleep Quality Index, REM = rapid eye movement, RLS = restless legs syndrome
Author contributions: all authors have contributed substantially to and are in agreement with the content of the manuscript. Conception/design: Y-KL and F-CY; provision of study materials: F-CY; collection and/or assembly of data: all authors; data analysis and interpretation: all authors; manuscript preparation: all authors; final approval of manuscript: all authors. The guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article: F-CY.
Funding: this study was supported in part by grants from Tri-Service General Hospital (TSGH-C101-159).The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Vos T, Barber RM, Bell B, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leonardi M, Raggi A. Burden of migraine: international perspectives. Neurol Sci 2013; 34 Suppl 1:S117–S118. [DOI] [PubMed] [Google Scholar]
- 3.Zeitlhofer J, Schmeiser-Rieder A, Tribl G, et al. Sleep and quality of life in the Austrian population. Acta Neurol Scand 2000; 102:249–257. [DOI] [PubMed] [Google Scholar]
- 4.Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache 2005; 45:904–910. [DOI] [PubMed] [Google Scholar]
- 5.Alberti A. Headache and sleep. Sleep Med Rev 2006; 10:431–437. [DOI] [PubMed] [Google Scholar]
- 6.Sahota P. Morning headaches in patients with sleep disorders. Sleep Med 2003; 4:377. [DOI] [PubMed] [Google Scholar]
- 7.Seidel S, Hartl T, Weber M, et al. Quality of sleep, fatigue and daytime sleepiness in migraine—a controlled study. Cephalalgia 2009; 29:662–669. [DOI] [PubMed] [Google Scholar]
- 8.Sadeghniiat K, Rajabzadeh A, Ghajarzadeh M, et al. Sleep quality and depression among patients with migraine. Acta Med Iran 2013; 51:784–788. [PubMed] [Google Scholar]
- 9.Karthik N, Kulkarni GB, Taly AB, et al. Sleep disturbances in ’migraine without aura’—a questionnaire based study. J Neurol Sci 2012; 321:73–76. [DOI] [PubMed] [Google Scholar]
- 10.Morgan I, Eguia F, Gelaye B, et al. Sleep disturbances and quality of life in Sub-Saharan African migraineurs. J Headache Pain 2015; 16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu C, Frederick IO, Sorensen T, et al. Sleep disturbances among pregnant women with history of migraines: a cross-sectional study. Cephalalgia 2015; 35:1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z, Fan X, Li X, et al. Prevalence and predictive factors for poor sleep quality among migraineurs in a tertiary hospital headache clinic. Acta Neurol Belg 2013; 113:229–235. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen BK. Migraine and tension-type headache in a general population: precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain 1993; 53:65–72. [DOI] [PubMed] [Google Scholar]
- 14.Odegard SS, Sand T, Engstrom M, et al. The long-term effect of insomnia on primary headaches: a prospective population-based cohort study (HUNT-2 and HUNT-3). Headache 2011; 51:570–580. [DOI] [PubMed] [Google Scholar]
- 15.Odegard SS, Sand T, Engstrom M, et al. The impact of headache and chronic musculoskeletal complaints on the risk of insomnia: longitudinal data from the Nord-Trondelag health study. J Headache Pain 2013; 14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhlig BL, Engstrom M, Odegard SS, et al. Headache and insomnia in population-based epidemiological studies. Cephalalgia 2014; 34:745–751. [DOI] [PubMed] [Google Scholar]
- 17.Lund N, Westergaard ML, Barloese M, et al. Epidemiology of concurrent headache and sleep problems in Denmark. Cephalalgia 2014; 34:833–845. [DOI] [PubMed] [Google Scholar]
- 18.Olesen J. ICHD-3 beta is published. Use it immediately. Cephalalgia 2013; 33:627–628. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, Monk TH, 3rd, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28:193–213. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67:361–370. [DOI] [PubMed] [Google Scholar]
- 22.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52:69–77. [DOI] [PubMed] [Google Scholar]
- 23.Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 2001; 56:S20–28. [DOI] [PubMed] [Google Scholar]
- 24.Malone CD, Bhowmick A, Wachholtz AB. Migraine: treatments, comorbidities, and quality of life, in the USA. J Pain Res 2015; 8:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schurks M, Winter A, Berger K, et al. Migraine and restless legs syndrome: a systematic review. Cephalalgia 2014; 34:777–794. [DOI] [PubMed] [Google Scholar]
- 26.Yang FC, Lin TY, Chen HJ, et al. Increased risk of restless legs syndrome in patients with migraine: a nationwide population-based cohort study. Medicine (Baltimore) 2016; 95:e2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ierusalimschy R, Moreira Filho PF. Precipitating factors of migraine attacks in patients with migraine without aura. Arq Neuropsiquiatr 2002; 60:609–613. [PubMed] [Google Scholar]
- 28.Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 2014; 311:183–192. [DOI] [PubMed] [Google Scholar]
- 29.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev 2006; 10:357–369. [DOI] [PubMed] [Google Scholar]
- 30.Tomim DH, Pontarolla FM, Bertolini JF, et al. The pronociceptive effect of paradoxical sleep deprivation in rats: evidence for a role of descending pain modulation mechanisms. Mol Neurobiol 2016; 53:1706–1717. [DOI] [PubMed] [Google Scholar]
- 31.Schrimpf M, Liegl G, Boeckle M, et al. The effect of sleep deprivation on pain perception in healthy subjects: a meta-analysis. Sleep Med 2015; 16:1313–1320. [DOI] [PubMed] [Google Scholar]
- 32.Panconesi A. Serotonin and migraine: a reconsideration of the central theory. J Headache Pain 2008; 9:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foo H, Mason P. Brainstem modulation of pain during sleep and waking. Sleep Med Rev 2003; 7:145–154. [DOI] [PubMed] [Google Scholar]
- 34.Murinova N, Krashin DL, Lucas S. Vascular risk in migraineurs: interaction of endothelial and cortical excitability factors. Headache 2014; 54:583–590. [DOI] [PubMed] [Google Scholar]
- 35.Vecchia D, Pietrobon D. Migraine: a disorder of brain excitatory-inhibitory balance? Trends Neurosci 2012; 35:507–520. [DOI] [PubMed] [Google Scholar]
- 36.Harnod T, Wang YC, Kao CH. Higher risk of developing a subsequent migraine in adults with nonapnea sleep disorders: a nationwide population-based cohort study. Eur J Intern Med 2015; 26:232–236. [DOI] [PubMed] [Google Scholar]
- 37.Holland PR. Headache and sleep: shared pathophysiological mechanisms. Cephalalgia 2014; 34:725–744. [DOI] [PubMed] [Google Scholar]
- 38.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005; 437:1257–1263. [DOI] [PubMed] [Google Scholar]
- 39.Claustrat B, Loisy C, Brun J, et al. Nocturnal plasma melatonin levels in migraine: a preliminary report. Headache 1989; 29:242–245. [DOI] [PubMed] [Google Scholar]
- 40.Murialdo G, Fonzi S, Costelli P, et al. Urinary melatonin excretion throughout the ovarian cycle in menstrually related migraine. Cephalalgia 1994; 14:205–209. [DOI] [PubMed] [Google Scholar]
- 41.Bruera O, Sances G, Leston J, et al. Plasma melatonin pattern in chronic and episodic headaches: evaluation during sleep and waking. Funct Neurol 2008; 23:77–81. [PubMed] [Google Scholar]
- 42.Hsu LK, Crisp AH, Kalucy RS, et al. Early morning migraine. Nocturnal plasma levels of catecholamines, tryptophan, glucose, and free fatty acids and sleep encephalographs. Lancet 1977; 1:447–451. [DOI] [PubMed] [Google Scholar]
- 43.Fox AW, Davis RL. Migraine chronobiology. Headache 1998; 38:436–441. [DOI] [PubMed] [Google Scholar]
- 44.Dexter JD, Weitzman ED. The relationship of nocturnal headaches to sleep stage patterns. Neurology 1970; 20:513–518. [DOI] [PubMed] [Google Scholar]
- 45.Vgontzas A, Cui L, Merikangas KR. Are sleep difficulties associated with migraine attributable to anxiety and depression? Headache 2008; 48:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz M, Mulero P, Pedraza MI, et al. From wakefulness to sleep: migraine and hypnic headache association in a series of 23 patients. Headache 2015; 55:167–173. [DOI] [PubMed] [Google Scholar]
- 47.Esposito M, Parisi P, Miano S, et al. Migraine and periodic limb movement disorders in sleep in children: a preliminary case-control study. J Headache Pain 2013; 14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esposito M, Roccella M, Parisi L, et al. Hypersomnia in children affected by migraine without aura: a questionnaire-based case-control study. Neuropsychiatr Dis Treat 2013; 9:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carotenuto M, Esposito M, Precenzano F, et al. Cosleeping in childhood migraine. Minerva Pediatr 2011; 63:105–109. [PubMed] [Google Scholar]
- 50.Carotenuto M, Guidetti V, Ruju F, et al. Headache disorders as risk factors for sleep disturbances in school aged children. J Headache Pain 2005; 6:268–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guidetti V, Dosi C, Bruni O. The relationship between sleep and headache in children: implications for treatment. Cephalalgia 2014; 34:767–776. [DOI] [PubMed] [Google Scholar]
- 52.Bruni O, Russo PM, Ferri R, et al. Relationships between headache and sleep in a non-clinical population of children and adolescents. Sleep Med 2008; 9:542–548. [DOI] [PubMed] [Google Scholar]
- 53.Esposito M, Pascotto A, Gallai B, et al. Can headache impair intellectual abilities in children? An observational study. Neuropsychiatr Dis Treat 2012; 8:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellini B, Arruda M, Cescut A, et al. Headache and comorbidity in children and adolescents. J Headache Pain 2013; 14:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esposito M, Verrotti A, Gimigliano F, et al. Motor coordination impairment and migraine in children: a new comorbidity? Eur J Pediatr 2012; 171:1599–1604. [DOI] [PubMed] [Google Scholar]
- 56.Hung HC, Lu FH, Ou HY, et al. Menopause is associated with self-reported poor sleep quality in women without vasomotor symptoms. Menopause 2014; 21:834–839. [DOI] [PubMed] [Google Scholar]
- 57.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 2003; 4:121–132. [DOI] [PubMed] [Google Scholar]
- 58.Kelman L. The premonitory symptoms (prodrome): a tertiary care study of 893 migraineurs. Headache 2004; 44:865–872. [DOI] [PubMed] [Google Scholar]
- 59.Kelman L. The postdrome of the acute migraine attack. Cephalalgia 2006; 26:214–220. [DOI] [PubMed] [Google Scholar]


