Abstract
Nonalcoholic fatty liver disease has been suggested to be associated with alanine aminotransferase (ALT) elevation in hepatitis B virus (HBV)-infected patients with HBe antigen (HBeAg)-negativity and a low HBV-DNA level. However, few studies have evaluated the association according to histological findings of the liver.
Among a total of 198 HBV-infected patients who received a percutaneous liver biopsy, we studied the histological and laboratory findings of HBeAg-negative patients without receiving nucleoside/nucleotide analogues treatment (N = 70) in order to evaluate whether hepatic steatosis and its related metabolic disorders were associated with an elevation in ALT levels in HBeAg-negative patients.
In HBeAg-negative patients with a high serum HBV-DNA level (≥2000 IU/mL), the level of HBV-DNA was the only significant factor related to ALT elevation. However, in HBeAg-negative patients with a low HBV-DNA level, the serum ferritin level, and histologically observed hepatic steatosis were significantly associated factors with ALT elevation. When we evaluated 2 metabolic variables (serum ferritin and fasting insulin) that are suggested to be relevant to the presence of progressive disease in Japanese patients, we found that the rate of metabolic disorders was significantly higher among patients with a high ALT level and a low HBV-DNA level than it was among those with other conditions. The triglyceride level and the frequency of moderate or severe hepatic steatosis were significantly higher in patients with a low HBV-DNA level than in those with a high HBV-DNA level.
Histologically proven hepatic steatosis and its related metabolic disorders are suggested to be involved in the elevation of aminotransferases of HBeAg-negative patients, particularly those with low HBV-DNA levels.
INTRODUCTION
Hepatitis B virus (HBV)-infected patients with seroconversion from HBe antigen (HBeAg) to anti-HBe antibody (anti-HBeAb) typically show low serum HBV-DNA levels and normal serum aminotransferase levels, and achieve a favorable clinical outcome with a low risk of hepatic failure and its complications (“asymptomatic carriers”).1–5 However, HBeAg-negative patients with a low HBV-DNA level occasionally show abnormal values of aminotransferases. In these patients, liver injury could be dependent on causes other than HBV infection, and the HBV-independent elevation of aminotransferases may influence their clinical courses.2,6
Hepatic steatosis due to nonalcoholic fatty disease (NAFLD) is one of major causes of alanine aminotransferase (ALT) elevation. Although some studies have reported the potential association between hepatic steatosis and ALT elevation in HBV-infected patients,7–9 these studies included patients who showed elevated HBV-DNA levels (≥2000 IU/mL) and/or HBeAg-positivity. Therefore, the clinical significance of hepatic steatosis in HBeAg-negative patients with low HBV-DNA levels has not been sufficiently clarified.
In 2014, Spradling et al10 reported that ALT elevation in patients with a high HBV-DNA level (≥2000 IU/mL) was mainly caused by the immune response to HBV infection, while NAFLD may be an important factor that contributes to ALT elevation in patients with a low HBV-DNA level (<2000 IU/mL). However, this study investigated a cohort of Alaskan patients, and due to the limited accession available only by air, NAFLD was mainly diagnosed by a few clinical characteristics, including the body mass index (BMI), the presence of diabetes mellitus, and the absence of a history of alcoholic consumption.
In the present study, we assessed the clinical variables and histological findings of HBeAg-negative patients and studied the associations between HBV-related markers and hepatic steatosis with ALT elevation in HBeAg-negative patients. In particular, we estimated the contribution of hepatic steatosis and its related metabolic disorders to ALT elevation in HBeAg-negative patients with a low HBV-DNA level.
PATIENTS AND METHODS
Patients
In a total of 198 HBV-positive patients who had undergone a percutaneous liver biopsy between January 2008 and August 2013 at our institution, patients who fulfilled the following conditions were enrolled in the present study: HBV infection diagnosed by a positive HBs antigen (HBsAg) status for at least 6 months and blood samples obtained on the same day as the liver biopsies under the fasting condition. Patients with the following conditions were excluded from the study: the presence of hepatocellular carcinoma, immunosuppressive therapy, or hepatitis C virus (HCV) coinfection. In the present study, patients treated with nucleoside analogs were excluded because the treatment could lead to reduced ALT levels. Patients whose ALT levels were considered to be affected by alcohol intake (≥20 g/day) were also excluded. A total of 70 patients with an HBeAg-negative status (seroconversion) were enrolled, and patients were classified into “low HBV-DNA level” and “high HBV-DNA level” groups defined as HBV-DNA <2000 IU/mL (=4.07 log copies/mL determined with the TaqMan PCR method) and HBV-DNA ≥2000 IU/mL, respectively. Patients were also classified into “high ALT” and “normal ALT” groups according to the presence or absence of an elevated ALT level (≥31 IU/mL) (Figure 1).
FIGURE 1.
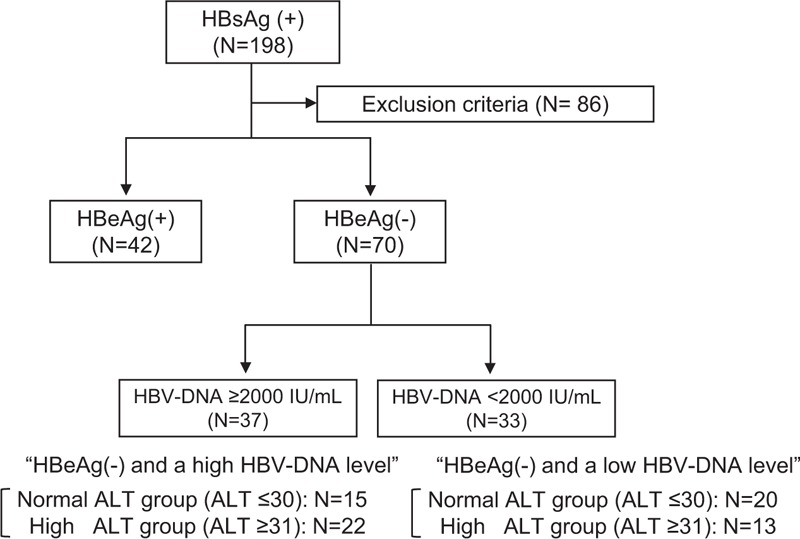
Algorithm for the classification of HBV-positive patients with a liver biopsy.
The study conformed to the ethical guidelines of the Declaration of Helsinki, and written informed consent regarding the liver biopsy and use of clinical data was obtained from all patients on admission. This study was approved by the ethics committee of the institutional review board (Nos. 1831 and Hi-92).
Liver Biopsy and Laboratory Data
General laboratory variables of HBV-infected patients, including platelet counts, the prothrombin time (PT), and liver function tests (ALT, aspartate aminotransferase (AST), alkaline phosphatase, albumin, and cholinesterase) were routinely measured. Regarding HBV-related markers, in addition to HBeAg, anti-HBeAb, and HBV-DNA, we also quantitated HBsAg, because the quantitative HBsAg level was recently reported to be clinical useful to predict the carrier status, the risk of hepatocellular carcinoma, and the probability of HBsAg loss.11
We measured the levels of the following metabolic parameters: glucose, immune reactive insulin (IRI), triglyceride, total cholesterol, and ferritin, since the serum level of ferritin (≥200 ng/mL [female]; ≥300 ng/mL [male]) and the fasting level of IRI (≥10 μU/mL) are suggested to be associated with the presence of progressive liver disease in Japanese NAFLD patients.12 We also evaluated insulin resistance by calculating the HOMA-IR (homeostatic model assessment insulin resistance: IRI × [plasma glucose]/405). All blood samples were collected on the day of liver biopsy under the fasting condition.
Liver biopsy examinations were carried out according to the standard techniques. All liver samples were evaluated with regard to the fibrosis stage and activity grade according to the METAVIR scoring system. Fibrosis was staged on a scale of F0–F4 (F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with rare septa; F3, numerous septa without cirrhosis; and F4, liver cirrhosis).13 In addition to the immune response to HBV infection, the presence of NAFLD was reported to be a cause of ALT elevation. Therefore, we histologically assessed the degree of hepatic steatosis in the present study. The histological severity of hepatic steatosis was classified as Grade 0 (<5%), Grade 1 (5–33%), Grade 2 (>33%–66%), and Grade 3 (>66%) according the report by Kleiner et al14 (Figure 2). Histological evaluations were performed by outside expert pathologists without information regarding the clinical data (Stelic Institute & Co., Inc., Tokyo, Japan).
FIGURE 2.
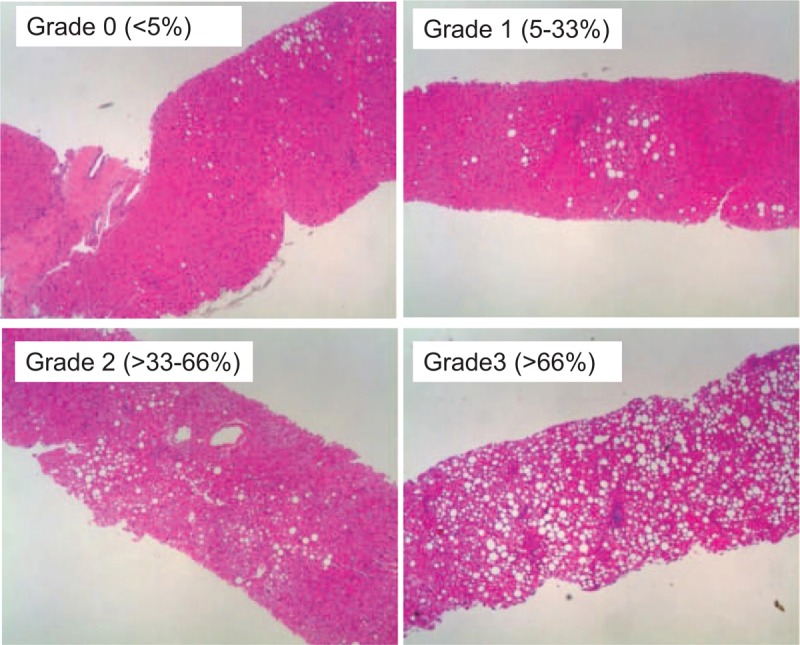
Histological findings of hepatic steatosis in HBV-positive patients. The histological degree of hepatic steatosis was classified from Grade 0 to Grade 3 according to the report by Kleiner et al,14 and representative results are shown.
Statistical Analysis
Quantitative variables are presented as the median values (range) and statistical analyses between 2 groups were evaluated using the Mann–Whitney U test. In the multivariate analysis, potential associated factors were selected (from those with a P-value <0.05) by the stepwise method and analyzed by the logistic regression model. To compare the frequency among 4 groups, the data were compared by the Chi-square test with Yates’ correction, and the group with a significantly higher or lower positive rate than the other groups was further determined by a residual analysis. A value of P < 0.05 was considered to be significant.
RESULTS
General Clinical Variables of HBeAg-Negative Patients
Among a total of 198 HBV-infected patients with a liver biopsy, 70 HBeAg-negative patients were included according to the criteria mentioned in the “Patients and Methods” section (Figure 1). The characteristics of the study population are summarized in Table 1. The population included 38 (54.2%) male patients and 32 (45.8%) female patients, and the age of the patients ranged from 28 to 77 years of age (median 49 years of age). The histological evaluation according to the METAVIR staging system showed that 14 (20.0%) patients had severe fibrosis (F3–F4) and 4 patients (4.7%) had cirrhosis (F4). All 4 cirrhotic patients had elevated ALT and high DNA levels.
TABLE 1.
The Characteristics of the HBeAg-Negative Patients
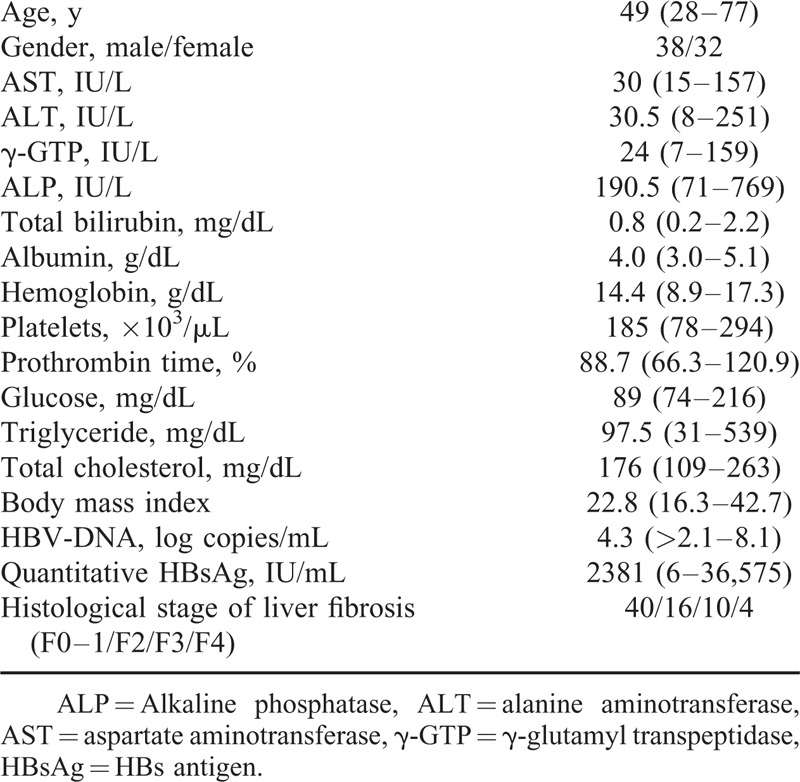
Regardless of the HBV-DNA levels, no significant difference was found in the basic laboratory variables of the patients with ALT elevation (including the aminotransferase, albumin, PT, and total bilirubin levels). Furthermore, there were no significant differences in the histological findings related to liver inflammation (Table 2). Since a recent study suggested that the immune response to HBV infection and NAFLD may be involved in ALT elevation in HBV-infected patients,10 we further investigated HBV-related markers and metabolic variables in HBeAg-negative patients.
TABLE 2.
Comparison of General Variables in HBeAg-Negative Patients With ALT Elevation Based on the HBV-DNA Levels

Clinical Data and Histological Findings of Hepatic Steatosis in HBeAg-Negative Patients With a High HBV-DNA Level
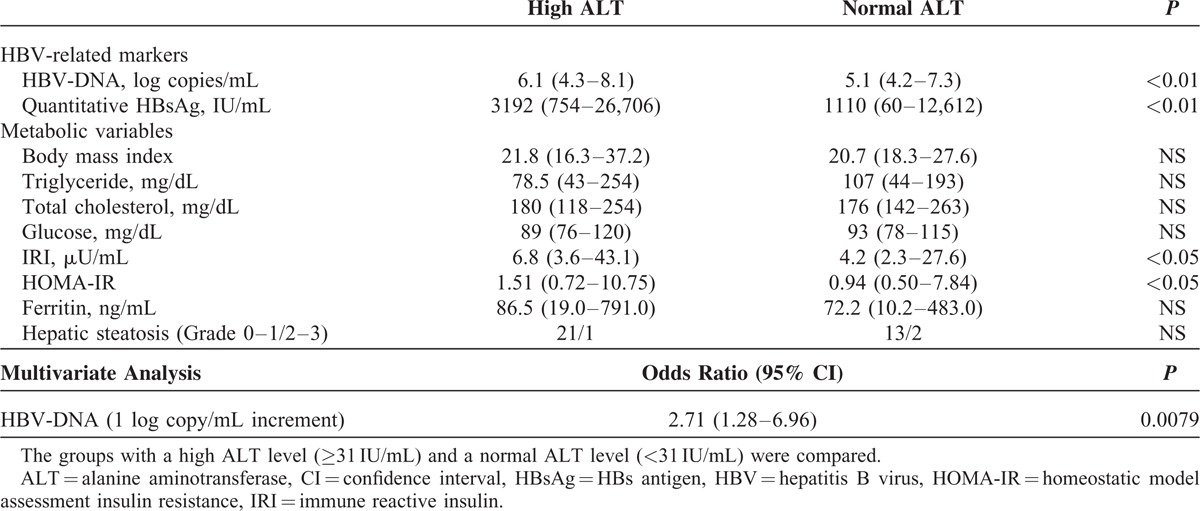
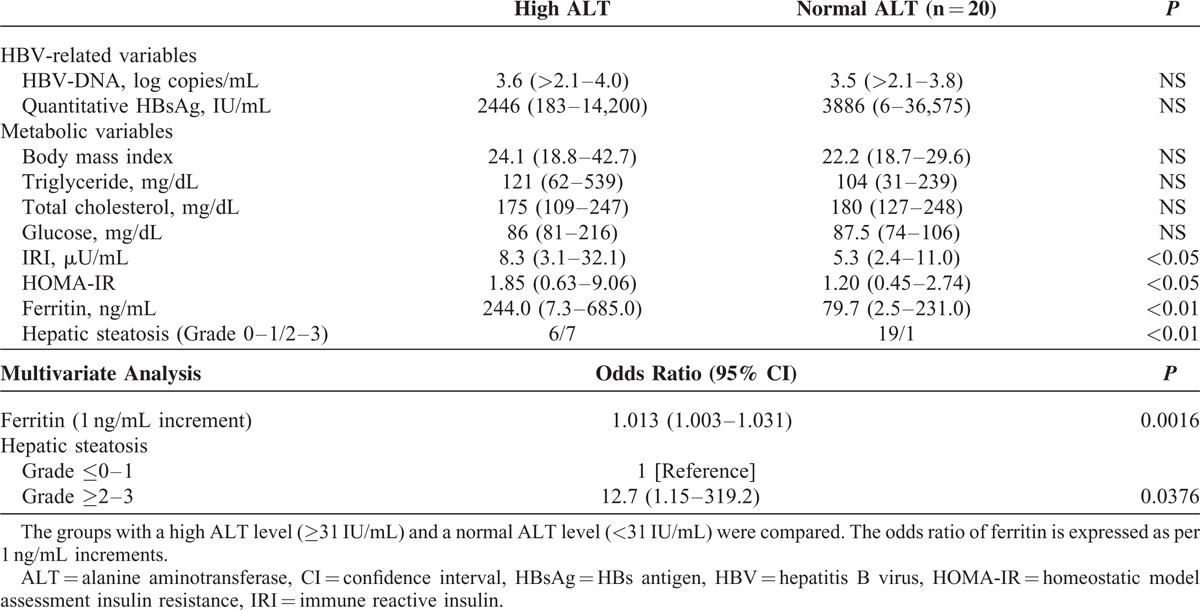
When we evaluated the HBV-related markers and metabolic variables in HBeAg-negative patients with a high DNA level (Table 3), a significant difference was found in the HBV-associated variables, including the levels of HBV-DNA and quantitative HBsAg between the 2 groups. However, the frequency of patients with moderate or severe hepatic steatosis (Grade 2 or 3) was not significantly different between the 2 groups. Although the HOMA-IR was increased with a mild significance according to a univariate analysis, a multivariate analysis showed that the level of HBV-DNA was the only significant factor associated with ALT elevation. These findings suggested that HBV infection is mainly associated with ALT elevation in HBeAg-negative patients with a high DNA level.
TABLE 3.
Comparison of HBV-Related Variables and Metabolic Variables in HBeAg-Negative Patients With a High HBV-DNA Level
Clinical Data and Histological Findings of Hepatic Steatosis in HBeAg-Negative Patients With a Low HBV-DNA Level
We next investigated the laboratory variables in HBeAg-negative patients with a low DNA level (Table 4). Unlike in patients with a high HBV-DNA level, no significant difference was found in the HBV-associated variables (the levels of HBV-DNA and quantitative HBsAg) between the 2 groups, thus suggesting that ALT elevation occurred independent of HBV infection. When we assessed the histological findings of hepatic steatosis and its related several clinical variables, the frequency of patients with moderate or severe hepatic steatosis (Grade 2 or 3) was significantly higher in patients with elevated ALT levels than in patients without. In addition, patients in the high ALT group had significantly higher levels of HOMA-IR and serum ferritin. Furthermore, the multivariate analysis showed that the histologically observed hepatic steatosis was a factor significantly associated with ALT elevation, suggesting the potential involvement of hepatic steatosis and its related metabolic disorders in ALT elevation.
TABLE 4.
Comparison of HBV-Related Variables and Metabolic Variables in HBeAg-Negative Patients With a Low HBV-DNA Level
A High Rate of Metabolic Disorders Was Observed in HBeAg-Negative Patients With a Low HBV-DNA Level
Our findings suggested that the presence of NAFLD-like hepatic steatosis and its associated metabolic disorders may contribute to the elevation of aminotransferases, particularly in HBeAg-negative patients with a low DNA level. NAFLD includes 2 types of diseases; 1 precedes a nonprogressive clinical course with a favorable prognosis, and the other is steatohepatitis which can progress to liver cirrhosis.15,16 These 2 conditions are histologically differentiated according to the presence of inflammation and/or fibrosis in the liver without hepatitis viral infection. However, the histological definition for steatohepatitis in HBV-infected patients has not been determined, thus leading to difficulty in precisely diagnosing the presence of progressive fatty liver disease. We therefore focused on two metabolic variables (serum ferritin and IRI) which have been suggested to relate to the presence of progressive liver disease in Japanese NALFD patients.12 When we investigated the whole study population, the frequency of abnormalities of at least one of these 2 metabolic variables (shown in “Patients and Methods” section) was significantly higher among patients with a high ALT level and a low HBV-DNA level than it was among those with other conditions (Figure 3). Therefore, hepatic steatosis and its related metabolic disorders with the probable association of progressive liver disease are suggested to be involved in ALT elevation in HBeAg-negative patients with a low HBV-DNA level.
FIGURE 3.
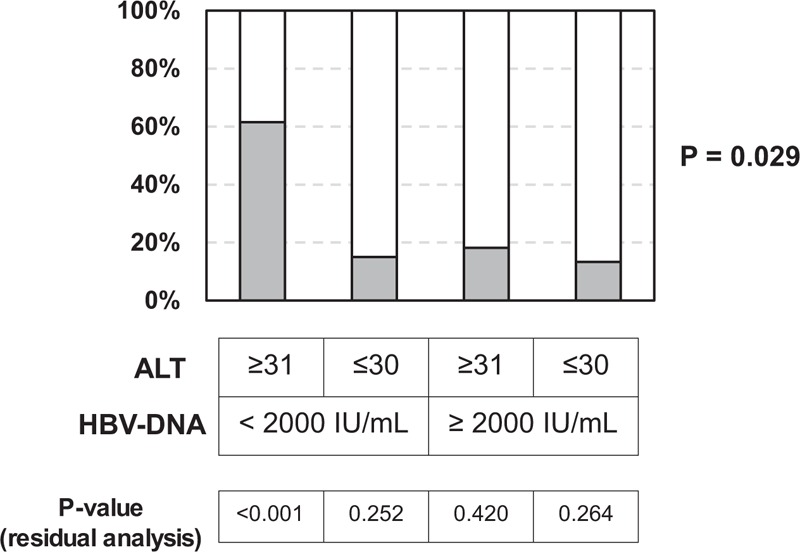
The rates of metabolic disorders among the HBeAg-negative patients. Since 2 metabolic variables (serum ferritin and IRI) are suggested to predict the presence of progressive disease in Japanese NAFLD patients,12 we focused on the levels of these variables. Gray boxes indicate the patients who had an abnormal level of at least one of the 2 metabolic variables (according to the criteria shown in the “Patients and Methods” section). The rate of metabolic disorders was significantly higher among patients with an elevated ALT level and a low HBV-DNA level than it was among the patients of the remaining three groups.
The Comparison of the Metabolic Variables in HBeAg-Negative Patients Based on HBV-DNA Levels
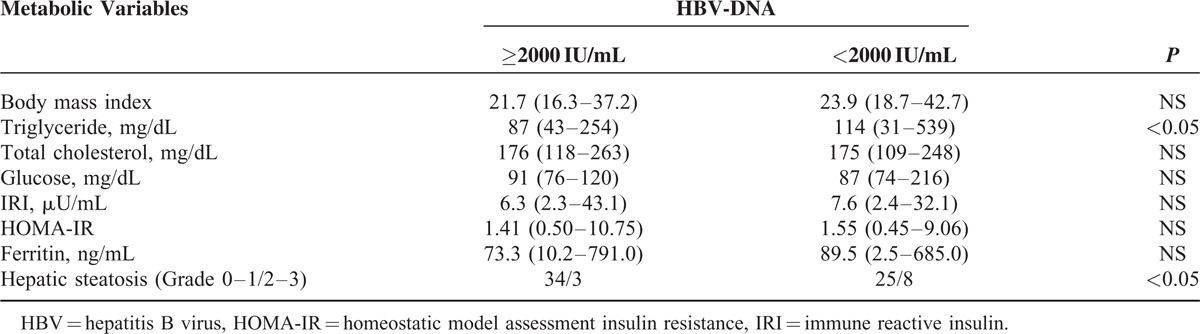
We further investigated whether the metabolic variables differed between patients with low HBV-DNA levels and those with high HBV-DNA levels. The triglyceride levels and the frequency of moderate or severe hepatic steatosis (Grade ≥2) were significantly higher among the patients with low HBV-DNA levels than among those with high HBV-DNA levels (Table 5).
TABLE 5.
Comparison of Metabolic Variables in HBeAg-Negative Patients Based on the HBV-DNA Levels
DISCUSSION
HBV infection is one of the most important etiologies of chronic liver disease worldwide. Previously, HBeAg-negative patients with low HBV-DNA levels were considered to be asymptomatic HBV carriers with presumed favorable clinical courses.1–5 Recent studies have revealed that approximately 10% to 30% of the patients may develop HBeAg-negative chronic hepatitis B (CHB) in whom HBV-DNA replicates, ALT level increases, and liver fibrosis progresses.1–3,17–19 Although HBeAg-negative CHB is an important condition that affects the natural course of HBV infection, HBeAg-negative patients with low HBV-DNA levels occasionally show the elevation of aminotransferases. We herein showed that hepatic steatosis and its related metabolic disorders may cause increased aminotransferase levels and subsequently affect the clinical courses of HBeAg-negative patients with a low DNA level.
Although previous studies reported the potential association of hepatic steatosis with ALT elevation,7–9 these studies included patients who had high HBV-DNA levels and/or HBeAg-positivity. A recent paper suggested that NAFLD can cause ALT elevation in patients with low HBV-DNA levels10; however, in this important report, due to the geographical challenge with the cohort studied, a liver biopsy was performed in 64 patients. In particular, a liver biopsy was performed only in 6 patients with normal ALT levels. We herein selected patients from a larger number of patients who received a liver biopsy (N = 198) and assessed the clinical conditions of HBeAg-negative patients not only by the laboratory variables, but also by the histological findings. We found that the presence of moderate or severe hepatic steatosis (≥Grade 2) was significantly related to the increased ALT level of HBeAg-negative patients with a low HBV-DNA level (Table 4). It remains controversial whether HBV infection itself can contribute to steatosis of the liver. Experimental studies suggest that HBV infection induces hepatic steatosis20,21; however, unlike HCV infection, recent reports suggest that HBV infection negatively correlates to dyslipidemia and hepatic steatosis.22,23 In addition, Ha et al24 showed that HBeAg positive and high levels of ALT were associated with lower prevalence of metabolic syndrome. Because the frequency of histological hepatic steatosis was low in patients with a high HBV-DNA level (Table 5), our results suggest an unlikely association between HBV infection and hepatic steatosis.
NAFLD is a major cause of chronic liver injury, and nonalcoholic steatohepatitis (NASH) is considered to lead to liver cirrhosis.25,26 Although NASH is diagnosed with a liver biopsy, it is impossible to perform a liver biopsy in all NAFLD patients. In addition, the diagnostic criteria of NASH in the presence of HBV infection have not been defined. Furthermore, it is difficult to determine precisely the contribution of NASH to the progression of liver fibrosis because HBV infection itself can cause liver fibrosis. Several excellent scoring systems for diagnosing NASH have been proposed27–32; however, Asian patients can develop NASH with a low BMI,33 and thus the applications of these scoring systems in our cohort is questionable. We therefore focused on two metabolic variables that are suggested to be associated with the presence of progressive fatty liver disease in Japanese NAFLD patients.12 Among the patients with low HBV-DNA levels, the rates of moderate or severe hepatic steatosis (Grade ≥2) were higher in the patients who showed ALT elevation (Table 4). Furthermore, the rate of metabolic disorders (elevated serum ferritin level and/or fasting insulin level) was higher among patients with elevated ALT levels and low HBV-DNA levels (Figure 3). These findings suggest that hepatic steatosis and its related metabolic disorders may be associated with increased aminotransferase levels and may cause the progression of liver disease in HBeAg-negative patients.
HBsAg loss and seroconversion to HBsAb may occur spontaneously in 1% to 3% of cases annually.1–4,34 Interestingly, HBV-infected patients with moderate to severe hepatic steatosis were reported to show a higher rate (more than 3-fold) of HBsAg seroclearance than those without hepatic steatosis.15,35 Therefore, presence of NAFLD may modulate host immunity and affect the efficiency to control viral replication and HBsAg production in the liver. Recently, several factors associated with the development of NASH (such as gene polymorphisms of the PNPLA3 gene) have been reported.36–39 If patients with HBeAg-negativity and a low DNA level have progressive fatty liver disease due to genetic characteristic(s), then these patients could be misdiagnosed with NASH-associated cirrhosis after HBsAg seroclearance. Although Asian patients can develop NASH with a low BMI, our findings show that some of the patients who have advanced liver fibrosis with hepatic steatosis could have an HBV-related fibrotic liver after HBsAg seroclearance (observed in 1–3% of HBV-infected patients per year as described above), because HBV infection is frequently observed in Asian individuals. Our findings suggest that hepatic steatosis should cause some NAFLD/NASH-like conditions with ALT elevation and may affect the clinical course of asymptomatic carriers. In addition, our results may also suggest that HBV infection can affect the diagnosis of NASH.
There are some limitations associated with the present study. Because some patients had elevated ALT levels in the absence of histological hepatic steatosis (Table 4), unrevealed causes other than hepatic steatosis may also contribute to ALT elevation in these patients. In addition, we enrolled 70 patients and classified them into four categories according to the presence or absence of AST elevation (≥31 IU/mL) and a high level of HBV-DNA (≥2000 IU/mL), resulting in small patient numbers among the groups (2 groups included less than 20 patients). Therefore, we were able to select a limited number of factors by the stepwise method to perform the multivariate analysis. Although our results suggested that histological hepatic steatosis could contribute to ALT elevation in HBeAg-negative patients with a low DNA level and may affect the natural history of asymptomatic carriers of HBV infection, we cannot assess the clinical course and prognosis of patients with a high ALT and a low HBV-DNA level, because most of these patients (10/13) received a liver biopsy in the past 5 years. Further studies with a larger number of patients and a long-term follow-up are important to confirm our present findings.
In summary, we showed histologically-proven hepatic steatosis could be involved in the increased aminotransferase levels in HBeAg-negative patients with a low HBV-DNA level. Hepatic steatosis and its related metabolic disorders may contribute to the progression of chronic liver disease in HBeAg-negative asymptomatic HBV carriers.
Acknowledgment
We thank N. Kanazawa, Y. Kasuya, S. Fujii, N. Tawara, and K. Minemoto (Hyogo College of Medicine) for their technical and secretarial assistance.
Footnotes
Abbreviations: ALT = alanine aminotransferase, anti-HBeAb = anti-HBe antibody, AST = aspartate aminotransferase, HBeAg = HBe antigen, HBsAg = HBs antigen, HBV = hepatitis B virus, HOMA-IR = homeostatic model assessment insulin resistance, IRI = immune reactive insulin, NAFLD = nonalcoholic fatty disease, NASH = nonalcoholic steatohepatitis, PT = prothrombin time
This study was in part supported by a Grant-in-Aid for Health and Labor Sciences Research from the Ministry of Health, Labour, and Welfare of Japan (the “Research Group for the Long-Term Prognosis of Anti-HBe Antibody Positive Asymptomatic HBV Carriers in Japan”).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009; 50:661–662. [DOI] [PubMed] [Google Scholar]
- 3.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016; 10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Franchis R, Meucci G, Vecchi M, et al. The natural history of asymptomatic hepatitis B surface antigen carriers. Ann Intern Med 1993; 118:191–194. [DOI] [PubMed] [Google Scholar]
- 5.Tai DI, Lin SM, Sheen IS, et al. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology 2009; 49:1859–1867. [DOI] [PubMed] [Google Scholar]
- 6.Kuo A, Gish R. Chronic hepatitis B infection. Clin Liver Dis 2012; 16:347–369. [DOI] [PubMed] [Google Scholar]
- 7.Peng D, Han Y, Ding H, et al. Hepatic steatosis in chronic hepatitis B patients is associated with metabolic factors more than viral factors. J Gastroenterol Hepatol 2008; 23 (7 Pt 1):1082–1088. [DOI] [PubMed] [Google Scholar]
- 8.Bondini S, Kallman J, Wheeler A, et al. Impact of non-alcoholic fatty liver disease on chronic hepatitis B. Liver Int 2007; 27:607–611. [DOI] [PubMed] [Google Scholar]
- 9.Lee IC, Huang YH, Chan CC, et al. Impact of body mass index and viral load on liver histology in hepatitis B e antigen-negative chronic hepatitis B. Clin Nutr 2011; 30:647–652. [DOI] [PubMed] [Google Scholar]
- 10.Spradling PR, Bulkow L, Teshale EH, et al. Prevalence and causes of elevated serum aminotransferase levels in a population-based cohort of persons with chronic hepatitis B virus infection. J Hepatol 2014; 61:785–791. [DOI] [PubMed] [Google Scholar]
- 11.Martinot-Peignoux, Lapalus M, Asselah T, et al. HBsAg quantification: useful for monitoring natural history and treatment outcome. Liver Int 2014; 34 Suppl. 1:97–107. [DOI] [PubMed] [Google Scholar]
- 12.Sumida Y, Yoneda M, Hyogo H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol 2011; 46:257–268. [DOI] [PubMed] [Google Scholar]
- 13.The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994; 20:15–20. [PubMed] [Google Scholar]
- 14.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 15.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55:2005–2023. [DOI] [PubMed] [Google Scholar]
- 16.Nascimbeni F, Pais R, Bellentani S, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol 2013; 59:859–871. [DOI] [PubMed] [Google Scholar]
- 17.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009; 49 5 Suppl.:S45–S55. [DOI] [PubMed] [Google Scholar]
- 18.Brunetto MR, Oliveri F, Coco B, et al. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol 2002; 36:263–270. [DOI] [PubMed] [Google Scholar]
- 19.Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e antigen negative chronic hepatitis B—natural history and treatment. Semin Liver Dis 2006; 26:130–141. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, Shin HJ, Kim K, et al. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPAR-γ. Gastroenterology 2007; 132:1955–1967. [DOI] [PubMed] [Google Scholar]
- 21.Wu YL, Peng XE, Zhu YB, et al. Hepatitis B virus X protein induces hepatic steatosis by enhancing the expression of liver fatty acid binding protein. J Virol 2015; Dec 4. pii: JVI.02604-15. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang CC, Tseng TC, Kao JH. Hepatitis B virus infection and metabolic syndrome: fact or fiction? J Gastroenterol Hepatol 2015; 30:14–20. [DOI] [PubMed] [Google Scholar]
- 23.Cheng YL, Wang YJ, Kao WY, et al. Inverse association between hepatitis B virus infection and fatty liver disease: a large-scale study in populations seeking for check-up. PLoS One 2013; 8:e72049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha M, Xia W, Tang D, et al. Hepatitis B e antigen-positive and high levels of alanine aminotransferase are associated with prevalence of metabolic syndrome in chronic HBV patients. Obes Res Clin Pract 2015; pii: S1871-403X(15)00163-5. [DOI] [PubMed] [Google Scholar]
- 25.Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis 1986; 8:283–298. [PubMed] [Google Scholar]
- 26.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44:865–873. [DOI] [PubMed] [Google Scholar]
- 27.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001; 121:91–100. [DOI] [PubMed] [Google Scholar]
- 28.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology 2000; 118:1117–1123. [DOI] [PubMed] [Google Scholar]
- 29.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45:846–854. [DOI] [PubMed] [Google Scholar]
- 30.Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008; 57:1441–1447. [DOI] [PubMed] [Google Scholar]
- 31.Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009; 50:165–173. [DOI] [PubMed] [Google Scholar]
- 32.Younossi ZM, Page S, Rafiq N, et al. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg 2011; 21:431–439. [DOI] [PubMed] [Google Scholar]
- 33.Wong RJ, Ahmed A. Obesity and non-alcoholic fatty liver disease: disparate associations among Asian populations. World J Hepatol 2014; 6:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Yang HI, Lee MH, et al. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology 2010; 139:474–482. [DOI] [PubMed] [Google Scholar]
- 35.Chu CM, Lin DY, Liaw YF. Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int J Obes (Lond) 2007; 31:871–875. [DOI] [PubMed] [Google Scholar]
- 36.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011; 53:1883–1894. [DOI] [PubMed] [Google Scholar]
- 37.Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014; 2:901–910. [DOI] [PubMed] [Google Scholar]
- 38.Wei JL, Leung JC, Loong TC, et al. Prevalence and severity of nonalcoholic fatty liver disease in non-obese patients: a population study using proton-magnetic resonance spectroscopy. Am J Gastroenterol 2015; 110:1306–1314. [DOI] [PubMed] [Google Scholar]
- 39.Khlaiphuengsin A, Kiatbumrung R, Payungporn S, et al. Association of PNPLA3 polymorphism with hepatocellular carcinoma development and prognosis in viral and non-viral chronic liver diseases. Asian Pac J Cancer Prev 2015; 16:8377–8382. [DOI] [PubMed] [Google Scholar]


