Abstract
Acquired ESR1 mutations are a major mechanism of resistance to aromatase inhibitors (AI). We developed ultra-high sensitivity multiplexed digital PCR assays for ESR1 mutations in circulating tumor DNA (ctDNA) and used these to investigate the clinical relevance and origin of ESR1 mutations in a cohort of 171 women with advanced breast cancer. ESR1 mutation status in ctDNA showed high concordance with contemporaneous tumor biopsies, and could be assessed in samples shipped at room temperature in preservative tubes without loss of accuracy. ESR1 mutations were found exclusively in patients with estrogen receptor positive breast cancer previously exposed to AI. Patients with ESR1 mutations had a substantially shorter progression-free survival on subsequent AI-based therapy (HR 3.1, 95%CI 1.9-23.1, log rank p=0.0041). ESR1 mutation prevalence differed markedly between patients that were first exposed to AI during the adjuvant and metastatic settings (5.8% (3/52) vs 36.4% (16/44) respectively, p=0.0002). In an independent cohort, ESR1 mutations were identified in 0% (0/32, 95%CI 0-10.9%) tumor biopsies taken after progression on adjuvant AI. In a patient with serial samples taken during metastatic treatment, ESR1 mutation was selected during metastatic AI therapy, to become the dominant clone in the cancer. ESR1 mutations can be robustly identified with ctDNA analysis and predict for resistance to subsequent AI therapy. ESR1 mutations are rarely acquired during adjuvant AI therapy, but are commonly selected by therapy for metastatic disease, providing evidence that the mechanisms of resistance to targeted therapy may be substantially different between the treatment of micro-metastatic and overt metastatic cancer.
Introduction
Cancer evolution and progression are driven by a sequence of somatic genetic and non-genetic alterations resulting in more favorable tumor cell growth and survival. Cancer genetic evolution is subject to intrinsic influences such as the tumor microenvironment, as well as extrinsic pressures such as drug therapy (1). The clinical pattern of ‘acquired’ resistance may in many circumstances represent outgrowth of resistant clones, which may have originally been present in the cancer at low frequency as a result of intra-tumoral genetic heterogeneity (2, 3) but grow out under the selective pressure of targeted therapy (1). For example, HER2 amplification is ‘acquired’ in about 2-5% of metastatic breast cancers that were originally HER2-nonamplified (4). MET amplification may be selected out as a mechanism of resistance to EGFR inhibitor therapy in non–small cell lung cancer (2), with the underlying biology behind selection of genetic events, at least in part, reflecting intra-tumoral heterogeneity and clonal selection.
With the potential of tumor genetics to evolve through treatment, repeat sampling of a tumor would be required to optimally guide therapy, because the mechanism of resistance may not be evident in analyses of the tumor before treatment. Yet, serial biopsies of recurrent, metastatic cancer would be invasive, risky, and unacceptable to many patients (5). Tumor-derived DNA is found in the plasma of patients with recurrent cancer, and high-depth analysis of circulating tumor DNA (ctDNA) presents a non-invasive way of analyzing tumor genetics and ‘acquisition’ of selected genetic events throughout the course of treatment (6, 7).
Around 75% of breast cancers express the estrogen receptor (ER), with hormone therapies being the mainstay of treatment for this group of patients. Hormone resistance is frequent in the treatment of early breast cancer, and inevitable in metastatic breast cancer. Recently, mutations in the estrogen receptor gene (ESR1) have been described in advanced breast cancers that had been exposed to prior therapy with aromatase inhibitors (AI) (8-11), drugs that suppress estrogen in post-menopausal women through inhibition of androgen aromatization. ESR1 mutations are only rarely detectable in primary breast cancer and are only found at appreciable frequency after the development of hormone resistance (11, 12). The majority of ESR1 mutations occur in a hotspot region within the ligand-binding domain (LBD) of ER, altering amino acid 536, 537, or 538 in helix 12 (p.Leu536Arg, p.Tyr537Ser, p.Tyr537Asn, p.Tyr537Cys, and p.Asp538Gly) (8-11). Functional studies of these LBD ESR1 mutations demonstrated that they constitutively activate the ER in a ligand-independent fashion (9). Hence cancers with these ESR1 mutations would be predicted to be resistant to AIs and ovarian suppression in premenopausal women, because these therapies work by depriving ligand. In contrast, in vitro and in vivo, LBD ESR1 mutations retain limited sensitivity to tamoxifen and fulvestrant (a selective ER modulator and a selective ER down-regulator, respectively) (8-11).
Prior studies of ESR1 mutations have been limited to patients with metastatic tumor biopsies and two small studies that have used plasma digital PCR (13) and plasma amplicon sequencing (14) to detect ESR1 mutations in plasma of patients with metastatic breast cancer. Here we used ctDNA analysis to study a large, unbiased series of consecutive patients with advanced breast cancer to determine whether these mutations can be detected noninvasively, and to examine the potential clinical importance and origin of ESR1 mutations.
Results
ESR1 mutations are detected in ctDNA of women with ER positive breast cancer
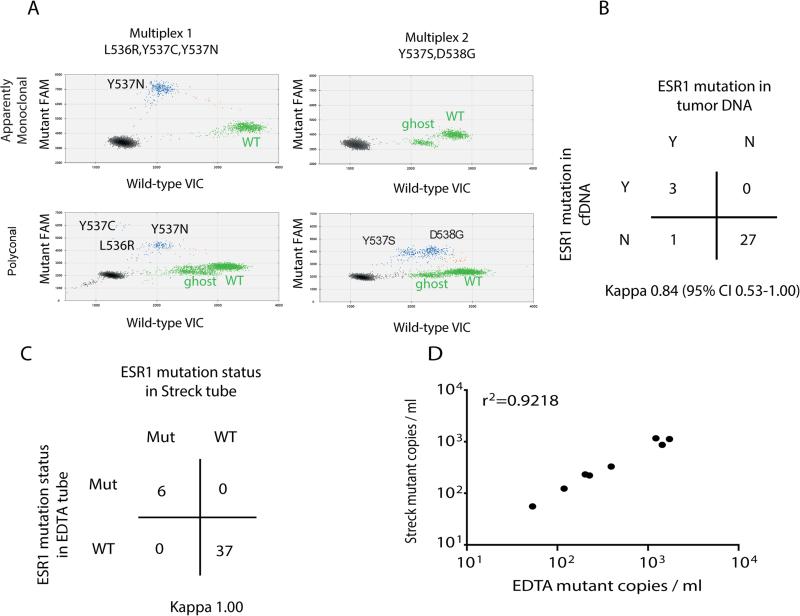
To investigate whether ESR1 mutations could be identified in the ctDNA, we recruited a cohort of 171 women with advanced breast cancer (Table S1), all of whom had plasma samples taken at the time of disease progression, because ctDNA can become undetectable in patients responding to therapy. We developed ultra-high sensitivity multiplex digital PCR assays for ESR1 LBD mutations and used these to assess for ESR1 mutations in plasma (Figure 1A and Table S2). To validate ctDNA assessment of ESR1 mutations, a subset of 31 women had a tumor biopsy contemporaneous to the plasma sample, and there was 97% agreement between tumor DNA and ctDNA analysis (Figure 1B, Kappa 0.84, 95% CI 0.53 to 1.00), with 75% sensitivity and 100% specificity for ctDNA analysis compared to tumor DNA. The single patient with an ESR1 mutation detected in tumor, but not in plasma, had a PIK3CA mutation in tumor that was also not detectable in plasma by digital PCR, suggesting that discordance reflected very low levels of ctDNA release. To assess technical reproducibility and the potential to mail samples in cell-free DNA preservative tubes, we separately collected blood samples into EDTA tubes and into DNA preservative Streck tubes. EDTA tubes were processed within 2 hours, whereas preservative tubes were mailed and processed 48-72 hours after venesection. There was 100% agreement between repeat plasma samples processed entirely separately, taken from the same patient at the same blood draw (Figure 1C, Kappa 1.00, 95% CI not estimable), with a high correlation of ESR1 mutation abundance (Figure 1D), providing evidence of both extremely high technical reproducibility and the ability to mail unprocessed samples in preservative tubes. In a dilution experiment, we showed that the digital PCR assays are able to detect as few as 3 copies of the mutant allele in an excess of WT DNA (Figure S1A).
Figure 1. Assessment of ESR1 in circulating tumor DNA with multiplex digital PCR assays has analytical and clinical validity.
A. High sensitivity multiplex digital PCR for ligand binding domain (LBD) ESR1 mutations. Representative digital PCR analysis of plasma DNA from two separate patients. Top panel: detection of an apparently monoclonal Y537N (c.1609T>A) mutation. Bottom panel: detection of polyclonal ESR1 mutations. The presence of all five mutations was confirmed by uniplex assays, with Y537C (c.1610A>G) and L536R (c.1607T>G) detectable in low amounts. In each plot, green dots represent VIC-labeled wild type DNA (except for the population labelled as “ghost,” which represents droplets with mutations different from those analyzed in the assay), blue dots represent FAM labeled mutant DNA, brown dots represent droplets containing both wild type and mutant DNA, and black dots are droplets with no DNA incorporated.
B. Comparison of ESR1 mutation calling between contemporaneous tumor biopsies and ctDNA plasma samples from 31 patients with advanced breast cancer, with overall 97% agreement between tumor DNA and ctDNA analysis, Kappa 0.84 (95% CI 0.53-1.0). Detection of ESR1 in ctDNA has 100% positive predictive value for tumor ESR1 mutation status and 96.4% negative predictive value.
C. Comparison of ESR1 mutation calling between repeat samples from the same 43 patients. 2 different tubes (1 EDTA tube and 1 cell free DNA Streck tube) were used at the same blood draw and processed entirely separately. There was 100% agreement between assays (Kappa 1.0, 95% CI not estimable), with exact concordance of the mutation called between samples.
D. Correlation between mutation abundance (mutant copies per ml of plasma) assessed in EDTA and Streck tubes in ESR1 mutant plasma samples. r2=0.92; Pearson's correlation coefficient.
ESR1 mutations were detected in the plasma of 10.5% (18/171, 95% CI 6%-16%) patients with advanced breast cancer, exclusively in patients with ER positive breast cancer (ER positive 14% vs ER negative 0%, p=0.0093, chi square test, Figure 2A). All patients with ESR1 mutations detected had prior AI exposure, with 23 months median duration of prior exposure (range 5.9-141.4) (Table 1). None (0/22, 95% CI 0-15%) of the patients with prior tamoxifen without AI exposure had detectable ESR1 mutations. ESR1 mutation ctDNA abundance was highly correlated between multiplex and uniplex mutation assays (Figure S1B), with a distribution of mutations that was highly similar to previously published data (Figure 2B) (8-11). ESR1 mutations were shown to be overtly polyclonal in 21% of ESR1 mutant patients, and apparently monoclonal in the remaining 79% of patients (Figure 2B).
Figure 2. Detection of ESR1 mutations in plasma predicts lack of sensitivity to subsequent AI therapy.
A. ESR1 ctDNA analysis by multiplex digital PCR in 171 patients with advanced breast cancer. ESR1 mutations are detected exclusively in plasma of patients with ER positive advanced breast cancer, p=0.0093 Chi Square test.
B. Left. Profile of ESR1 mutations detected in ctDNA. Right Percentage of cases with apparently monoclonal (79%) or polyclonal (21%) ESR1 mutations.
C. Progression-free survival on AI therapy after ctDNA analysis, for patients with ESR1 mutant and wild type ctDNA (HR 3.1, 95%CI 1.9-23.1, p=0.0041 log rank test).
Table 1.
Characteristics of patients with advanced ER positive breast cancer grouped by ESR1 status.
| ESR1 Wild type | ESR1 Mutant | P value | |
|---|---|---|---|
| n | 109 | 19 | |
| Median age | 58 | 64 | 0.9* |
| Grade | |||
| 1 | 8 | 1 | 0.89 |
| 2 | 59 | 10 | |
| 3 | 38 | 5 | |
| HER2 positive | 17% | 11% | 0.61 |
| Disease presentation+ | |||
| Relapsed | 90 | 15 | 0.74 |
| De novo | 19 | 4 | |
| Visceral disease | 60% | 79% | 0.07 |
| Bone disease | 65% | 84% | 0.12 |
| Prior chemotherapy | |||
| Adjuvant | 50% | 42% | 0.62 |
| Courses metastatic chemo | |||
| 0 | 68 | 9 | 0.63 |
| 1 | 22 | 5 | |
| 2 | 13 | 3 | |
| 3+ | 6 | 2 | |
| Prior endocrine therapy | |||
| Adjuvant | |||
| tamoxifen+/−OvS | 56% | 53% | 0.8 |
| aromatase inhibitor | 45% (49/109) | 16% (3/19) | 0.017 |
| Advanced | |||
| tamoxifen+/−OvS | 19% (20/109) | 48% (9/19) | 0.0053 |
| aromatase inhibitor | 46% (50/109) | 95% (18/19) | <0.0001 |
| First exposure to AI | |||
| Adjuvant | 49 | 3 | 0.0002 |
| Advanced | 28 | 16 | |
| No AI exposure | 32 | 0 | |
| Median cumulative duration of prior AI exposure | 31.8 month | 23.4 month | 0.42* |
P values are chi square test except
unpaired t test
presentation of advanced disease is defined as de novo: advanced at first presentation or relapsed: relapsed after prior presentation with early stage cancer.
OvS: ovarian suppression.
Detection of ESR1 mutations predicts for relative resistance to subsequent AI-based therapy
We investigated sensitivity to hormonal therapy after ctDNA assessment. Patients with ESR1 mutation(s) detected in plasma had a substantially shorter progression-free survival (PFS) on subsequent AI-based therapy, whether given as therapy after disease progression (Figure S2, HR 3.7, 95% CI 1.9-76.9, p=0.008 log rank test) or including the duration of AI given as maintenance therapy after response to prior chemotherapy (Figure 2C, HR 3.1, 95%CI 1.9-23.1, p=0.0041 log rank test). There were not enough patients who had exposure to the ER degrader fulvestrant after ctDNA assessment to assess the activity of fulvestrant in this population.
ESR1 mutations are predominantly acquired during the treatment of metastatic disease
To investigate the factors that associated with detection of ESR1 mutations in ctDNA, we looked for differences in clinical and pathological factors between ER positive cancers that were ESR1 wild-type and mutant in ctDNA (Table 1). There was no difference in grade, HER2 status, prior chemotherapy exposure, cumulative duration of prior AI therapy, or sites of metastasis. Although all patients with detectable ESR1 mutation had prior AI exposure, patients with detectable ESR1 mutations had less frequent AI exposure in the adjuvant setting (ESR1 mutant 16% vs ESR1 wild type 45%, p=0.0216), and more frequent exposure prior AI exposure in the metastatic setting (ESR1 mutant 95% vs ESR1 wild type 36%, p<0.0001, Table 1). Within the subset of patients who had AI exposure only in the metastatic setting, there was no difference in cumulative duration of prior AI exposure (23.3 months ESR1 wild type versus 23.0 months ESR1 mutant, p=0.82 Mann Whitney U test), number of courses of prior endocrine therapy (median 2.0 versus 1.5 respectively, p=0.39), or duration of first AI exposure (13.2 versus 18.1 months respectively, p=0.47).
We further investigated whether the timing of prior AI exposure influenced the prevalence of ESR1 mutations. Strikingly, patients who had received prior AI in the adjuvant setting for treatment of micro-metastatic disease, whether followed by further therapy in the metastatic setting or not, had a substantially lower prevalence of ESR1 mutation(s) compared to patients who first received prior AI only for treatment of metastatic breast cancer (5.8% versus 36.4%, Figure 3A, p=0.0002 chi square test). The subset of patients who had ctDNA samples taken at the time of recurrence on adjuvant AI also had a low rate of ESR1 mutation in ctDNA (4.8% 1/21), suggesting that the difference did not reflect loss of ESR1 mutation due to time between exposure to AI and taking of plasma sample for ctDNA analysis. A further confounding factor could be that plasma samples contained no ctDNA in patients exposed to AI in the adjuvant setting. To address this, we assessed the prevalence of hotspot PIK3CA mutations in plasma DNA and found similar rates of PIK3CA mutation across all groups (Figure 3B), suggesting that differences in ctDNA abundance did not explain the observation.
Figure 3. ESR1 mutations are rarely acquired during adjuvant AI, and frequently during metastatic AI therapy.
A. ESR1 ctDNA mutation rate split by timing of patients’ exposure to AI therapy, or no prior exposure to AI therapy. P<0.0001 Chi Square test overall, and p=0.0002 Chi Square test comparing metastatic AI only to prior exposure first in the adjuvant setting.
B. PIK3CA ctDNA mutation rate, assessed with multiplex digital PCR assay, split by timing of patients’ exposure to AI therapy, or no prior exposure to AI therapy. Samples from 19 patients included in part A were not analyzed because plasma was exhausted. p=0.66, Chi squared test.
We assessed the rate of ESR1 mutations only in patients with known detectable ctDNA, by assessing the rate of ESR1 mutation only in samples with another mutation detected in plasma DNA. To extend the power of this analysis beyond PIK3CA mutations identified with digital PCR, we sequenced plasma samples from 76 patients with massive parallel sequencing (MPS) using a custom Ion Torrent amplicon sequencing panel (Table S3), calling mutations with an allele frequency >3%. Mutations with an allele frequency 3-10% were additionally confirmed by digital PCR. We sequenced plasma from 7 patients with ESR1 mutations identified by digital PCR and found the corresponding mutation in 4 (57%) of these patients, reflecting the higher sensitivity of digital PCR for low allele frequencies. Of the 69 patients without an ESR1 LBD mutation by digital PCR, none had an ESR1 LBD mutation by MPS (p<0.0001 chi square test). Using MPS, we identified an additional mutation (other than PIK3CA) in 13 patients. We next assessed the rate of ESR1 mutations only in the 49 patients who had experimental evidence of ctDNA being present, either by PIK3CA digital PCR or plasma DNA MPS. ESR1 mutations were found in 7% (1/15) of patients exposed to AI in the adjuvant setting only, 14% (2/14) of patients with exposure in both adjuvant and metastatic settings, and 42% (5/12) of patients with AI exposure only in the metastatic setting (Figure 4A). Our data suggest that ESR1 mutations were present at a higher rate in patients exposed to AI in the metastatic setting and only rarely occur in patients exposed to AI in the adjuvant setting.
Figure 4. Validation and independent series confirm the importance of timing of prior AI exposure for ESR1 mutation selection.
A. ESR1 mutation rate assessed only in patients with detection of a mutation other than ESR1 in plasma DNA. p=0.061 Chi Square test overall and p=0.035 adjuvant AI only vs metastatic AI only.
B. Assessment of ESR1 mutation rate in an independent series of 49 breast tumor biopsies that had recurred after prior AI therapy. No ESR1 mutations were identified in breast tumor biopsies relapsing after adjuvant AI (0%, 95% CI 0-10.9%).
C. Reassessment of a second independent series of ESR1 mutant positive cancers, with timing of prior AI therapy (9).
To confirm these observations, we examined three independent cohorts. The first independent cohort was from 49 patients with tumor biopsies taken at the time of recurrence or at progression on AI. We identified no mutations in patients exposed to AI in the adjuvant setting (0/32, 95% CI 0%-10.9%, Figure 4B), confirming the low incidence of ESR1 mutations in patients treated in the adjuvant setting. Conversely, mutations were identified in 12% of patients exposed to AI in the metastatic setting (2/17). For the second cohort, we collected plasma samples from an additional independent set of 28 patients with metastatic breast cancer, 18 of who had prior AI treatment. In those with prior AI therapy in the adjuvant setting, we identified ESR1 mutations in 0% (0/7) patients, whilst in those who had received prior AI only in the metastatic setting we identified ESR1 mutations in 36% (4/11) patients. For the third independent cohort, we re-examined the timing of AI exposure on the previously published index series of ESR1 mutant cancers, none of which had prior exposure to AI in the adjuvant setting alone (Figure 4C)(9).
ESR1 mutations can be selected to become the dominant clone in the cancer
We assessed the relative clonality of ESR1 mutations in ctDNA, comparing the abundance of ESR1 mutation to other common driver mutations identified with PIK3CA digital PCR and ctDNA MPS. In a patient with sequential samples taken during metastatic treatment, an ESR1 mutation was shown to be selected by prior AI treatment for metastatic cancer to become the dominant clone (Figure 5A). We assessed the relative clonal dominance of ESR1 mutation, comparing the allele frequency of the ESR1 mutation with allele frequency of PIK3CA on the same plasma sample. We assumed that the majority of PIK3CA mutations are clonal in ER positive breast cancer, and therefore that the relative allele frequency would give an assessment of clonal dominance (within wide limits to take account of potential copy number variation in the tumor). We included an additional patient with a PTEN mutation identified by MPS. This analysis suggested that the ESR1 mutation may be clonally dominant in 4 patients’ tumors, whereas the ESR1 mutation was likely subclonal in 2 patients’ tumors (Figure 5B). These data suggest that assessment of ESR1 mutation clonality is likely to be important in the development of therapeutics targeting the mutant estrogen receptor. Finally, we investigated for evidence of loss of ESR1 mutation with time after AI exposure (15). The rate of detection of ESR1 mutation was unaltered between patients with samples taken at the time of completing AI therapy for metastatic cancer or >6 months later (samples <6 months from completing AI therapy 22% (11/50) versus >6 months 41% (7/17) ESR1 mutant, p=0.12 chi square test), suggesting that once selected by prior AI therapy, the ESR1 mutation may persist in the tumor through subsequent therapy.
Figure 5. ESR1 mutations are selected to become the dominant clone in the cancer.
A. Serial ctDNA analysis in a patient with plasma samples taken before and after exposure to AI for metastatic breast cancer. Tumor PIK3CA mutation is detected in both plasma samples, whilst ESR1 mutation is only detected after developing resistance to AI. OvS: ovarian suppression (goserelin, followed by bilateral salpyngo-oophorectomy); tam: tamoxifen.
B. Relative abundance of ESR1 mutations in ctDNA compared to abundance of other tumor derived mutations detected in ctDNA. Below dotted line (0.25) suggests likely sub-clonal mutations.
Discussion
Circulating tumor DNA analysis has the potential to allow non-invasive analysis of tumor genetic alterations in advanced cancer. Here we demonstrate that ctDNA analysis has analytical and clinical validity in the identification of hotspot LBD ESR1 mutations in the plasma of advanced breast cancer patients, and has possible clinical utility in identifying a group of patients who derive very limited benefit from further AI therapy.
Our data provide evidence that the mechanism of resistance to targeted therapy, specifically aromatase inhibitors, may differ substantially depending on the setting of exposure. ESR1 mutations are selected frequently during treatment for metastatic breast cancer, likely through selection of rare ESR1 mutant sub-clones that were present in low amounts before therapy as a result of genetic intra-tumoral heterogeneity and clonal diversity in the cancer (Figure S3). However, our data suggest that in patients treated in the adjuvant setting with micro-metastatic disease, ESR1 mutations are selected/acquired only rarely, potentially due to a lack of genetic diversity in micro-metastatic disease. We speculate that the tumor bulk of micro-metastatic disease may be sufficiently low that rare ESR1 mutant sub-clones are not present, and cannot therefore be selected by therapy (Figure S3). We were unable to provide direct evidence that ESR1 mutations pre-exist before therapy in the metastatic setting. However, we do show that ESR1 mutations are frequently monoclonal, are therefore likely derived from a single cell, arise over a median of 23 months’ exposure, and frequently become the dominant clone in the cancer. Given the doubling rate of ER-positive breast cancer (16), it is relatively unlikely that a single cell could repopulate the metastatic cancer in this time, and therefore our data suggest that ESR1 mutations likely pre-exist in the tumor before exposure. However, it is also possible that in cancers with a longer duration of prior AI exposure the mutation could be acquired during therapy, initiated through mutagenic processes such as the APOBEC enzymes (17).
Our data illustrate the potential value of ctDNA analysis and the ability to access large unbiased cohorts of patients with pre-treated advanced cancer without the potential selection biases inherent in tissue biopsy cohorts, where patients are selected on the basis of suitability for biopsy. Nevertheless, our study has a number of limitations. The studied population is heterogeneously treated, and the sample size does not allow us to fully examine whether ESR1 mutation detection predicts sensitivity to specific hormone therapies or combinations, for example fulvestrant or everolimus plus exemestane, respectively. We could not assess the lead-time between emergence of ESR1 mutations in ctDNA and clinical progression of the disease, because of limited longitudinal sampling. In this study, we did not examine less frequent ESR1 mutations (for example E380Q or S463P), partly because their function has not been studied extensively yet. In line with prior publications, we found D538G to be the most common mutation identified in plasma DNA, although we had insufficient data to address whether there were clinical differences between different ESR1 mutations in the LBD. Finally, in lung cancer, T790M EGFR mutations selected by prior EGFR targeted therapy can be lost during subsequent treatment, possibly because the T790M clone may proliferate more slowly and be ‘overtaken’ by residual wild-type EGFR clones once the selective pressure of EGFR targeted therapy is removed. A similar result has been reported for selected KRAS mutations in colorectal cancer treated with EGFR inhibitors (15). This could provide an explanation for the low rates of ESR1 mutations in the adjuvant AI setting, if there was intervening non-AI treatment before sampling. We provide two controls to suggest that this is not the main explanation: the subset of patients with a ctDNA sample taken at the time of relapse on AI had very low ESR1 mutation detection, and we observed no mutations in the independent series of tumor biopsies taken at relapse on AI therapy. In addition, our data suggest that ESR1 mutations remain readily detectable in ctDNA many months and years after stopping AI therapy, suggesting possible differences between selected ESR1 mutations in breast cancer and KRAS in colorectal cancer.
Our data provide evidence for the clinical validity of ctDNA ESR1 mutation testing and support ctDNA screening to select patients for prospective clinical trials with drugs that target ESR1 mutations, such as oral estrogen receptor degraders, which are in early clinical development. ESR1 mutations are rarely acquired during adjuvant AI therapy, but are commonly selected by therapy for metastatic disease, providing evidence that the mechanisms of resistance to targeted therapy may be substantially different between the treatment of micro-metastatic and overt metastatic cancer.
Materials and Methods
Study design
We collected a series of plasma DNA samples from patients with advanced breast cancer to assess the potential utility of ESR1 mutation analysis in ctDNA. We developed multiplex digital PCR assays for hot-spot ligand binding domain mutations and used these assays to screen for ESR1 mutations. In parallel, a subset of patients had biopsy samples obtained contemporaneously with plasma samples to assess agreement with tumor biopsy, and repeat plasma sampling to assess reproducibility and the ability to mail samples in preservative tubes. We investigated the relationship between detection of ESR1 mutation and sensitivity to subsequent hormonal therapy. We also identified the clinical and pathological features that associated with the presence of an ESR1 mutation and validated these associations in an independent series of 49 tumor biopsies taken on progression on aromatase inhibitor therapy.
Patients
We analysed 171 consecutive patients with advanced breast cancer at the Royal Marsden Hospital (RMH), who consented to tissue collection studies approved by multi-center research ethics committees (REC Ref No: 10/H0805/50, REC Ref No: 11/LO1595). All patients had recently relapsed or progressed after prior therapy.
Estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) were assessed in a single laboratory at the Royal Marsden Histopathology department (or reviewed by the RMH when reported from a referring hospital) using standard criteria.
For patients who had biopsy of recurrent cancer, this biopsy was used to define the receptor status, and for the remaining patients the pathology of the primary cancer was used. For patients who had biopsies from multiple metachronous metastatic sites, the most recent biopsy was used to define the receptor status. Patients who presented with primary breast cancer simultaneously with metastatic disease were recorded as having biopsy of recurrent cancer. Table 1 illustrates the main clinico-pathological characteristics of ER-positive patients in this cohort, and Table S1 the whole cohort.
Patients with prior aromatase inhibitor (AI) exposure were divided by time of first exposure to AI in the adjuvant setting (treatment with either pre-operative AI or adjuvant AI after surgery for early breast cancer or isolated local recurrence and before metastatic relapse) or the metastatic setting (first treatment with an AI after metastatic relapse). Patients were considered to have had plasma samples taken ‘at time of relapse on AI therapy’ when blood was taken within 6 months of stopping adjuvant AI. To analyze associations with subsequent AI-based therapy, progression-free survival (PFS) was defined as the time from starting AI to documented disease progression or death. Patients receiving AI and an anti-HER2 agent concomitantly were excluded from this analysis. For patients who received chemotherapy after the blood test and then went onto maintenance AI therapy, the baseline date for PFS analysis was the starting date of AI.
An independent validation cohort consisted of a separate series of 49 patients with recurrent breast cancer who were all pre-treated with an AI before the recurrent disease biopsy (18). For 43/49 of these cases, a biopsy sample obtained before AI therapy was also available for assessment. First exposure to AI was in the adjuvant setting for 32 of these patients and in the metastatic setting for 17 patients using the above definitions. A further, entirely independent, set of plasma samples was also collected from an additional 28 patients with metastatic breast cancer for additional independent validation.
Processing and DNA extraction from tumor tissue
Thirty-one patients in our cohort had tumor biopsies contemporaneous with the plasma samples. Archival formalin-fixed and paraffin-embedded (FFPE) tissue blocks were retrieved, and sections (4-8 × 4 μm) were cut from these blocks, stained with Nuclear Fast Red (NFR), and microdissected to achieve >70% tumor cell purity, using an H&E stained slide to guide manual microdissection. Tumor DNA was isolated using the QIAamp DNA FFPE Tissue Kit (Qiagen) as per manufacturer's instructions. DNA was eluted in 50-100 μl ATE buffer, depending on the amount of tumor on the slides, and stored at −20°C until quantification. For the validation cohort, tumor DNA was isolated using the Qiagen AllPrep DNA/RNA FFPE Kit as per manufacturer's instructions.
Processing of plasma and extraction of circulating DNA
Blood collected in Vacutainer EDTA Blood Collection Tubes (BCT) was processed within two hours of sample collection and centrifuged at 1600 g for 20 min to separate the plasma from the peripheral blood cells. Plasma was stored at −80°C until DNA extraction. DNA was extracted from 2 ml aliquots of plasma using the QIAamp circulating nucleic acid kit (Qiagen) according to the manufacturer's instructions. The DNA was eluted into 50 μl buffer AVE and stored at −20°C until quantification. For a subset of patients, to test concordance and alternative methods of blood processing, blood was also placed in Streck Cell-Free DNA BCT tubes at the same visit as the EDTA sample, and shipped at room temperature, with plasma separated 48-72 hours after venesection.
DNA quantifications from tissue and/or plasma
DNA isolated from tissue or plasma was quantified on a Bio-Rad QX200 droplet ddPCR system using RNase P as the reference gene. 1 μl of eluate was added to a ddPCR reaction containing 10 μl ddPCR Supermix for probes (Bio-Rad) and 1 μl of TaqMan Copy Number Reference Assay, human, RNase P (Life Technologies) in a total volume of 20 μl. The reaction was partitioned into ~14,000 droplets per sample in a QX200 droplet generator according to manufacturer's instructions. Emulsified PCR reactions were run on a 96-well plate on a G-Storm GS4 thermal cycler, incubating the plates at 95°C for 10 min followed by 40 cycles at 95°C for 15 sec and 60°C for 60 sec, then 10 min incubation at 98°C. The temperature ramp increment was 2.5°C/sec for all steps. Plates were read on a Bio-Rad QX200 droplet reader with QuantaSoft v1.6.6.0320 software from Bio-Rad. At least two negative control wells with no DNA were included in every run. The amount of amplifiable RNase P DNA was calculated from the concentration provided by the software.
Development of ligand binding domain (LBD) ESR1 mutations digital PCR assays
We designed primer probe combinations for each of the most common ESR1 mutations (p.L536R, p.Y537S, p.Y537N, p.Y537C, and p.D538G) (Supplementary Table 2) using Primer 3 plus or Life Technologies’ custom SNP genotyping assay tool. Primers and probes were analyzed for the presence of hairpins, secondary structures, or hetero/homo dimer formation. Primers were analyzed for specificity of the primer pair to their amplicon using University of California, Santa Cruz ePCR tool (http://genome.ucsc.edu/cgi-bin/hgPcr?command=start). Digital PCR conditions were optimized with a temperature gradient to identify the optimal annealing/extension temperature using wild-type DNA spiked with a mutant synthetic oligonucleotide (Table S2) on a QX200 droplet digital PCR system (Bio-Rad) using Taqman chemistry. We developed multiplex assays varying the concentration of the fluorescent probes to differentiate mutations on the basis of fluorescence intensity (19). We selected the two optimal multiplex assay combinations (Multiplex 1 and 2) and probe concentrations as shown in Table S2.
Limit of Detection of ESR1 assays
Genomic DNA (gDNA) was extracted from the ESR1 WT human mammary gland cell line CAMA-1 (ATCC HTB-21) with DNeasy Blood and Tissue Kit (Qiagen) as per manufacturer instructions. DNA was quantified using Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies) as per manufacturer instructions. We spiked 15,000 genomes of STR-typed CAMA-1 gDNA with 200, 100, 5, and 3 copies of mutant D538G DNA and ran a uniplex digital PCR with the primers and probes for this mutation (Table S2) as described above. We calculated the number of copies assayed versus the frequency of mutation detected after digital PCR cycling and plotted it to assess the lower limit of detection for this assay.
Hotspot PIK3CA mutation digital PCR assays
Digital PCR assays for the 4 most common occurring mutations in the PIK3CA gene: E542K (c.1624G>A), E545K (c.1633G>A), H1047L (c.3140A>T), and H1047R (c.3140A>G) were developed as for ESR1 LBD assays, using Taqman probes (FAM (fluorescein) - labeled for mutant and VIC-labeled for WT), and have been described previously (20). We developed a multiplex assay varying the concentration of the fluorescent probes to differentiate mutations on the basis of fluorescence intensity as above, and we selected the optimal multiplex assay combination and probe concentrations, which enabled us to test for the 4 hotspot mutations in one single reaction. PIK3CA mutation testing in ctDNA was performed in a subgroup of the cohort (109/128 ER-positive patients) who had sufficient residual plasma for analysis.
Digital PCR analysis of circulating free DNA (cfDNA) and tumor tissue DNA
Digital PCR was performed on a QX200 digital PCR system (Bio-Rad) using the assays described in table S2. For plasma samples, circulating free DNA (cfDNA) equivalent to 250 μl plasma was used per multiplex assay. For tumor tissue DNA, 1 ng was used per multiplex assay. PCR reactions were prepared with digital PCR Supermix for probes (Bio-Rad) and partitioned into a median of ~14,000 droplets per well in a QX200 droplet generator according to manufacturer's instructions. Emulsified PCR reactions were run on a 96 well plate on a G-Storm GS4 thermal cycler, incubating the plates at 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec, then 10 min incubation at 98°C. The temperature ramp increment was 2.5°C/sec for all steps. Plates were read on a Bio-Rad QX200 droplet reader with QuantaSoft v1. 6.6.0320 software from Bio-Rad to assess the number of droplets positive for mutant DNA, wild-type DNA, both, or neither. At least two negative control wells with no DNA were included in every run. A mutation was considered positive with at least 2 ESR1 mutant (or PIK3CA) droplets. The multiplex dPCR was performed for mutation detection, and the individual mutation or mutations present were subsequently confirmed with uniplex dPCR assays.
Digital PCR analysis
The concentration of mutant DNA (copies of mutant DNA per droplet) was estimated from the Poisson distribution. Number of mutant copies per droplet was calculated as Mmu = -ln (1-(nmu/n)), where nmu = number of droplets positive for mutant-FAM probe and n = total number of droplets. The DNA concentration in the reaction was estimated as follows: MDNAconc = -ln (1-(nDNAconc/n)), where nDNAconc = number of droplets positive for mutant-FAM probe and wild type-VIC probe and n = total number of droplets. The mutation allele fraction = Mmu/MDNAconc. The number of mutant copies per ml of plasma was estimated from the mutation allele fraction by taking into account the number of wells run for the sample and the volume equivalent of plasma run, and the mean volume of a droplet (0.89 pl) using the following formula:
Ion Torrent Proton sequencing of plasma samples
Sequencing libraries were prepared with a custom Ion AmpliSeq Breast Cancer Panel targeting 14 known breast cancer driver and focal mutations (table S3) using the Ion AmpliSeq Library Preparation protocol with 3 ng of cfDNA, according to manufacturer's instructions. After barcoding, libraries were quantified using qPCR, diluted to 100 pM, and pooled. Libraries were templated with the Ion OneTouch2 system (Life Technologies), and sequenced on a PI chip using the Ion PI OT2 200 Kit (Life Technologies), 520 flows, and an average amplicon length of 97 bases to a mean depth of x9183. The sequencing resulted in 1042543-5763164 reads per sample.
Ion torrent Variant caller v4.0-r73742 with no Hotspot region and configuration “Germ Line Low Stringency” was used for calling variants. Read counts for all positions were computed using pileup (samtools v1.1(21)) and this data was analysed for possible variants using custom perl and R scripts. Variants at > 3% reported by both analysis methods and not reported in 1000 Genomes Project database (www.1000genomes.org) were identified as possible somatic mutations. The data was cross referenced against the Cosmic database v70 (cancer.sanger.ac.uk) to identify possible hotspot mutations Variants not appearing in the 1000 genomes database were taken forward for development of digital PCR assays.
Statistical analysis
All statistical analysis was performed with GraphPad Prism version 6.0 or Microsoft Excel. Unless stated otherwise, p values were two tailed and considered significant if p<0.05.
Supplementary Material
One Sentence Summary.
ESR1 mutations evolve during the treatment of metastatic breast cancer
Acknowledgments
We thank N. Orr, K. Tomczyk, D. Novo, and F. Daley for technical assistance. Funding: This research was funded by NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and the ICR, Cancer Research UK C30746/A16642, Breast Cancer Now with generous support from the Mary-Jean Mitchell Green Foundation, the Cridlan Trust, and the Susan G. Komen Foundation
Footnotes
Author contributions: GS and NCT designed the study and set up the database. GS, IES, NCT enrolled the patients. RR, L-AM, MD selected and collected the samples for the validation cohort. GS, SH, IG-M designed the assays. GS, SH, IG-M, AP, NT, E-LK conducted the experiments. GS, SH, IG-M analysed the data and GS and NCT did the statistical analyses. KF and IK performed the NGS experiment. RJC performed the NGS analysis GS, IG-M and NCT wrote the manuscript with assistance and final approval from all authors.
Competing interests: NCT has received advisory board honoraria from Roche/Genentech and AstraZeneca. MD is on the scientific advisory board of Radius and has paid consulting relationships with GTx, Roche/Genentech, Genoptix, Nanostring, Pfizer, GSK, AstraZeneca, and Ventana. All other authors declare that they have no competing interests.
References and Notes
- 1.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13:e178–185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 2.Turke AB, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell PJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houssami N, Macaskill P, Balleine RL, Bilous M, Pegram MD. HER2 discordance between primary breast cancer and its paired metastasis: tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res Treat. 2011;129:659–674. doi: 10.1007/s10549-011-1632-x. [DOI] [PubMed] [Google Scholar]
- 5.Ding L, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz LA, Jr., et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–540. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misale S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–536. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merenbakh-Lamin K, et al. D538G Mutation in Estrogen Receptor-alpha: A Novel Mechanism for Acquired Endocrine Resistance in Breast Cancer. Cancer research. 2013;73:6856–6864. doi: 10.1158/0008-5472.CAN-13-1197. [DOI] [PubMed] [Google Scholar]
- 9.Toy W, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson DR, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nature genetics. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeselsohn R, et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20:1757–1767. doi: 10.1158/1078-0432.CCR-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sefrioui D, et al. Short report: Monitoring ESR1 mutations by circulating tumor DNA in aromatase inhibitor resistant metastatic breast cancer. Int J Cancer. 2015;137(10):2513–9. doi: 10.1002/ijc.29612. [DOI] [PubMed] [Google Scholar]
- 14.Guttery DS, et al. Noninvasive Detection of Activating Estrogen Receptor 1 (ESR1) Mutations in Estrogen Receptor-Positive Metastatic Breast Cancer. Clin Chem. 2015;61:974–982. doi: 10.1373/clinchem.2015.238717. [DOI] [PubMed] [Google Scholar]
- 15.Siravegna G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:795–801. doi: 10.1038/nm.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008;10:R41. doi: 10.1186/bcr2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Bruin EC, et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science. 2014;346:251–256. doi: 10.1126/science.1253462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnedos M, et al. Biomarker changes associated with the development of resistance to aromatase inhibitors (AIs) in estrogen receptor-positive breast cancer. Ann Oncol. 2014;25:605–610. doi: 10.1093/annonc/mdt575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taly V, et al. Multiplex picodroplet digital PCR to detect KRAS mutations in circulating DNA from the plasma of colorectal cancer patients. Clin Chem. 2013;59:1722–1731. doi: 10.1373/clinchem.2013.206359. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Knowles E, et al. Relationship of PIK3CA mutation and pathway activity with antiproliferative response to aromatase inhibition. Breast cancer research : BCR. 2014;16:R68. doi: 10.1186/bcr3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.