Abstract
The etiology of sudden sensorineural hearing loss (SSNHL) remains unclear in most cases. This study aimed to assess abnormal magnetic resonance imaging (MRI) findings in patients with SSNHL and evaluate the value of MRI in identifying the cause of SSNHL.
A retrospective analysis of the charts and MRI findings of 291 patients with SSNHL was performed.
In 291 patients, MRI abnormality, which was considered a cause of SSNHL, was detected in 13 patients. Vestibular schwannoma involving the internal auditory canal (IAC) and/or cerebellopontine angle was observed in 9 patients. All 9 patients had intrameatal tumors, and 6 of the 9 patients displayed extrameatal extension of their tumors. The tumor was small (<1 cm) or medium-sized (1.1–2.9 cm) in these 6 patients. Intralabyrinthine schwannoma, labyrinthine hemorrhage, IAC metastasis, and a ruptured dermoid cyst were each observed in 1 patient.
The most commonly observed MRI abnormality in patients with SSNHL was vestibular schwannoma, and all of the lesions were small or medium-sized tumors involving the IAC.
INTRODUCTION
The etiology of sudden sensorineural hearing loss (SSNHL), defined as sensorineural hearing loss of at least 30 dB in 3 contiguous audiometric frequencies occurring over less than 3 days, remains unclear. Viral neurolabyrinthitis, intralabyrinthine ischemia, labyrinthine hemorrhage, and disruption of the cochlear membrane have been suggested as possible causes of SSNHL.
Magnetic resonance imaging (MRI) has been accepted as a sensitive imaging method to identify lesions in the cerebellopontine angle (CPA), internal auditory canal (IAC), and inner ear in patients with SSNHL.1 The American Academy of Otolaryngology—Head and Neck Surgery recommended that patients with SSNHL undergo audiometry and MRI of the middle and inner ear.2
In this study, we evaluated the MRI findings of 291 patients with SSNHL and assessed the frequency and characteristics of the detected abnormalities.
MATERIALS AND METHODS
A retrospective analysis of the charts and MRI findings of 291 patients with SSNHL who were admitted to the Department of Otorhinolaryngology—Head and Neck Surgery at tertiary referral hospital from January 2007 to December 2012 was performed. The patients met the clinical diagnostic criteria for SSNHL, which is defined as sensorineural hearing loss of 30 dB or more over at least 3 contiguous frequencies in pure tone audiometry (PTA) that develops within 3 days.3 All patients had a complete history and underwent a neuro-otological examination. No patient had a history of familial deafness or metabolic diseases. The mean PTA thresholds in the conversational frequencies (0.5, 1, 2, and 4 kHz) were calculated and used to define each patient's hearing level.4 MRI was performed within 10 days after the onset of sudden hearing loss in all patients, excluding 1 patient who underwent MRI 18 days after the onset of sudden hearing loss (See Results). MRI was conducted using a 1.5 or 3.0 T MRI (Signa HDx: GE healthcare, Milwaukee, WI) with a phased-array head coil. Our IAC MRI protocol included 2-mm thick T1 (TR/TE on 1.5 T and 3.0 T MRI; 600/14 ms and 800/2 ms) and T2 (TR/TE; 4000/92 ms and 4000/107 ms) weighted axial images, 3D balanced steady-state gradient echo sequence (FIESTA) for cranial nerves, and postcontrast 3D volumetric T1-weighted images (SPGR). The parameters for acquiring FIESTA data for cranial nerves on 1.5/3.0 T MRI were the following: TR, 6.1/7.28 ms; TE, 1.7/2.30 ms; FOV, 180 mm; flip angle, 65°/60°; matrix, 512 × 256; acquiring slice thickness, 1 mm; reconstructed slice thickness, 0.5 mm. Postcontrast 3D volumetric T1-weighted images (TR/TE, 8.85/3.95 ms; FOV, 180 mm; flip angle, 12°; matrix, 256 × 256; acquiring slice thickness, 1.2 mm; reconstructed slice thickness, 0.6 mm) were obtained at 1 minute after an intravenous bolus injection of a standard dose of gadobutrol (Gadovist; Schering, Berlin, Germany; 0.1 mmol/kg of body weight) through the antecubital vein using a power injector with a rate of 1 mL per second. All injections were followed by a saline flush of up to 20 mL. The scanning encompassed the region from the mastoid to the upper edge of the petrous bone. All patients were administered systemic high-dose steroids (prednisolone 1 mg/kg/day for 4 days, tapered off over the next 10 days). The Institutional Review Board of Konkuk University Medical Center approved the study (KUH1110056).
RESULTS
Among 291 patients, 153 patients were women and 138 patients were men (mean age, 45.7 years; range, 11–81 years). SSNHL was unilateral in all patients, and 275 of 291 patients complained of tinnitus on the side of SSNHL. MRI abnormality, which was considered a cause of SSNHL, was detected in 13 patients (Table 1). Other 278 patients who did not show MRI abnormality as a cause of SSNHL were finally diagnosed with idiopathic SSNHL, although some patients showed abnormal MRI findings such as “mild chronic ischemic changes in bilateral cerebral white matter” which were not considered causes of SSNHL.
TABLE 1.
Summary of Abnormal MRI Findings in Patients With SSNHL (n = 13)
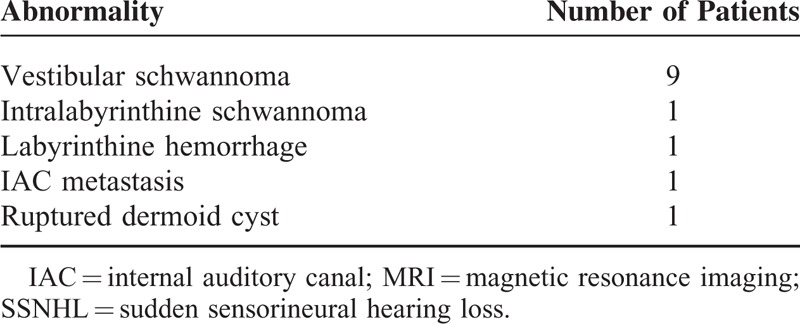
The most common finding was vestibular schwannoma involving the IAC and/or CPA (n = 9, Table 2, Figure 1). The ages of these 9 patients (5 men and 4 women) ranged from 40 to 65 years, and the right and left side were involved in 4 and 5 patients, respectively. Three patients exhibited only intrameatal tumors, and 6 patients had intrameatal tumors with medial extension out of the porous. All 6 of these patients had small (<1 cm) to medium-sized (1.1–2.9 cm) tumors. When measuring the size of the tumors, intrameatal tumors and the largest extrameatal diameter were used according to the recommendation of the Consensus Meeting on Systems for Reporting Results in Vestibular Schwannoma.4 The mean PTA threshold was 56 ± 23 dB, and the mean speech discrimination score was 63% ± 34%. Contralesional ear did not show hearing loss. The patients with serviceable hearing loss (better than 50 dB PTA average/50% speech discrimination score) were followed-up with serial audiometry and MRI imaging, which revealed no change of tumor size for 2.5 to 3.7 years. Two patients with nonserviceable hearing received gamma knife surgery, and after surgery hearing was aggravated (110 dB in patient no. 2 and 99 dB in patient no. 8). One patient displayed a well-defined nodular enhancing lesion in the left IAC fundus and basal turn of the cochlea that was compatible with intralabyrinthine schwannoma (Figure 2). This patient showed low-frequency mixed hearing loss with PTA average of 46 dB in air conduction and 35 dB in bone conduction on the left side, and contralateral side showed normal hearing. The patient did not undergo surgery, and was followed-up with serial audiometry and MRI imaging, which revealed no aggravation of hearing loss nor tumor growth over 3 years.
TABLE 2.
Clinical Characteristics of Patients With Vestibular Schwannoma Presenting With Sudden Sensorineural Hearing Loss
FIGURE 1.
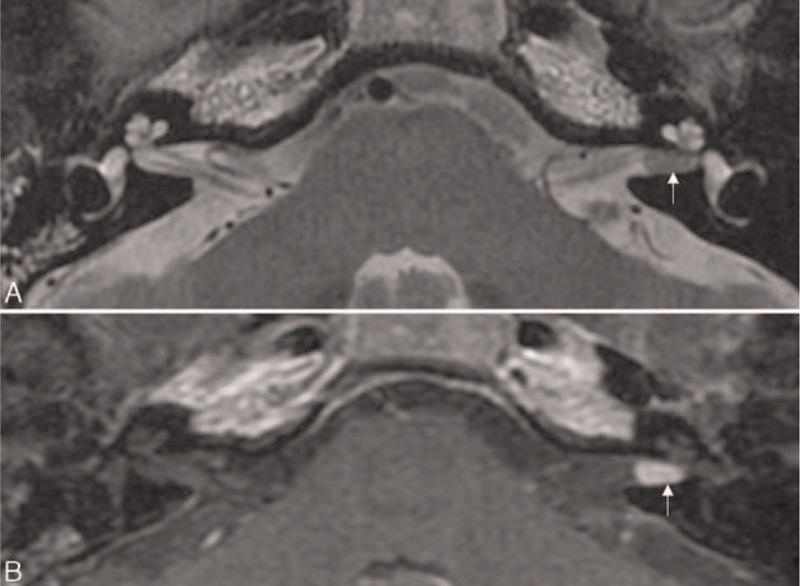
Intrameatal vestibular schwannoma. An axial T2-weighted image (A) reveals a mass with low signal intensity in the left internal auditory canal (arrow). Post-contrast axial T1-weighted image (B) demonstrates a well-enhancing mass in the left internal auditory canal (arrow).
FIGURE 2.
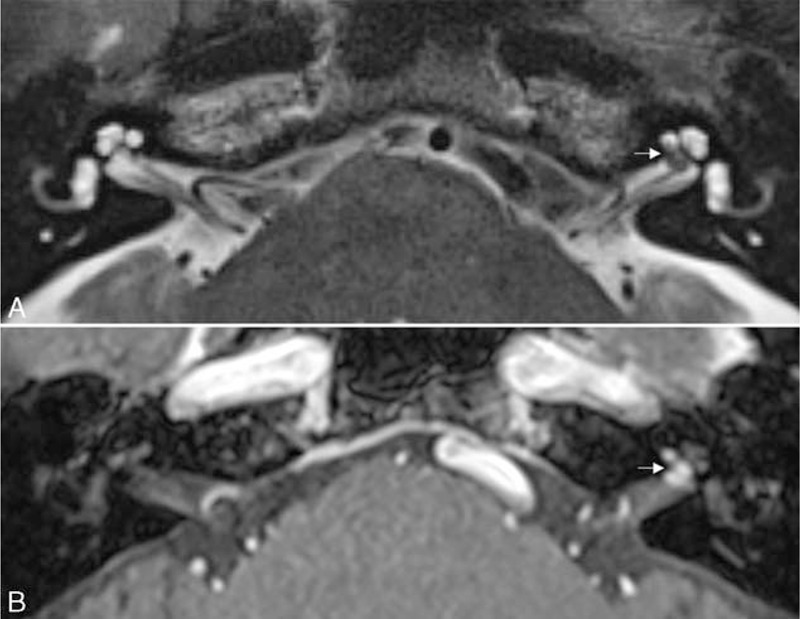
Intralabyrinthine schwannoma. (A) An axial T2-weighted image shows a hypointense filling defect in the basal turn of the left cochlea and fundus of the internal auditory canal (arrow). (B) An axial contrast-enhanced T1-weighted image demonstrates an enhancing mass in the basal turn of the left cochlea with extension into the fundus of the internal auditory canal (arrow).
In a previously healthy, 14-year-old patient, increased signal intensity was noted on precontrast T1-weighted and T2 fluid-attenuated inversion recovery (FLAIR) images in the right cochlea and vestibule (Figure 3A and D). Plain T2-weighted images revealed symmetric signal intensity in both labyrinths (Figure 3C). Contrast-enhanced T1-weighted images revealed no change in signal intensity (Figure 3B). Based on the MRI findings, a diagnosis of subacute labyrinthine hemorrhage was made. Vertigo was accompanied by SSNHL, and PTA revealed an average threshold of 106 dB on the right side. The left ear showed normal hearing level. MRI was performed 18 days after symptom onset. The right ear showed no hearing improvement after treatment using systemic corticosteroids.
FIGURE 3.
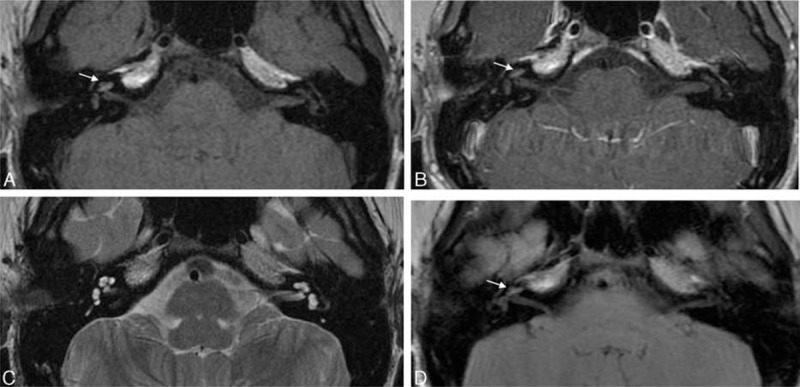
Labyrinthine hemorrhage. An axial precontrast T1-weighted image (A) reveals slightly higher signal intensity in the right labyrinth (arrow). An axial contrast-enhanced T1-weighted image (B) demonstrates no appreciable increase in the high-intensity signal in the right labyrinth (arrow). An axial T2-weighted image (C) shows symmetric signal intensity in both labyrinths. A T2 fluid-attenuated inversion recovery image (D) shows slightly increased signal intensity in the right labyrinth (arrow).
Distant metastasis into the IAC from stomach cancer was detected in a 56-year-old patient who suddenly developed total hearing loss on the right side, although hearing level on the left side was normal. MRI revealed evidence of leptomeningeal carcinomatosis with enhancing lesions in the right IAC (Figure 4). The patient received whole-brain radiotherapy and palliative chemotherapy, but the right hearing loss did not recover.
FIGURE 4.
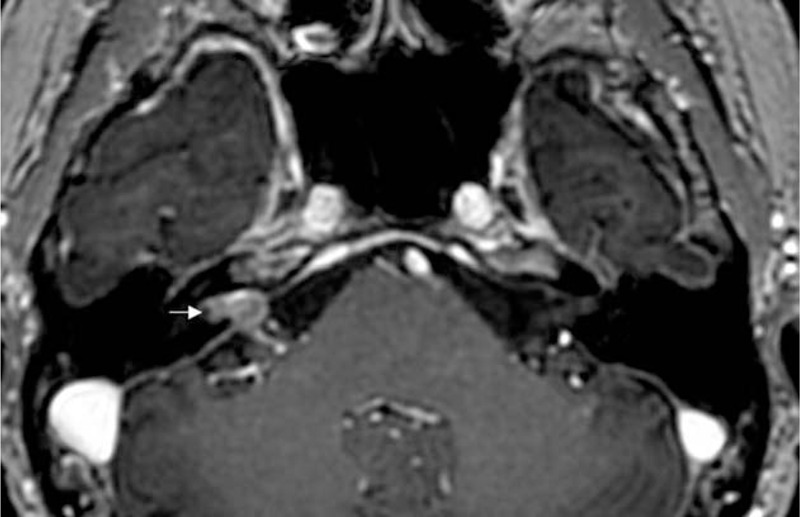
Distant metastasis into the internal auditory canal from stomach cancer. Post-contrast axial view of a 3-dimensional fast spoiled gradient-echo image reveals soft tissue mass with heterogeneous enhancement in the internal auditory canal and cerebellopontine angle (arrow).
In a 17-year-old patient with SSNHL, MRI demonstrated a mass with heterogeneous hyperintensity between the sigmoid sinus and cerebellum on both T1- (Figure 5A and B), and T2-weighted images (Figure 5C), which led to a radiological diagnosis of dermoid cyst.5 Contrast-enhanced T1-weighted images exhibited no change in signal intensity (Figure 5D). The right vestibular aqueduct was slightly dilated and filled with high-signal-intensity lesions (Figure 5A). High-resolution T1- and T2-weighted images revealed foci of high signal intensity in the cochlea and vestibule (Figures 5B–D). Based on the MRI findings, a diagnosis of a dermoid cyst that had ruptured into the endolymphatic sac and then into the cochlea and vestibule was made. Preoperative PTA average was 71 dB and speech discrimination score was 24% on the right side, and the left ear showed normal hearing level. The patient underwent surgery, and histopathologic examination uncovered keratinous debris and sebaceous glands lined by stratified keratinizing squamous epithelium consistent with the diagnosis of a dermoid cyst. Postoperative PTA average was 96 dB and speech discrimination score was 12% on the right side.
FIGURE 5.
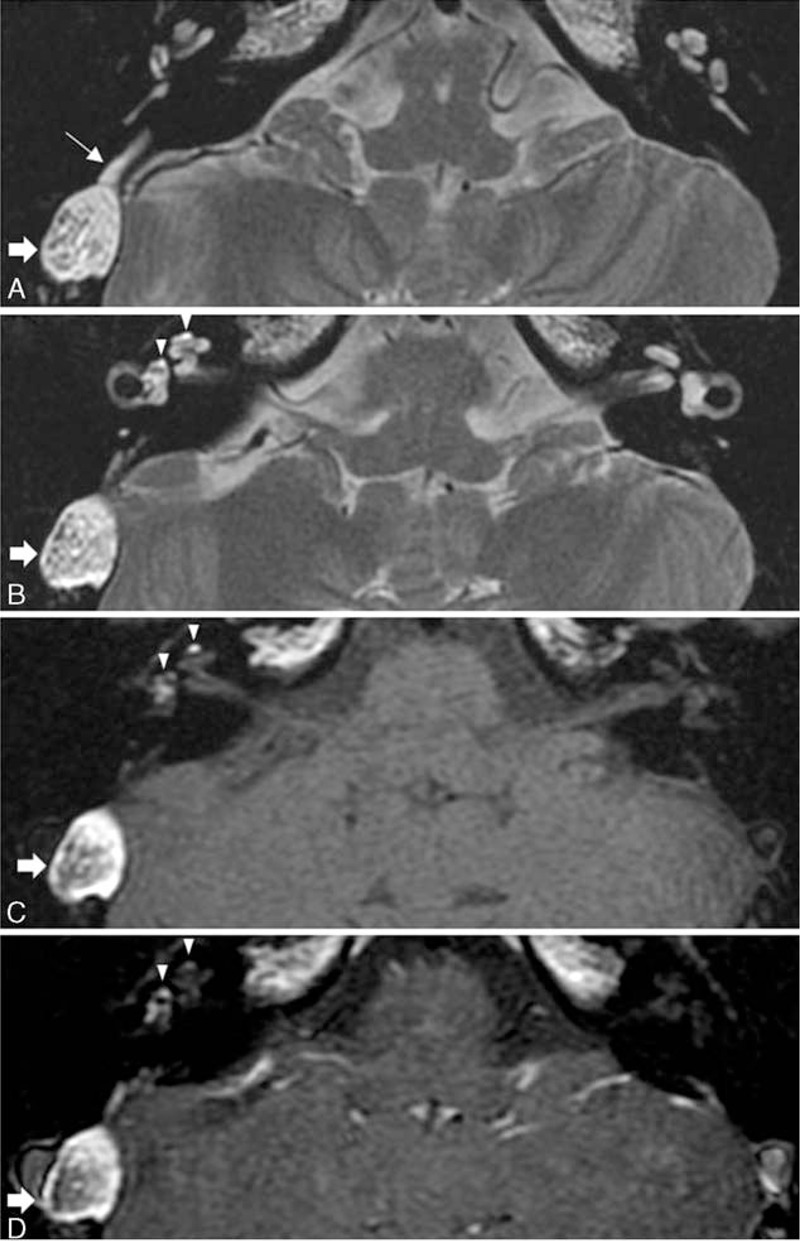
Ruptured dermoid cyst into the endolymphatic sac, cochlea, and vestibule. A mass with heterogeneous hyperintensity is located between the sigmoid sinus and cerebellum on axial T2-weighted (A and B), precontrast T1-weighted (C), and postcontrast T1-weighted (D) images (thick arrow). A T2-weighted image at the level of vestibular aqueduct (A) shows that the right vestibular aqueduct is dilated and filled with hyperintense material (thin arrow). Foci of high signal intensity (arrowheads) are demonstrated in the right cochlea and vestibule on axial T2-weighted (B), precontrast T1-weighted (C), and postcontrast T1-weighted (D) images.
DISCUSSION
The present study demonstrated that 4.5% (13 of 291) of patients with SSNHL exhibited abnormality on IAC MRI, and the most common abnormality detected in these patients was vestibular schwannoma.
Patients with vestibular schwannoma may have variable symptoms including hearing loss, dizziness, and tinnitus, and SSNHL has been reported as a presenting symptom of vestibular schwannoma.6–8 Possible mechanisms of SSNHL such as mechanical compression of the cochlear nerve, microvascular compromise in the cochlea, endolymphatic hydrops, and biochemical changes of inner ear fluids have been suggested.9,10 Although tumor size is not believed to be correlated with the severity of hearing loss,9 the correlation between tumor size and the incidence of SSNHL is controversial. It has been reported that SSNHL is more frequently encountered in small tumors (<1 cm) than in medium-sized (1.1–2.9 cm) and large tumors (>3 cm).6 However, no relationship was found between tumor size and the incidence of SSNHL in another study.11 Lateral tumors originating from or extending to the IAC have been more frequently associated with hearing loss than medial tumors,7,12 and this finding was believed to be associated with compression of the cochlear nerve because of an elevation of IAC pressure induced by the tumor. In our study, vestibular schwannoma involved the IAC in all 9 patients, and the tumors were small or medium sized, which was consistent with previous studies.7,8,12
Concerning intralabyrinthine schwannoma, 99% of patients exhibit hearing loss, and the pattern of hearing loss is variable.13 Intralabyrinthine schwannoma may present with progressive (61% of the patients), fluctuating (7% of the patients), or sudden (39% of the patients) patterns of hearing loss.14 Considering that 38% of intralabyrinthine schwannomas did not involve the cochlea or IAC even though hearing loss was observed in 99% of the patients,14 pathophysiologic mechanisms of hearing loss other than isolated neural damage have been suggested. Blockage of endolymphatic flow by a tumor may lead to endolymphatic hydrops,14 and abnormal metabolites or interruption of K+ cycling within the labyrinth may disrupt the function of hearing.15 As observed in our patient, intralabyrinthine schwannoma can display characteristic MRI findings of a lack of normal fluid density on T2-weighted imaging, with corresponding enhancement observed on gadolinium-enhanced T1-weighted scans.
Temporal bone histopathologic examination in patients with SSNHL and vertigo revealed spontaneous labyrinthine hemorrhage. A massive collection of blood was observed in the perilymphatic space, and a smaller hemorrhage was observed in the endolymphatic space.16 Endolymphatic hemorrhage and destruction of the stria vascularis were also observed in a patient with SSNHL and vertigo who died of leukemia.17 Labyrinthine hemorrhage has been previously observed in MRI studies of patients with SSNHL and vertigo.18–21 Normal inner ear structures are isointense with cerebrospinal fluid on both T1- and T2-weighted MRI images. Abnormal high-intensity signals in the inner ear organs on precontrast T1-weighted images that do not change after enhancement may indicate the presence of a labyrinthine hemorrhage. High signal intensity on precontrast T1-weighted images is likely caused by intracellular methemoglobin and an increased concentration of protein macromolecules within the endolymph and perilymph after hemorrhage.16 Intracellular methemoglobin is normally converted from deoxyhemoglobin, which is generated from oxyhemoglobin. These conversion processes are delayed by the relatively high oxygen tension in inner ear fluid. Thus, high signal intensity on precontrast T1-weighted images might become apparent after the subacute stage of a hemorrhagic event.16 High signal intensity on T2 FLAIR images is another characteristic of labyrinthine hemorrhage.22 MRI was performed in our patient with vertigo-associated SSNHL 18 days after the onset of sudden hearing loss. Because labyrinthine hemorrhage exhibits high signal intensity on T1-weighted MRI only in the subacute or chronic stage,18,20 MRI performed in the early stage may not reveal the findings of labyrinthine hemorrhage in patients with SSNHL who might have labyrinthine hemorrhage.
SSNHL caused by IAC metastasis is a rare initial presenting symptom in patients with leptomeningeal carcinomatosis. Leptomeningeal involvement occurs in approximately 5% of all patients with malignant tumors, most commonly in those with breast, lung, renal, stomach, or prostate cancer.23 Cranial nerves III, V, and VII are the most commonly affected cranial nerves in patients with leptomeningeal carcinomatosis,24 and 10% of patients have cranial nerve VIII involvement.25 Gadolinium-enhanced MRI findings of heterogeneous nodular enhancement in the IAC and CPA with leptomeningeal enhancement may facilitate a differential diagnosis.
Because dermoid cysts contain lipid metabolites, decomposed epithelial cells, cholesterol crystals, and hair, they display MRI findings similar to those of fat. Rupture of dermoid cysts, although rare, has been described, and elevation of intracystic pressure because of increased glandular secretion, which may be caused by age-dependent hormonal changes, movements of the head, or brain pulsation, were suggested as possible mechanisms.26 In our patient, a dermoid cyst was interdurally located between the dural leaflets of the sigmoid sinus and the posterior fossa, and as a dermoid cyst grows, the thin wall of the adjacent extraosseous part of the endolymphatic sac might be eroded. The foci of high signal intensity observed in the vestibular aqueduct, cochlea, and vestibule on precontrast T1-weighted MRI may indicate lipid metabolites within the endolymphatic space because of the spillage of lipid metabolites into the endolymphatic system after the rupture of a dermoid cyst, which might have disrupted endolymphatic homeostasis, leading to SSNHL.
Although the present study has certain limitations because a definitive diagnosis by histopathologic examination was not made in most patients, this study may provide various examples of MRI abnormalities that are associated with SSNHL.
CONCLUSION
Various causes of SSNHL such as vestibular schwannoma, labyrinthine hemorrhage, IAC metastasis, ruptured dermoid cyst, and intralabyrinthine schwannoma could be identified from MRI findings. The most commonly observed MRI abnormality in patients with SSNHL was vestibular schwannoma, and all of the lesions were small or medium-sized tumors involving the IAC.
Footnotes
Abbreviations: CPA = cerebellopontine angle, FLAIR = fluid-attenuated inversion recovery, IAC = internal auditory canal, MRI = magnetic resonance imaging, PTA = pure tone audiometry, SSNHL = sudden sensorineural hearing loss
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2015R1C1A1A01055849).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hegarty JL, Patel S, Fischbein N, et al. The value of enhanced magnetic resonance imaging in the evaluation of endocochlear disease. Laryngoscope 2002; 112:8–17. [DOI] [PubMed] [Google Scholar]
- 2.Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg 2012; 146:S1–35. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker S. Idiopathic sudden hearing loss. Am J Otol 1980; 1:180–183. [PubMed] [Google Scholar]
- 4.Kanzaki J, Tos M, Sanna M, et al. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol 2003; 24:642–648. [DOI] [PubMed] [Google Scholar]
- 5.Koh YC, Choi JW, Moon WJ, et al. Intracranial dermoid cyst ruptured into the membranous labyrinth causing sudden sensorineural hearing loss: CT and MR imaging findings. Am J Neuroradiol 2012; 33:E69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanagihara N, Asai M. Sudden hearing loss induced by acoustic neuroma: significance of small tumors. Laryngoscope 1993; 103:308–311. [DOI] [PubMed] [Google Scholar]
- 7.Sauvaget E, Kici S, Kania R, et al. Sudden sensorineural hearing loss as a revealing symptom of vestibular schwannoma. Acta Otolaryngol 2005; 125:592–595. [DOI] [PubMed] [Google Scholar]
- 8.Lee JD, Lee BD, Hwang SC. Vestibular schwannoma in patients with sudden sensorineural hearing loss. Skull Base 2011; 21:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pensak ML, Glasscock ME, 3rd, Josey AF, et al. Sudden hearing loss and cerebellopontine angle tumors. Laryngoscope 1985; 95:1188–1193. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y, Kanzaki J, Ogawa K. Vestibular schwannoma presenting as sudden deafness. J Laryngol Otol 2000; 114:589–592. [DOI] [PubMed] [Google Scholar]
- 11.Moffat DA, Baguley DM, von Blumenthal H, et al. Sudden deafness in vestibular schwannoma. J Laryngol Otol 1994; 108:116–119. [DOI] [PubMed] [Google Scholar]
- 12.Berrettini S, Ravecca F, Sellari-Franceschini S, et al. Acoustic neuroma: correlations between morphology and otoneurological manifestations. J Neurol Sci 1996; 144:24–33. [DOI] [PubMed] [Google Scholar]
- 13.Van Abel KM, Carlson ML, Link MJ, et al. Primary inner ear schwannomas: a case series and systematic review of the literature. Laryngoscope 2013; 123:1957–1966. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy RJ, Shelton C, Salzman KL, et al. Intralabyrinthine schwannomas: diagnosis, management, and a new classification system. Otol Neurotol 2004; 25:160–167. [DOI] [PubMed] [Google Scholar]
- 15.DeLozier HL, Gacek RR, Dana ST. Intralabyrinthine schwannoma. Ann Otol Rhinol Laryngol 1979; 88:187–191. [DOI] [PubMed] [Google Scholar]
- 16.Schuknecht HF. Vascular disorders. In: Pathology of the ear 2nd edition. Malvern, Pennsylvania, United States: Lea & Febiger; 1993. [Google Scholar]
- 17.Sando I, Egami T. Inner ear hemorrhage and endolymphatic hydrops in a leukemic patient with sudden hearing loss. Ann Otol Rhinol Laryngol 1977; 86:518–524. [DOI] [PubMed] [Google Scholar]
- 18.Vakkalanka S, Ey E, Goldenberg RA. Inner ear hemorrhage and sudden sensorineural hearing loss. Am J Otol 2000; 21:764–765. [PubMed] [Google Scholar]
- 19.Weissman JL, Curtin HD, Hirsch BE, et al. High signal from the otic labyrinth on unenhanced magnetic resonance imaging. Am J Neuroradiol 1992; 13:1183–1187. [PMC free article] [PubMed] [Google Scholar]
- 20.Naganawa S, Ishihara S, Iwano S, et al. Detection of presumed hemorrhage in the ampullar endolymph of the semicircular canal: a case report. Magn Reson Med Sci 2009; 8:187–191. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Chen K, Sun L, et al. Magnetic resonance imaging-detected inner ear hemorrhage as a potential cause of sudden sensorineural hearing loss. Am J Otolaryngol 2014; 35:318–323. [DOI] [PubMed] [Google Scholar]
- 22.Cervantes SS, Barrs DM. Sudden sensorineural hearing loss associated with intralabyrinthine hemorrhage. Otol Neurotol 2014; 36:e134–e135. [DOI] [PubMed] [Google Scholar]
- 23.Streitmann MJ, Sismanis A. Metastatic carcinoma of the temporal bone. Am J Otol 1996; 17:780–783. [PubMed] [Google Scholar]
- 24.Morgan MK, Zammit-Maempel I, Hill J. Meningeal carcinomatosis: an unusual cause of deafness. J R Coll Surg Edinb 1998; 43:119–121. [PubMed] [Google Scholar]
- 25.Alberts MC, Terrence CF. Hearing loss in carcinomatous meningitis. J Laryngol Otol 1978; 92:233–241. [DOI] [PubMed] [Google Scholar]
- 26.Liu JK, Gottfried ON, Salzman KL, et al. Ruptured intracranial dermoid cysts: clinical, radiographic, and surgical features. Neurosurgery 2008; 62:377–384. [DOI] [PubMed] [Google Scholar]


