Abstract
Currently, limited data are available regarding the efficacy and safety of pegylated interferon alpha-2a (PEG-IFN α-2a) in Korean patients with chronic hepatitis B (CHB), in whom hepatitis B virus (HBV) genotype C is the most common type.
We collected data from 439 patients (HBeAg positive, n = 349; HBeAg negative, n = 90) with CHB who were treated with PEG-IFN α-2a as a first-line therapy from 18 institutions. Treatment responses at the end of treatment (ET) and at 6 months posttreatment (PT6) were compared between the patients who were treated for 24 weeks versus 48 weeks, and adverse events (AEs) were evaluated.
In HBeAg-positive patients, those who received PEG-IFN α-2a for 48 weeks showed significantly higher HBV DNA suppression (HBV DNA < 2000 IU/mL) than those who were treated for 24 weeks (48 weeks vs 24 weeks; at ET, 44.4% vs 36.7%, P = 0.035; at PT6, 35.9% vs 13.3%, P = 0.035). The HBeAg seroconversion rate at ET was 18.1% in 48-week treatment group, which is significantly higher than the 2.2% (P < 0.001) that was seen in 24-week treatment group. This finding also continued at PT6 (29.0% vs 10.0%, P < 0.001). Following 48 weeks of treatment in HBeAg-negative patients, HBV DNA suppression at ET was higher than in HBeAg-positive patients (87.8% vs 44.4%). AEs were typical of those associated with PEG-IFN α-2a.
In naïve Korean HBeAg-positive CHB patients treated with PEG-IFN α-2a, higher rates of HBV DNA suppression and HBeAg seroconversion were achieved in the 48-week treatment group than in the 24-week treatment group without additional risk of AEs.
INTRODUCTION
Chronic hepatitis B (CHB) infection is a global health problem affecting approximately 300 million people worldwide and also a major concern in Asian countries including Korea.1 As CHB is associated with fatal complications such as cirrhosis, liver failure, and hepatocellular carcinoma, effective therapy is necessary.2,3 Currently, there are 2 treatment options for CHB patients: pegylated interferon (PEG-IFN) or oral nucleos(t)ide analogs (NA).4 The advantage of PEG-IFN over NA is the dual action of immune-modulation and antiviral effects, which together result in a relatively high chance of achieving hepatitis B virus surface antigen (HBsAg) clearance in patients with undetectable HBV DNA.5,6 However, PEG-IFN has several drawbacks when compared to NAs, including lower potency for vial suppression, inconvenient administration, and several side effects.4
For this reason, PEG-IFN has been used in only a small number of highly selected patients. Therefore, efficacy and safety data based on treatment experiences in Korea are lacking. Although there is one published study showing the efficacy and safety of PEG-IFN α-2a in Korean patients with CHB, the study included only a small number of patients (n = 88).7 In addition, even worldwide, only a few small studies have investigated the proper dose and duration of PEG-IFN α-2a treatment in patients with CHB.8–10 Recently, after publication of the NEPTUNE study,11 all guidelines are recommending the use of PEG-IFN α-2a 180 μg for 48 weeks in hepatitis B virus endogenous antigen (HBeAg) positive patients as well as in HBeAg-negative patients.4,12 However, there has been no study validating the result that PEG-IFN α-2a 180 μg for 48 weeks is superior over 24 weeks of treatment in a Korean patient cohort.
Thus, this study aimed to investigate the efficacy and safety of PEG-IFN α-2a in Korean patients with CHB who are receiving the treatment in a real life setting. Specifically, we compared treatment responses (hepatitis B virus [HBV] DNA suppression, virological response [VR], HBsAg seroconversion, HBeAg seroconversion in HBeAg-positive patients, alanine transaminase [ALT] normalization) between the 24- and 48-week regimens at the end of treatment (ET) and at 6 months posttreatment (PT6). We also evaluated adverse events (AEs) and serious adverse events (SAEs).
MATERIALS AND METHODS
Patients
From May 2005 to May 2011, 640 patients who showed HBV DNA > 20 000 IU/mL in HBeAg-positive patients and HBV DNA > 2 000 IU/mL in HBeAg-negative patients and who were being treated with PEG-IFN α-2a as the first-line therapy in 18 Korean hospitals were considered for inclusion in this cohort study. In practice, treatment regimens with PEG-IFN α-2a 180 μg/week are usually either 24 or 48 weeks long based upon the clinician's decision and factors such as the patients’ VR and clinical condition, safety, and medical insurance. Exclusion criteria were as follows: patients with other concurrent chronic hepatitis (hepatitis C, hepatitis D, autoimmune, or heavy alcoholic liver disease) or human immunodeficiency virus positivity (n = 5), patients with previous interferon or oral NA therapy (n = 30), patients with no available DNA level was available at the beginning, end, or PT6 (n = 68), patients who were lost to follow-up (n = 10), and patients who discontinued treatment during the intended treatment period (n = 88, AEs [n = 30]; patient refusal [n = 28]; unknown causes [n = 18]; and suboptimal response [n = 12]). After excluding 201 patients, data from 439 patients was analyzed (Figure 1). For the safety analysis, a total of 527 patients were analyzed, including patients who were lost to follow-up (n = 10), who discontinued treatment (n = 88), and who were in the final cohort (n = 439).
FIGURE 1.

Study population. Among 640 patients who were being treated with PEG-IFN α-2a in 18 Korean hospitals, 201 patients were excluded, and data from 439 patients was analyzed.
This study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from each patient. The institutional review boards of each institute approved this study.
Laboratory Assay and Definitions
Complete blood count, HBeAg, antibody to HBeAg (anti-HBe), HBV DNA, and ALT levels were checked at baseline and every 4 to 12 weeks until the ET and at PT6. HBV DNA levels were quantified using the PCR assay of each respective institute; however, detection limits varied from 5 to 20 IU/mL. HBV DNA suppression was defined as serum HBV DNA less than 2000 IU/mL and VR was defined as serum HBV DNA less than 20 IU/mL, both of which were assessed at ET and PT6. For defining normalization of ALT levels, a threshold of 40 IU/L was used as the upper normal limit (UNL). HBeAg seroconversion was defined as the loss of HBeAg with the development of anti-HBe on at least 2 consecutive follow-up evaluations. HBsAg seroconversion was defined as the loss of HBsAg with the development of antibodies to HBsAg (anti-HBs). AE was defined as any abnormal physical sign, symptom, or disease associated with the patients’ treatment with PEG-IFN based on Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0., and SAE was defined as any AE that resulted in death, was life-threatening, required prolonged hospitalization, or caused persistent and significant disability or incapacity. In case of laboratory abnormalities (ie, ALT elevation, neutropenia, or thrombocytopenia), PEG-IFN was reduced in dosage or discontinued depending upon the clinicians’ decisions.
Study Endpoints
Treatment responses at ET and PT6 were compared between patients who were treated for 24 weeks versus 48 weeks. The primary endpoint was HBV DNA suppression at ET. The secondary endpoints included VR, HBsAg seroconversion, HBeAg seroconversion in HBeAg-positive patients, ALT normalization, and the development of AEs and SAEs.
Statistical Analysis
Data are expressed as means ± SD or medians (range) as appropriate. To compare the parameters between groups, the Student t test was used for continuous variables and the χ2 test was used for categorical variables. Univariate regression analyses were performed to evaluate the predictive factors for HBV DNA suppression at ET. Then, a multivariate regression analysis was performed using factors found to be significant by univariate analysis.
Data analysis was performed using SAS software version 18.0 (SAS Institute, Cary, NC) and 2-sided P values < 0.05 were considered significant.
RESULTS
Baseline Characteristics
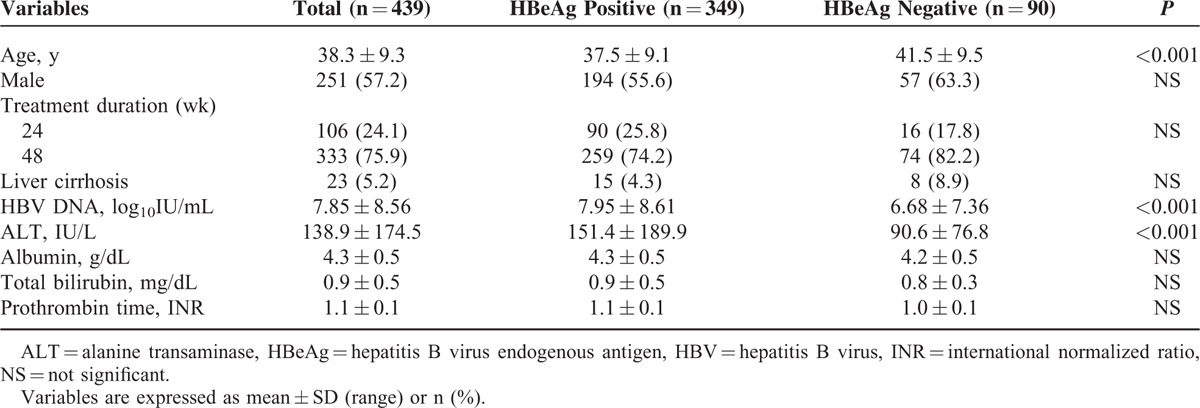
The baseline characteristics of the 439 patients who finished the PEG-IFN α-2a for the intended treatment period are summarized in Table 1. Three hundred forty-nine (79.5%) patients were HBeAg positive, and the other 90 (20.5%) patients were HBeAg negative. The mean age was 38 years and 57.2% of patients were male. Seventy-six percent of patients were treated for 48 weeks and 5.2% of patients had liver cirrhosis. Among the 349 patients with HBeAg positivity, 90 (25.8%) patients were treated for 24 weeks and 259 (74.2%) patients were treated for 48 weeks. Among patients who showed HBeAg negativity (n = 90), 16 (17.8%) patients were treated for 24 weeks and 74 (82.2%) patients were treated for 48 weeks. HBV DNA and ALT level were significantly higher in HBeAg-positive patients than in HBeAg-negative patients (HBV DNA; 7.95 ± 8.61 vs 6.68 ± 7.36 log10 IU/mL; P < 0.001, ALT; 151.4 ± 189.9 vs 90.6 ± 76.8 U/L; P < 0.001).
TABLE 1.
Baseline Characteristics
Efficacy Analysis
Response rates at ET and PT6 are depicted in Tables 2 and 3, respectively. In HBeAg-positive patients, HBV DNA suppression at ET was significantly greater in the 48-week treatment group compared to the 24-week treatment group (44.4% vs 36.7%, P = 0.035) (Figure 2A). VR at ET was also better achieved in patients who underwent the longer treatment regimen (48 weeks vs 24 weeks; 22.0% vs 11.1%, P = 0.029). At PT6, the HBV DNA suppression rate and VR decreased compared to ET, however, the 48-week treatment group showed significantly higher rates in these parameters than the 24-week treatment group (HBV DNA suppression, 35.9% vs 13.3%, P = 0.035; VR, 13.9% vs 8.9%, P = 0.004). The HBeAg seroconversion rate at ET of 18.1% in the 48-week treatment group was significantly higher than the 2.2% (P < 0.001) that occurred in the 24-week treatment group. This finding also continued at PT6 (29.0% vs 10.0%, P < 0.001). ALT normalization was also significantly higher in the 48-week treatment group compared to the 24-week treatment group at both ET and PT6 (ET, 57.1% vs 43.3%, P = 0.005; PT6, 71.4% vs 50.0%, P = 0.008). Collectively, the rate of achieving all targets, namely HBV DNA suppression, ALT normalization, and HBeAg seroconversion, at ET and PT6 was higher in the longer treatment group (48 weeks vs 24 weeks; at ET, 10.0% vs 2.2%; P < 0.001; at PT6, 13.5% vs 4.4%; P = 0.002). HBsAg loss was present in only 1 (0.4%) patient at ET and 2 patients (0.8%) at PT6 in the 48-week treatment group. In contrast, no patients achieved HBsAg loss at any time point in the 24-week treatment group.
TABLE 2.
Comparison of Response Rates Between 24 Weeks Versus 48 Weeks of Treatment at the End of Treatment
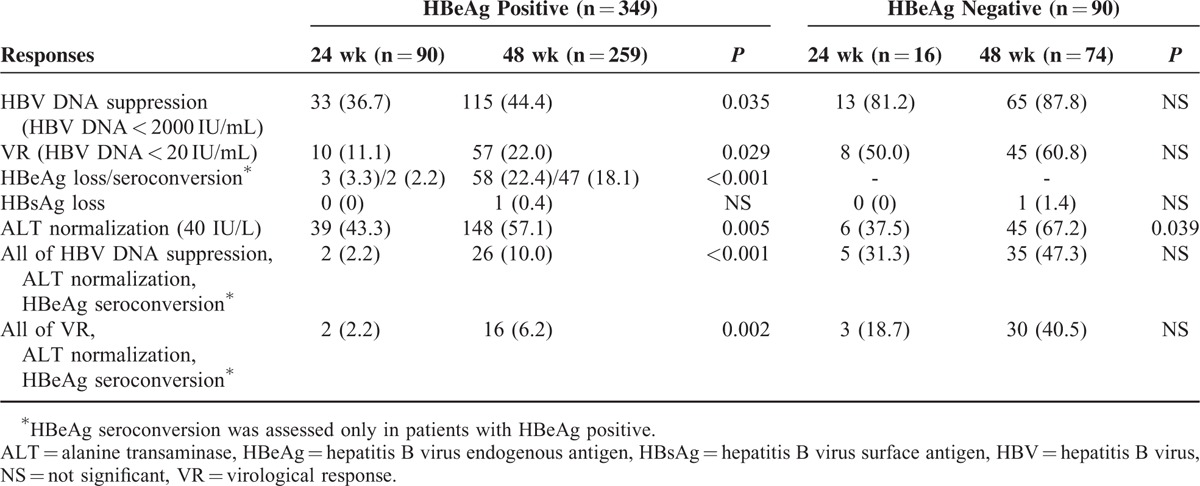
TABLE 3.
Comparison of Response Rates Between 24 Weeks Versus 48 Weeks of Treatment at 6 Months Posttreatment
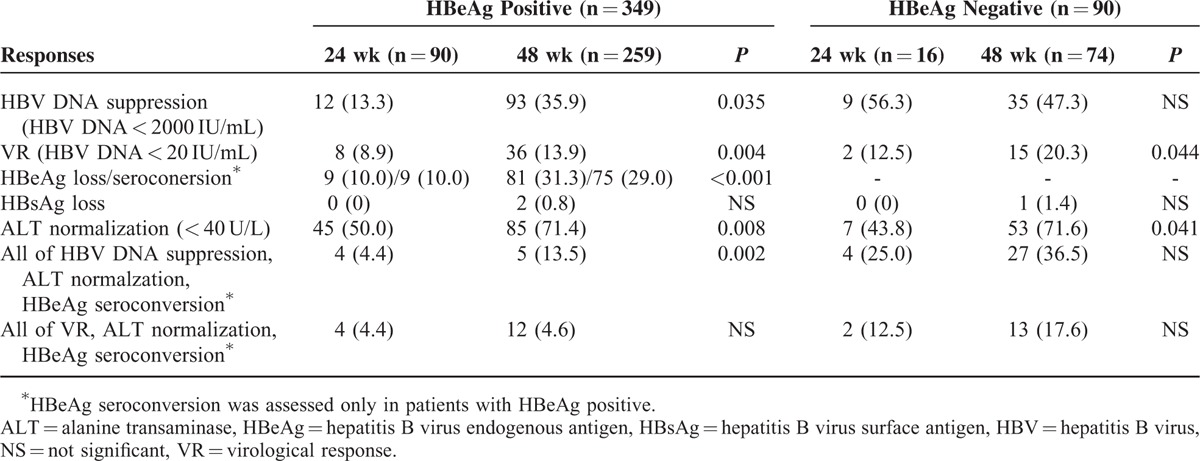
FIGURE 2.
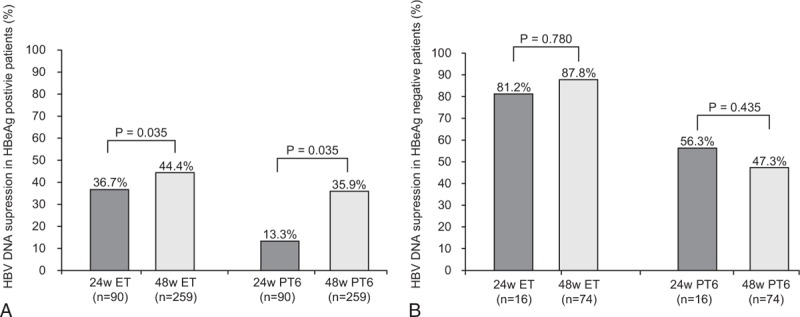
HBV DNA suppression according to treatment length at the end of treatment and at 6 months posttreatment in (A) HBeAg-positive patients and (B) HBeAg-negative patients. (A) In HBeAg-positive patients, HBV DNA suppression at ET and PT6 was significantly greater in the 48-week treatment group compared to the 24-week treatment group (at ET, 44.4% vs 36.7%, P = 0.035; at PT6, 35.9% vs 13.3%, P = 0.035). (B) In HBeAg-negative patients, 48-week treatment group compared to 24-week treatment group, did not show statistically superior treatment responses in terms of HBV DNA suppression (at ET, 87.8% vs 81.2%, P = 0.780; at PT6, 47.3% vs 56.3%, P = 0.435). ET, end of treatment; PT6, 6 months posttreatment.
Following 48 weeks of treatment in HBeAg-negative patients, HBV DNA suppression and VR at ET was higher than in HBeAg-positive patients (HBeAg-negative vs HBeAg positive; HBV DNA suppression, 87.7% vs 44.4%; VR, 60.8% vs 22.0%). In HBeAg-negative patients at ET, 48-week treatment group compared to 24-week treatment group, did not show statistically superior treatment responses in terms of HBV DNA suppression (87.8% vs 81.2%, P = 0.780) (Figure 2B) and VR (60.8% vs 50.0%, P = 0.437). Only the ALT normalization rate at ET was significantly higher in the 48-week treatment group compared to the 24-week treatment group (67.2% vs 37.5%, P = 0.039). At PT6, HBV DNA suppression was similar between 24-week treatment group and 48-week treatment group (56.3% vs 47.3%, P = 0.435). VR (20.3% vs 12.5%, P = 0.044), and ALT normalization (71.6% vs 43.8% P = 0.041) were significantly greater in the 48-week treatment group compared to the 24-week treatment group. In HBeAg-negative patients, only 1 (1.4%) patient in the 48-week treatment group showed HBsAg loss during the treatment period and at the PT6 time point.
Durability
At ET, 33 (36.7%) and 115 (44.4%) of the HBeAg-positive patients who were treated for 24 and 48 weeks, respectively, achieved HBV DNA suppression. Among them, 12 (36.6%) and 93 (35.9%) patients in each group showed sustained HBV DNA suppression at PT6. In HBeAg-negative patients, 9 out of 13 (69.2%) patients in the 24-week treatment group and 35 out of 65 (53.8%) patients in the 48-week treatment group maintained sustained response for 6 months after achieving HBV DNA suppression at ET.
The number of patients demonstrating HBeAg seroconversion increased from 2 (2.2%) at ET to 9 (10.0%) at PT6 in the 24-week treatment group. In the 48-week treatment group, among the 47 patients who achieved HBeAg seroconversion at ET, 2 experienced seroreversion at PT6. However, as 30 patients additionally achieved HBeAg seroconversion at 6 months after treatment, an overall of 75 (29.0%) patients possessed HBeAg seroconversion at PT6.
A total of 325 patients (24-week treatment group, n = 60; 48-week treatment group, n = 265) were followed for longer than PT 6. In the 24-week treatment group (HBeAg positive, n = 55; HBeAg negative n = 5), 6 (12.7%) HBeAg-positive patients and 3 (60%) HBeAg-negative patients maintained sustained HBV DNA suppression during the median follow-up time frame of 52 (range, 27–96) weeks and 48 (range, 30–48) weeks, respectively. At PT6, 6 out of 55 HBeAg-positive patients were HBeAg seroconverted and no further HBeAg seroconversion or reversion cases were noted during further follow-up periods.
In the 48-week treatment group (HBeAg positive, n = 202; HBeAg negative, n = 63), 59 (26.2%) HBeAg-positive patients and 19 (30.1%) HBeAg-negative patients maintained sustained HBV DNA suppression during the median follow-up periods of 96 (range, 60–144) weeks and 96 (range, 72–124) weeks, respectively. At PT6, 58 out of 202 (28.7%) patients were HBeAg seroconverted, however 8 (13.8%) of these patients showed HBeAg seroreversion during the subsequent follow-up period.
During the period between PT6 to the last follow-up, 218 out of 325 patients showed HBV DNA > 2000 IU/mL. Among them, 34 (15.8%) patients started entecavir for rescue therapy and 28 (82.3%) achieved VR within one year.
Predictive Factors for HBV DNA Suppression at ET
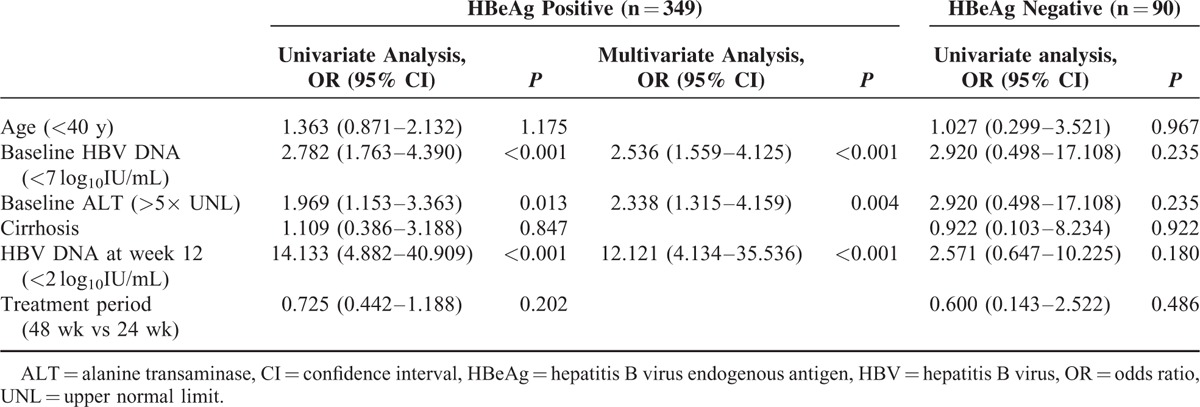
In HBeAg-positive patients, baseline HBV DNA (<7 log10IU/mL) and baseline ALT (>5× UNL) were predictive factors for HBV DNA suppression at ET. During treatment, HBV DNA at week 12 was another favorable predictor. These univariate predictors were entered into a multivariate logistic regression model. Ultimately, lower baseline HBV DNA (<7 log10IU/mL), higher baseline ALT (>5× UNL), and lower HBV DNA at week 12 (<2 log10IU/mL) were significantly associated with achieving HBV DNA suppression at ET with the following odds ratios: 2.54 (95% CI, 1.56–4.13) for baseline HBV DNA (<7 log10IU/mL vs >7 log10IU/mL), 2.34 (95% CI, 1.32–4.16) for baseline ALT level (>5× UNL vs <5× UNL), and 12.12 (95% CI, 4.13–35.54) for HBV DNA at week 12 (<2 log10IU/mL vs >2 log10IU/mL) (Table 4). No significant predictors for HBV DNA suppression at ET for HBeAg-negative patients were found.
TABLE 4.
Predictive Factors for the Suppression of HBV DNA (HBV DNA < 2000 IU/mL) at the End of Treatment
Safety
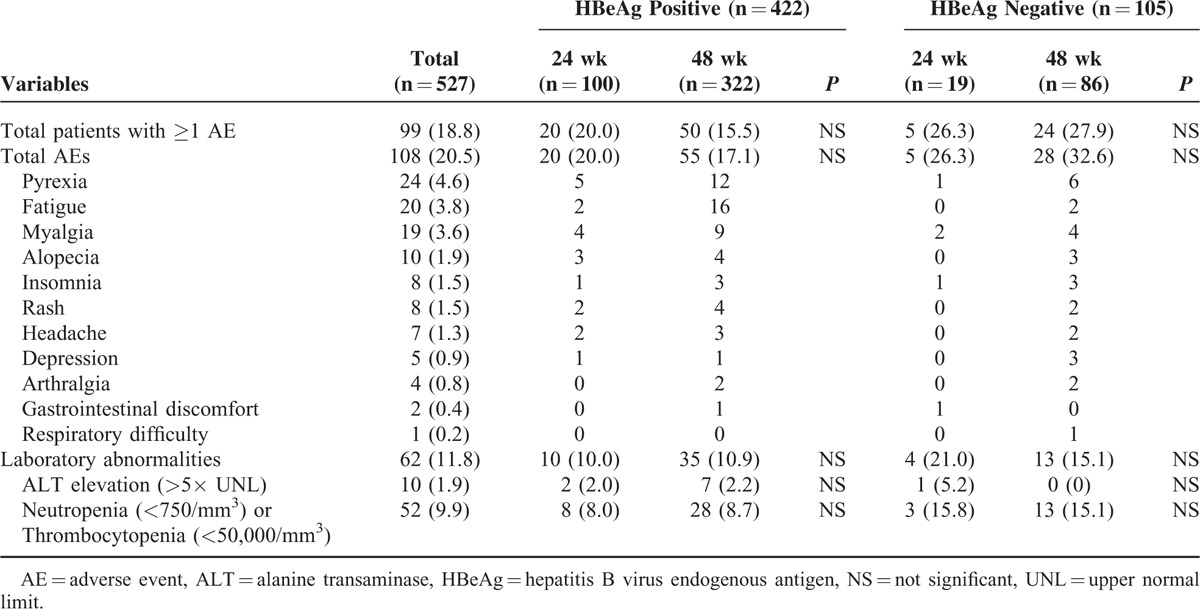
The safety profile of PEG-IFN α-2a was investigated in 527 patients (439 patients from the efficacy analysis plus 88 patients who discontinued treatment) (Table 5). The total number of AEs reported was 108 (20.5%) and the total number of patients who experienced at least 1 AE was 99 (18.8%). The most common AEs were as follows: pyrexia (4.6%), fatigue (3.8%), myalgia (3.6%), and alopecia (1.9%). No patients experienced SAEs such as death, life-threatening events, or disabilities. A total of 62 (11.8%) patients showed laboratory abnormalities (ALT elevation (>5× UNL) in 10 (1.9%) patients and neutropenia (<750/mm3) or thrombocytopenia (<50,000/mm3) in 52 (9.9%) patients). There was no patient who stopped PEG-IFN treatment because of ALT elevation more than 10× UNL, however, in 10 patients with ALT level of 5 to 10× UNL, PEG-IFN was stopped until recovery for median of 48 (19–113) days and was retried thereafter. In 52 patients with neutropenia (<750/mm3) or thrombocytopenia (<50,000/mm3), PEG-IFN was reduced from 180 μg/week to 135 μg/week, and then to 90 μg/week depending upon the patients’ clinical course. There was no patient who discontinued treatment ultimately due to laboratory abnormalities.
TABLE 5.
Safety Profiles of Pegylated Interferon α-2a
DISCUSSION
To date, PEG-IFN is accepted as a first-line drug for the treatment of naïve CHB patients because it has both direct antiviral activity and immunomodulatory action.5 Recently, all international guidelines have adopted the results of a large clinical trial (the NEPTUNE study), which proved that antiviral efficacy was maximized when PEG-IFN α-2a 180 μg/week was given for 48 weeks.4,12 However, in general, the outcomes of HBV treatment from “real-life” situations are believed to be different from those derived from large clinical trials because clinical practice involves various conditions including attenuated monitoring and poor compliance due to individual circumstances. Our present study validated the efficacy of PEG-IFN α-2a in a “real-life” setting and specifically investigated the appropriateness of 180 μg/week dosing and 48 week treatment periods in Korean naïve CHB patients. In addition, this study monitored certain key side effects of PEG-IFN α-2a that often cause clinicians and patients to hesitate when first considering its use. This multicenter study provided valuable insights regarding PEG-IFN therapy in Korean naïve CHB patients in a real clinical setting.
In HBeAg-positive patients, HBV DNA suppressions (HBV DNA < 2000 IU/mL) at ET and PT6 after 48 weeks of treatment, were 44.4% and 35.9%, respectively. Although direct comparison is difficult, these “real-life” results are comparable with those from a well-controlled phase III clinical trial by Lau et al9 (HBV DNA < 100,000 copies/mL; 52.0% at ET and 32.0% at PT6). HBeAg loss was also comparable between the 2 studies (this study vs Lau et al; 22.4% vs 27.0% at ET; 29.0% vs 32.0% at PT6). Contrary to the results of previous literature, which indicated that genotype C or B HBV is associated with poorer outcomes than genotype A HBV,13 treatment response rates in our Korean HBeAg-positive patients who were exclusively infected with genotype C HBV were not inferior to another large study by Lau et al, which enrolled 80% non-Asians with predominantly genotype A or D HBV. Moreover, HBeAg seroconversion and HBV DNA suppression rates in our study were not inferior to another large study by Janssen et al,14 which enrolled 80% non-Asians with predominantly genotype A or D HBV. When treatment periods (48 weeks vs 24 weeks) were compared in our study, outcomes at ET in terms of HBV DNA suppression, HBeAg loss/seroconversion, ALT normalization, and combined response were all significantly greater in the 48-week treatment group than in the 24-week treatment group. This tendency toward higher response rates with longer treatment time was also maintained at PT6. All of the above findings awaken expectations to follow the protocol of the NEPTUNE study11 and to use PEG-IFN α-2a at a dose of 180 μg/week for 48 weeks in the treatment of Korean HBeAg-positive CHB patients.
In HBeAg-negative patients, HBV DNA suppression was well achieved at ET after 48 weeks of treatment and this result was sustained at PT6. HBV DNA suppression after 48 weeks of treatment (87.8% at ET; 47.3% at PT6) was comparable to results seen in a large clinical trial by Marcellin et al10 (HBV DNA < 20,000 copies/mL; at ET, 81.0%; at PT6, 43.0%) and a previous study by Kwon et al,7 which included a small number of Koreans (HBV DNA < 2000 IU/mL; at ET, 71.4%; at PT6, 47.6%). In terms of HBV DNA suppression at ET and at PT6, 48-week treatment group showed similar results to 24-week treatment group. Considering the small number of sample size in HBeAg-negative patients, comparison of efficacy analyses between 48 and 24-week treatment of PEG-IFN α-2a in Korean HBeAg-negative CHB patients should be investigated in the future study.
The HBeAg seroconversion rate further increased during the 6 months after stopping treatment (2.2–10.0% in the 24-week treatment group, 18.1–29.0% in the 48-week treatment group), suggesting high durability of PEG-IFN. In a large study by Buster et al,5 HBeAg negativity was sustained for 3 years in 81% patients who showed HBeAg negativity at 24 weeks post-PEG-IFN treatment. Similarly, in HBeAg-negative patients, the biochemical and VR were sustained for 3 years in approximately 25% of patients who were given a 48-week course of PEG-IFN α-2a.15 In short, PEG-IFN α-2a provided a more favorable sustained posttreatment response than NA, probably due to the drug's off-treatment immune control over HBV.16 Moreover, the HBsAg clearance that is induced by PEG-IFN therapy will be associated with long-term complication-free survival,15 supporting the use of PEG-IFN α-2a therapy as a primary option for the treatment of CHB patients.
However, antiviral efficacy of PEG-IFN in terms of HBV DNA suppression is moderate compared to that of third generation NAs such as ETV17–19 or TDF20–22 (VR at week 48 in naïve HBeAg-negative patients treated with ETV, 88–99%; TDF, 89–95%; VR at week 48 in naïve HBeAg-positive patients treated with ETV, 48–75%; TDF, 59–76%). In addition, uncomfortable subcutaneous injection and frequent need of laboratory monitoring made clinicians to use PEG-IFN only for selected patients. The desire to determine which patients would derive the most benefit from PEG-IFN α-2a therapy drove clinicians to investigate predictors for favorable VR. In HBeAg-negative patients, a study by Bonino et al23 showed high baseline ALT, low baseline HBV DNA, younger age, female gender, and genotype B or C rather than genotype D as independent predictors of a combined ALT and HBV DNA response at 24 weeks posttreatment. In the PARC trial, which included 102 genotype D dominant HBeAg-negative CHB patients, no decline in HBsAg level plus no reduction in HBV DNA ≥ 2 log10 level at week 12 was established as a stopping rule for PEG-IFN α-2a therapy24; this criteria was also externally validated in subsequent studies.25,26 In HBeAg-positive patients, low baseline HBV DNA, the presence of the precore G1896A mutation or the basal core promoter A1762T/G1764A mutation,27 and HBV genotype B rather than genotype C,28 are known to be associated with good PEG-IFN response. In addition, declines in HBsAg and HBeAg levels have also been reported as on-treatment predictors.29,30 In our study, baseline low HBV DNA, high ALT level, and low HBV DNA at week 12 (<2 log10IU/mL) turned out to be significant predictors for achieving HBV DNA suppression at ET in Korean HBeAg-positive patients. Thus, HBV DNA and ALT should be measured before treatment and taken into account when considering the initiation of PEG-IFN. Also, HBV DNA at week 12 should be checked and factored into the decision of whether to continue therapy. In our study, 82.3% (28 of 34) of patients who did not achieve HBV DNA suppression after completion of PEG-IFN therapy and started entecavir achieved VR within 1 year, suggesting the possible effectiveness of sequential PEG-IFN and NA treatment. In a study by Iannazzo et al,31 the cost-effectiveness of applying the 12-week HBV DNA/HBsAg stopping rule for PEG-IFN therapy in HBeAg-negative CHB and switching to an effective NA treatment was demonstrated. The effectiveness of response-guided first-line treatment with PEG-IFN followed by a switch to NA, which enables individualized treatment for CHB, should be further validated in future studies.
Regarding safety, there was no difference between the 48-week treatment group and the 24-week treatment group in the total number or the character of AEs, suggesting that the 48-week PEG-IFN treatment was not only more efficient but also carried no increased risk of AEs in Koreans. No SAE was reported up to 6 months after treatment in our study cohort. Compared with studies that included patients of various races, the prevalence of AE and the proportion of moderate to severe laboratory abnormalities were lower in Koreans, suggesting that Koreans may tolerate the PEG-IFN-based treatment better than patients in other race groups.9,10,14,32 However, the profile of AE was similar between our study and other studies and systemic symptoms such as pyrexia, fatigue, and myalgia were the most common complaints.9,10,14,32
This study has several limitations, which are delineated as follows: As this is a retrospective, multicenter study, many applicants were lost during enrollment and follow-up. In addition, these data did not reflect the typical response rates and safety profile of that have been seen in primary and secondary hospitals of Korea. However, this study is the largest study that enrolled many homogenous Korean CHB patients with genotype C and demonstrated efficacy and safety in a real-life setting during the designated follow-up period of PT6. Therefore, it is our hope that future studies will investigate the same primary and secondary endpoints during extended follow-up periods as well as the clinical course and treatment options for patients with virological breakthrough after PEG-IFN treatment.
In conclusion, the efficacy and the safety of PEG-IFN in a real-world situation were equivalent to results seen in clinical trials. In addition, higher HBV DNA suppression was achieved in the 48-week treatment group than in the 24-week treatment group without additional risk of AEs in HBeAg-positive CHB patients. However, considering the moderate efficacy of PEG-IFN α-2a in terms of direct viral suppression, PEG-IFN α-2a should be carefully applied to selected naïve Korean CHB patients. Pretreatment and on-treatment predictors such as baseline HBV DNA, baseline ALT level, or HBV DNA during treatment will allow individualization of therapy and should be further validated in the future in order to maximize beneficial patient outcomes.
Acknowledgments
The authors are grateful to Prof. Do young Kim and Prof. Seung Up Kim from Yonsei University for their help in enrollment of subjects. We also appreciate Dong-Su Jang (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the figures.
Footnotes
Abbreviations: AE = adverse event, ALT = alanine transaminase, anti-HBe = antibody to HBeAg, anti-HBs = antibodies to HBsAg, CHB = chronic hepatitis B, CTCAE = Common Terminology Criteria for Adverse Events, ET = end of treatment, HBeAg = hepatitis B virus endogenous antigen, HBsAg = hepatitis B virus surface antigen, HBV = hepatitis B virus, NA = nucleos(t)ide analogs, PEG-IFN α-2a = pegylated interferon alpha-2a, PT6 = 6 months posttreatment, SAE = serious adverse event, UNL = upper normal limit, VR = virological response.
This study was supported by partial research grants from F Hoff mann-La Roche (Korea).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung KS, Kim SU, Song K, et al. Validation of hepatitis B virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology 2015; 62:1757–1766. [DOI] [PubMed] [Google Scholar]
- 4.EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185. [DOI] [PubMed] [Google Scholar]
- 5.Buster EH, Flink HJ, Cakaloglu Y, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology 2008; 135:459–467. [DOI] [PubMed] [Google Scholar]
- 6.Kao JH. HBeAg-positive chronic hepatitis B: why do I treat my patients with pegylated interferon? Liver Int 2014; 34 Suppl 1:112–119. [DOI] [PubMed] [Google Scholar]
- 7.Kwon JH, Kim YS, Kim SG, et al. The efficacy and safety of peginterferon-alpha-2a in Korean patients with chronic hepatitis B: a multicenter study conducted in a real clinical setting. Gut Liver 2013; 7:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooksley WG, Piratvisuth T, Lee SD, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat 2003; 10:298–305. [DOI] [PubMed] [Google Scholar]
- 9.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005; 352:2682–2695. [DOI] [PubMed] [Google Scholar]
- 10.Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004; 351:1206–1217. [DOI] [PubMed] [Google Scholar]
- 11.Liaw YF, Jia JD, Chan HL, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology 2011; 54:1591–1599. [DOI] [PubMed] [Google Scholar]
- 12.Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 2012; 6:531–561. [DOI] [PubMed] [Google Scholar]
- 13.Buster EH, Hansen BE, Lau GK, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 2009; 137:2002–2009. [DOI] [PubMed] [Google Scholar]
- 14.Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005; 365:123–129. [DOI] [PubMed] [Google Scholar]
- 15.Marcellin P, Bonino F, Lau GK, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology 2009; 136:2169–2179.e2161-2164. [DOI] [PubMed] [Google Scholar]
- 16.Ratnam D, Dev A, Nguyen T, et al. Efficacy and tolerability of pegylated interferon-alpha-2a in chronic hepatitis B: a multicenter clinical experience. J Gastroenterol Hepatol 2012; 27:1447–1453. [DOI] [PubMed] [Google Scholar]
- 17.Zoutendijk R, Reijnders JG, Brown A, et al. Entecavir treatment for chronic hepatitis B: adaptation is not needed for the majority of naive patients with a partial virological response. Hepatology 2011; 54:443–451. [DOI] [PubMed] [Google Scholar]
- 18.Yuen MF, Seto WK, Fung J, et al. Three years of continuous entecavir therapy in treatment-naive chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol 2011; 106:1264–1271. [DOI] [PubMed] [Google Scholar]
- 19.Ono A, Suzuki F, Kawamura Y, et al. Long-term continuous entecavir therapy in nucleos(t)ide-naive chronic hepatitis B patients. J Hepatol 2012; 57:508–514. [DOI] [PubMed] [Google Scholar]
- 20.Petersen J, Heyne R, Mauss S, et al. Effectiveness and safety of tenofovir disoproxil fumarate in chronic hepatitis B: a 3-year prospective field practice study in Germany. Dig Dis Sci 2015. [DOI] [PubMed] [Google Scholar]
- 21.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455. [DOI] [PubMed] [Google Scholar]
- 22.Ahn SS, Chon YE, Kim BK, et al. Tenofovir disoproxil fumarate monotherapy for nucleos(t)ide-naive chronic hepatitis B patients in Korea: data from the clinical practice setting in a single-center cohort. Clin Mol Hepatol 2014; 20:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonino F, Marcellin P, Lau GK, et al. Predicting response to peginterferon alpha-2a, lamivudine and the two combined for HBeAg-negative chronic hepatitis B. Gut 2007; 56:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rijckborst V, Hansen BE, Cakaloglu Y, et al. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology 2010; 52:454–461. [DOI] [PubMed] [Google Scholar]
- 25.Goulis I, Karatapanis S, Akriviadis E, et al. On-treatment prediction of sustained response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B patients. Liver Int 2015; 35:1540–1548. [DOI] [PubMed] [Google Scholar]
- 26.Rijckborst V, Hansen BE, Ferenci P, et al. Validation of a stopping rule at week 12 using HBsAg and HBV DNA for HBeAg-negative patients treated with peginterferon alfa-2a. J Hepatol 2012; 56:1006–1011. [DOI] [PubMed] [Google Scholar]
- 27.Yang HC, Chen CL, Shen YC, et al. Distinct evolution and predictive value of hepatitis B virus precore and basal core promoter mutations in interferon-induced hepatitis B e antigen seroconversion. Hepatology 2013; 57:934–943. [DOI] [PubMed] [Google Scholar]
- 28.Wai CT, Chu CJ, Hussain M, et al. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology 2002; 36:1425–1430. [DOI] [PubMed] [Google Scholar]
- 29.Sonneveld MJ, Rijckborst V, Boucher CA, et al. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology 2010; 52:1251–1257. [DOI] [PubMed] [Google Scholar]
- 30.Sonneveld MJ, Hansen BE, Piratvisuth T, et al. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology 2013; 58:872–880. [DOI] [PubMed] [Google Scholar]
- 31.Iannazzo S, Coco B, Brunetto MR, et al. Individualized treatment of HBeAg-negative chronic hepatitis B using pegylated interferon-alpha2a as first-line and week-12 HBV DNA/HBsAg stopping rule: a cost-effectiveness analysis. Antivir Ther 2013; 18:623–633. [DOI] [PubMed] [Google Scholar]
- 32.Buster EH, Hansen BE, Buti M, et al. Peginterferon alpha-2b is safe and effective in HBeAg-positive chronic hepatitis B patients with advanced fibrosis. Hepatology 2007; 46:388–394. [DOI] [PubMed] [Google Scholar]


