Supplemental Digital Content is available in the text
Abstract
Malaria and human immunodeficiency virus (HIV) infections are globally important public health concerns. The objectives of this study were (i) to determine the prevalence of malaria and HIV co-infections in people living in endemic countries, and (ii) to assess the effect of co-infection on anemia.
Studies were searched on electronic databases including PubMed, Embase, Medline, Google Scholar, and African Journals Online. Observational studies, assessing the prevalence of co-infection and reporting its association with anemia, were included. The methodological quality of included studies was assessed using a tool called the risk of bias assessment for non-randomized studies. Heterogeneity among studies was investigated with the I-square test. Pooled prevalence of the co-infection and its 95% confidence interval (CI) were estimated using the random-effect model, reflected on heterogeneity among studies. Summary odds ratio (OR), summary standardized mean difference (SMD), and their corresponding 95% CIs were estimated, as appropriate. Subgroup analysis and meta-regression were performed for robustness of results. Publication bias was assessed by visualization of a funnel plot.
Twenty-three studies were included in the present study. Overall, the pooled prevalence of co-infection was 19% (95% CI: 15–23%, I2: 98.1%), showing 26% (95% CI: 20–32%, I2: 98.7%) in adults, 12% (95% CI: 7–17%, I2: 95.0) in pregnant women, and 9% (95% CI: 6–11%, I2: 68.6%) in children. Anemia was comparable between the monoinfected and co-infected adults (summary OR: 1.49, 95% CI: 0.93–2.37) and increased by 49% in co-infected pregnant women (summary OR: 1.49, 95% CI: 1.14–1.94). The mean hemoglobin concentration was significantly lower in the co-infected group than the monoinfected group (summary SMD: −0.47, 95% CI: −0.61 to −0.33). The results of meta-regression on the prevalence of co-infection using the publication year and total population as covariates showed the I2 value remained high implying a de facto random distribution of heterogeneity. An asymmetrical funnel plot indicated the presence of publication bias. Due to heterogeneity of the studies in this review, the results have to be interpreted with caution.
The findings of this study suggest that the prevalence of malaria and HIV co-infection, particularly in pregnant women, requires special attention from healthcare personnel. Better understanding of the co-infection is crucial for designing treatment strategies. Future well-powered, prospective designs assessing the interaction between malaria and HIV are recommended.
INTRODUCTION
A substantial progress in malaria control has already been achieved in 55 of the 106 endemic countries in the context of target 6 in Millennium Development Goal. However, malaria remains endemic in all 6 World Health Organization (WHO) regions and the heaviest burden is in the African Region which claimed ∼ 90% of all malaria deaths.1 Sub-Saharan Africa also accounted for an estimated 25 million adults and children living with HIV/AIDS. Globally, malaria and HIV are the 2 major health problems and together they contribute to >4 million deaths per year.2 People residing in regions where HIV and malaria are endemic are prone to develop co-infection. As such, there is a growing interest in whether this dual infection intensifies each other and attributes to complications in the clinical spectra and treatment outcomes.
Anemia is a significant consequence of malaria infection in terms of morbidity. Co-infection with HIV could lead to worsening of anemia through nutritional or immunological interactions. Empirical studies on malaria-HIV co-infection and particularly its effects on anemia showed varied results. Some studies reported this dual infection adversely affects maternal hemoglobin (Hb) concentration,3 whereas others reported differently.4–6 There is limited information on the collective impact of malaria-HIV co-infection on the Hb levels in general population.7 Hence, a better understanding of the impact of co-infections could help in prevention and control of malaria. Earlier reviews in this field were either merely narrative8,9 or not up-to-date.10 However, since there has been a continuous flow of publications on the co-infection in endemic areas, it is timely to synthesis evidence by a meta-analysis.11 Taken together, the objectives of the present study were (i) to determine the prevalence of malaria and HIV co-infections in people living in endemic countries, and (ii) to assess the effect of co-infection on anemia.
METHODS
The present study was carried out following the guideline on Meta-analyses of Observational Studies in Epidemiology (MOOSE)12 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)13 (See supplemental checklist). The protocol of this study is available in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42015020248).
Study Search
Studies on the co-infection between malaria and HIV up to the end of June 2015 were searched in databases such as Embase Medline, Global Health, Google scholar, Scientific Electronic Library Online (SciELO), and African Journals Online (AJOL). To maximize the search, only 2 subject headers were used: malaria and HIV or AIDS and co-infections. Subsequently, specific terms such as plasmodium and HIV/AIDS were used. Only malaria and HIV were used for the search in SciELO and AJOL. Only studies on humans, published in English language were considered. The references of the identified papers and relevant reviews were reviewed to track any study that had not been captured by the electronic search.
Selection of Studies
Studies were included if they were (i) done on participants co-infected with malaria and HIV, and (ii) observational studies which compared the outcomes of anemia between those who were co-infected with malaria and HIV and those with monoinfection. To be eligible, malaria was confirmed by microscopy of Giemsa-stained blood films or rapid-onsite diagnostic test. Subsequent PCR-based analysis for confirmation of the species was advantageous, if done. Severe falciparum malaria and anemia were defined according to the WHO guideline.14,15 Anemia was defined as Hb < 13 g/dL in men and <12 g/dL in women, whereas severe anemia as Hb < 7 g/dL in adults and <8 g/dL in children.15 Placental malaria was diagnosed, based on analysis of placenta blood, either by PCR, by blood smear or both. Anemia defined in terms of low packed cell volume (PCV) or hematocrit (HCT) was accepted. If Hb concentration were specified as <5 g/dL or 11 g/dL in the same group, then it was collectively grouped under Hb < 11 g/dL. HIV was confirmed by an HIV RNA analysis/concentration, CD4+ cell counts, and rapid-onsite immunochromatographic test (RDT). For case-control studies, controls were not to be co-infected either with HIV or malaria, or vice versa. HIV in this study is HIV-1. Studies which did not meet the inclusion criteria were excluded.
Data Extraction and Quality Assessment
Two authors independently screened the citations, retrieved articles potentially eligible for the present study and extracted data from the studies, using a piloted data extraction sheet. Any discrepancies were resolved by consensus. The 2 investigators independently determined the quality of studies following the risk of bias assessment for nonrandomized studies (RoBANS) checklist, which consists of 6 domains.16
Statistical Analysis
For the variance stabilizing transformation of the proportions in estimating the prevalence of malaria and HIV co-infection, the Freeman–Tukey double arcsine transformation method was used as described elsewhere17 and metaprop command in Stata. Details of this Stata command are available elsewhere.18 Data were pooled using DerSimonian and Laird random-effect model, according to the results of heterogeneity testing.19 For pooling of effect estimates for the effect of co-infection on anemia, summary odds ratio (OR) and its 95% CI for dichotomous data, and summary standardized mean difference (SMD) and its standard deviation (SD) for continuous data were used. The heterogeneity between these studies was assessed with the I2 test. An I2 value of >50% indicated substantial heterogeneity. For pooling of the results, a more conservative random-effect model was used as heterogeneity was substantial.20
Sensitivity Analysis
The prevalence of co-infections was stratified into infants, children, adults, and pregnant mothers. Due to paucity of data, stratification by Plasmodium species (P vivax, P falciparum, and mixed infections), CD4 counts in HIV, parasite density, or by gravidae were not possible. To account for within-study heterogeneity, metaregression on the prevalence of co-infection was performed using the publication year and total population as covariates. As a minimum number of 10 studies were available to investigate the publication bias,20 a funnel plot was drawn with data of the effect size on anemia. This includes the regression line corresponding to the regression test for funnel-plot asymmetry.21 Data entry was done in spreadsheet and analysis was with STATA 14.0 (Stata Corp, Txt). This study was approved by the Institutional Ethical Review Board ([BMS1–1/2015 [05]).
RESULTS
The study selection process was shown in the flowchart (Figure 1). The initial search yielded 2109 citations, of which 47 potentially met the inclusion criteria. A final of 23 studies (with 24 datasets) (n = 9159) were included in quantitative synthesis. Around one-third (37.5%) of the included studies were published between 2013 and 2015.
FIGURE 1.
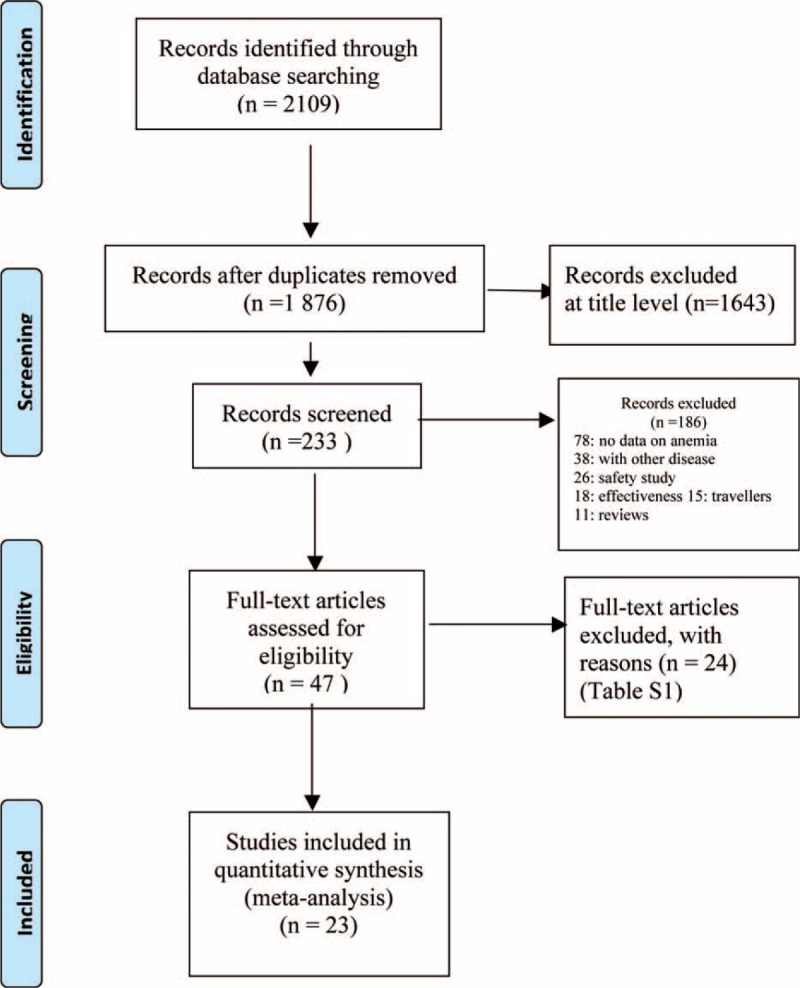
PRISMA flow diagram indicating the study selection process.
Characteristics of the Included Studies
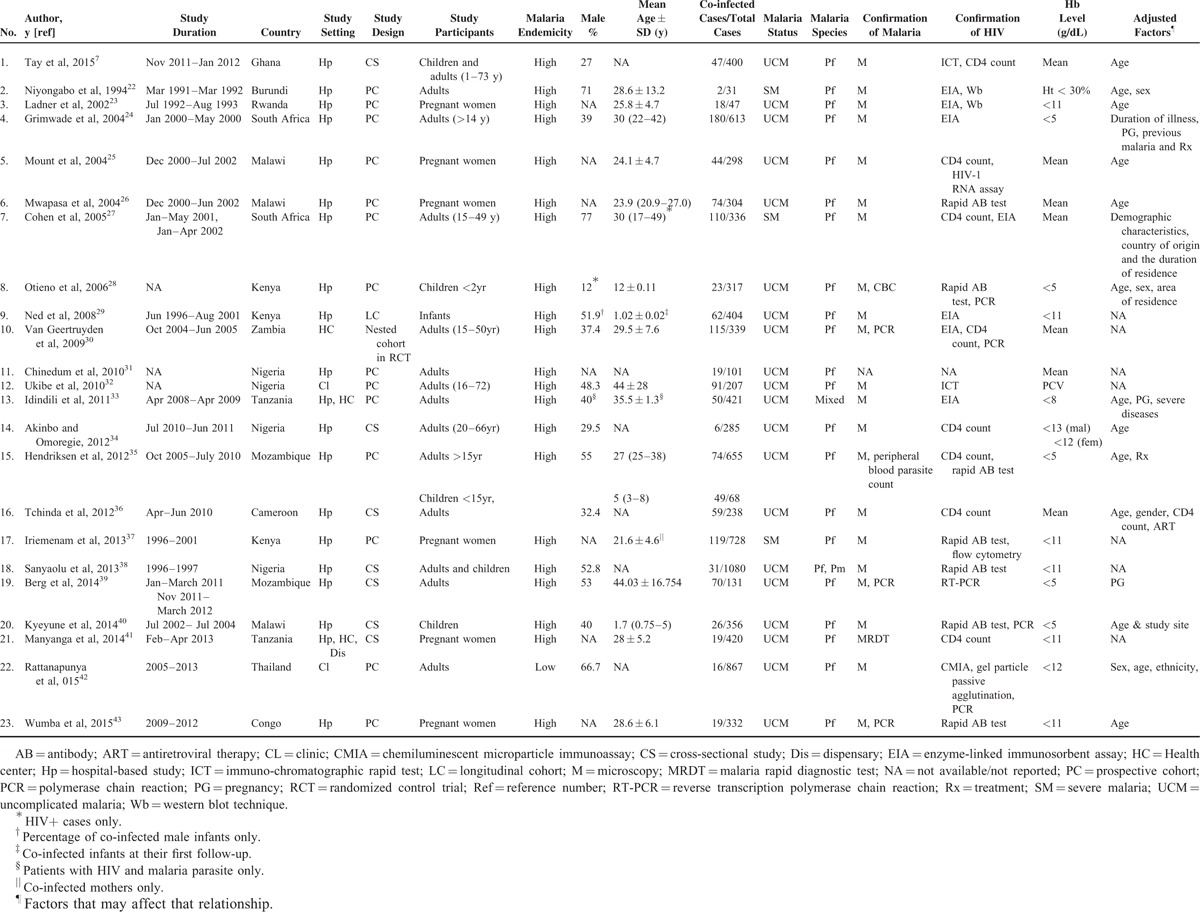
The main characteristics of the 23 included studies7,21–43 were presented in Table 1. Among the included studies, all but one study were carried out in the African countries. The remaining 1 was from Thailand in the WHO South-East Asia region.42 A summary of the 24 excluded studies were provided44–63 (see, Supplemental Table 1). Sixteen of the 23 included studies used cohort designs; of them, 13 studies were prospective cohort designs, 2 studies were longitudinal cohort studies, and 1 study was nested-cohort study. Malaria transmission was high in all these African countries where studies in this review were done. Thailand has low malaria transmission potential. Many studies showed low or unclear risk of bias (see, Supplemental Table 2).
TABLE 1.
Characteristics of the Included Studies
Prevalence of Malaria and HIV Co-Infection
All 23 studies included in the present review provided data on prevalence of co-infection. Among these, 13 studies reported prevalence of HIV in malaria-infected patients,22–24,27,28,30,35,37–40,42,43 whereas 10 studies on prevalence of malaria in HIV positives.7,25,26,29,31–34,36,41
Overall, prevalence of co-infection among individual studies ranged between 2% and 72% and pooled prevalence of co-infection was 19% (95% CI: 15–23%, I2: 98.1%). The pooled analysis of 13 studies with HIV in malaria yielded a prevalence of 22% (95% CI: 16–27%, I2: 98.5%). The pooled analysis of 10 studies with malaria in HIV-infected individuals yielded a prevalence of 16% (95% CI: 10–22%, I2: 96.8%) (Figure 2).
FIGURE 2.
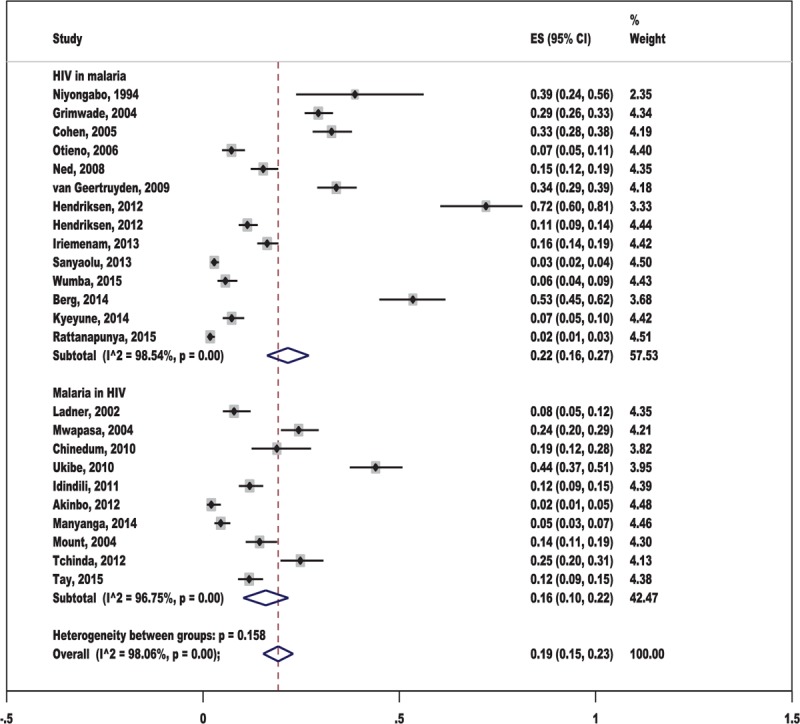
Forest plot showing overall prevalence of co-infections.
Anemia in Co-infection
All studies measured anemia in terms Hb except for 2 which measured in terms of PCVs32 and hematocrit.22 Nine studies (with 10 datasets) compared mean Hb levels between those who had dual infection and those who had malaria or HIV infection.7,25,26,30–32,35,36,40 Thirteen studies compared proportions of anemia between those who were co-infected and those who had monoinfected.22,24,27–29,33,34,37–39,41–43 Anemia was comparable between the monoinfected and co-infected adults (summary OR: 1.49, 95% CI: 0.93–2.37) and increased by 49% in co-infected pregnant women (summary OR: 1.49, 95% CI: 1.14–1.94) (Figure 3). Only 1 study on children reported that anemia was 5.5 times more in the co-infected group.28
FIGURE 3.
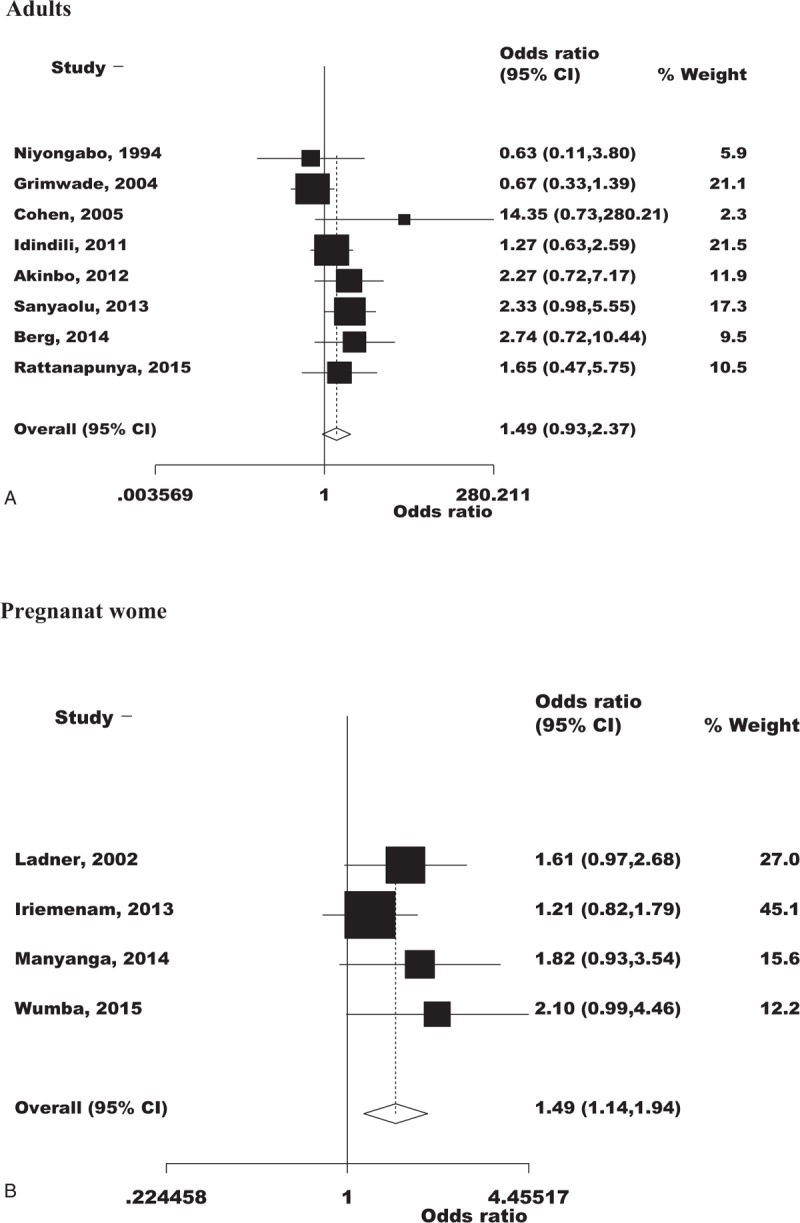
Overall anemia comparing between the co-infection and the monoinfection groups.
When stratified by infection groups, studies with malaria in HIV showed significant lower level of Hb concentrations in the co-infected group than in the individuals infected with malaria alone (summary SMD:−0.47, 95% CI:−0.61 to −0.33). On further breakdown, this was (summary SMD: −0.33, 95% CI: −0.51 to −0.14) among adults and (summary SMD: −0.66, 95% CI: −0.87 to −0.45) among pregnant women (Figure 4). As expected, a fall in Hb concentration was more pronounced in pregnant women than in the nonpregnant adults.
FIGURE 4.
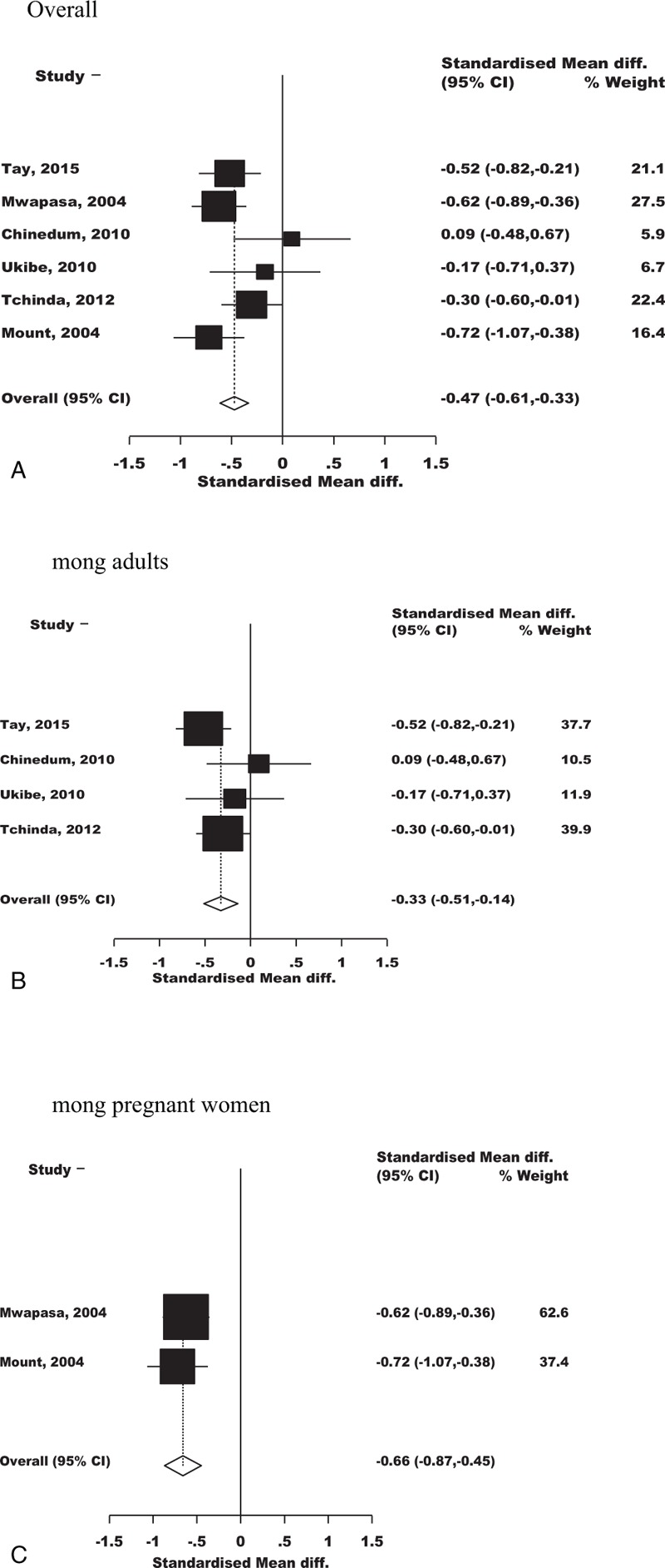
Forest plot indicating the overall effect of malaria in HIV on anemia.
Subgroup Analysis on Prevalence of Co-infection
Studies identified for the current analysis were done in different subgroups. By age groups, prevalence of co-infection was 26% (95% CI: 20–32%, I2: 98.7%) in adults, 12% (95% CI: 7–17%, I2: 95.0) in pregnant women and 9% (95% CI: 6–11%, I2: 68.6%) in children (see, Supplemental Figure 1, which shows prevalence of co-infections stratified by age groups). Only 1 study on infants reported the prevalence of co-infection was 15% (95% CI: 12–19%).29 The prevalence of co-infection among severe/complicated malaria cases was 28% (95% CI: 14–42%, I2: 94.5%) and 18% (95% CI: 14–22%, I2: 98.0%) among uncomplicated malaria cases (data not shown).
The metaregression plots obtained show that the publication year of the included studies could explain only 4.1% of the heterogeneity (figure not shown) and size of the denominator population of the included studies could attribute to 29.2% of the heterogeneity (Figure 5). The funnel plot of studies assessing effect on anemia showed an asymmetrical plot in the presence of publication bias among studies. This might be a result of the smaller studies producing smaller effect size21 (Figure 6).
FIGURE 5.
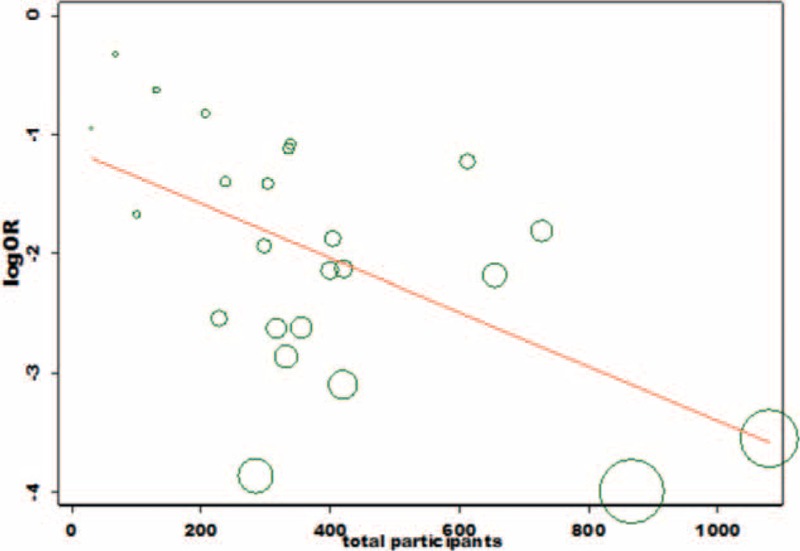
Meta-regression plot on prevalence of co-infection and size of denominator population.
FIGURE 6.
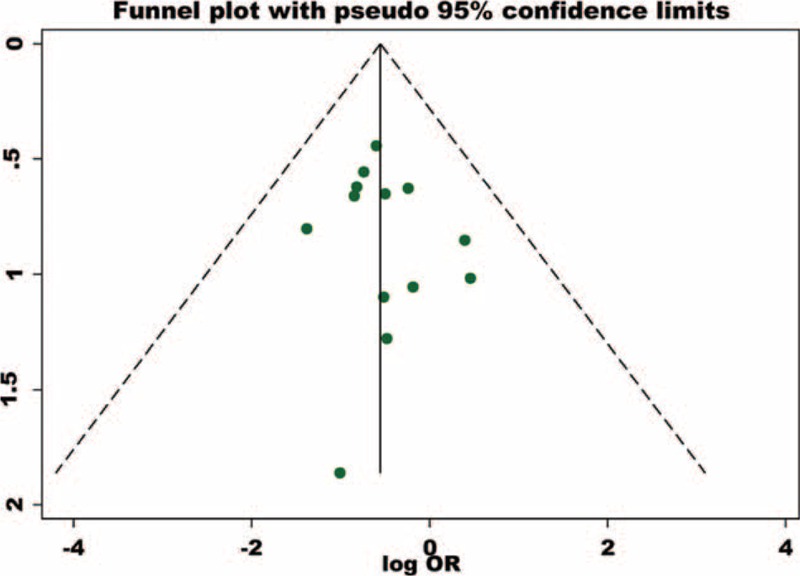
Asymmetrical funnel plot in the presence of publication bias.
DISCUSSION
The current meta-analysis provides information on the prevalence of malaria and HIV co-infections and its impact on anemia in endemic countries.
Prevalence of Co-infection
As shown, the prevalence of HIV in malaria cases was higher (16–27%) than that of the malaria in HIV-infected patients (15–23%). This implies that the 2 pathogens could interact synergistically in human hosts.64 Thus, the communities in these regions suffer a double burden of these 2 infections, with the higher burden being the HIV infections. HIV infection could impair immune responses to malaria parasites, leading to a decreased ability to control parasitemia,27 whereas malaria infection can modulate HIV progression65 and HIV RNA replication.29,64,66
It has been established that both humoral and cellular immune responses play protective roles against malaria infection.65 During a humoral response the HIV-associated decline in antibody responses to variant surface antigens (VSAs) could partially explain the increased susceptibility of HIV-infected pregnant women to malaria. Hence, HIV infection impairs antimalarial immunity, most notably in immunosuppressed women. This increases the susceptibility of women to malaria during pregnancy. Studies have documented that the placenta provide a unique and hospitable niche for HIV-1 replications.63,67
Effect on Anemia
The cause of anemia is multifactorial, involving a number of mechanisms. Both malaria and HIV could individually cause anemia; the present analysis has documented that anemia was comparable between the monoinfected and co-infected adults. Both infections can lead to loss of appetite. Furthermore, advanced HIV/AIDS cases may cause diseases of the gastrointestinal tract, leading to malabsorption of nutrients necessary for the formation of Hb, which subsequently results in anemia.58 Also, the ability of HIV to infiltrate the bone marrow68 could accentuate the progression of anemia in co-infected individuals.
It has been well established that the primary target of human plasmodium species is the red blood cells (RBCs). In both vivax and falciparum malaria, parasitized and possibly nonparasitized RBCs are hypothesized to be more fragile than RBCs in noninfected individuals and more prone to damage from shear stress.69 Malaria causes anemia through increased destruction of infected RBCs as well as uninfected RBCs.70
Studies had documented multiple factors that can contribute to ineffective erythropoiesis in severe malaria anemia including (i) hemozoin-induced repression of macrophage function, (ii) secretion of proinflammatory cytokines from macrophages, and (iii) perturbation of erythroblast metabolism with the resultant decrease in RBC output from the bone marrow.71 Details of these mechanisms are described elsewhere.70,71 An empirical study showed that HIV-infected multigravidae were less likely to clear infection than primigravidae. The altered immune-recognition in the setting of HIV could have contributed to it.59 This implies that in malaria endemic regions, anemia could be more pronounced in multigravidae women co-infected with HIV/AIDS.
Study Limitations
A small number of participants in some studies identified for this review may not be adequately powered to reach the statistically significant level between the co-infected group and the mono-infected group. Sampling bias could occur if the participant groups, whether HIV-positive and HIV-negative or malaria positive and malaria negative were recruited at different times of the year. It is also important to note antiretroviral therapy (ART) such as zidovudine may cause anemia in HIV/AIDS patients as well as malaria and HIV co-infected patients. Other confounding factor includes the possibility of co-medication effects and other comorbidity conditions (e.g., tuberculosis, hepatitis B, hepatitis C, diabetes mellitus, renal failure, and chronic liver disease) in the participants in primary studies. These factors should be kept in mind when interpreting the findings. Furthermore the quality of included studies varied because many of these studies were not primarily aimed to assess the effect of anemia in co-infections. This could have attributed to ascertainment bias among these studies. As there was publication bias, findings in the current analysis should be interpreted with caution.
Public Health Implications
Anemia caused by P falciparum remains the frequent indication for blood transfusion in Sub-Saharan Africa.72 As HIV can affect the course of malaria infection in many ways, screening of HIV for blood transfusion is important. Moreover, routine screening of pregnant women for both HIV and malaria at the first antenatal visits and education on malaria prevention (e.g., insecticide-treated nets and intermittent prophylactic treatment) should be initiated.
CONCLUSION
The findings of this study suggest that the prevalence of malaria and HIV co-infection, particularly in pregnant women, requires special attention from healthcare personnel. Better understanding of the co-infection is crucial for designing treatment strategies. Future well-powered, prospective designs assessing the interaction between malaria and HIV are recommended.
Supplementary Material
Supplementary Material
Acknowledgments
The authors are grateful to the participants and researches of the primary studies included in the current meta-analysis. They thankfully acknowledge the anonymous reviewers and editors for the comments and valuable inputs to improve the quality of our manuscript. They also thank the International Medical University, Kuala Lumpur, Malaysia for allowing them to perform this study [BMSI-1/2015 (05)].
Footnotes
Abbreviations: AJOL = African Journals Online, ART = antiretroviral therapy, CI = confidence interval, Hb = hemoglobin, HCT = haematocrit, HIV = human immunodeficiency virus, MOOSE = Meta-analyses of Observational Studies in Epidemiology, OR = odds ratio, PCV = packed cell volume, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses, PROSPERO = International Prospective Register of Systematic Reviews, RoBANS = Risk of Bias Assessment for Non-randomized Studies, SciELO = Scientific Electronic Library Online, SMD = standardised mean difference, VSA = variant surface antigen, WHO = World Health Organization.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Global technical strategy for malaria 2016–2030. World Health Organization [Online]. 2015 [cited 2015 Sept 12]; Available at: http://www.who.int/malaria/areas/global_technical_strategy/en/ (accessed September 12, 2015). [Google Scholar]
- 2.Malaria and HIV interactions and their implications for public health policy. World Health Organization [Online]. 2004 [cited 2015 Sept 12]; Available at: http://www.who.int/hiv/pub/prev_care/malariahiv.pdf (accessed September 12, 2015). [Google Scholar]
- 3.Guyatt HL, Snow RW. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am J Trop Med Hyg 2001; 64 (1–2 Suppl):36–44. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen-Dinh P, Greenberg AE, Mann JM, et al. Absence of association between Plasmodium falciparum malaria and human immune-deficiency virus infection in children in Kinshasa, Zaire. Bull World Health Organ 1987; 65:607–613. [PMC free article] [PubMed] [Google Scholar]
- 5.Cuadros DF, Branscum AJ, García-Ramos G. No evidence of association between HIV-1 and malaria in populations with low HIV-1 prevalence. PLoS One 2011; 6:e23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouala C, Houzé S, Guiguet M, et al. Imported malaria in HIV-infected patients enrolled in the ANRS CO4 FHDH study. J Acquir Immune Defic Syndr 2008; 49:55–60. [DOI] [PubMed] [Google Scholar]
- 7.Tay SCK, Badu K, Mensah AA, et al. The prevalence of malaria among HIV seropositive individuals and the impact of the co- infection on their hemoglobin levels. Ann Clin Microbiol and Antimicrob 2015; 14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewitt K, Steketee R, Mwapasa V, et al. Interactions between HIV and malaria in non-pregnant adults: evidence and implications. AIDS 2006; 20:1993–2004. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg L, Frean J. Malaria control in South Africa—challenges and successes. S Afr Med J 2007; 97:1193–1197. [PubMed] [Google Scholar]
- 10.Ter Kuile FO, Parise ME, Verhoeff FH, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in Sub-Saharan Africa. Am J Trop Med Hyg 2004; 71:41–54. [PubMed] [Google Scholar]
- 11.Petitti DB. Meta-analysis, Decisions Analysis and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. 2nd edNew York: Oxford University Press; 2000. [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PloS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). Severe falciparum malaria. Trans R Soc Trop Med Hyg 2000; 94:1–31. [PubMed] [Google Scholar]
- 15.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. World Health Organisation [Online]. 2011. [cited 2015 Sept 12]. Available at: http://apps.who.int/iris/bitstream/10665/85839/3/WHO_NMH_NHD_MNM_11.1_eng.pdf (accessed September 12, 2015). [Google Scholar]
- 16.Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013; 66:408–414. [DOI] [PubMed] [Google Scholar]
- 17.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist 1950; 21:607–611. [Google Scholar]
- 18.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. West Sussex, England: Wiley-Blackwell; 2011. [Google Scholar]
- 21.Egger M, Smith GD, Schneider M, et al. Bias in meta-anaysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niyongabo T, Deloron P, Aubry P, et al. Prognostic indicators in adult cerebral malaria: a study in Burundi, an area of high prevalence of HIV infection. Acta Trop 1994; 56:299–305. [DOI] [PubMed] [Google Scholar]
- 23.Ladner J, Leroy V, Simonon A, et al. HIV infection, malaria and pregnancy: a prospective cohort study in Kigali, Rwanda. Am J Trop Med Hyg 2002; 66:56–60. [DOI] [PubMed] [Google Scholar]
- 24.Grimwade K, French N, Mbatha DD, et al. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS 2004; 18:547–554. [DOI] [PubMed] [Google Scholar]
- 25.Mount AM, Mwapasa V, Elliott SR, et al. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet 2004; 363:1860–1867. [DOI] [PubMed] [Google Scholar]
- 26.Mwapasa V, Rogerson SJ, Molyneux ME, et al. The effect of Plasmodium falciparum malaria onperipheral and placental HIV-1 RNA concentrations in pregnant Malawian women. AIDS 2004; 18:1051–1059. [DOI] [PubMed] [Google Scholar]
- 27.Cohen C, Karstaedt A, Frean J, et al. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin Infect Dis 2005; 41:1631–1637. [DOI] [PubMed] [Google Scholar]
- 28.Otieno RO, Ouma C, Ong’echa JM, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS 2006; 20:275–280. [DOI] [PubMed] [Google Scholar]
- 29.Ned RM, Price AE, Crawford SB, et al. Effect of placental malaria and HIV infection on the antibody responses to Plasmodium falciparum in infants. J Infect Dis 2008; 198:1609–1619. [DOI] [PubMed] [Google Scholar]
- 30.van Geertruyden JP, van Eijk E, Yosaatmadja F, et al. The relationship of Plasmodium falciparum humeral immunity with HIV-1 immunosuppression and treatment efficacy in Zambia. Malar J 2009; 8:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chinedum CO, Chidiebere FE, Adamma RA, et al. Serum iron markers in HIV and HIV-malaria infected participants residing in malaria endemic area of South-Eastern Nigeria. Int J Biol Chem Sci 2010; 4:2409–2414. [Google Scholar]
- 32.Ukibe NR, Onyenekwe C, Ahaneku J, et al. Packed cell volume and serum iron in subjects with HIV-malaria co-infection in Nnewi, South-Eastern Nigeria. Int J Biol Chem Sci 2010; 4:471–478. [Google Scholar]
- 33.Idindili B, Jullu B, Hattendorft J, et al. HIV and parasitic co-infections among patients seeking care at health facilities in Tanzania. Tanzan J Health Res 2011; 13:75–85. [DOI] [PubMed] [Google Scholar]
- 34.Akinbo FO, Omoregie R. Plasmodium falciparum infection in HIV-infected patients on highly active antiretroviral therapy (HAART) in Benin City, Nigeria. J Rest Health Sci 2012; 12:15–18. [PubMed] [Google Scholar]
- 35.Hendriksen IC, Ferro J, Chhanganlal KD, et al. Diagnosis, clinical presentation, and in-hospital mortality of severe malaria in HIV-co-infected children and adults in Mozambique. Clin Infect Dis 2012; 55:1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tchinda GG, Atashili J, Achidi EA, et al. Impact of malaria on hematological parameters in people living with HIV/AIDS attending the Laquintinie Hospital in Douala, Cameroon. PLoS One 2012; 7:e40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iriemenam NC, Pandey JP, Williamson J, et al. Association between immunoglobulin GM and KM genotypes and placental malaria in HIV-1 negative and positive women in western Kenya. PLoS One 2013; 8:e53948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanyaolu AO, Fagbenro-Beyioku AF, Oyibo WA, et al. Malaria and HIV co-infection and their effect on haemoglobin levels from three health-care institutions in Lagos, Southwest Nigeria. Afr Health Sci 2013; 13:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg A, Patel S, Aukrust P, et al. Increased severity and mortality in adults co-infected with malaria and HIV in Maputo, Mozambique: a prospective cross-sectional study. PLoS One 2014; 9:e88257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kyeyune FX, Calis JC, Phiri KS, et al. The interaction between malaria and human immunodeficiency virus infection in severely anaemic Malawian children: a prospective longitudinal study. Trop Med Int Health 2014; 19:698–705. [DOI] [PubMed] [Google Scholar]
- 41.Manyanga VP, Minzi O, Ngasala B, et al. Prevalence of malaria and anaemia among HIV infected pregnant women receiving co-trimoxazole prophylaxis in Tanzania: a cross sectional study in Kinondoni Municipality. BMC Pharmacol Toxicol 2014; 15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rattanapunya S, Kuesap J, Chaijaroenkul W, et al. Prevalence of malaria and HIV co-infection and influence of HIV infection on malaria disease severity in population residing in malaria endemic area along the Thai–Myanmar border. Acta Trop 2015; 145:55–60. [DOI] [PubMed] [Google Scholar]
- 43.Wumba RD, Zanga J, Aloni MN, et al. Interactions between malaria and HIV infections in pregnant women: a first report of the magnitude, clinical and laboratory features, and predictive factors in Kinshasa, the Democratic Republic of Congo. Malar J 2015; 14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simooya OO, Mwendapole RM, Siziya S, et al. Relation between falciparum malaria and HIV seropositivity in Ndola, Zambia. BMJ 1988; 297:30–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenberg AE, Nsa W, Ryder RW, et al. Plasmodium falciparum malaria and perinatally acquired human immunodeficiency virus type 1 infection in Kinshasa, Zaire. New Engl J Med 1991; 325:105–109. [DOI] [PubMed] [Google Scholar]
- 46.Kalyesubula I, Musoke-Mudido P, Marum L, et al. Effects of malaria infection in human immunodeficiency virus type-1 infected Ugandan children. Pediatr Infect Dis J 1997; 16:876–881. [DOI] [PubMed] [Google Scholar]
- 47.Chandramohan D, Greenwood BM. Is there an interaction between human immunodeficiency virus and Plasmodium falciparum? Int J Epidemiol 1998; 27:296–301. [DOI] [PubMed] [Google Scholar]
- 48.Bastos FI, Barcellos C, Lowndes CM, et al. Co-infection with malaria and HIV in injecting drug users in Brazil: a new challenge to public health? Addiction 1999; 94:1165–1174. [DOI] [PubMed] [Google Scholar]
- 49.French N, Gilks CF. Fresh from the field: some controversies in tropical medicine and hygiene. HIV and malaria, do they interact? Trans R Soc Trop Med Hyg 2000; 94:223–237. [DOI] [PubMed] [Google Scholar]
- 50.Whitworth J, Morgan D, Quigley M, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet 2000; 356:1051–1056. [DOI] [PubMed] [Google Scholar]
- 51.Rowland-Jones SL, Lohman B. Interactions between malaria and HIV infection—an emerging public health problem? Microbes Infect 2002; 4:1265–1270. [DOI] [PubMed] [Google Scholar]
- 52.Ayisi JG, Branch OH, Rafi-Janajreh A, et al. Does infection with human immunodeficiency virus affect the antibody responses to Plasmodium falciparum antigenic determinants in asymptomatic pregnant women? J Infect 2003; 46:164–172. [DOI] [PubMed] [Google Scholar]
- 53.Kassa D, Petros B, Messele T, et al. Parasito-haematological features of acute Plasmodium falciparum and P. vivax malaria patients with and without HIV co-infection at Wonji Sugar Estate, Ethiopia. Ethiop J Health Dev 2005; 19:132–139. [Google Scholar]
- 54.Kublin JG, Patnaik P, Jere CS, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet 2005; 365:233–240. [DOI] [PubMed] [Google Scholar]
- 55.Patnaik P, Jere CS, Miller WC, et al. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J Infect Dis 2005; 192:984–991. [DOI] [PubMed] [Google Scholar]
- 56.Bronzan RN, Taylor TE, Mwenechanya J, et al. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV co-infection, and outcome. J Infect Dis 2007; 195:895–904. [DOI] [PubMed] [Google Scholar]
- 57.Jaworowski A, Kamwendo DD, Ellery P, et al. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis 2007; 196:38–42. [DOI] [PubMed] [Google Scholar]
- 58.Mehta S, Manji KP, Young AM, et al. Nutritional indicators of adverse pregnancy outcomes and mother-to-child transmission of HIV among HIV-infected women. Am J Clin Nutr 2008; 87:1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newman PM, Wanzira H, Tumwine G, et al. Placental malaria among HIV-infected and uninfected women receiving anti-folates in a high transmission area of Uganda. Malar J 2009; 8:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imani PD, Musoke P, Byarugaba J, et al. Human immunodeficiency virus infection and cerebral malaria in children in Uganda: a case-control study. BMC Pediatr 2011; 11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wariso KT, Nwauche CA. The prevalence of malaria antigen in the serum of HIV seropositive patients in Port Harcourt. Nigerian Health Journal 2011; 11: [Google Scholar]
- 62.Laar AK, Grant FE, Addo Y, et al. Predictors of fetal anemia and cord blood malaria parasitemia among newborns of HIV-positive mothers. BMC Res Notes 2013; 6:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turner AN, Tabbah S, Mwapasa V, et al. Severity of maternal HIV-1 disease is associated with adverse birth outcomes in Malawian women: a cohort study. J Acquir Immune Defic Syndr 2013; 64:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orlov M, Vaida F, Finney OC, et al. P. falciparum enhances HIV replication in an experimental malaria challenge system. PLoS One 2012; 7:e39000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ned RM, Moore JM, Chaisavaneeyakorn S, et al. Modulation of immune responses during HIV-malaria co-infection in pregnancy. Trends Parasitol 2005; 21:284–292. [DOI] [PubMed] [Google Scholar]
- 66.Ismaili J, van der Sande M, Holland MJ, et al. Plasmodium falciparum infection of the placenta affects new-born immune responses. Clin Exp Immunol 2003; 133:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar SB, Handelman SK, Voronkin I, et al. Different regions of HIV-1 subtype C env are associated with placental localization and in utero mother-to-child transmission. J Virol 2011; 85:7142–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moses AV, Williams S, Heneveld ML, et al. 1996. Human immunodeficiency virus infection of bone marrow endothelium reduces induction of stromal hematopoietic growth factors. Blood 1996; 87:919–925. [PubMed] [Google Scholar]
- 69.Douglas NM, Anstey NM, Buffet PA, et al. The anaemia of Plasmodium vivax malaria. Malar J 2012; 11:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lamikanra AA, Brown D, Potocnik A, et al. Malarial anemia: of mice and men. Blood 2007; 110:18–28. [DOI] [PubMed] [Google Scholar]
- 71.Lamikanra AA, Merryweather-Clarke AT, Tipping AJ, et al. Distinct mechanisms of inadequate erythropoiesis induced by tumor necrosis factor alpha or malarial pigment. PLoS One 2015; 10:e0119836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Idemyor V. Human immunodeficiency virus (HIV) and malaria interaction in sub-Saharan Africa: the collision of two Titans. HIV Clin Trials 2007; 8:246–253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


